Abstract
Streptococcus thermophilus is one of the most widely used lactic acid bacteria in the dairy industry, in particular in yoghurt manufacture, where it is associated with Lactobacillus delbrueckii subsp. bulgaricus. This bacterial association, known as a proto-cooperation, is poorly documented at the molecular and regulatory levels. We thus investigate the kinetics of the transcriptomic and proteomic modifications of S. thermophilus LMG 18311 in response to the presence of L. delbrueckii subsp. bulgaricus ATCC 11842 during growth in milk at two growth stages. Seventy-seven different genes or proteins (4.1% of total coding sequences), implicated mainly in the metabolism of nitrogen (24%), nucleotide base (21%), and iron (20%), varied specifically in coculture. One of the most unpredicted results was a significant decrease of most of the transcripts and enzymes involved in purine biosynthesis. Interestingly, the expression of nearly all genes potentially encoding iron transporters of S. thermophilus decreased, whereas that of iron-chelating dpr as well as that of the fur (perR) regulator genes increased, suggesting a reduction in the intracellular iron concentration, probably in response to H2O2 production by L. bulgaricus. The present study reveals undocumented nutritional exchanges and regulatory relationships between the two yoghurt bacteria, which provide new molecular clues for the understanding of their associative behavior.
Streptococcus thermophilus is one of the most widely used lactic acid bacteria (LAB) in the dairy fermentation industry. This bacterium is traditionally used in combination with Lactobacillus delbrueckii subsp. bulgaricus for the manufacture of yoghurt. When both bacteria gain a mutual benefit, this association is known as proto-cooperation (57), and it often results in higher acidification rates (2, 7, 8, 25, 46, 50), a lower final pH (46, 53), a more abundant S. thermophilus population (10, 46, 53), stimulation of aromatic compound production (10, 15, 24, 30), improved stability of the final product (24), and an increase of exopolysaccharide production (11) compared to monocultures. This cooperation effect improves the yield of fermentation and is therefore of industrial interest. The characterization of this phenomenon so far evidenced that it depends on the strains of each species which is associated and is, at least partly, based on nutritional exchanges. In milk, the amounts of several free amino acids (AA) are growth limiting for LAB such as S. thermophilus and L. delbrueckii subsp. bulgaricus, which have to degrade caseins into peptides and AA in order to fulfill their AA requirements. L. delbrueckii subsp. bulgaricus has a cell wall proteinase, PrtB, enabling the degradation of caseins, whereas only a few strains of S. thermophilus exhibit PrtS, an extracellular protease ortholog of PrtB (55). The peptides and AA released from caseins by L. delbrueckii subsp. bulgaricus (1, 7, 25, 50, 53) stimulate the growth of S. thermophilus, and PrtS does not play a significant role in the presence of PrtB+ L. delbrueckii subsp. bulgaricus (16). In turn, S. thermophilus stimulates the growth of L. delbrueckii subsp. bulgaricus by the production of formic acid under anaerobic conditions (10, 64) and of carbon dioxide (22). Carbon dioxide results from the decarboxylation of urea catalyzed by urease, present and widely distributed only in S. thermophilus among LAB, which are generally recognized as safe (36, 47). This association has thus mainly been studied in terms of nutritional exchanges, but it remains poorly documented at the global level.
It is only recently and in a very limited number of studies that postgenomic approaches were used for the analysis of simple ecosystems such as bacterial cocultures. Initial work on Pseudomonas aeruginosa revealed that, in the presence of Staphylococcus aureus, the transcription of iron-regulated genes decreased in coculture, indicating that the presence of S. aureus increased iron availability for P. aeruginosa in this environment (45). A transcriptomic analysis of the hyperthermophilic bacterium Thermotoga maritima demonstrated that 15.5% of its genome was differentially expressed in coculture with Methanococcus jannaschii compared to monoculture (35). More recently, a DNA microarray analysis identified Streptococcus gordonii genes regulated in response to coaggregation with Actinomyces naeslundii (34). The only study of a dual bacterial culture by combining transcriptomics and proteomics reported up to now described the interactions of two dental plaque bacteria, Streptococcus mutans and Veillonella parvula (37). This study pointed out that growth in a biofilm together with a nonpathogenic bacterium such as V. parvula changes the physiology of S. mutans and gives this bacterium an advantage in surviving antimicrobial treatment.
The recent completion of the genome sequences of S. thermophilus CNRZ 1066, LMG 18311 (9), and LMD-9 (44) and L. delbrueckii subsp. bulgaricus ATCC 11842 (62) allows us to reexamine the association between these bacteria at a molecular level. We recently investigated the physiology of S. thermophilus LMG 18311 during the late stage of milk fermentation (32), using proteomic and transcriptomic approaches. We revealed the upregulation of nitrogen metabolism (transport and biosynthetic pathways, notably for sulfur AA) and of the metabolism of various sugars. In the present work, we explored the physiology of the same strain of S. thermophilus (LMG 18311) cultivated in the presence of its yoghurt partner, L. delbrueckii subsp. bulgaricus (ATCC 11842), during growth in milk, by identifying the proteome and transcriptome modifications that were specific for the coculture. This study revealed an ambiguous relationship providing evidence that S. thermophilus benefits but also protects itself from compounds produced by L. delbrueckii subsp. bulgaricus. In addition, undocumented nutritional exchanges and hints of regulators altered during the coculture growth were evidenced, providing new molecular clues for the understanding of this dairy ecosystem.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
S. thermophilus LMG 18311 was obtained from the BCCM collection (Belgium), and L. delbrueckii subsp. bulgaricus ATCC 11842 from ATCC (USA). Stock cultures were prepared in reconstituted 10% (wt/vol) Nilac skim milk (NIZO, Ede, The Netherlands) as described previously (32). Cocultures of S. thermophilus with L. delbrueckii subsp. bulgaricus (ratio of 1:1 CFU/ml) were grown at 42°C in Marguerite milk (La Laiterie, Villefranche sur Saône, France) and sterilized by microfiltration. Before use, the milk was skimmed by centrifugation (4°C, 5,000 × g, 30 min). A total of 500 ml of milk was then inoculated with 106 CFU/ml of stock cultures of each strain and incubated at 42°C. For coculture assays in the presence of catalase, bovine catalase (Sigma) was added to cultures after 2 h 30 min of growth at a final concentration of 1,000 U/ml.
The pH was measured until it reached a value of 4.9; the acidification rate was calculated as ΔpH/Δt between pH 6.2 and 5.2, i.e., before milk coagulation. Every 20 min, cell chains of S. thermophilus were disrupted by a 40-s treatment with a mechanical blender (Turax X620, Labo-Moderne, France), and culture dilutions were plated on M17 agar lactose (1%) (for S. thermophilus counts) or MRS agar lactose (2%) acidified to pH 5.2 (for L. delbrueckii subsp. bulgaricus) with an automatic spiral platter (AES Laboratoires, Combourg, France). Colonies were counted after 16 h of incubation (S. thermophilus) or 36 h (L. delbrueckii subsp. bulgaricus) in anaerobic conditions (Anaerocult A; Merck, Darmstadt, Germany) at 42°C. All cultures were prepared in three independent experiments.
Transcriptomic analysis.
Total RNA was extracted from Marguerite milk cocultures with the Trizol method as described previously (32). RNA was extracted at 2 h 30 min (as a control) and at 5 h 30 min (with or without catalase).
Genome-wide expression profiles were established using a commercial DNA microarray (EGT-K40C, Eurogentec) containing 92% of S. thermophilus LMG 18311 genes (spotted in duplicate), according to the method described by Hervé-Jiménez et al. (32). The cross-hybridization of L. delbrueckii subsp. bulgaricus ATCC 11842-labeled cDNA with the S. thermophilus LMG 18311 microarray was expected to be minimal, since L. delbrueckii subsp. bulgaricus RNA comprised a small fraction of the coculture total RNA (less than 10%, checked by real-time quantitative reverse transcription-PCR [RT-qPCR]). RNA from L. delbrueckii subsp. bulgaricus was labeled using the protocols described above, and by hybridizing the labeled samples to the S. thermophilus microarrray, we verified that L. delbrueckii subsp. bulgaricus RNA did not cross-hybridize.
A total of 10 μg of total RNA was reverse transcribed by random priming, using the Pronto! plus direct system (Corning-Promega, United States) and labeled by incorporation of Cy3- or Cy5-dCTP nucleotides (Amersham Biosciences, United Kingdom). A total of 100 pmol of each labeled cDNA was used for overnight hybridization at 42°C. The arrays were scanned on a microarray scanner (Agilent, United States). The statistical analysis was based on dye swap. For each array, the raw data comprised the logarithm of the median feature pixel intensity at wavelengths of 635 and 532 nm. No background was subtracted. Arrays were normalized with the Anapuce package (http://cran.r-project.org/web/packages/anapuce/), using general loess and a block effect correction. In order to determine differentially expressed genes, we used the Varmixt method, which is based on a variance mixture analysis (19). Finally, the raw P values were adjusted by the Bonferroni method, which controls the family-wise error rate. We considered genes with both a P value of ≤0.05 and a ratio higher than 2 to be differentially expressed.
