Abstract
The type I interferon (IFN) response represents one of the first lines of defense against influenza virus infections. In this study, we assessed the protective potential of exogenous IFN-α against seasonal and highly pathogenic influenza viruses in ferrets. Intranasal treatment with IFN-α several hours before infection with the H1N1 influenza A virus strain A/USSR/90/77 reduced viral titers in nasal washes at least 100-fold compared to mock-treated controls. IFN-treated animals developed only mild and transient respiratory symptoms, and the characteristic fever peak seen in mock-treated ferrets 2 days after infection was not observed. Repeated application of IFN-α substantially increased the protective effect of the cytokine treatment. IFN-α did not increase survival after infection with the highly pathogenic H5N1 avian influenza A virus strain A/Vietnam/1203/2004. However, viral titers in nasal washes were significantly reduced at days 1 and 3 postinfection. Our study shows that intranasal application of IFN-α can protect ferrets from seasonal influenza viruses, which replicate mainly in the upper respiratory tract, but not from highly pathogenic influenza viruses, which also disseminate to the lung. Based on these results, a more intensive evaluation of IFN-α as an emergency drug against pandemic influenza A is warranted.
Influenza A virus is a leading cause of seasonal upper respiratory tract infections resulting in more than 250,000 deaths annually (28). This number is expected to increase considerably in the case of a pandemic caused by a newly emerging virus against which there is no preexisting immunity in the human population (5). Seasonal influenza A viruses infect mainly the upper respiratory tract, where the virus primarily replicates in epithelial cells carrying α-2,6-linked sialic acids, which serve as viral receptor (39). The disease is characterized by a sudden onset of fever, fatigue, and malaise, followed by classical signs of upper respiratory tract disease, including congestion, coryza, and sneezing (21). Uncomplicated cases resolve within 4 to 7 days, while complications mainly associated with secondary bacterial infections can result in severe pneumonia and death (32, 37). In contrast, highly pathogenic influenza A virus strains also target the lower respiratory tract. The resulting severe inflammation of the lung is responsible for the increased lethality associated with these infections (52).
Immunization with matched inactivated or live vaccines is the best prophylactic option for protection from and control of influenza (29, 43). However, to bridge the gap between the emergence of a new antigenic virus variant and the production of a matched vaccine, efficient antiviral drugs are of crucial importance (27). Currently available drugs, the ion channel blockers amantadine and rimantadine and the neuraminidase inhibitors oseltamivir and zanamivir, act directly on viral proteins (20), resulting in rapid emergence of resistance due to the high natural mutation rate of influenza virus (23, 38). In contrast, drugs that target host factors may avoid this problem. Furthermore, such drugs are expected to be active against a large panel of pathogenic reassortants that may arise in the future.
Upon viral infection, most cells start to express interferon (IFN) genes, which ultimately results in a general antiviral response through the activation of a broad range of effector molecules (12, 33, 42, 51). One group of these antiviral molecules are the IFN-induced Mx GTPases, which are found in many species, including birds, fish, and mammals (8, 13). The Mx proteins show antiviral activity against several RNA viruses such as influenza A virus (13). In mice, the presence of a functional Mx1 gene confers resistance to lethal challenge with mouse-adapted or highly pathogenic nonadapted influenza A virus strains (11, 45). The human MxA protein also inhibits the replication of influenza viruses (30, 53). In spite of promising initial clinical studies with IFN-α for the treatment of respiratory diseases, side effects such as irritation of the nasal mucosa and occasional nose bleeding have prevented the in-depth evaluation of IFN-α as a treatment for these diseases (6, 15, 19, 25). If intranasally applied IFN were effective against influenza A viruses, the benefit would in many cases outweigh the reported mild side effects of the treatment.
Ferrets have traditionally been employed for influenza virus research because of their natural susceptibility to virus strains isolated from humans and because of the similarity between ferrets and humans with respect to disease severity and clinical signs (24). The model is regularly used to assess the efficacy of new vaccines and antiviral agents (47). Recent studies further demonstrated the value of ferrets for the characterization of antiviral host immune responses (40). In this report, we evaluated the feasibility and efficacy of IFN-α as influenza prophylaxis. IFN-α was highly effective against a seasonal strain, which replicates in the upper respiratory tract. It was much less effective against a highly pathogenic H5N1 strain, presumably because the drug was not successfully delivered to the lower respiratory tract.
MATERIALS AND METHODS
Cells and viruses.
Madin-Darby canine kidney (MDCK) cells (ATCC CCL-34), 293T cells (ATCC CRL-1573), and T98G cells (ATCC CRL-1690) were maintained in Dulbecco's modified Earle's medium (DMEM) supplemented with Earle's salts (Invitrogen) in the presence of 5% fetal bovine serum (FBS; Invitrogen). Vero cells (ATCC CCL-81) and L929 cells (ATCC CCL-1) were cultivated in DMEM with 10% FBS. Primary ferret alveolar cells were isolated following the protocol outlined in reference 10. Briefly, lungs from an adult ferret were perfused with 0.9% saline solution and lavaged five times with sterile 0.9% saline solution. Eighty units of elastase (Worthington Biochemical Corporation) at a concentration of 2 U/ml in digestion buffer (133 mM NaCl, 5.2 mM KCl, 6 mM Na2HPO4, 1 mM NaH2PO4, 10.3 mM HEPES, 5.6 mM glucose) was then instilled into the trachea. The treated organ was suspended in digestion buffer and incubated at 37°C for 20 min. Subsequently, the lung was finely minced, the material was filtered through gauze, and the filtrate was layered on a discontinuous Percoll (Amersham) gradient and centrifuged at 250 × g for 20 min. The cell band was harvested and digested for 20 min at 4°C with 30 ml of DNase IV (Sigma) at a concentration of 50 μg/ml in digestion buffer, followed by centrifugation at 250 × g for 10 min and suspension in DMEM containing 10% FBS. The resulting cells were seeded into six-well plates. Two days after isolation, the cells were transfected with pRSV40T (36), coding for the large T antigen of simian virus 40 and passaged regularly to encourage proliferation. The resulting ferret alveolar epithelial cell line (FtAEpC) was maintained in DMEM containing 10% FBS.
