Abstract
The endoplasmic reticulum (ER) is a key organelle involved in sensing and responding to stressful conditions, including those resulting from infection of viruses, such as human cytomegalovirus (HCMV). Three signaling pathways collectively termed the unfolded protein response (UPR) are activated to resolve ER stress, but they will also lead to cell death if the stress cannot be alleviated. HCMV is able to modulate the UPR to promote its infection. The specific viral factors involved in such HCMV-mediated modulation, however, were unknown. We previously showed that HCMV protein pUL38 was required to maintain the viability of infected cells, and it blocked cell death induced by thapsigargin. Here, we report that pUL38 is an HCMV-encoded regulator to modulate the UPR. In infection, pUL38 allowed HCMV to upregulate phosphorylation of PKR-like ER kinase (PERK) and the α subunit of eukaryotic initiation factor 2 (eIF-2α), as well as induce robust accumulation of activating transcriptional factor 4 (ATF4), key components of the PERK pathway. pUL38 also allowed the virus to suppress persistent phosphorylation of c-Jun N-terminal kinase (JNK), which was induced by the inositol-requiring enzyme 1 pathway. In isolation, pUL38 overexpression elevated eIF-2α phosphorylation, induced ATF4 accumulation, limited JNK phosphorylation, and suppressed cell death induced by both thapsigargin and tunicamycin, two drugs that induce ER stress by different mechanisms. Importantly, ATF4 overexpression and JNK inhibition significantly reduced cell death in pUL38-deficient virus infection. Thus, pUL38 targets ATF4 expression and JNK activation, and this activity appears to be critical for protecting cells from ER stress induced by HCMV infection.
Cell death, such as apoptosis, is intimately involved in the biology of many viruses. Apoptosis can be beneficial to some viruses, such as measles virus and influenza virus, to promote their infection and spread under certain conditions (8, 9, 44). However, apoptosis is detrimental to many other viruses, including herpesviruses such as human cytomegalovirus (HCMV), because it functions as a cellular antiviral response to eliminate infected cells, acts to elicit the immune response, or is a deleterious but inevitable consequence due to the stress inflicted by viruses on host cells. To survive, these viruses have developed many strategies to prevent premature cell death of host cells (reviewed in reference 2). HCMV is a member of the betaherpesvirus family and is a widespread opportunistic pathogen that causes severe disease and death in newborns and immunocompromised individuals. HCMV is a slow-growing virus, has a long replication cycle, and requires 48 to 72 h to complete an infection cycle in cultured human fibroblasts and thus would be highly exposed to many cellular defense responses. Not surprisingly, HCMV encodes an array of viral factors that allow it to modulate the host antiviral responses, including cell death, in order to replicate and disseminate.
Viruses have the ability to prevent cell death induced through either the extrinsic or the intrinsic pathways. The extrinsic pathway is commonly activated by extracellular death ligands as an integral part of the host immune response. These death ligands, such as interferon, tumor necrosis factor alpha, and Fas ligand, bind to their cognate receptors on the cell surface and activate the apoptotic program within the target cells. The intrinsic pathway is induced by stress signals within cells, such as DNA damage or endoplasmic reticulum (ER) stress caused by virus infection. These stress signals will activate the apoptotic program through mitochondrion- or endoplasmic reticulum (ER)-mediated pathways, leading to the demise of the cell. HCMV has been reported to encode multiple cell death inhibitors to block the extrinsic and the mitochondrion-mediated intrinsic pathway. Viral protein pUL36 (vICA) directly binds to procaspase 8 and prevents its activation (39), whereas pUL37x1 (vMIA) disrupts the mitochondrial network, binds to Bax, and sequesters this important antiapoptotic molecule at the mitochondria (1, 10, 22, 32). In addition, HCMV proteins IE1 and IE2 protect cells from apoptosis by activating the Akt-mediated signaling pathway (20, 50, 52). Furthermore, HCMV produces a highly abundant 2.7-kb noncoding viral RNA during infection that interacts with mitochondrial respiratory chain complex I, maintains the mitochondrial function under stress conditions, and blocks mitochondrion-mediated apoptosis (35). Finally, we have also reported that HCMV protein pUL38 is able to suppress cell death induced by thapsigargin, a pharmaceutical agent that perturbs Ca2+ homeostasis of the ER (42).
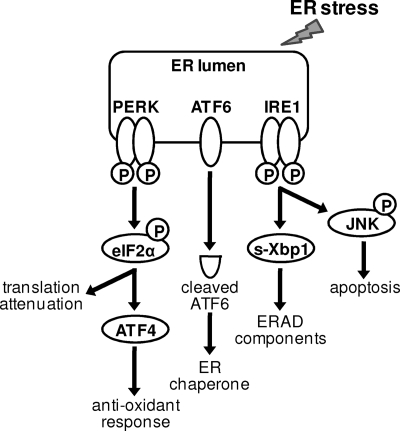
Cells initiate a set of signaling pathways that are collectively termed the unfolded protein response (UPR) when various insults and stresses occur to the ER, such as the accumulation of a large amount of glycoproteins or perturbations in Ca2+ homeostasis in the ER. The UPR is initiated by three ER lumen-located sensor molecules, PKR-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1), when ER stress is detected (21, 36) (Fig. 1). PERK is activated by phosphorylation, and activated PERK phosphorylates the α subunit of eukaryotic translation initiation factor 2 (eIF-2α), resulting in attenuation of global translation. It also selectively induces ATF4 translation, leading to expression of proteins involved in the antioxidant response to facilitate oxidative protein folding in the ER (11, 12, 27). Activated ATF6 induces transcriptional activation of ER-resident chaperone proteins, such as GRP78 (Bip) and GRP94 (29, 38, 47). Activated IRE1 has both endoribonuclease and kinase activities (7, 26). Mediated by tumor necrosis factor receptor-associated factor 2, the kinase activity of IRE1 activates c-Jun N-terminal kinase (JNK) by phosphorylation (43). The endoribonuclease activity of IRE1 mediates splicing of the X-box binding protein 1 (XBP-1) transcript, thus promoting translation of the spliced form of XBP-1 protein (XBP-1s), an active transcriptional factor that induces expression of a subset of the ER resident chaperones and enzymes involved in ER-associated degradation (ERAD) (e.g., ER degradation-enhancing α-mannosidase-like protein [EDEM]) (6, 17, 24, 48). Collectively, the UPR attenuates synthesis of nascent proteins, induces degradation of misfolded proteins, and enhances the ER folding capacity, thus overcoming ER stress and restoring ER homeostasis. Therefore, the short-term induction of the UPR helps cells adapt to stressful conditions and maintains their viability. However, a prolonged UPR may lead to cell death when damage to the ER is too great to overcome or the ER homeostasis cannot be restored. Under these conditions, molecular events induced by the UPR, such as CHOP/GADD153 expression (23) and JNK phosphorylation and activation, lead to cell death. In particular, activated JNK phosphorylates and inhibits antiapoptotic Bcl-2 protein and activates proapoptotic BH3 proteins, thus tipping the balance of the UPR toward cell death (18, 33, 43). Therefore, the prosurvival or prodeath nature of the UPR is dependent on the context of the cellular environment and stress conditions.
FIG. 1.
Diagram of signaling pathways activated in the UPR. Three ER lumen-located sensor molecules, PERK, ATF6, and IRE-1, are activated in response to ER stress. Activated PERK phosphorylates eIF-2α and attenuates global translation but selectively induces ATF4 translation, leading to the expression of proteins involved in the antioxidant response to facilitate oxidative protein folding in the ER. Activated ATF6 induces transcription of ER resident chaperone proteins. Activated IRE1 has both kinase and endoribonuclease activities. Its endoribonuclease activity mediates the removal of an intron from the transcript of XBP-1 to generate the spliced mRNA, s-Xbp1, that encodes an active transcription factor inducing expression of components involved in ER stress-associated protein degradation (i.e., ERAD). In addition, the kinase activity of IRE1 phosphorylates and activates JNK, which could lead to apoptosis.