RT-qPCR was carried out using cDNA synthesized from 3 μg of RNA samples by PowerScript reverse transcriptase (ClonTech, Saint-Quentin-en-Yvelines, France) according to the supplier's protocol. All gene-specific primers were designed using Primer3 software (54) and are reported in Table S1 in the supplemental material. For each condition, the measures were done in triplicate, with cDNAs synthesized from two independent RNA samples. Data were recorded as threshold cycles (CT), expressed as means ± standard deviations, and computed using the comparative critical threshold (2−ΔΔCT) method (43). The results were normalized using stu1254 for S. thermophilus and gmk1 for L. delbrueckii subsp. bulgaricus as references, as they were expressed at a constant level under our conditions (microarray data, present work).
Proteomic analysis.
Cytoplasmic proteins were extracted from 300 ml of milk cocultures at two different growth stages (early [2 h 30 min] and late exponential [5 h 30 min]) and separated by two-dimensional gel electrophoresis (2-DE) as described previously (32). Briefly, 300 μg of proteins was precipitated with the 2-DE clean-up kit in 10% trichloroacetic acid (GE Healthcare, Saclay, France) and loaded on 24-cm (pH 4 to 7) IPG strips (Bio-Rad, Hercules, CA), which were rehydrated at 50 V for 12 h, and isoelectric focusing was carried out for 60,000 V/h at a maximum of 10,000 V using an Ettan IPGphor (GE Healthcare); 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used for the second migration. Gels were stained with BioSafe colloidal Coomassie blue (Bio-Rad); they were digitized using an Epson Expression 1640XL scanner set (at 256 gray levels) controlled by the Silver Fast software and analyzed using the Phoretix 2-DE software package (GE Healthcare).
The 2-DE images obtained at the two growth stages and from three independent experiments were compared and an analysis of variance was performed using the statistical software R as described previously (32). Only differences with P values of <0.05 and at least twofold volume variations between the two conditions were further analyzed. When a value was missing for one of the triplicate experiments, it was set to the mean value of the two other values, as proposed in reference 14. Proteins that were significantly altered in abundance between the two growth stages were identified by mass spectrometry analyses using a Voyager-DE-STR mass spectrometer (Applied Biosystems, Framingham, MA) on our proteomic platform (http://www.jouy.inra.fr/unites/proteines/papss/), according to Guillot et al. (29), except that the monoisotopic mass lists were searched against local S. thermophilus LMG 18311 (9) and L. delbrueckii subsp. bulgaricus ATCC 11842 (62) databases using a local version of the MS-FIT program (http://prospector.ucsf.edu).
The codon adaptation index was calculated for all open reading frames of the S. thermophilus LMG 18311 genome with the synonymous codon usage analysis program with Codonmixture 1.0 (P. Nicolas, personal communication).
Determination of the Eh and partial pressure of dissolved O2.
Monocultures of S. thermophilus and L. delbrueckii subsp. bulgaricus were cultivated in the Biostat Q fermentor (B. Braun Biotech International, Melsunge, Germany) in 1-liter vessels with a working volume of 500 ml skim Marguerite milk. The temperature was set at 42°C, and the cultures were stirred at 22 rpm. The Em (redox electrode, Einstabmesskelte, EasyFerm plus K8RX; Hamilton, Switzerland), partial pressure of dissolved O2 (Oxyferm O2 sensor FDA 160; Hamilton, Switzerland), pH (pH sensor EasyFerm Plus K8; Hamilton, Switzerland), and temperature were continuously monitored as previously reported (39) with the software MFCS Shell/win 2.0 (B. Braun Biotech, International Sartorius Group). Corrections of the pH and temperatures of Em measures were performed with the hydrogen electrode as a reference. The final value of Eh (mV) was expressed as Eh = Em + Eref, where Eref = +198 mV at 37°C.
RESULTS
The coculture in milk of S. thermophilus LMG 18311 and L. delbrueckii subsp. bulgaricus ATCC 11842 favors S. thermophilus growth.
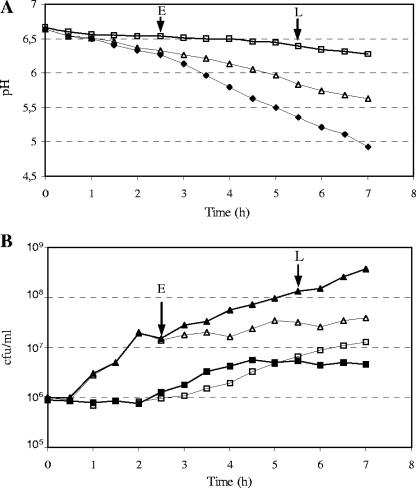
We first characterized the growth of both LAB in mono- and cocultures in milk by monitoring the pH and species-specific bacterial counts. Compared to results with the monocultures, the acidification of milk was enhanced when the two LAB were cultivated together (Fig. 1A). The coculture presented significantly higher ΔpH values (0.38 ± 0.02) (between 0 and 4 h) than S. thermophilus (0.31 ± 0.02) and L. delbrueckii subsp. bulgaricus (0.11 ± 0.02) monocultures. Similarly, the acidification rate (ΔpH/Δt) of the coculture (0.31 h−1 ± 0.02) was 1.8- and 1.3-fold higher than that of S. thermophilus (0.17 h−1 ± 0.02) and of L. delbrueckii subsp. bulgaricus (0.23 h−1 ± 0.02) monocultures, respectively.
FIG. 1.
Milk growth of S. thermophilus LMG 18311 in mono- and cocultures with L. delbrueckii subsp. bulgaricus ATCC 11842. (A) pH evolution of monocultures of S. thermophilus LMG 18311 (Δ) and L. delbrueckii subsp. bulgaricus ATCC 11842 (□) and of coculture (⧫). (B) Evolution of bacterial counts of S. thermophilus (Δ) and L. delbrueckii subsp. bulgaricus (□) in monoculture and of S. thermophilus (▴) and L. delbrueckii subsp. bulgaricus (▪) in coculture. These graphs are representative of the curves obtained in three independent experiments. At early (E) (2 h 30 min) and late (L) (5 h 30 min) exponential phases (arrows), bacteria were harvested for proteomic and transcriptomic analysis.
Consistently, species-specific bacterial counts differed between the mono- and the cocultures (Fig. 1B). During the first 2 h to 2 h 30 min, the growth curves of the mono- or cocultures superimposed. After 2 h 30 min, the cocultures resulted in higher bacterial counts than each of the monocultures. The bacterial counts revealed that the stimulatory effect was transitory for L. delbrueckii subsp. bulgaricus, which stopped growing after 4 h 30 min, but lasted until the end of fermentation for S. thermophilus. For L. delbrueckii subsp. bulgaricus, the coculture resulted in a threefold decrease of the final bacterial counts (6.0 × 106 CFU/ml, compared to 2.0 × 107 CFU/ml in monoculture). For S. thermophilus, the association with L. delbrueckii subsp. bulgaricus resulted in a 10-fold-higher final population (3.7 × 108 CFU/ml) than that of the monoculture (3.9 × 107 CFU/ml). At the end of fermentation (5 h 30 min), LMG 18311 significantly dominated L. delbrueckii subsp. bulgaricus ATCC 11842 in the coculture, with 60-fold more cells.
Transcriptome and proteome analysis of LMG 18311 cultivated in milk with ATCC 11842.
The effects of L. delbrueckii subsp. bulgaricus on the physiology of LMG 18311 were further analyzed using transcriptomics and proteomics on samples harvested at two stages of growth, where S. thermophilus development was stimulated by the presence of L. delbrueckii subsp. bulgaricus: early (2 h 30 min) and late (5 h 30 min) exponential phase. The evolution of the gene expression and protein abundance of S. thermophilus at these two stages was thus established and compared to that of S. thermophilus monoculture (32). In mono- as well as in coculture, we observed the upregulation of (i) peptides, AA transporters, and specific AA biosynthetic pathways, notably for sulfur AA, and (ii) genes and proteins involved in the metabolism of various sugars, although the effect on sugar metabolism was less pronounced in coculture. The variations that were obtained strictly in coculture were thus considered specifically related to the presence of L. delbrueckii subsp. bulgaricus and are reported in the present work (Tables 1 and 2).
TABLE 1.