Like many other viruses, HCMV infection has a profound impact on the ER (3, 13, 15, 40, 41). During infection, HCMV produces an abundant amount of viral glycoproteins, promotes the release of Ca2+ from the ER to the cytosol (37), and induces multiple cellular stress responses. Alwine and coworkers have elegantly shown that HCMV modulates the UPR by targeting all three signaling pathways during infection (13). HCMV induces the phosphorylation of PERK/eIF-2α and translation of ATF4 but prevents the attenuation of global translation. HCMV blocks ATF6 cleavage, but the virus induces expression of a subset of downstream target genes, such as Bip and GRP94, in an ATF6-independent manner. HCMV also modulates the IRE1 pathway by promoting the endoribonuclease activity of IRE1 and inducing splicing of the XBP-1 transcript. However, the virus suppresses the expression of genes normally transactivated by XBP-1s, such as EDEM. It is conceivable that HCMV uses such a modulatory strategy to take advantage of some aspects of the UPR that are beneficial but block others that are potentially detrimental to virus infection (13). It is unclear, however, which specific viral factors are encoded by HCMV to allow it to modulate the UPR and how the virus deals with the apoptotic nature of the UPR, such as IRE1-mediated JNK phosphorylation, in response to prolonged ER stress during infection.
In screening an HCMV mutant library, we have recently identified viral protein pUL38 as a novel factor required for maintaining the viability of host cells during HCMV infection (42). pUL38 appears to play multiple roles in HCMV infection as it interacts with an array of cellular and viral proteins, and it also activates the mammalian target of rapamycin complex 1 (mTORC1), an important cellular sensor regulating cell growth, proliferation, and metabolism (25). We found that the mutant virus lacking the UL38 gene induced drastic premature cell death during infection, which could be inhibited in pUL38-expressing cells (42). While the expression of pUL38 alone failed to protect human fibroblasts from anti-Fas induced cell death, it blocked cell death induced by thapsigargin, a drug that induced the ER stress by disrupting the calcium homeostasis of the ER. This led us to hypothesize that pUL38 plays an important role in blocking the ER stress-mediated cell death pathway during HCMV infection (42).
In the present study, we investigated the engagement of pUL38 activity in the UPR and the mechanism by which pUL38 inhibits ER-stress induced cell death. We present evidence that pUL38 induces robust ATF4 accumulation but inhibits persistent JNK phosphorylation. This activity of pUL38 reprograms the UPR to protect host cells from cell death induced by ER stress inflicted by HCMV infection.
MATERIALS AND METHODS
Plasmids and reagents.
The plasmid pCG-ATF4 containing the human ATF4 cDNA was kindly provided by Tsonwin Hai at Ohio State University (19). pYD-C163, pYD-C245, and pYD-C453 were retroviral overexpression vectors derived from pRetro-EBNA (16). pYD-C163 carried the UL38 open reading frame (ORF) (42) and pYD-C245 contained a DsRed gene whose expression was driven by an internal ribosome entry site. pYD-C453 was created by cloning the human ATF4 ORF upstream of the internal ribosome entry site of pYD-C245. pYD-C255 carried a GalK/kanamycin dual expression cassette that was used for the first step of linear recombination (34) (see below).
The JNK inhibitor SP600125 and ER-stress inducers tunicamycin and thapsigargin were purchased from EMD Chemicals and Sigma, respectively. The primary antibodies used in the present study included anti-cleaved poly(ADP-ribose) polymerase (PARP) (19F4), anti-phosphorylated JNK, and anti-total JNK (Cell Signaling); anti-total eIF-2α, anti-Bip (H129), anti-GRP94 (9G10), and anti-ATF4 (C-20) (Santa Cruz Biotechnology); anti-cleaved caspase 3 (MAB835; R&D Systems); anti-phosphorylated PERK (BioLegend); anti-phosphorylated eIF-2α (Invitrogen); anti-β actin (AC15; Abcam); and anti-pUL38 (42), anti-pUL36 (31), anti-pUL37x1 (37), and anti-IE1 (42) (gifts from Thomas Shenk at Princeton University). 35S protein labeling mix (Perkin-Elmer) was used for the metabolic pulse-labeling experiment as described in Fig. 6B.
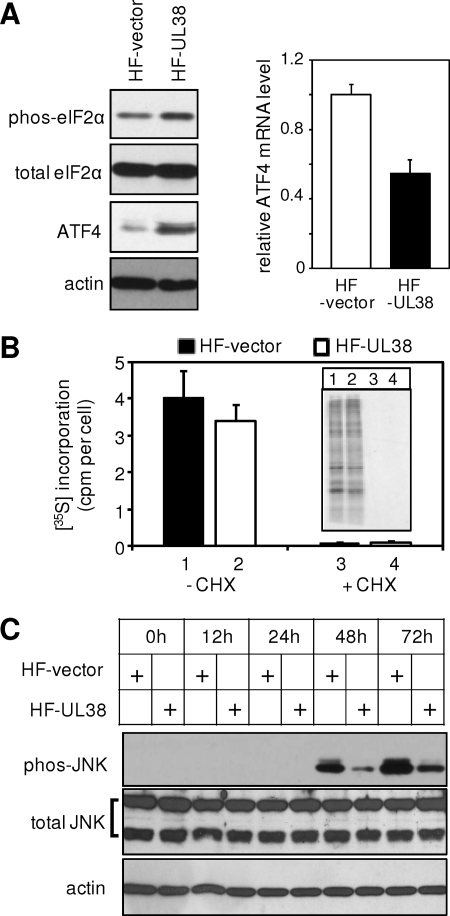
FIG. 6.
pUL38 expression alone induced ATF4 translation and blocked JNK phosphorylation. (A) Immunoblotting analysis of the eIF-2α (both total and phosphorylated) and ATF4 proteins and real-time RT-PCR analysis of the ATF4 transcript in HF-vector or HF-UL38 cells. (B) Global protein translation measured by [35S]methionine and [35S]cysteine incorporation as described previously (13) in the presence or absence of 100 μg of cycloheximide/ml (CHX). [35S]incorporation was quantitated by trichloroacetic acid precipitation using a scintillation counter. Protein lysate from equal numbers of cells was also separated by SDS-PAGE and detected by autoradiography (shown in the inset). (C) HF-vector or HF-UL38 cells were treated with 2 μg of tunicamycin/ml, and the total and the phosphorylated levels of JNK at different times posttreatment were examined by immunoblotting.
Cells and viruses.
Primary human foreskin fibroblasts (HFs) were propagated in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum. To create cells expressing pUL38 (HF-UL38) or the human ATF4 protein (HF-ATF4), HFs were transduced three times with retrovirus reconstituted from pYD-C163 or pYD-C453 as previously described (34). Control cells (HF-vector) for HF-UL38 or HF-ATF4 were made by transduction with retrovirus reconstituted from pRetro-EBNA or pYD-C245.
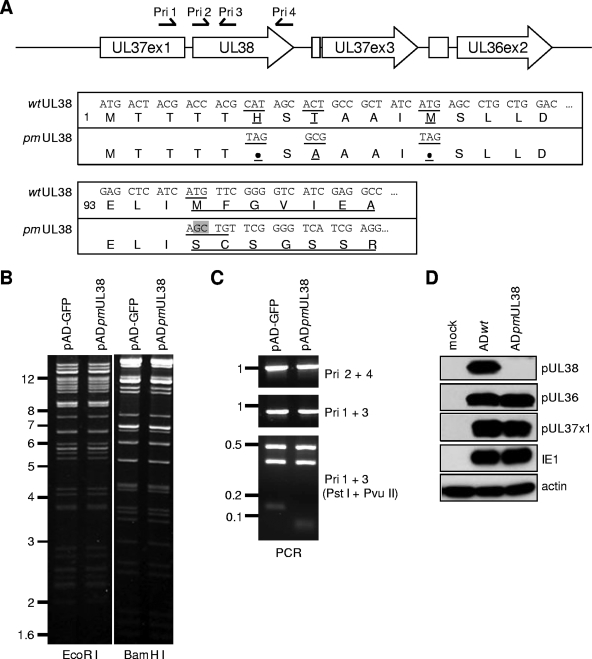
Three BAC-HCMV clones were used in the present study to reconstitute recombinant HCMV viruses. pAD-GFP carried the green fluorescent protein (GFP)-tagged genome of the HCMV AD169 strain and was used to produce wild-type virus ADwt (34). pADdlUL38 lacked the entire UL38 coding sequence as previously described (42). pADpmUL38 carried multiple point mutations engineered to specifically disrupt pUL38 expression and was constructed by two-step linear recombination as previously described (34). Briefly, the PCR fragment containing a GalK/kanamycin dual marker cassette was used to recombine the marker cassette into the UL38 region of pAD-GFP, and the marker cassette was subsequently replaced by the PCR fragment of the UL38 gene that contained the desired mutations. The mutations introduced include nonsense mutations at His6 and Met12, along with a three-base pair substitution (ACT to GCG) at Thr8 to introduce an EagI restriction site, and a 2-bp insertion (GC) at Met96 to introduce frameshift mutation and a PvuII restriction site (Fig. 2).
FIG. 2.
Construction and analysis of the BAC-HCMV clone pADpmUL38 that carried point mutations abrogating pUL38 expression. (A) Viral genomic region encoding UL36-UL38 and the sequence of the UL38 coding sequence where the mutations were introduced. The first line is the schematic structure of the viral genomic region. Viral ORFs are indicated by boxed arrows. Also indicated are the positions of primers used for PCR analysis in panel C. The nucleotide and the corresponding amino acid sequences of the 5′ terminus of the UL38 gene are shown below. The codons 1 and 93 of the UL38 ORF are also indicated. Underlined are the codons and the corresponding amino acids that are targeted by mutagenesis. The 2-bp insertion at codon 96, which results in a frameshift, is indicated by the shadowed box. (B) EcoRI and BamHI restriction digestion analysis. (C) PCR analysis of the BAC-HCMV clones. For PCR analysis, primer pairs used for each reaction and restriction endonucleases used for digestion are indicated. Also shown are molecular markers (in kb). (D) Analysis of viral proteins expressed from the UL36-UL38 locus. HFs were infected with wild-type virus (ADwt) or UL38 point mutant virus (ADpmUL38) at an MOI of 1, and the accumulation of pUL36, pUL37x1, and pUL38 was analyzed by immunoblotting at 24 h postinfection. The antibodies to the viral protein IE1 and cellular protein β actin were used as the infection control and the loading control, respectively.
To reconstitute virus, 2 μg of the BAC-HCMV DNA and 1 μg of the pp71-expression plasmid were transfected into HFs by electroporation as described previously (49). Culture medium was changed 24 h later, and virus stock was prepared by harvesting cell-free culture supernatant when the entire monolayer of cells was lysed. Alternatively, virus stocks were produced by collecting cell-free culture medium from infection at the multiplicity of infection (MOI) of 0.05.
Cell survival assays.
Cell survival was measured by ATP-based viability assay or trypan blue exclusion assay. ATP viability assay was performed according to the manufacturer's instructions (Cell Titer-Glo; Promega) in a 96-well plate format using Microtest Optilux plates (BD Biosciences) and analyzed by using a Wallac Victor II plate reader (Perkin-Elmer). For trypan blue exclusion assay, cells were treated with trypsin and stained with trypan blue (Sigma) for 10 min at room temperature. Stain-negative, live cells were scored with a hemacytometer. Experiments were performed in triplicate well, and the sample from each well was counted twice.
Reverse transcription-quantitative PCR analysis.
Total RNA was extracted by using the TRIzol reagent (Invitrogen) and treated with the Turbo DNA-free reagent (Ambion) to remove genomic DNA contaminants. cDNA was reverse transcribed from 1.0 mg of total RNA with random hexamer primers using the a High Capacity cDNA reverse transcription kit (Applied Biosystems). Cellular cDNA was quantified by real-time quantitative PCR using the SYBR Advantage qPCR Premix (Clontech) and primer pairs specific for the human ATF4 gene (5′-CCC TAG TCC AGG AGA CTA ATA AGC A-3′ and 5′-ACT TTC TGG GAG ATG GCC AAT-3′), the human EDEM gene (5′-CCG TCC AAG TCT TTG AGG CCA CG-3′ and 5′-GTG CAT GTC TCA TTA TTG GTG TCA GGA GG-3′), and the human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene (5′-CTC CAT CCT GGC CTC GCT GT-3′ and 5′-GCT GTC ACC TTC ACC GTT CC-3′). The amounts of ATF4 and EDEM were normalized using GAPDH as the internal control.
To measure splicing of the XBP-1 transcript, total RNA was extracted as described above, and the total XBP-1 transcripts (spliced and unspliced) were quantitatively amplified by reverse transcription-PCR (RT-PCR) with a primer pair spanning the splicing region (5′-CCT TGT AGT TGA GAA CCA GG-3′ and 5′-GGG GCT TGG TAT ATA TGT GG-3′) (14). PCR products amplified from the spliced and the unspliced transcripts were resolved by agarose electrophoresis and quantified by ImageJ software (http://rsbweb.nih.gov/ij/).
Immunoblotting analysis.
Proteins were analyzed by immunoblotting as described previously (42). Briefly, cells were collected, washed, and lysed in the sodium dodecyl sulfate (SDS)-containing sample buffer. Proteins from equal cell numbers were resolved by electrophoresis on an SDS-containing polyacrylamide gel, transferred to a polyvinylidene difluoride membrane, hybridized with primary antibodies, reacted with horseradish peroxidase-conjugated secondary antibodies, and visualized by using SuperSignal West Pico chemiluminescent substrate (Thermo Scientific).
RESULTS
Characterization of the recombinant HCMV virus in which pUL38 expression is ablated by point mutations.
We have previously reported that the HCMV recombinant virus lacking the entire UL38 gene induced the premature death of infected cells, identifying pUL38 as a novel cell death-inhibitory protein encoded by HCMV (42). Intriguingly, the neighboring genes UL37x1 and UL36 also encode cell death inhibitors (10, 39). In order to dissect the mechanistic basis of pUL38 activity without the potential complication involving pUL37x1 and pUL36, we used a bacterial artificial chromosome (BAC)-based approach to create a recombinant HCMV virus in which pUL38 expression was disrupted by point mutations. The parental BAC-HCMV clone, pAD-GFP, was used to produce wild-type virus, ADwt. The recombinant BAC clone, pADpmUL38, carried multiple point mutations in the UL38 coding region. They included two nonsense mutations at codons His6 and Met12, a 3-bp substitution at Thr8 to introduce an EagI restriction site, and a 2-bp insertion at Met96 to introduce frameshift and a PvuII restriction site (Fig. 2A). The frameshift mutation was necessary in addition to nonsense mutations in order to completely abolish pUL38 expression. If mutations were introduced only at codons 6 to 12 but Met96 was preserved, a partially active form of a truncated UL38 protein was produced, likely because any one of four Mets between codons 12 and 96 could be used as an alternative translation site, thus possibly complicating our analysis of the mutant virus (data not shown).
Multiple assays were performed to validate the sequence of pADpmUL38. The overall sequence of the BAC clone appeared intact since the EcoRI and the BamHI restriction endonuclease digestion patterns of pADpmUL38 were identical to those of pAD-GFP (Fig. 2B). The UL38 gene was further examined by PCR analysis with 4 locus-specific primers (i.e., Pri1 was localized in the UL37x1 coding sequence, whereas Pri2 to Pri4 were located within the pUL38 coding sequence) (Fig. 2A and C). The 5′-half and the 3′-half of the UL38 coding region were examined by using the primer pairs Pri1/Pri3 and Pri2/Pri4, respectively. As anticipated, PCR amplification on the wild-type BAC and the UL38 recombinant BAC with the same pair of primers generated a product with the identical size (Fig. 2C). However, the wild-type and the mutant UL38 loci were distinguished by restriction endonuclease digestion of the PCR product generated with Pri1 and Pri3. UL38 Mutations created a PvuII restriction site within the PCR product (Fig. 2A). As a result, PstI/PvuII double endonuclease restriction digestion cleaved this 930-bp wild-type PCR product into 124-, 343-, and 463-bp fragments, whereas it cleaved the UL38 mutant PCR product into 54-, 70-, 343-, and 463-bp fragments (Fig. 2C). Finally, the UL38 locus of pADpmUL38 was also examined by direct sequencing (data not shown). pADpmUL38 contained the precise intended modifications in UL38.