Changes in S. thermophilus mRNA levels in coculture with L. delbrueckii subsp. bulgaricus between early and late exponential phases
| Gene name or locus tag (stu no.)d | Functional category | Description | Fold change L vs Ea
|
Putative operon structureb | |
|---|---|---|---|---|---|
| Microarray | RT-qPCR | ||||
| 0158 | AA and peptide transporter | Polar AA ABC transporter, ATP binding protein | 3.9 | stu0159-0158 | |
| 0159 | Polar AA ABC transporter, permease | 2.4c | stu0159-0158 | ||
| 0605 | Polar AA ABC transporter, permease | 4.6 | stu0605-0606 | ||
| 1579 | Polar AA ABC transporter, substrate binding protein | 2.7c | stu1582-1581-1580-1579 | ||
| 1580 | Polar AA ABC transporter, ATP binding protein | 4.4 | stu1582-1581-1580-1579 | ||
| 1652 | Polar AA ABC transporter, ATP binding protein | 3.5 | stu1653-1652 | ||
| 1653 | Polar AA ABC transporter, permease | 3.9 | stu1653-1652 | ||
| argR | AA metabolism | Arginine repressor | 3.7 | argR-stu0049-mutS1 | |
| argC | N-Acetyl-gamma-glutamyl-phosphate reductase | 2.7c | argCJBD | ||
| argJ | Bifunctional ornithine acetyltransferase/N-acetylglutamate synthase protein | 6.3 | argCJBD | ||
| argB | Acetylglutamate kinase | 4.5 | argCJBD | ||
| argD | Acetylornithine aminotranferase | 4.9 | argCJBD | ||
| ilvD | Dihydroxy-acid dehydratase | 3.0c | 2.0 ± 0.2 | ilvD | |
| argH | Arginosuccinate lyase | 3.2c | 57.5 ± 10 | argH | |
| ilvC | Ketol-acid reductoisomerase | 5.6 | 2.1 ± 0.4 | ilvC | |
| ilvB | Acetolactate synthase, large subunit | NV | NV | ilvBN | |
| leuB | 3-Isopropylmalate dehydrogenase | NV | NV | leuAB | |
| thrB | Homoserine kinase | 2.8c | thrB-rarD | ||
| aldB | Alpha-acetolactate decarboxylase | NV | NV | als-aldB | |
| 1413 | Bifunctional glutamate-cysteine ligase | 6.6 | stu1413 | ||
| 0336 | Purine and pyrimidine | Putative MFS transporter, xanthine/uracil permease | 2.4c | 1.8 ± 0.40 | stu0336 |
| prsA1 | metabolism | Ribose-phosphate pyrophosphokinase | 0.24 | 0.14 ± 0.05 | prsA1 |
| purC | Phosphoribosylaminoimidazole-succinocarboxamide synthase | 0.12 | 0.39 ± 0.10 | purCLFMNH | |
| purL | Phosphoribosylformylglycinamidine synthase II | 0.14 | 0.03 ± 0.01 | purCLFMNH | |
| purF | Amidophosphoribosyltransferase | 0.19 | purCLFMNH | ||
| purM | Phosphoribosylformylglycinamide cyclo-ligase | 0.36c | purCLFMNH | ||
| purN | Phosphoribosylformylglycinamide formyl transferase | 0.3c | purCLFMNH | ||
| purH | Phosphoribosylaminoimidazole carboxamide formyl transferase | 0.26c | purCLFMNH | ||
| purD | Phosphoribosylamine-glycine lyase | 0.3c | purDEK | ||
| purE | Phosphoribosylaminoimidazole carboxylase I | 0.36c | purDEK | ||
| purB | Adenylosuccinate lyase | 0.59 | 0.22 ± 0.08 | purB | |
| purA | Adenylosuccinate synthetase | NV | 0.40 ± 0.14 | purA | |
| purR | Purine operon repressor | NV | 1.90 ± 0.40 | purR | |
| pyrC | Dihydroorotase | 5.8 | ung-pyrC | ||
| feoA | Metal ion metabolism | Ferrous iron uptake transporter, protein A | NV | 0.33 ± 0.15 | feoABC |
| feoB | Ferrous iron uptake transporter, protein B | NV | feoABC | ||
| feoC | Hypothetical protein | NP | 0.43 ± 0.2 | feoAB | |
| mntH | Mn2+ transporter | 3.6 | 1.7 ± 0.2 | mntH | |
| dpr | Peroxide resistance protein, non-heme-containing ferritin | 18.3 | 13.7 ± 4.0 | dpr-fur | |
| fur | Ferric transport regulator protein | 8.1 | dpr-fur | ||
| tatA | Sec-independent protein translocase | NV | 0.14 ± 0.02 | stu1024-1023-1022-tatC-tatA | |
| tatC | Sec-independent protein translocase | 0.4c | stu1024-1023-1022-tatC-tatA | ||
| 1022 | Predicted membrane protein of the lead (Pb2+) uptake porter (PbrT) family | 0.3 | stu1024-1023-1022-tatC-tatA | ||
| 1023 | Hypothetical protein, putative iron-dependent peroxidase | 0.4c | stu1024-1023-1022-tatC-tatA | ||
| 1024 | Hypothetical protein (predicted lipoprotein) | 0.4c | stu1024-1023-1022-tatC-tatA | ||
| fatB | Iron complex ABC transporter, substrate binding protein | 0.4c | fatDCAB | ||
| fatA | Iron complex ABC transporter, ATP binding protein | 0.2 | fatDCAB | ||
| fatC | Iron complex ABC transporter, permease | 0.4c | fatDCAB | ||
| hrcA | Stress | Heat-inducible transcription repressor | NV | 0.16 ± 0.07 | hrcA-grpE-dnaK |
| grpE | Heat shock protein, chaperonine | 0.3 | hrcA-grpE-dnaK | ||
| dnaK | Molecular chaperone | NV | hrcA-grpE-dnaK | ||
| htpX | Heat shock protein | 4.5 | htpX | ||
| htrA | Exported serine protease | 4 | htrA | ||
| 0808 | C metabolism | Simple sugar transport system, substrate-binding protein | 0.3 | 0.11 ± 0.01 | stu0806-0807-0808-0809- 0810-0811 |
| manN | Mannose PTS system, component IID | 4.4 | manLMN-stu0330 | ||
| manM | Mannose PTS system, component IIC | NV | manLMN-stu0330 | ||
| manL | Mannose PTS system, component IIAB | 3.6 | manLMN-stu0330 | ||
| mutS1 | DNA and RNA metabolism | DNA mismatch repair protein | 2.4c | argR-stu0049-mutS1 | |
| polC | DNA polymerase III | 5.0 | polC | ||
| ung | Uracyl DNA glycosylase | 8.7 | ung-pyrC | ||
| rhe | ATP-dependent RNA helicase | 6.0 | rheA | ||
| ssbB | Single-strand binding protein | 3.5 | rpsF-ssbB-rpsR | ||
| mutY | A/G-specific adenine glycosylase | 13.2 | mutY | ||
| radA | DNA repair protein | 0.3 | radA | ||
| rplU | Ribosome and translation | 50S ribosomal protein L21 | 4.2 | rplU-rpmA | |
| rpmA | 50S ribosomal protein L27 | 5.8 | rplU-rpmA | ||
| gatB | Glutamyl-tRNA Gln amidotransferase, subunit B | 10.5 | gatCAB | ||
| rpsF | 30S ribosomal protein S6 | 3.4 | rpsF-ssbB-rpsR | ||
| 0049 | Other | Hypothetical protein | 5.6 | argR-stu0049-mutS1 | |
| 0103 | Hypothetical protein | 3.6 | stu0103 | ||
| 0110 | Hypothetical protein poly-gamma glutamate synthesis (capsule biosynthesis) | 0.2 | stu0110-0112-0113 | ||
| 0161 | Hypothetical protein | 5.0 | stu0161 | ||
| rr01 | Response regulator | NV | 0.26 ± 0.18 | rr01-hk01 | |
| rarD | Chloramphenicol sensitivity protein | 3.5 | thrB-rarD | ||
| fhs | Formate-tetrahydrofolate ligase | 0.44c | fhs | ||
| 0800 | Methyl transferase | 0.3 | stu0800-0801 | ||
| 0912 | Hypothetical protein | 4.1 | stu0912 | ||
| oxlT | Oxalate/formate antiporter | 0.2 | oxlT | ||
| ksgA | Dimethyladenosine transferase | 4.9 | tatD-stu1800-ksgA | ||
| 1896 | Predicted membrane protein | 4.5 | stu1896 | ||
E, early exponential phase (2 h 30 min); L, late exponential phase (5 h 30 min); L versus E, ratio between the relative mRNA levels at 5 h 30 min and 2 h 30 min; NV, no variation; NP, not spotted on the microarray.
Determined with ProFinder software (http://www.softberry.com/all.htm).
Significantly differentiated genes without Bonferroni correction belonging to the putative operons identified.
Underlined genes are genes with similar variations, as determined by microarray and RT-qPCR analyses.
TABLE 2.