The UL38 point-mutant virus, ADpmUL38, was reconstituted from transfection of pADpmUL38 in UL38-expressing HFs (HF-UL38) (42). Expression of three neighboring cell-death inhibitory proteins, pUL38, pUL37x1, and pUL36, were examined by immunoblotting analysis (Fig. 2D). As anticipated, expression of pUL38 was abrogated in cells infected with ADpmUL38, whereas the expression of pUL37x1 and pUL36 were comparable to those in cells infected with wild-type virus.
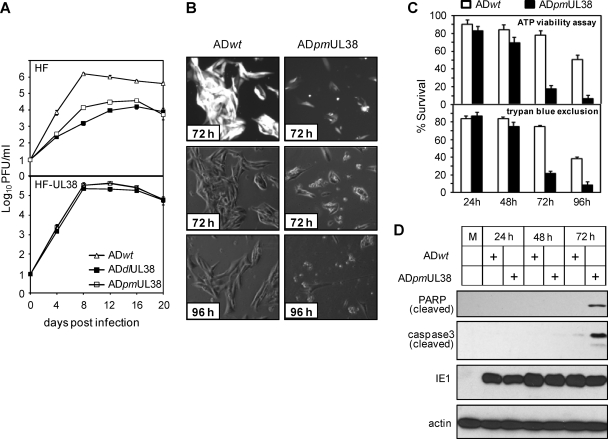
ADpmUL38 was further examined for its growth kinetics and ability to maintain the viability of infected cells. Growth of wild-type virus (ADwt), the UL38 deletion mutant (ADdlUL38) (42), and the point-mutant virus (ADpmUL38) was examined in normal fibroblasts or UL38-expressing fibroblasts at an MOI of 0.2 (Fig. 3A). Like the deletion mutant virus, the point mutant virus grew significantly slower, produced peak yield ∼100-fold lower than that of wild-type virus, and never reached the titer of wild-type virus. Both the deletion mutant virus and the point mutant virus, however, replicated with wild-type kinetics in UL38-expressing fibroblasts, a finding consistent with the previous report (42). Importantly, microscopy analysis demonstrated that, like the deletion mutant virus, the point mutant virus induced tremendous cell death at late times postinfection (42) (Fig. 3B). Cell death during HCMV infection was quantified by trypan blue exclusion assay or an ATP-based viability assay that measured the amount of ATP proportional to the number of viable cells in culture (5, 46). The results from both assays were consistent and confirmed the cell death observed morphologically in ADpmUL38 infection (Fig. 3C). At early times, the levels of cell death were low in HCMV infection, and the virus was able to maintain the viability of infected cells efficiently even in the absence of pUL38 by 48 h postinfection. However, both assays indicated markedly more viable cells in infection with wild-type virus than infection of the mutant virus at 72 and 96 h, substantiating a major role of pUL38 in preventing cell death at late times in HCMV infection. As infection with the UL38 deletion mutant virus, elevated cell death induced by ADpmUL38 infection correlated with the induction of two major biochemical markers of apoptosis, proteolytic cleavage of caspase 3 and PARP (Fig. 3D) (42). Thus, the point mutant virus ADpmUL38 recapitulated the growth defect and the cell death induction phenotype observed with infection of the deletion virus ADdlUL38, providing us with an excellent tool to dissect the function of pUL38 in HCMV infection. Since the results obtained from multiple cell death-viability assays were reproducibly consistent, the quantitative and sensitive ATP-based assay was used to measure cell death-viability for the remainder of the present study except where experimental conditions do not permit.
FIG. 3.
The UL38 mutant HCMV virus ADpmUL38 was severely attenuated in growth and induced caspase 3-dependent death of infected cells. (A) Normal HFs or fibroblasts transduced with the pUL38-expressing retroviral vector (HF-UL38) were infected with indicated recombinant HCMV viruses at an MOI of 0.2. Culture medium was collected at different days postinfection, and yields of cell-free virus were determined by plaque assay. ADwt and ADpmUL38 are described in the legend to Fig. 2, and ADdlUL38 lacks the entire UL38 coding sequence (42). (B and C) HFs were infected with indicated recombinant HCMV viruses at an MOI of 3; at the indicated times postinfection the cell morphology was examined under a phase-contrast microscope (B), and cell survival was quantified by ATP viability assay and trypan blue exclusion assay (C). The top panel of panel B shows HCMV-infected cells indicated by GFP that was expressed from the virus genome. The cell viability in panel C was normalized to that at the time of infection. (D) HFs were infected at an MOI of 1, and cleavage of caspase 3 and PARP at different times postinfection was examined by immunoblotting analysis.
pUL38 expression in isolation suppresses ER stress-induced cell death.
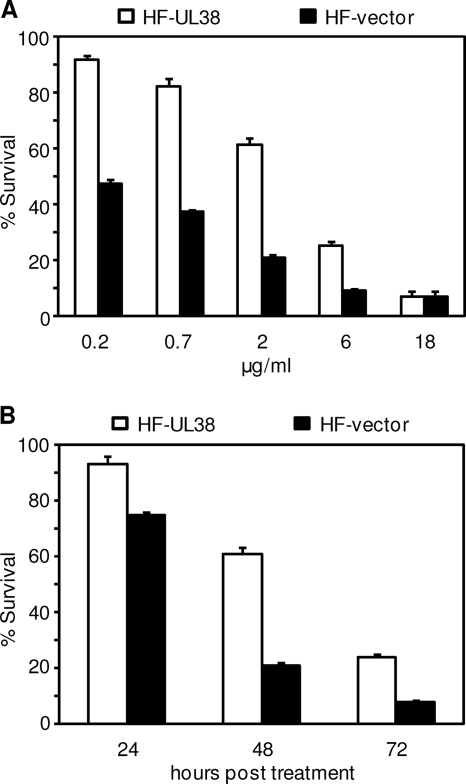
We previously found that expression of pUL38 in isolation failed to prevent anti-Fas antibody-induced extrinsic cell death pathway but protected cells from the treatment of thapsigargin, an agent that causes ER stress by destabilizing intracellular calcium homeostasis (42). We confirmed this observation with the ATP-based viability assay (data not shown), and we hypothesized that one pUL38 function was to block cell death that resulted from ER stress inflicted by viral activities, such as the production of a large amount of viral glycoproteins that need to be processed by the ER or perturbations in the calcium homeostasis in the ER. To further test our hypothesis, we used another ER stress inducer, tunicamycin, a chemical agent that induces ER stress by inhibiting the synthesis of N-linked glycoproteins. We first examined how pUL38 modulated cell death in response to different doses of tunicamycin. pUL38 expression clearly protected fibroblasts from cell death induced by tunicamycin at the concentration up to the 6 μg/ml (Fig. 4A). At 2 μg of tunicamycin/ml, a concentration that is commonly used to induce ER stress, >60% of the pUL38-expressing fibroblasts were viable compared to merely 20% viable cells that expressed vector only at 48 h posttreatment. The protective effect provided by pUL38 was maintained even when tunicamycin was reduced to 0.2 μg/ml, a concentration that was 10-fold less than the one commonly used, thus further minimized any nonspecific effect of the agent. In addition, pUL38 protected cells from death at all times tested in the presence of 2 μg of tunicamycin/ml (Fig. 4B). The greatest difference of cell death in control and pUL38-expressing cells was observed at 48 h, and pUL38 was still able to provide substantial protection even at 72 h, with threefold more cells viable when pUL38 was expressed compared to the control. In addition, consistent with the previous report (42), pUL38 expression also protected HFs from cell death induced by thapsigargin in a similar manner to that seen with tunicamycin (data not shown). Thus, pUL38 protected cells from death induced by two different types of ER stress inducers, thapsigargin and tunicamycin. We conclude that pUL38 is able to suppress ER stress-induced cell death.
FIG. 4.
pUL38 expression alone blocked cell death induced by the stress on the endoplasmic reticulum. (A) HFs transduced with empty retroviral vector (HF-vector) or with pUL38-expressing retroviral vector (HF-UL38) were treated with tunicamycin at the indicated concentrations, and cell survival was measured by ATP viability assay at 48 h posttreatment. (B) HF-vector or HF-UL38 cells were treated with 2 μg of tunicamycin/ml, and cell survival was measured at different times posttreatment as indicated. Cell viability was normalized to that of untreated cells.
pUL38-deficient virus fails to induce robust ATF4 translation or suppress persistent JNK phosphorylation at late times during infection.
ER stress activates the UPR, which is initiated by three sensor molecules (i.e., PERK, ATF6, and IRE1), enhances the ER capacity, and restores the ER homeostasis. The UPR temporarily sustains cell viability, but it ultimately leads to cell death if the ER stress cannot be resolved. HCMV induces the UPR but is able to modulate it in a manner that benefits virus infection (13). The ability of pUL38 expression in isolation to block ER stress-induced cell death led us to hypothesize that pUL38 plays a similar role in virus infection by modulating the virus-induced ER stress response and blocking the resulting cell death. pUL38 may elevate the UPR in a manner that enhances the ability of the ER to deal with stress and/or directly block death signaling activated by the persistent ER stress during HCMV infection.
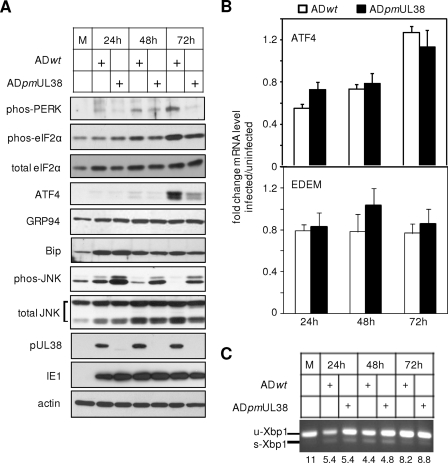
We tested this hypothesis by systematically examining PERK, ATF6, and IRE1 signaling pathways of the UPR in response to ADpmUL38 infection. HFs were infected with ADpmUL38 or ADwt and harvested at 24, 48, and 72 h postinfection, and representative markers of these three pathways were examined by immunoblotting analysis (Fig. 5A). For the PERK pathway, the phosphorylation of PERK and eIF-2α, as well as the expression of ATF4 were examined. In ADwt infection, the increased accumulation of phosphorylated PERK and eIF-2α became visible at 48 h, continued to elevate at 72 h, and the abundant accumulation of the ATF4 protein was evident at 72 h. In addition, the level of total eIF-2α was also elevated as early as 24 h during HCMV infection. Therefore, the overall modulation of the PERK pathway by HCMV infection was consistent with the previous study (13). However, the levels of phosphorylated PERK and eIF-2α appeared to reduce in cells infected with ADpmUL38 compared to those with ADwt at 48 to 72 h. Most strikingly, the accumulation of the ATF4 protein was substantially lower in cells infected with ADpmUL38 than those with ADwt, most evident at 72 h. Since the translation of ATF4 is known to be specifically induced by phosphorylated eIF-2α, we then determined whether the observed differential regulation of ATF4 accumulation was at the level of translation or transcription. Consistent with the previous report, despite a substantial increase of ATF4 at the protein level, there was only a small (∼2-fold) increase at the transcript level during the course of infection (13) (Fig. 5B). More importantly, at any given time no substantial difference in the ATF4 transcriptional level was observed in cells infected with ADwt or with ADpmUL38. This suggests that ATF4 expression was induced at the translational level by elevated eIF-2α phosphorylation in the presence of pUL38 during HCMV infection. Therefore, pUL38 was required for HCMV to efficiently activate the PERK/eIF-2α/ATF4 pathway at late times, which is critical to enhance the ER capacity in response to virus-activated ER stress.
FIG. 5.
pUL38 was required for HCMV to modulate the unfolded protein response during virus infection. HFs were infected with ADwt or ADpmUL38 at an MOI of 1, and at different times postinfection major molecular components of the signaling pathway initiated by PERK (i.e., PERK, eIF-2α, and ATF4), ATF6 (i.e., GRP94 and Bip), or IRE1 (i.e., XBP-1, EDEM, and JNK) were examined by immunoblotting (A), real-time RT-PCR (B), and RT-PCR (C). In panel B, the fold change represented the amount of transcript in HCMV-infected cells relative to that in mock-infected cells from the same time point. In panel C, the unspliced (u-Xbp1) and the spliced (s-Xbp1) transcript of XBP-1 were simultaneously and quantitatively amplified by a pair of primers spanning the splicing junction. The amounts of the unspliced transcript relative to that of the spliced at different times postinfection were indicated.
To determine the potential involvement of pUL38 in the ATF6 pathway, we analyzed levels of GRP94 and Bip in response to virus infection. Consistent with the previous report (13), the elevated level of GRP94 was observed as early as 24 h and persisted through the course of infection, whereas the level of Bip peaked at 24 h and gradually decreased after (Fig. 5A). Importantly, no substantial difference was observed in GRP94 and Bip levels in ADpmUL38 infection or ADwt infection. Thus, pUL38 is unlikely to be involved in the ATF6 branch of the UPR.
Finally, we analyzed the IRE1 pathway of the UPR by examining XBP-1 splicing, EDEM transcription, and JNK phosphorylation. HCMV infection has been reported to induce splicing of the XBP-1 transcript, an event entirely dependent on the endo-RNase activity of IRE1; however, the infection does not induce the transcription of EDEM, a downstream event that is activated by the XBP-1 splicing in a prototypical UPR response (13). Similarly, we found that XBP-1 splicing was initiated at as early as 24 h (Fig. 5C), and the transcript level of EDEM remained constant at 24, 48, and 72 h postinfection (Fig. 5B). However, no detectable difference was observed in cells infected with ADpmUL38 or with ADwt at all times examined (Fig. 5B and C). We next analyzed the phosphorylation of JNK, another key event that was activated by the IRE1 pathway and was dependent on the kinase activity of IRE1. The total JNK level appeared to slightly increase, if at all, during HCMV infection, and cells infected with ADpmUL38 accumulated the same amount of JNK as those with ADwt at any time examined (Fig. 5A). A detectable level of phosphorylated JNK was present in mock-infected fibroblasts, likely because HCMV infection experiments in the present study were carried out 24 h after cells were seeded, a condition where JNK was activated by serum stimulation (45, 51). Intriguingly, wild-type virus infection appeared to elevate JNK phosphorylation at 24 h but subsequently blocked it at 48 to 72 h postinfection (Fig. 5A). On the other hand, the pUL38-deficient virus infection not only induced a greater JNK phosphorylation at 24 h but also failed to suppress it at 48 and 72 h postinfection as efficiently as did wild-type virus. Since JNK phosphorylation in response to ER stress is dependent on the kinase activity of IRE1, this suggests that HCMV infection activates the endo-RNase activity of IRE1 but antagonizes its kinase activity to prevent persistent JNK phosphorylation, and modulation of the latter is pUL38 dependent.
Our results confirmed a previous report that HCMV infection modulated the UPR (13), and our study further suggested that at late times during infection pUL38 was required for HCMV to efficiently activate the PERK/eIF-2α/ATF4 pathway and reduce persistent JNK phosphorylation that was likely to be dependent on the kinase activity of IRE1.
pUL38 expression alone is sufficient to activate ATF4 translation and attenuate JNK phosphorylation.