Changes in S. thermophilus protein abundance in coculture with L. delbrueckii subsp. bulgaricus between early and late exponential phases
| Gene name or locus tag (stu no.)d | Functional category | Description | Spot | Fold change L vs Eb
|
||
|---|---|---|---|---|---|---|
| 2-DE | Array | RT-qPCR | ||||
| ilvC | AA metabolism | Ketol-acid reductoisomerase | 232 | 2.4 | 5.6 | 2.1 ± 0.4 |
| 239a | 1.7 | |||||
| ilvB | Acetolactate synthase, large subunit | 92 | 4.8 | NV | 1.6 ± 0.4 | |
| leuB | 3-Isopropylmalate dehydrogenase | 227 | 2.0 | NV | 1.4 ± 0.2 | |
| purC | Purine and pyrimidine metabolism | Phosphoribosylaminoimidazole- succinocarboxamide | 330 | 0.5 | 0.12 | 0.39 ± 0.10 |
| purL | Phosphoribosylformylglycinamidine synthase II | 12 | NV | 0.14 | 0.03 ± 0.01 | |
| 13 | NV | |||||
| 16 | 0.48 | |||||
| 17a | 0.46 | |||||
| purH | Phosphoribosylaminoimidazole carboxamide formyl transferase | 103 | 0.24 | 0.26 | ||
| 105a | 0.18 | |||||
| purD | Phosphoribosylamine-glycine lyase | 205 | 0.5 | 0.3 | ||
| 204a | 0.45 | |||||
| purB | Adenylosuccinate lyase | 178 | 0.26 | 0.59 | 0.22 ± 0.08 | |
| purA | Adenylosuccinate synthetase | 179a | 0.21 | 0.40 ± 0.14 | ||
| 201 | NV | |||||
| purR | Purine operon repressorc | 303 | 14 | NV | 1.90 ± 0.40 | |
| guaA | GMP synthase | 106 | 0.37 | NV | ||
| 109a | 0.5 | |||||
| 131 | 0.56 | |||||
| pyrC | Dihydroorotase | 191a | 1.7 | 5.8 | ||
| 206 | NV | |||||
| gor | Stress | Glutathione reductase | 172 | 0.35 | NV | |
| gpsA | C metabolism | Glycerol-3-phosphate dehydrogenase [NAD(P)+] | 242 | 0.42 | NV | |
| galU | UDP-glucose pyrophosphorylase | 264 | 0.24 | NV | ||
| exoA | DNA and RNA metabolism | 3′ exo DNase III | 293 | 3.3 | NV | |
| ileS | t-RNA synthetase | Isoleucyl-tRNA synthetase | 22 | 5.3 | NV | |
| valS | Valyl-tRNA synthetase | 28 | 6.9 | NV | ||
| pepT | Peptidase, protease | Peptidase Tc | 156 | 0.33 | NV | |
| rr01 | Transcriptional regulator | Response regulator | 341 | 2.0 | NV | 0.26 ± 0.18 |
| folP | Other | Dyhydropteroate synthase | 327 | 2.7 | NV | |
| fhs | Formate-tetrahydrofolate ligase | 115 | 0.38 | 0.44 | ||
| 118a | 0.48 | |||||
| 0113 | Hypothetical protein | 249a | 0.13 | 0.43 | ||
| 274 | NV | |||||
Most abundant form of the protein.
E, early exponential phase (2 h 30 min); L, late exponential phase (5 h 30 min); L versus E, ratio between the relative mRNA levels at 5 h 30 min and 2 h 30 min; NV, no variation.
Spot of low intensity and missing in one of the three repetitions at the early exponential phase (2 h 30 min).
Boldface type indicates genes with similar variations by 2-DE and transcriptomic analyses. P < 0.05 for 2-DE and microarray assays.
First, to identify S. thermophilus genes differentially expressed during the coculture in milk, the expression of 1,759 coding sequences (92% of the genome) was assessed with LMG 18311 microarrays before and during the stimulatory effect of L. delbrueckii subsp. bulgaricus, i.e., at 2 h 30 min and 5 h 30 min of coculture, respectively. According to our statistical analysis, the levels of expression of 67 genes of S. thermophilus LMG 18311 (3.8% of the spotted genes) varied in the presence of L. delbrueckii subsp. bulgaricus at 5 h 30 min of growth compared to 2 h 30 min. A total of 43 and 24 genes corresponding to 48 putative transcriptional units were upregulated and downregulated, respectively. Of these, approximately 47% showed more than a fourfold alteration in transcript levels. In order to independently confirm microarray results, the transcript levels of 21 genes, representatives of the main pathways modulated during S. thermophilus growth with L. delbrueckii subsp. bulgaricus, were measured by RT-qPCR. For 13 of them, RT-qPCR results confirmed microarray data (underlined in Table 1), while for the others, RT-qPCR data showed significant variations not detected by microarrays; feoC was not spotted on the microarray, and expression of mntH did not significantly vary in RT-qPCR. These discrepancies probably reflect differences in the relative sensitivities and specificities of the two methods.
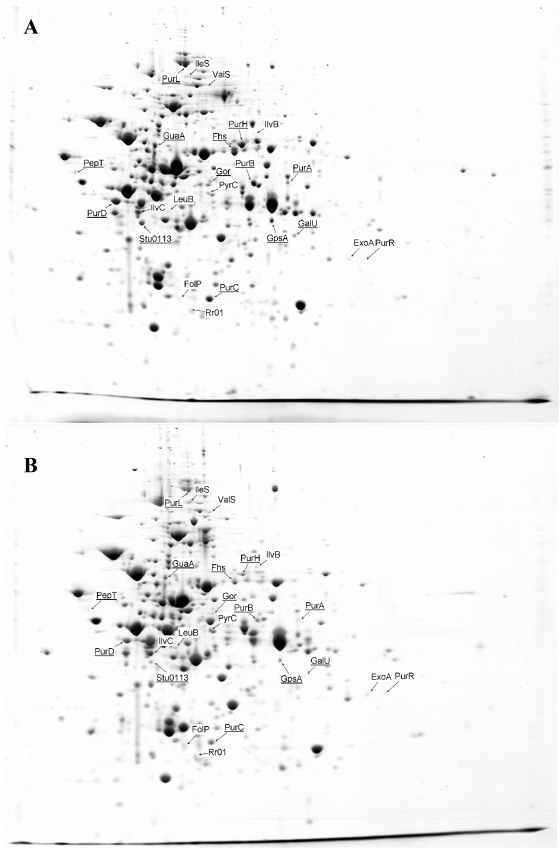
In parallel, a proteomic analysis based on 2-DE was performed on the same samples as those used for transcriptomics. The proteomes of three independent cultures at 2 h 30 min and 5 h 30 min of growth in milk were compared. The statistical analysis revealed that 21 proteins were significantly altered between the two growth stages (P value of <0.05 and at least twofold volume variations): 8 and 13 became more and less abundant, respectively (Table 2 and Fig. 2). Of these proteins, approximately 35% (8/21 [in bold in Table 2]) varied in the same way at the RNA level, indicating a correlation between transcriptomic and proteomics data.
FIG. 2.
2-DE (pH gradient 4 to 7) of cytosolic proteins of S. thermophilus LMG 18311 cocultivated in milk with L. delbrueckii subsp. bulgaricus at early (2 h 30 min) (A) and late (5 h 30 min) (B) exponential phases. A total of 300 μg of proteins were loaded in the first dimension. Proteins whose abundance significantly varied between the two conditions are shown by the arrows as well as the name of their corresponding genes (proteins whose abundance decreases or increases at the early versus late exponential phase are underlined or not underlined, respectively).
Taken together, the proteomic and transcriptomic analyses revealed 77 different genes or proteins (4.1% of total coding sequences) that significantly varied during the growth of S. thermophilus in coculture with L. delbrueckii subsp. bulgaricus. These variations triggered mostly nitrogen metabolism (24% of the altered genes/proteins), nucleotide base metabolism (21%), and iron metabolism (20%) (see below). The other modifications affected nucleic acid metabolism (9%), translation (6%), stress responses (6%), and carbon metabolism (mannose metabolism) (3%).
Main modifications in LMG 18311 during growth with ATCC 11842. Nitrogen metabolism.
In the coculture between 2 h 30 min and 5 h 30 min, the AA transport systems and biosynthesis pathways were upregulated. Although the specificities of the four polar AA transporters which were overexpressed were not experimentally established, one of them, encoded by stu0158-0159 and sharing homologies with a Streptococcus sanguinis Arg/His transporter, could be involved in the transport of arginine.
Regarding the AA biosynthesis, the branched-chain AA (BCAA) and Arg pathways were massively induced, while the overexpression of the thrB gene indicated that the Thr biosynthesis may also be modified. Concerning the BCAA, four enzymes (IlvBN, IlvC, IlvD1, and BcaT) are needed to produce Val and Ilv from pyruvate, while to produce Leu, the LeuA, LeuD, and LeuB enzymes are required. The transcription of ilvC and ilvD1 and the abundance of IlvC, IlvB, and LeuB increased between 2 h 30 min and 5 h 30 min, suggesting a higher production of BCAA in the cells. Interestingly, we observed a concomitant increase of IleS and ValS proteins (tRNA synthetases), necessary to load Ilv and Val AA on their respective tRNAs during translation.
Several evidences indicated that the requirement for de novo Arg biosynthesis increased during LMG 18311 growth in coculture. From glutamate, four enzymes, ArgJ, ArgB, ArgC, and ArgD, are involved in the biosynthesis of ornithine, which is then transformed into Arg by three enzymes, ArgF, ArgG, and ArgH. Note that the last enzyme coproduces fumarate and Arg from arginosuccinate. During the growth of LMG 18311, the transcription of argCJBD and argH increased, and RT-qPCR confirmed a 57-fold overexpression of argH. Consistently, fumarate was produced in the coculture medium; we measured 45 μM of fumarate at time zero and 131 μM after 5 h of growth. We also observed a 3.7-fold increased expression of argR, the putative Arg regulator, suggesting an activator role for ArgR.
Nucleic base metabolism.