The data obtained during HCMV infection led us to hypothesize that pUL38 expression was sufficient to activate ATF4 translation and inhibit persistent JNK phosphorylation. To test the hypothesis, we first examined the status of eIF-2α and ATF4 in fibroblasts expressing pUL38 or control fibroblasts expressing vector only (Fig. 6A). Experiments were carried out 48 h after cell seeding to reduce basal levels of JNK phosphorylation. The protein levels of phosphorylated eIF-2α and, in particular, ATF4, were elevated in pUL38-expressing cells. However, the transcript level of ATF4 in pUL38-expressing cells was actually somewhat lower than that in control cells, suggesting that ATF4 translation was activated by phosphorylated eIF-2α. Intriguingly, global translation in UL38-expressing cells as measured by 35S incorporation was comparable to that in control cells despite the elevated eIF-2α phosphorylation (Fig. 6B). (This 35S incorporation represented nascent protein synthesis because it was blocked in the presence of cycloheximide.) It is conceivable that pUL38-mediated activation of mTORC1 (25), a pathway known to elevate protein translation, may compensate for the potential protein translation attenuation caused by eIF-2α phosphorylation. We also treated cells with tunicamycin to induce JNK phosphorylation and determined the potential inhibitory effect of pUL38 (Fig. 6C). Whereas the total JNK level remained constant after the tunicamycin treatment, the levels of phosphorylated JNK started to increase at 48 h and persisted to 72 h (Fig. 6C), a finding consistent with the marked death of HF-vector cells observed at 48 to 72 h (Fig. 4B). Importantly, JNK phosphorylation was significantly reduced in pUL38-expressing cells compared to that in control cells. Finally, we also analyzed other aspects of the tunicamycin-induced UPR in pUL38-expressing cells. We did not observe a measurable difference in ATF6 signaling, as indicated by the accumulation of Bip and GRP94, or the IRE1 endoribonuclease-dependent XBP-1 splicing in the presence or absence of pUL38, a finding consistent with the role of pUL38 during HCMV infection (data not shown).
These results suggest that pUL38 expression alone was sufficient to activate eIF-2α-dependent ATF4 translation and attenuate persistent JNK phosphorylation and activation.
Overexpression of ATF4 or inhibition of JNK activation enhances the viability of cells infected with pUL38-deficient virus.
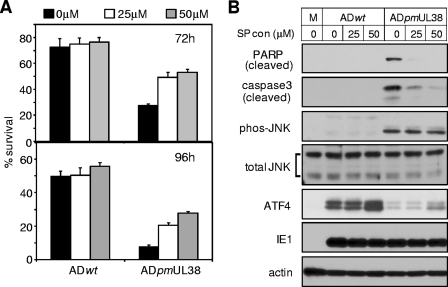
The pUL38-mediated modulation of two critical components of the UPR led us to further hypothesize that induction of ATF4 expression and inhibition of persistent JNK activation contribute to the ability of pUL38 to suppress cell death in HCMV infection. We predicted that overexpression of ATF4 or inhibition of JNK activation would protect cells from death induced by the pUL38-deficient mutant virus. To test the involvement of JNK activity, we treated HFs with a JNK inhibitor SP600125 that inhibits the kinase activity of activated JNK, infected cells with HCMV, and measured cell viability at 72 and 96 h postinfection. Since SP600125 is an ATP analog that could interfere with the ATP viability assay, we used trypan blue exclusion assay to score viable cells. Neither ATF4 expression nor total or phosphorylated levels of JNK was altered in the presence of SP600125 (Fig. 7B). However, as anticipated to directly inhibit the kinase activity of activated JNK, SP600125 significantly enhanced the viability of cells infected with pUL38-deficient virus at concentrations of 25 and 50 μM, whereas it showed a minimal effect on cells infected with wild-type virus (Fig. 7A). A SP600125 concentration of 50 μM enhanced the number of viable cells infected with pUL38-deficient virus by 100 and 300% at 72 and 96 h postinfection, respectively. Consistently, the cleavage of caspase 3 and PARP was also substantially inhibited by SP600125 at both concentrations (Fig. 7B).
FIG. 7.
Inhibition of JNK activity rescued the viability of cells infected with pUL38-deficient virus. HFs were infected with ADwt or ADpmUL38 at an MOI of 3 in the presence of the JNK inhibitor SP600125 at 0, 25, or 50 μM; cell survival at 72 and 96 h was measured by trypan blue exclusion assay (A), and caspase 3, PARP, JNK, and ATF4 at 72 h were examined by immunoblotting (B). Cell viability was normalized to that at the time of infection.
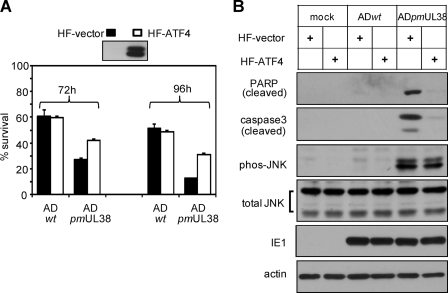
To test the involvement of ATF4 in cell death induced by pUL38-deficient virus, control fibroblasts (HF-vector) or fibroblasts overexpressing ATF4 (HF-ATF4) were prepared by transduction with empty or ATF4-expressing retroviral vector, and the overexpression of ATF4 in HF-ATF4 cells was confirmed by immunoblotting (Fig. 8A). Cells were then infected with wild-type virus or pUL38-deficient virus, cell viability was analyzed by trypan blue exclusion assay, and the cleavage of caspase 3 and PARP was assessed by immunoblotting analysis. ATF4 overexpression enhanced the number of viable cells infected with pUL38-deficient virus by 55 and 150% at 72 and 96 h, respectively, whereas it has no detectable effect on cells infected with wild-type virus (Fig. 8A). Consistently, the cleavage of caspase 3 and PARP in pUL38-deificient virus-infected cells was greatly reduced by ATF4 expression (Fig. 8B). However, JNK phosphorylation induced by infection of pUL38-deificient virus was not inhibited by ATF4 overexpression. This result, together with the observation that the inhibition of JNK activity failed to alter ATF4 expression (Fig. 7B), suggests that JNK inhibition and ATF4 induction are likely to be independently modulated by pUL38.
FIG. 8.
Overexpression of ATF4 rescued the viability of cells infected with pUL38-deficient virus. (A) HFs were transduced with empty retroviral vector (HF-vector) or retroviral vector expressing human ATF4 (HF-ATF4), confirmed for ATF4 overexpression, and infected with ADwt or ADpmUL38 at an MOI of 3, and then cell survival at 72 and 96 h was measured by trypan blue exclusion assay. (B) Caspase 3, PARP, and JNK at 72 h were examined by immunoblotting. Cell viability was normalized to that at the time of infection.
Collectively, our results demonstrate that pUL38 plays an important role in protecting cells from ER stress caused by HCMV infection, at least in part, by activating eIF-2α-dependent ATF4 expression and suppressing persistent JNK phosphorylation and activation.
DISCUSSION
Viruses need to activate cellular responses that are beneficial and overcome many that are detrimental in order to establish a successful infection. Cell death is a central cellular response that HCMV needs to target because it is highly effective in controlling slow-growing viruses such as HCMV and because it can be activated by multiple signals that are a result of HCMV infection. Not surprisingly, several HCMV-encoded cell death-inhibitory proteins, such as pUL36, pUL37x1, IE1, and IE2, have been characterized and shown to inhibit apoptosis induced by extrinsic or mitochondrion-mediated intrinsic pathways. We have previously identified HCMV protein pUL38 as a novel cell death inhibitor and demonstrated that it protected cells from the ER stress inducer thapsigargin (42). In the present study we investigated the mechanism underscoring this activity of pUL38. This viral protein deregulated the ER stress-induced cell death because its expression alone protected cells from thapsigargin and tunicamycin, two drugs that induced the ER stress via different modes (Fig. 4 and data not shown) (42). To understand how pUL38 modulated the UPR during infection, we took advantage of the recombinant HCMV virus in which pUL38 expression was specifically disrupted by point mutations (Fig. 2). Systematic and comparative analysis of the UPR induced and modulated by UL38 point-mutant virus or wild-type virus indicated that pUL38 targeted at least two critical components of the UPR: ATF4 translation and JNK phosphorylation/activation (Fig. 5).
Like UL38 deletion mutant virus (42), UL38 point mutant virus was severely growth defective and induced massive death of infected fibroblasts, and its growth was restored in pUL38-expressing cells (Fig. 3). We have previously also reported that viral late gene expression and viral DNA synthesis were delayed during HCMV infection in the absence of pUL38 (42). Thus, the infection cycle progression of UL38 mutant virus appeared to be delayed compared to that of wild-type virus. Could the aberrant ATF4 and JNK modulation simply reflect a delayed modulation of the UPR due to the slower progression of mutant virus infection? We think that this is unlikely because the alteration in the UPR induced in mutant virus infection was multifaceted rather than a simple delay (Fig. 5). Although mutant virus failed to robustly activate PERK/eIF-2α/ATF4, it induced expression of Bip/GRP94 and splicing of XBP-1 mRNA as efficiently as the wild-type virus. Furthermore, mutant virus induced persistent JNK phosphorylation to a greater level than wild-type virus did. More importantly, pUL38 expression alone inhibited JNK phosphorylation and activated ATF4 induction (Fig. 6). Collectively, our results support a direct role of pUL38 in modulating the UPR in HCMV infection.
pUL38 shares no apparent homologues to any known viral or cellular proteins involved in cell death pathways, but it interacts with several cellular and viral proteins, suggesting that it may play multiple roles during HCMV infection (25). One of the pUL38-interacting partners is a component of the tuberous sclerosis protein complex (TSC1/2) that is the negative regulator of mTORC1 (25). Thus, one function of pUL38 appears to inhibit TSC, activate mTORC1, and promote downstream events such as protein synthesis and ribosome biosynthesis to create a favorable metabolic environment for HCMV replication (25). However, it is unclear whether and how activation of mTORC1 engages ER stress-induced cell death. Intriguingly, the loss of TSC1/2 activates mTORC1 but also renders cells hypersensitive to ER stress induced by tunicamycin and thapsigargin (30). Inhibition of mTORC1 by rapamycin protects cells from ER stress-induced cell death (28). Therefore, activation of mTORC1 may protect cells from certain stress but sensitize cells to the ones on the ER. The fact that pUL38 interacts with an array of cellular proteins involved in different biological pathways (25) led us to favor the view that pUL38 is a multifunctional protein. It may use multiple mechanisms to protect host cells from various stresses imposed by HCMV infection, and the ability of pUL38 to block ER stress-induced cell death may be independent of its effect on mTORC1 activation. Nonetheless, we cannot rule out the possibility that pUL38 may modulate mTORC1 in a way to subvert this pathway to antagonize the ER stress-induced cell death.
As anticipated, the JNK inhibitor SP600125 and ATF4 overexpression substantially enhanced the viability of cells infected with UL38-deficient virus (Fig. 7 and 8). However, perhaps due to multiple reasons, such treatments did not completely restore the cell viability to the level seen in wild-type virus infection nor recover the growth of mutant virus (data not shown). Notably, JNK and ATF4 were tightly regulated in wild-type virus infection. JNK activation was, in fact, transiently induced at early times and gradually decreased thereafter, whereas ATF4 induction was mostly evident only at late times (Fig. 5), raising the possibility that such temporary regulation may be critical for efficient HCMV replication, and therefore the approach of constitutive JNK inhibition or ATF4 induction may also have a detrimental effect on HCMV infection. Moreover, the multifunctional nature of pUL38 suggests that this protein engages in several cellular or viral processes and may employ multiple mechanisms in addition to those identified in the present study to block cell death and facilitate virus growth. Aside from its aforementioned role in mTORC1 activation, pUL38 facilitates viral DNA synthesis (42) and interacts with nucleosome remodeling and histone deacetylation complex, suggesting a possible role in regulating viral and cellular gene transcription (25). It is conceivable that these functions of pUL38, in addition to its modulation of UPR, are all required for HCMV growth. Nonetheless, our results revealed the protective role of JNK inhibition and ATF4 expression under HCMV-induced stress conditions, and we concluded that pUL38-mediated modulation of these two components of the UPR contributes, at least in part, to its role as a cell death-inhibitory protein in HCMV infection.
The simultaneous targeting of multiple cellular responses by one viral protein has an advantage in that it may also allow HCMV to maintain the beneficial aspects of these responses and at the same time limit the adverse effects of these responses. For instance, short-term induction of the UPR protects cell from ER stress, but the prolonged induction results in attenuated protein synthesis and ultimately cell death. It is plausible that pUL38 targets the mTORC1 and the UPR simultaneously to enhance the ER capacity and at the same time maintain the global protein translation (Fig. 6B) and limited the detrimental effect of UPR in HCMV infection. With such a strategy, it is conceivable that the virus is able to modulate the cellular environment effectively and precisely to facilitate its infection.
Currently, it is unclear how pUL38 simultaneously targets the PERK/eIF-2α/ATF4 pathway and the IRE1/JNK pathway, whether pUL38 has a direct kinase/phosphatase activity, and what its proximal cellular targets are in order to regulate these pathways. It is possible that modulation of one pathway by pUL38 leads to the modulation of the other because of the potential cross talk between the two pathways. As ATF4 controls the expression of genes that help cells adapt to stress conditions, it may prevent the activation of late signaling events that lead to cell death, such as JNK phosphorylation. However, it is equally possible that pUL38 modulates ATF4 translation and JNK phosphorylation/activation through distinct mechanisms. In HCMV infection, the modulation on JNK phosphorylation (i.e., 24 h postinfection) was observed earlier than that on eIF-2α phosphorylation and ATF4 expression (i.e., 48 to 72 h postinfection) (Fig. 5A). Thus, it is possible that the inhibition of JNK at early times may not depend on the induction of eIF-2α/ATF4. It is intriguing to speculate that pUL38 may prevent JNK activation that is potentially induced by multiple stresses (4) at early times in addition to ER stress that results from late viral activities (e.g., producing and overloading viral glycoproteins to the ER) during infection. Further studies are required to elucidate how pUL38 integrates its regulatory activities on different cellular pathways and how multiple functions of pUL38 cross talk to counteract various stresses and to facilitate HCMV growth.
Acknowledgments
We thank Herbert Virgin and the members of his laboratory for helpful discussions and invaluable advice, Thomas Shenk for the generous gifts of the antibodies, and members of Yu lab for their critical reading of the manuscript.
This study was supported by a Howard Temin Award (CA-101957) and a Public Health Service grant (CA-120768) from the National Cancer Institute and a grant from the American Heart Association (0755804Z). Z.Q. was a Morse/Berg Fellow of the Department of Molecular Microbiology, Washington University School of Medicine.
Footnotes
Published ahead of print on 4 February 2009.