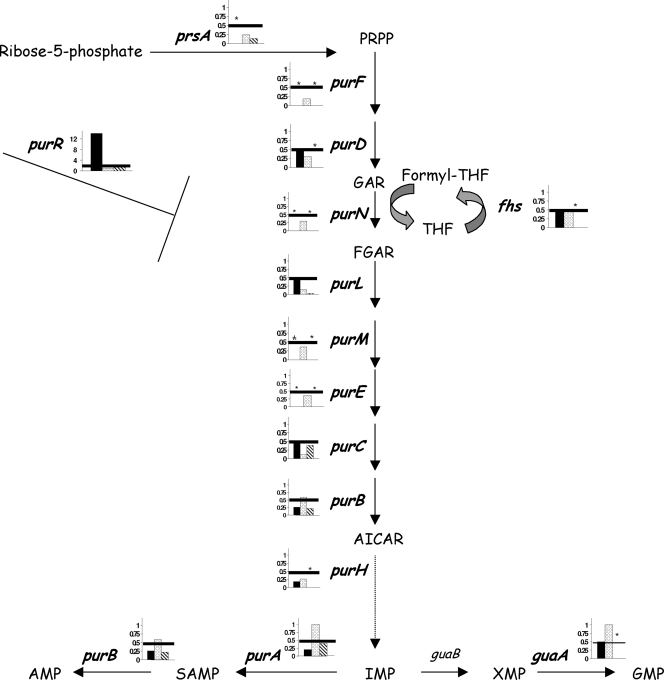
In all organisms, nucleotides are essential; they are substrates for RNA and DNA synthesis and are the main energy donors for cellular processes. In milk, purine nucleotides are growth limiting for S. thermophilus and the purine biosynthesis pathway is essential for its optimal growth (27). Thus, one of the most unexpected results of our analysis was the downregulation of the vast majority of the genes and corresponding enzymes involved in purine biosynthesis. In silico analysis of LMG 18311 indicated that the purine nucleotides are synthesized from PRPP (5-phosphoribosyl-α-1-pyrophosphate) to IMP via nine different enzymes; the pathway then split in two, leading to AMP or GMP, thanks to two enzymes for each of them (Fig. 3). Between 2 h 30 min and 5 h 30 min of growth, 10 genes and seven proteins involved in this pathway were downregulated. Importantly, we also observed a decrease in the transcription of prsA1 and fhs and in the level of the Fhs protein, which are required for the purine biosynthesis for the supply of PRPP and formyl groups, respectively. All together, these results show that during the growth of LMG 18311 with L. delbrueckii subsp. bulgaricus in milk, the biosynthesis of purines was switched off at the transcription level. Consistently, a 14-fold increase of the putative purine repressor PurR was observed. It is noteworthy that the expression of stu0336, a homolog of the nucleobase transporter pbuX of Lactococcus lactis, increased 2.4-fold.
FIG. 3.
Comparative analysis of protein abundance (black) and gene expression (dots for microarray analysis and hatching for RT-qPCR analyses) for the purine biosynthesis pathway between 2 h 30 min and 5 h 30 min of growth of S. thermophilus. *, not detected on 2-DE gels or on microarrays, or not measured by RT-qPCR; #, quantification not possible because of the presence of two proteins in the same spot; bar, a variation ≥ 2. purA, adenylosuccinate synthetase; purB, adenylosuccinate lyase; purC, phosphoribosylaminoimidazole-succinocarboxamide synthetase; purD, phosphoribosylamine-glycine ligase; purE, phosphoribosylaminoimidazole carboxylase I; purF, amidophosphoribosyltransferase; purH, phosphoribosylaminoimidazolecarboxamide formyltransferase; purM, phosphoribosylformylglycinamide cyclo-ligase; purN, phosphoribosylglycinamide (GAR) formyltransferase; purl, phosphoribosylformylglycinamidine synthase II; prsA, ribose-phosphate pyrophosphokinase; purR, purine operon repressor; guaA, GMP synthase; guaB, IMP dehydrogenase; fhs, formate-tetrahydrofolate ligase; PRPP, phosphoribosyl pyrophosphate; GAR, glycinamide ribonucleotide; FGAR, formylglycinamide ribonucleotide; AICAR, aminoimidazole; SAMP, adenylosuccinate.
Iron metabolism.
The transcription of the following five of the seven genes of LMG 18311 potentially involved in iron transport was downregulated between 2 h 30 min and 5 h 30 min of growth: an ABC transporter of iron complexes (fatA, B, and C), a ferrous iron transporter (feoA), and a putative high-affinity iron permease (stu1022). Interestingly, the dpr gene, which codes for an intracellular ferritin (i.e., an iron chelator) and is involved in H2O2 tolerance in streptococci (52), was the highest induced gene between 2 h 30 min and 5 h 30 min, with an induction factor of ∼18. In LMG 18311, dpr forms a putative operon with a perR-like gene homolog (annotated fur), the putative regulator of iron transport, which was induced ∼eightfold. All together, our data establish that at the late growth stage, LMG 18311 induced several systems to limit its intracellular iron concentration. It was previously reported that L. delbrueckii subsp. bulgaricus ATCC 11842 can produce H2O2 during its growth in M17 (61). We therefore wondered whether the observed modification of the LMG 18311 iron metabolism could be a coordinated response aimed at limiting the production of damageable reactive oxygen species by the Fenton reaction, which happens with H2O2 in the presence of an iron catalyst.
Stress.
It is obvious that among the genes or proteins altered during the LMG 18311 growth in coculture, several are involved in stress adaptation. Intriguingly, several were upregulated, while others were downregulated. It is therefore difficult to conclude whether LMG 18311 is at least partly stressed or not at 5 h 30 min of growth in the presence of L. delbrueckii subsp. bulgaricus. Beyond the upregulation of dpr, the upregulation of htrA (serine protease) and htpX (heat shock protein) could be related to stress, as is the upregulation of the mutS, mutY, and ung genes, coding for DNA glycosylases involved in DNA repair relative to oxidative stress damages (18, 66). However, we observed a downregulation of Gor, the glutathione reductase, and radA, which belong to the oxidative stress response. Moreover, the transcription of hrcA and grpE, coding for stress responsive proteins, decreased by threefold between 2 h 30 min and 5 h 30 min. In many Firmicutes, HrcA is the repressor of the hrcA-grpE-dnaK and groEL-groES operons, which encode chaperonins involved in different stress responses (heat shock, acid stress, salt stress, and oxidative stress) (5, 63). The expression of rr01 also decreased ∼3.8-fold, whereas the corresponding protein increased 2.2-fold. This two-component response regulator (RR01) is homologous to the CovR protein described for other streptococci (28, 41) and thus may be involved in the regulation of general stress responses.
Additional data which strengthen the hypothesis of H2O2 production by L. delbrueckii subsp. bulgaricus.
We hypothesized that L. delbrueckii subsp. bulgaricus produces H2O2 during growth in milk. Since the production of H2O2 by ATCC 11842 was probably too low (<100 μM) to be directly measured in milk by classical means (TiCl4 or peroxydase methods, data not shown), we monitored the oxido-reduction potential (Eh) of monocultures in milk. In contrast to the S. thermophilus monoculture, the L. delbrueckii subsp. bulgaricus monoculture presented an increase of Eh from 2 to 5 h, indicating the production of a molecule more oxidant than O2, which is likely to be H2O2 (data not shown). The expression of the L. delbrueckii subsp. bulgaricus genes potentially involved in H2O2 production (from in silico analysis, pox1, pyrD1, and pyrD2) was determined by qPCR in cocultures at 2 h 30 min and 5 h 30 min of growth. While the pyruvate oxidase (pyruvate + phosphate + O2 ⇒ acetyl phosphate + CO2 + H2O2) pox1 expression did not vary during the growth of L. delbrueckii subsp. bulgaricus in coculture, those of the two dihydroorotate dehydrogenase (dihydroorotate + O2 ⇒ orotate + H2O2)-encoding genes pyrD1 and pyrD2 increased by factors of 6.4 (± 0.3) and 7.7 (± 0.43), respectively. Furthermore, we observed an increase (by a factor of 13.2 ± 0.6) in the expression of the gene pyrF, coding for an orotidine-5′-phosphate decarboxylase involved in the following steps of orotate metabolism. These results demonstrated that the H2O2 production pathway of L. delbrueckii subsp. bulgaricus is activated in coculture.
To test our hypothesis which links the downregulation of iron transporters and the induction of dpr observed in coculture to the presence of H2O2 in the medium, the coculture was supplemented with catalase, an enzyme which converts H2O2 into O2 and H2O. The expression of the dpr and feoA genes was measured by RT-qPCR at 2 h 30 min and 5 h 30 min in the presence or absence of catalase. As expected, in the control without catalase, dpr and feoA were up- and downregulated, respectively, between the two growth stages. However, when catalase was added to the coculture at 2 h 30 min, the transcription of dpr and feoA stayed constant during growth (ratio between 2 h 30 min and 5 h 30 min equaled 0.92 [±0.2] for dpr and 0.8 [±0.5] for feoA). This observation established that the modulation of expression of these genes correlated with the presence of H2O2, thereby confirming our hypothesis.