REFERENCES
- 1.Arnoult, D., L. M. Bartle, A. Skaletskaya, D. Poncet, N. Zamzami, P. U. Park, J. Sharpe, R. J. Youle, and V. S. Goldmacher. 2004. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA 1017988-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedict, C. A., P. S. Norris, and C. F. Ware. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 31013-1018. [DOI] [PubMed] [Google Scholar]
- 3.Bitko, V., and S. Barik. 2001. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J. Cell Biochem. 80441-454. [DOI] [PubMed] [Google Scholar]
- 4.Bogoyevitch, M. A., and B. Kobe. 2006. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 701061-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce, M., K. F. Bryant, C. Jousse, K. Long, H. P. Harding, D. Scheuner, R. J. Kaufman, D. Ma, D. M. Coen, D. Ron, and J. Yuan. 2005. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307935-939. [DOI] [PubMed] [Google Scholar]
- 6.Calfon, M., H. Zeng, F. Urano, J. H. Till, S. R. Hubbard, H. P. Harding, S. G. Clark, and D. Ron. 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 41592-96. [DOI] [PubMed] [Google Scholar]
- 7.Cox, J. S., C. E. Shamu, and P. Walter. 1993. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 731197-1206. [DOI] [PubMed] [Google Scholar]
- 8.Esolen, L. M., S. W. Park, J. M. Hardwick, and D. E. Griffin. 1995. Apoptosis as a cause of death in measles virus-infected cells. J. Virol. 693955-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldmacher, V. S., L. M. Bartle, A. Skaletskaya, C. A. Dionne, N. L. Kedersha, C. A. Vater, J. W. Han, R. J. Lutz, S. Watanabe, E. D. Cahir McFarland, E. D. Kieff, E. S. Mocarski, and T. Chittenden. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 9612536-12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding, H. P., Y. Zhang, A. Bertolotti, H. Zeng, and D. Ron. 2000. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell 5897-904. [DOI] [PubMed] [Google Scholar]
- 12.Harding, H. P., Y. Zhang, and D. Ron. 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397271-274. [DOI] [PubMed] [Google Scholar]
- 13.Isler, J. A., A. H. Skalet, and J. C. Alwine. 2005. Human cytomegalovirus infection activates and regulates the unfolded protein response. J. Virol. 796890-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joo, J. H., G. Liao, J. B. Collins, S. F. Grissom, and A. M. Jetten. 2007. Farnesol-induced apoptosis in human lung carcinoma cells is coupled to the endoplasmic reticulum stress response. Cancer Res. 677929-7936. [DOI] [PubMed] [Google Scholar]
- 15.Jordan, R., L. Wang, T. M. Graczyk, T. M. Block, and P. R. Romano. 2002. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J. Virol. 769588-9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinsella, T. M., and G. P. Nolan. 1996. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 71405-1413. [DOI] [PubMed] [Google Scholar]
- 17.Lee, A. H., N. N. Iwakoshi, and L. H. Glimcher. 2003. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 237448-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei, K., and R. J. Davis. 2003. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA 1002432-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang, G., and T. Hai. 1997. Characterization of human activating transcription factor 4, a transcriptional activator that interacts with multiple domains of cAMP-responsive element-binding protein (CREB)-binding protein. J. Biol. Chem. 27224088-24095. [DOI] [PubMed] [Google Scholar]
- 20.Lukac, D. M., and J. C. Alwine. 1999. Effects of human cytomegalovirus major immediate-early proteins in controlling the cell cycle and inhibiting apoptosis: studies with ts13 cells. J. Virol. 732825-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marciniak, S. J., and D. Ron. 2006. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 861133-1149. [DOI] [PubMed] [Google Scholar]
- 22.McCormick, A. L., V. L. Smith, D. Chow, and E. S. Mocarski. 2003. Disruption of mitochondrial networks by the human cytomegalovirus UL37 gene product viral mitochondrion-localized inhibitor of apoptosis. J. Virol. 77631-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCullough, K. D., J. L. Martindale, L. O. Klotz, T. Y. Aw, and N. J. Holbrook. 2001. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 211249-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meusser, B., C. Hirsch, E. Jarosch, and T. Sommer. 2005. ERAD: the long road to destruction. Nat. Cell Biol. 7766-772. [DOI] [PubMed] [Google Scholar]
- 25.Moorman, N. J., I. M. Cristea, S. S. Terhune, M. P. Rout, B. T. Chait, and T. Shenk. 2008. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe 3253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori, K., W. Ma, M. J. Gething, and J. Sambrook. 1993. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74743-756. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen, T., P. J. Sherratt, and C. B. Pickett. 2003. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 43233-260. [DOI] [PubMed] [Google Scholar]
- 28.Ogata, M., S. Hino, A. Saito, K. Morikawa, S. Kondo, S. Kanemoto, T. Murakami, M. Taniguchi, I. Tanii, K. Yoshinaga, S. Shiosaka, J. A. Hammarback, F. Urano, and K. Imaizumi. 2006. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 269220-9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada, T., H. Yoshida, R. Akazawa, M. Negishi, and K. Mori. 2002. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem. J. 366585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozcan, U., L. Ozcan, E. Yilmaz, K. Duvel, M. Sahin, B. D. Manning, and G. S. Hotamisligil. 2008. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell 29541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson, C. E., and T. Shenk. 1999. Human cytomegalovirus UL36 protein is dispensable for viral replication in cultured cells. J. Virol. 737126-7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poncet, D., N. Larochette, A. L. Pauleau, P. Boya, A. A. Jalil, P. F. Cartron, F. Vallette, C. Schnebelen, L. M. Bartle, A. Skaletskaya, D. Boutolleau, J. C. Martinou, V. S. Goldmacher, G. Kroemer, and N. Zamzami. 2004. An antiapoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 27922605-22614. [DOI] [PubMed] [Google Scholar]
- 33.Putcha, G. V., S. Le, S. Frank, C. G. Besirli, K. Clark, B. Chu, S. Alix, R. J. Youle, A. LaMarche, A. C. Maroney, and E. M. Johnson, Jr. 2003. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron 38899-914. [DOI] [PubMed] [Google Scholar]
- 34.Qian, Z., B. Xuan, T. T. Hong, and D. Yu. 2008. The full-length protein encoded by human cytomegalovirus gene UL117 is required for the proper maturation of viral replication compartments. J. Virol. 823452-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves, M. B., A. A. Davies, B. P. McSharry, G. W. Wilkinson, and J. H. Sinclair. 2007. Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science 3161345-1348. [DOI] [PubMed] [Google Scholar]
- 36.Schroder, M., and R. J. Kaufman. 2005. The mammalian unfolded protein response. Annu. Rev. Biochem. 74739-789. [DOI] [PubMed] [Google Scholar]
- 37.Sharon-Friling, R., J. Goodhouse, A. M. Colberg-Poley, and T. Shenk. 2006. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc. Natl. Acad. Sci. USA 10319117-19122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen, J., X. Chen, L. Hendershot, and R. Prywes. 2002. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 399-111. [DOI] [PubMed] [Google Scholar]
- 39.Skaletskaya, A., L. M. Bartle, T. Chittenden, A. L. McCormick, E. S. Mocarski, and V. S. Goldmacher. 2001. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA 987829-7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su, H. L., C. L. Liao, and Y. L. Lin. 2002. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. J. Virol. 764162-4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tardif, K. D., K. Mori, and A. Siddiqui. 2002. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J. Virol. 767453-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terhune, S., E. Torigoi, N. Moorman, M. Silva, Z. Qian, T. Shenk, and D. Yu. 2007. Human cytomegalovirus UL38 protein blocks apoptosis. J. Virol. 813109-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urano, F., X. Wang, A. Bertolotti, Y. Zhang, P. Chung, H. P. Harding, and D. Ron. 2000. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287664-666. [DOI] [PubMed] [Google Scholar]
- 44.Wurzer, W. J., O. Planz, C. Ehrhardt, M. Giner, T. Silberzahn, S. Pleschka, and S. Ludwig. 2003. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 222717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia, Y., C. Makris, B. Su, E. Li, J. Yang, G. R. Nemerow, and M. Karin. 2000. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc. Natl. Acad. Sci. USA 975243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu, L., J. Zhu, X. Hu, H. Zhu, H. T. Kim, J. LaBaer, A. Goldberg, and J. Yuan. 2007. c-IAP1 cooperates with Myc by acting as a ubiquitin ligase for Mad1. Mol. Cell 28914-922. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida, H., K. Haze, H. Yanagi, T. Yura, and K. Mori. 1998. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J. Biol. Chem. 27333741-33749. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida, H., T. Matsui, A. Yamamoto, T. Okada, and K. Mori. 2001. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107881-891. [DOI] [PubMed] [Google Scholar]
- 49.Yu, D., G. A. Smith, L. W. Enquist, and T. Shenk. 2002. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J. Virol. 762316-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu, Y., and J. C. Alwine. 2002. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3′-OH kinase pathway and the cellular kinase Akt. J. Virol. 763731-3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yujiri, T., S. Sather, G. R. Fanger, and G. L. Johnson. 1998. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science 2821911-1914. [DOI] [PubMed] [Google Scholar]
- 52.Zhu, H., Y. Shen, and T. Shenk. 1995. Human cytomegalovirus IE1 and IE2 proteins block apoptosis. J. Virol. 697960-7970. [DOI] [PMC free article] [PubMed] [Google Scholar]