DISCUSSION
The proto-cooperation between S. thermophilus and L. delbrueckii subsp. bulgaricus has been described so far in terms of nutritional exchanges, with L. delbrueckii subsp. bulgaricus supplying peptides and AA to S. thermophilus and, in turn, with S. thermophilus producing formic acid and carbon dioxide, which stimulate the L. delbrueckii subsp. bulgaricus growth. In this work, this phenomenon was analyzed for the first time through global monitoring of transcription and protein abundance at two stages of the growth of the LMG 18311 and ATCC 11842 coculture. It revealed an alteration of 4.1% of the S. thermophilus transcriptome and proteome, triggering modifications of specific cellular metabolisms. For each experiment, an LMG 18311 monoculture was grown in parallel to the coculture, and the samples were harvested at the same times and analyzed via the same methods (32). Comparison of the modifications observed in the coculture and in the monoculture revealed common transcriptomic and proteomic modifications. The main similarity was that sulfur AA metabolism was stimulated in both types of cultures, as was galactose metabolism. Several changes were specific to the coculture and therefore related to the association of S. thermophilus with L. delbrueckii subsp. bulgaricus, among them, the purine biosynthesis pathway, iron metabolism, and two AA biosynthetic pathways (Arg and BCAA).
Upregulation of BCAA and Arg biosynthesis in the presence of L. delbrueckii subsp. bulgaricus.
The upregulation of BCAA and Arg synthesis pathways in coculture is consistent with previous studies establishing that for optimal growth in milk, S. thermophilus requires BCAA and arginine (12, 26). These observations also suggest that the AA requirements differed between mono- and coculture. Since we showed that the coculture of S. thermophilus LMG 18311 and L. delbrueckii subsp. bulgaricus ATCC 11842 improved the growth of LMG 18311 (Fig. 1), we propose that the increased requirement for Arg and BCAA observed for LMG 18311 in the coculture reflects a higher level of protein synthesis because of probable growth-limiting free intracellular AA. Indeed, BCAA and Arg are the most abundant AA in the predicted proteins of LMG 18311, as they account for 24.4% and 7.38% of the residues, respectively (http://www.cbs.dtu.dk/services/GenomeAtlas/). On the other hand, we cannot rule out that S. thermophilus and L. delbrueckii subsp. bulgaricus compete in coculture, in particular for these AA, which would lead to the induction of Arg and BCAA pathways in S. thermophilus. In silico analysis of the two sequenced strains of L. delbrueckii subsp. bulgaricus (including ATCC 11842) indeed indicated that the Arg and BCAA pathways are not present, and the auxotrophy for these AA has been demonstrated for four other strains of L. delbrueckii subsp. bulgaricus (42). This upregulation of AA biosynthesis and thus of protein biosynthesis possibly attested for the better growth of S. thermophilus when associated with L. delbrueckii subsp. bulgaricus, as well as the overexpression of several genes involved in nucleic acid metabolism (polC and ssbB) and in translation (ribosomal proteins, tRNA synthetases, and gatB, a subunit of the Glu-tRNAGln amidotransferase).
Purine downregulation in the presence of L. delbrueckii subsp. bulgaricus.
All the enzymes needed for the purine biosynthesis are present in the genome of S. thermophilus (9). However, it is well established that for optimal growth of S. thermophilus in milk, supplementation with purines is required (21, 27). In the coculture, LMG 18311 grew better than in the monoculture, but paradoxically, the purine biosynthesis pathway was downregulated. We propose that L. delbrueckii subsp. bulgaricus ATCC 11842 provided purines or their precursors to LMG 18311. Several evidences support this hypothesis: (i) ATCC 11842 possesses a complete purine biosynthesis pathway and grows in purine-free medium (62); (ii) the LMG 18311 genome encodes putative transporters for purines or purine precursors, such as the gene encoding a putative xanthine/uracil permease (stu0336), which was overexpressed in the coculture, but also the four genes encoding phosphoribosyl transferases (Hpt, Apt, Xpt, and HprT) involved in the phosphorylation of the nucleobases (9); (iii) the addition of purines to a culture of LMG 18311 in milk caused a downregulation of PurM, PurH, and Fhs (21), demonstrating that exogenous purines are internalized and downregulate the corresponding biosynthesis pathway (4); and (iv) we observed an overexpression of PurR and a downregulation of prsA1 during the growth of LMG 18311 with L. delbrueckii subsp. bulgaricus.
The increase of PurR is consistent with repressor activity, as in Bacillus subtilis (23) and Streptococcus pneumoniae (49). In B. subtilis, the addition of purines in the medium results in an inhibition of PrsA1, the PRPP synthase (6), leading to a decrease of the intracellular PRPP pool which is perceived by the PurR repressor and leading to the repression of purine biosynthesis (23). The PurR protein of LMG 18311 possesses PRPP and DNA binding domains highly similar to its B. subtilis and L. lactis homologs (40). We propose that a similar regulatory scheme could be involved in purine metabolism control in S. thermophilus and that this regulatory system is operative during the growth of LMG 18311 in coculture. The former hypothesis is strengthened by the presence of the PurR-box motif (AWWWCCGAACWWWT), which is involved in the PurR-dependent activation in L. lactis and in the promoter regions of the S. thermophilus purC, purD, and fhs clusters.
Reduction of the intracellular iron in response to H2O2 production?
We propose that in coculture, LMG 18311 encountered H2O2 produced by L. delbrueckii subsp. bulgaricus ATCC 11842 (as previously reported [61]) possibly via its dihydroorotase dehydrogenase proteins (PyrD1 and PyrD2). Note that the expression of LMG 18311 Nox2 and SodA, the two potential enzymes leading to H2O2 production from O2 in LMG 18311, did not vary at the mRNA and protein level during growth. As S. thermophilus (as other streptococci) lacks H2O2-degrading enzymes, such as catalase and NADH peroxidase, it probably develops other means of protection against the membrane diffusible H2O2, which lead, through the Fenton reaction (H2O2 + Fe2+ ⇒ Fe3+ + −OH + ·OH), to the production of reactive oxygen species, highly damageable, notably for DNA. Our data show that S. thermophilus avoids these damageable reactions by inducing the gene encoding Dpr, which sequesters iron and is involved in bacterial H2O2 tolerance (3, 33, 65), and downregulating almost all of the genes potentially involved in iron import in LMG 18311 (feoA, fatABC, and stu1022). In addition, we established that the modulation in expression of at least feoA and dpr correlated with the presence of H2O2, as it disappeared after the addition of catalase in the coculture medium. Uncommonly among bacteria, S. thermophilus dpr constitutes an operon with fur (perR), the putative ferric transport regulator which was also upregulated during the coculture, as in E. coli (67) and in B. subtilis (31), in response to H2O2. Here, we observed that S. thermophilus Fur shared high homologies with PerR from B. subtilis, which is the major regulator of the inducible peroxide stress response, in particular of the dpr gene (31). In fact, S. thermophilus Fur is 55 and 39% identical to PerR from S. pyogenes and B. subtilis, respectively, with, in particular, a highly conserved 12-AA region (at positions 57 to 68) which could be specific for PerR proteins, as it is not conserved in other Fur proteins (13). The LMG 18311 Fur/PerR protein also potentially contained a structural Zn(Cys)4 site, which is a distinctive feature of the PerR-like metalloregulators in bacteria (60), and we propose that the S. thermophilus LMG 18311 Fur-annotated protein is a PerR protein. Interestingly, we compared the upstream regions of the fatD and dpr (which is in an operon with fur [perR]) genes with those of genes regulated by S. aureus, Streptococcus pyogenes, and B. subtilis perR and identified a consensus region (TTANAAWNATTNTWA) (http://weblogo.berkeley.edu/; WebLogo, a sequence logo generator) which could constitute a common PerR box. By using the i-Momi program (51), we showed that 11 S. thermophilus LMG 18311 genes possess this consensus sequence; among them, 3 are involved in iron metabolism: dpr, fatD, and stu0164, which is clustered with genes involved in oxidative stress response via the (Fe-S) cluster formation in S. thermophilus (58).
Higher intracellular Mn concentrations have been involved in oxidative stress resistance (4), probably thanks to the H2O2-quenching activity of Mn(II) (56). In enteric bacteria, peroxide stress induces the transcription of a Mn(II) transporter, MntH (38), which was also the case for S. thermophilus mntH in coculture. In addition, the RR01 protein, a CovR homolog, could be part of the probable S. thermophilus H2O2 response, as recently demonstrated with S. mutans (20).
Finally, we did not observe any upregulation of several genes usually involved in oxidative stress response and in particular in H2O2 response (gor, trxAB, recA, uvrABC, clpC, and radA) (48) but, to the contrary, did observe a downregulation of some of them (radA and gor). We propose that in coculture with L. delbrueckii subsp. bulgaricus, S. thermophilus sets up an adaptative H2O2 response rather than a response to an oxidative shock. Indeed, the H2O2 production by L. delbrueckii subsp. bulgaricus is not only most probably very low compared to the concentrations that are usually used for the study of H2O2 stress responses in bacteria but also very progressive, occurring during the course of L. delbrueckii subsp. bulgaricus growth.
Overall, from the present results, one can hypothesize that S. thermophilus possesses, as previously suggested (59), an inducible and efficient defense system based on iron homeostasis, which avoids the potential damageable effect of H2O2 and would provide an indirect system for this microaerophilic catalase-negative bacterium to tolerate H2O2.
In conclusion, the present study revealed specific physiological changes in S. thermophilus during growth stimulation due to the presence of L. delbrueckii subsp. bulgaricus. The combination of transcriptomic and proteomic analyses not only revealed undocumented nutritional effects on the BCAA, Arg, and purine metabolisms with their regulators but also evidenced other unexpected effects, such as the adaptation to H2O2, indicating that this bacterial proto-cooperation is more complex and much more ambiguous than previously reported.
Supplementary Material
Acknowledgments
We thank C. Gitton for proteomics advice, C. Henry for MALDI-TOF assistance, S. Tachon for redox measurement assistance, S. Pollet and J. Y. Coppée for open access to scanners, D. Olivier and B. Schaeffer for statistical advice, V. Loux for codon adaptation index calculation, and P. Nicolas for Codonmixture 1.0 software.
We acknowledge M. van de Guchte, M.-Y. Mistou, and R. Gardan for fruitful discussions. L.H.-J. was supported by the MICA department of INRA and Ile-de-France Region. P.H. is Research Associate at FNRS.
Footnotes
Published ahead of print on 29 December 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Accolas, J. P., M. Veaux, and J. Auclair. 1971. Etude des interactions entre diverses bactéries lactiques thermophiles et mésophiles, en relation avec la fabrication des fromages à pâte cuite. Lait 51:249-272. [Google Scholar]
- 2.Amoroso, M. J., M. C. Manca de Nadra, and G. Oliver. 1988. Glucose, galactose, fructose, lactose and sucrose utilization by Lactobacillus bulgaricus and Streptococcus thermophilus isolated from commercial yoghurt. Milchwiss 43:626-631. [Google Scholar]
- 3.Antelmann, H., S. Engelmann, R. Schmid, A. Sorokin, A. Lapidus, and M. Hecker. 1997. Expression of a stress- and starvation-induced dps/pexB-homologous gene is controlled by the alternative sigma factor σB in Bacillus subtilis. J. Bacteriol. 179:7251-7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archibald, F. S., and I. Fridovich. 1981. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J. Bacteriol. 146:928-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arena, S., C. D'Ambrosio, G. Renzone, R. Rullo, et al. 2006. A study of Streptococcus thermophilus proteome by integrated analytical procedures and differential expression investigations. Proteomics 6:181-192. [DOI] [PubMed] [Google Scholar]
- 6.Arnvig, K., B. Hove-Jensen, and R. L. Switzer. 1990. Purification and properties of phosphoribosyl-diphosphate synthetase from Bacillus subtilis. Eur. J. Biochem. 192:195-200. [DOI] [PubMed] [Google Scholar]
- 7.Bautista, E. S., R. S. Dahiya, and M. L. Speck. 1966. Identification of compounds causing symbiotic growth of Streptococcus thermophilus and Lactobacillus bulgaricus in milk. J. Dairy Res. 33:299-307. [Google Scholar]
- 8.Beal, C., and G. Corrieu. 1991. Influence of pH, temperature, and inoculum composition on mixed cultures of Streptococcus thermophilus 404 and Lactobacillus 398. Biotechnol. Bioeng. 38:90-98. [DOI] [PubMed] [Google Scholar]
- 9.Bolotin, A., B. Quinquis, P. Renault, A. Sorokin, S. D. Ehrlich, S. Kulakauskas, A. Lapidus, E. Goltsman, M. Mazur, G. D. Pusch, M. Fonstein, R. Overbeek, N. Kyprides, B. Purnelle, D. Prozzi, K. Ngui, D. Masuy, F. Hancy, S. Burteau, M. Boutry, J. Delcour, A. Goffeau, and P. Hols. 2004. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat. Biotechnol. 22:1554-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottazzi, V., B. Battistotti, and G. Montescani. 1973. Influence des souches seules et associées de L. bulgaricus et Str. thermophilus ainsi que des traitements du lait sur la production d'aldéhyde acétique dans la yaourt. Lait 53:295-308. [Google Scholar]
- 11.Bouzar, F., J. Cerning, and M. Desmazeaud. 1997. Exopolysaccharide production and texture-promoting abilities of mixed-strain starter cultures in yogurt production. J. Dairy Sci. 80:2310-2317. [Google Scholar]
- 12.Bracquart, P., and D. Lorient. 1977. Effet des acides aminés sur la croissance de Streptococcus thermophilus. Milchwiss 32:221-224. [Google Scholar]
- 13.Bsat, N., A. Herbig, L. Casillas-Martinez, P. Setlow, and J. D. Helmann. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189-198. [DOI] [PubMed] [Google Scholar]
- 14.Chich, J. F., O. David, F. Villers, et al. Statistics for proteomics: experimental design and 2-DE differential analysis. J. Chromatogr. B 849:261-272. [DOI] [PubMed]
- 15.Courtin, P., and F. Rul. 2004. Interactions between microorganisms in a simple ecosystem: yogurt bacteria as a study model. Lait 84:125-134. [Google Scholar]
- 16.Courtin, P., V. Monnet, and F. Rul. 2002. Cell-wall proteinases PrtS and PrtB have a different role in Streptococcus thermophilus/Lactobacillus bulgaricus mixed cultures in milk. Microbiology 148:3413-3421. [DOI] [PubMed] [Google Scholar]
- 17.Dalton, T. L., R. I. Hobb, and J. R. Scott. 2006. Analysis of the role of CovR and CovS in the dissemination of Streptococcus pyogenes in invasive skin disease. Microb. Pathog. 40:221-227. [DOI] [PubMed] [Google Scholar]
- 18.Delaney, S., W. L. Neeley, J. C. Delaney, and J. M. Essigmann. 2007. The substrate specificity of MutY for hyperoxidized guanine lesions in vivo. Biochemistry 46:1448-1455. [DOI] [PubMed] [Google Scholar]
- 19.Delmar, P., S. Robin, and J. J. Daudin. 2005. VarMixt: efficient variance modelling for the differential analysis of replicated gene expression data. Bioinformatics 21:502-508. [DOI] [PubMed] [Google Scholar]
- 20.Deng, D. M., M. J. Liu, J. M. ten Cate, and W. Crielaard. 2007. The VicRK system of Streptococcus mutans responds to oxidative stress. J. Dent. Res. 86:606-610. [DOI] [PubMed] [Google Scholar]
- 21.Derzelle, S., A. Bolotin, M.-Y. Mistou, and F. Rul. 2005. Proteome analysis of Streptococcus thermophilus grown in milk reveals pyruvate formate-lyase as the major upregulated protein. Appl. Environ. Microbiol. 71:8597-8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Driessen, F. M., F. Kingma, and J. Stadhouders. 1982. Evidence that Lactobacillus bulgaricus in yogurt is stimulated by carbon dioxide produced by Streptococcus thermophilus. Neth. Milk Dairy J. 36:135-144. [Google Scholar]
- 23.Ebbole, D. J., and H. Zalkin. 1989. Interaction of a putative repressor protein with an extended control region of the Bacillus subtilis pur operon. J. Biol. Chem. 264:3553-3561. [PubMed] [Google Scholar]
- 24.El-Abbassy, M. Z., and M. Sitohy. 1993. Metabolic interaction between Streptococcus thermophilus and Lactobacillus bulgaricus in single and mixed starter yogurts. Nahrung 37:53-58. [Google Scholar]
- 25.El-Soda, M. A., S. A. Abou-Donia, H. K. El-Shafy, R. Mashaly, and A. A. Ismail. 1986. Metabolic activities and symbiosis in zabady isolated cultures. Egypt. J. Dairy Sci. 14:1-10. [Google Scholar]
- 26.Garault, P., C. Letort, V. Juillard, and V. Monnet. 2000. Branched-chain amino acid biosynthesis is essential for optimal growth of Streptococcus thermophilus in milk. Appl. Environ. Microbiol. 66:5128-5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garault, P., C. Letort, V. Juillard, and V. Monnet. 2001. Branched-chain amino acids and purine biosynthesis: two pathways essential for optimal growth of Streptococcus thermophilus in milk. Lait 81:83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham, M. R., L. M. Smoot, C. A. L. Migliaccio, K. Virtaneva, D. E. Sturdevant, S. F. Porcella, M. J. Federle, G. J. Adams, J. R. Scott, and J. M. Musser. 2002. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. USA 99:13855-13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guillot, A., C. Gitton, P. Anglade, and M.-Y. Mistou. 2003. Proteomic analysis of Lactococcus lactis, a lactic acid bacterium. Proteomics 3:337-354. [DOI] [PubMed] [Google Scholar]
- 30.Hamdan, I. Y., J. E. Kunsman, and D. D. Deane. 1971. Acetaldehyde production by combined yogurt cultures. J. Dairy Sci. 54:1080-1082. [Google Scholar]
- 31.Helmann, J. D., M. F. Wu, A. Gaballa, P. A. Kobel, M. M. Morshedi, P. Fawcett, and C. Paddon. 2003. The global transcriptional response of Bacillus subtilis to peroxide stress is coordinated by three transcription factors. J. Bacteriol. 185:243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herve-Jimenez, L., I. Guillouard, E. Guedon, C. Gautier, S. Boudebbouze, P. Hols, V. Monnet, F. Rul, and E. Maguin. 2008. Physiology of Streptococcus thermophilus during the late stage of milk fermentation with special regard to sulfur amino-acid metabolism. Proteomics 8:4273-4286. [DOI] [PubMed] [Google Scholar]
- 33.Higuchi, M., Y. Yamamoto, and Y. Kamio. 2000. Molecular biology of oxygen tolerance in lactic acid bacteria: functions of NADH oxidases and Dpr in oxidative stress. J. Biosci. Bioeng. 90:484-493. [PubMed] [Google Scholar]
- 34.Jakubovics, N. S., S. R. Gill, S. E. Iobst, M. M. Vickerman, and P. E. Kolenbrander. 2008. Regulation of gene expression in a mixed-genus community: stabilized arginine biosynthesis in Streptococcus gordonii by coaggregation with Actinomyces naeslundii. J. Bacteriol. 190:3646-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson, M. R., S. B. Conners, C. I. Montero, C. J. Chou, K. R. Shockley, and R. M. Kelly. 2006. The Thermotoga maritima phenotype is impacted by syntrophic interaction with Methanococcus jannaschii in hyperthermophilic coculture. Appl. Environ. Microbiol. 72:811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juillard, V., M. J. Desmazeaud, and H. E. Spinnler. 1988. Mise en évidence d'une activité uréasique chez Streptococcus thermophilus. Can. J. Microbiol. 34:818-822. [Google Scholar]
- 37.Kara, D. 2007. PhD thesis. Interactions between oral biofilm bacteria. University of Amsterdam, Amsterdam, The Netherlands.
- 38.Kehres, D. G., M. L. Zaharik, B. B. Finlay, and M. E. Maguire. 2000. The NRAMP proteins of Salmonella typhimurium and Escherichia coli are selective manganese transporters involved in the response to reactive oxygen. Mol. Microbiol. 36:1085-1100. [DOI] [PubMed] [Google Scholar]
- 39.Kieronczyk, A., R. Cachon, G. Feron, and M. Yvon. 2006. Addition of oxidizing or reducing agents to the reaction medium influences amino acid conversion to aroma compounds by Lactococcus lactis. J. Appl. Microbiol. 101:1114-1122. [DOI] [PubMed] [Google Scholar]
- 40.Kilstrup, M., and J. Martinussen. 1998. A transcriptional activator, homologous to the Bacillus subtilis PurR repressor, is required for expression of purine biosynthetic genes in Lactococcus lactis. J. Bacteriol. 180:3907-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamy, M. C., M. Zouine, J. Fert, M. Vergassola, E. Couve, E. Pellegrini, P. Glaser, F. Kunst, T. Msadek, P. Trieu-Cuot, and C. Poyart. 2004. CovS/CovR of group B streptococcus: a two-component global regulatory system involved in virulence. Mol. Microbiol. 54:1250-1268. [DOI] [PubMed] [Google Scholar]
- 42.Letort, C. 2001. PhD thesis. Relation entre croissance et nutrition azotée de deux bactéries lactiques thermophiles: Streptococcus thermophilus et Lactobacillus delbrueckii subsp. bulgaricus. University of Poitiers, Poitiers, France.
- 43.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCt) method. Methods Enzymol. 25:402-408. [DOI] [PubMed] [Google Scholar]
- 44.Makarova, K., A. Slesarev, Y. Wolf, et al. 2006. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. USA 103:15611-15616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mashburn, L. M., A. M. Jett, D. R. Akins, and M. Whiteley. 2005. Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J. Bacteriol. 187:554-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moon, N. J., and G. W. Reinbold. 1976. Commensalism and competition in mixed cultures of Lactobacillus bulgaricus and Streptococcus thermophilus. J. Milk Food Technol. 39:337-341. [Google Scholar]
- 47.Mora, D., M. G. Fortina, C. Parini, G. Ricci, M. Gatti, G. Giraffa, and P. L. Manachini. 2002. Genetic diversity and technological properties of Streptococcus thermophilus strains isolated from dairy products. J. Appl. Microbiol. 93:278-287. [DOI] [PubMed] [Google Scholar]
- 48.Mostertz, J., C. Scharf, M. Hecker, and G. Homuth. 2004. Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Microbiology 150:497-512. [DOI] [PubMed] [Google Scholar]
- 49.Ng, W.-L., K. M. Kazmierczak, G. T. Robertson, R. Gilmour, and M. E. Winkler. 2003. Transcriptional regulation and signature patterns revealed by microarray analyses of Streptococcus pneumoniae R6 challenged with sublethal concentrations of translation inhibitors. J. Bacteriol. 185:359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pette, J. W., and H. Lolkema. 1950. Symbiosis and antibiosis in mixed cultures Lb. bulgaricus and S. thermophilus. Neth. Milk Dairy J. 4:197-208. [Google Scholar]
- 51.Pons, N., J.-M. Batto, S.-D. Ehrlich, and P. Renault. 2008. Development of software facilities to characterize regulatory binding motifs and application to streptococcaceae. J. Mol. Microbiol. Biotechnol. 14:67-73. [DOI] [PubMed] [Google Scholar]
- 52.Pulliainen, A. T., S. Haataja, S. Kähkönen, and J. Finne. 2003. Molecular basis of H2O2 resistance mediated by streptococcal Dpr. Demonstration of the functional involvement of the putative ferroxidase center by site-directed mutagenesis in Streptococcus suis. J. Biol. Chem. 278:7996-8005. [DOI] [PubMed] [Google Scholar]
- 53.Rajagopal, S. N., and W. E. Sandine. 1990. Associative growth and proteolysis of Streptococcus thermophilus and Lactobacillus bulgaricus in skim milk. J. Dairy Sci. 73:894-899. [DOI] [PubMed] [Google Scholar]
- 54.Rozen, S., and H. J. Skaletsky. 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 132:365-386. [DOI] [PubMed] [Google Scholar]
- 55.Shahbal, S., D. Hemme, and M. Desmazeaud. 1991. High cell wall-associated proteinase activity of some Streptococcus thermophilus strains (H-strains) correlated with a high acidification rate in milk. Lait 71:351-357. [Google Scholar]
- 56.Stadtman, E. R., B. S. Berlett, and P. B. Chock. 1990. Manganese-dependent disproportionation of hydrogen peroxide in bicarbonate buffer. Proc. Natl. Acad. Sci. USA 87:384-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamime, A. Y., and R. K. Robinson. 1999. Yoghurt. Science and technology. Woodhead Publishing Limited, Cambridge, United Kingdom.
- 58.Thibessard, A., F. Borges, A. Fernandez, B. Gintz, B. Decaris, and N. Leblond-Bourget. 2004. Identification of Streptococcus thermophilus CNRZ368 genes involved in defense against superoxide stress. Appl. Environ. Microbiol. 70:2220-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thibessard, A., A. Fernandez, B. Gintz, N. Leblond-Bourget, and B. Decaris. 2001. Hydrogen peroxide effects on Streptococcus thermophilus CNRZ368 cell viability. Res. Microbiol. 152:593-596. [DOI] [PubMed] [Google Scholar]
- 60.Traoré, D. A., A. El Ghazouani, S. Ilango, J. Dupuy, L. Jacquamet, J.-L. Ferrer, C. Caux-Thang, V. Duarte, and J.-M. Latour. 2006. Crystal structure of the apo-PerR-Zn protein from Bacillus subtilis. Mol. Microbiol. 61:1211-1219. [DOI] [PubMed] [Google Scholar]
- 61.van de Guchte, M., S. D. Ehrlich, and E. Maguin. 2001. Production of growth-inhibiting factors by Lactobacillus delbrueckii. J. Appl. Microbiol. 91:147-153. [DOI] [PubMed] [Google Scholar]
- 62.van de Guchte, M., S. Penaud, C. Grimaldi, V. Barbe, K. Bryson, P. Nicolas, C. Robert, S. Oztas, S. Mangenot, A. Couloux, V. Loux, R. Dervyn, R. Bossy, A. Bolotin, J.-M. Batto, T. Walunas, J.-F. Gibrat, P. Bessières, J. Weissenbach, S. D. Ehrlich, and E. Maguin. 2006. The complete genome sequence of Lactobacillus bulgaricus reveals extensive and ongoing reductive evolution. Proc. Natl. Acad. Sci. USA 103:9274-9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van de Guchte, M., P. Serror, C. Chervaux, T. Smokvina, S. D. Ehrlich, and E. Maguin. 2002. Stress responses in lactic acid bacteria. Antonie van Leeuwenhoek 82:187-216. [PubMed] [Google Scholar]
- 64.Veringa, H. A., T. E. Galesloot, and H. Davelaar. 1968. Symbiosis in yoghourt (II). Isolation and identification of a growth factor for Lactobacillus bulgaricus produced by Streptococcus thermophilus. Neth. Milk Dairy J. 22:114-120. [Google Scholar]
- 65.Yan, Z. Y., Z. J. Xu, G. Z. Xu, B. Tian, and Y. J. Hua. 2007. Construction of a dps mutant and its functional analysis in Deinococcus radiodurans. Wei Sheng Wu Xue Bao 47:610-615. [PubMed] [Google Scholar]
- 66.Zhao, J., and M. E. Winkler. 2000. Reduction of GC → TA transversion mutation by overexpression of MutS in Escherichia coli K-12. J. Bacteriol. 182:5025-5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng, M., X. Wang, L. J. Templeton, D. R. Smulski, R. A. LaRossa, and G. Storz. 2001. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J. Bacteriol. 183:4562-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.