The influenza A virus strains H1N1 A/USSR/90/77 (USSR/77), H3N2 A/Port Chalmers/1/73 (PC/73) (40), and H5N1 A/Vietnam/1203/2004 (Vietnam/04) were grown in MDCK cells in DMEM with 2 μg/ml tolylsulfonyl phenylalanyl chloromethyl ketone-trypsin (Sigma). The recombinant enhanced green fluorescent protein (EGFP)-expressing vesicular stomatitis virus strain (VSV-GFP) (4) was amplified in Vero cells, and the titer was determined on Vero cells by fluorescence microscopy. The titers of USSR/77 and PC/73 were determined by the limited-dilution method and expressed as 50% tissue culture infectious doses (TCID50), while the titer of Vietnam/04 was determined by plaque assay and expressed as PFU per ml.
IFN-α production.
A eukaryotic expression plasmid encoding ferret IFN-α (ftIFN-α) was produced by transferring the previously cloned open reading frame (41) into pcDNA3.1 (Invitrogen). A human IFN-αB/D-expressing plasmid (26) was generated by combining the open reading frames of IFN-αD (GenBank accession no. CQ847555) and IFN-αB (GenBank accession no. X03125) by overlap extension PCR (16) and subsequently transferring the resulting PCR product into pCDNA3.1. The sequences of all plasmids were verified.
Recombinant ftIFN-α and human IFN-αB/D were produced by transient transfection of human 293T cells using Nanofectin (PAA Laboratories). Their antiviral activity was determined on FtAEpCs or L929 cells, respectively, treated with serial 10-fold dilutions for 16 h before infection with recombinant VSV-GFP at a multiplicity of infection of 1. After 15 h of incubation, virus titers in supernatants were determined on Vero cells by serial 10-fold dilutions. Purified human hybrid IFN-αB/D produced in Escherichia coli was used as a positive control (18, 45).
Animal experiments.
Male 16-week-old ferrets (Marshall Farms) without antibodies against circulating H1N1 and H3N2 strains were used for the experiments. In an initial dose-response study, groups of two animals were anesthetized with isoflurane and inoculated intranasally with 300 μl of 293T supernatant containing ftIFN-α or mock supernatant at 20 and 4 h before the animals were killed, and lung, nasal turbinates, and trachea were collected. Groups of six animals were used for all infection experiments. Ferrets were pretreated with either 107 U of purified E. coli-produced human IFN-αB/D or 293T cell supernatants containing or lacking ftIFN-α as outlined above, anesthetized with ketamine (1 mg/kg of body weight) and midazolam (10 mg/kg of body weight), and infected intranasally with 105 TCID50 of PC/73 or USSR/77 or 104 PFU of Vietnam/04, respectively. An additional group of animals received 2.5 mg of oseltamivir (Tamiflu; Roche), corresponding to 2.2 to 2.5 mg/kg of body weight, orally twice daily for 5 days, starting at 4 h before infection. This dose and treatment regimen has been previously shown to be effective in preventing clinical signs and mortality in ferrets infected with highly pathogenic viruses (2). For the experiment with PC/73 and the second USSR/77 experiment, animals received additional doses of IFN-αB/D or ftIFN-α, respectively, at 24 h, and for USSR/77 also at 48 h postinfection.
Clinical signs, body temperature, and treadmill endurance were assessed daily, and the animals were weighed every second day. Rectal body temperature of animals infected with PC/73 or Vietnam/04 was measured manually every 24 h. For the USSR/77 experiments, body temperature was measured by using data loggers (SubCue) implanted under aseptic conditions into the abdominal cavity. The temperature was monitored continuously and recorded at 15-min intervals, starting 5 days before infection. For the daily assessment of respiratory signs, sneezing, nose exudates, and congestion were scored using a 0-1-2 scale. Zero indicates minimal deviations of the physiologic state, 1 indicates moderate nasal discharge, congestion, and/or occasional sneezing, and 2 indicates severe nasal discharge and/or labored breathing, dyspnea, and frequent sneezing. Nasal washes were collected every day for the first 4 days and every second day thereafter. Briefly, 500 μl of phosphate-buffered saline (Invitrogen) was instilled in one nostril and expectorate was collected in 50-ml centrifuge tubes. This procedure was repeated twice to obtain a minimal volume of 400 μl. Cells in the nasal wash fluid were counted, and the virus titer was determined by the limited dilution method. In the first experiment, chest X-rays of three animals of each group were taken under ketamine/midazolam anesthesia on days 0, 2, and 4 postinfection.
The study involving Vietnam/04 was performed in the biosafety level 4 containment laboratory of the National Microbiology Laboratory in Winnipeg. Clinical signs, body temperature, and weight were assessed daily, and nasal washes were collected on days 1, 3, and 6 or 7 postinfection. Nasal wash titers were determined by the limited-dilution method and expressed as TCID50. Clinical signs were scored as follows. For posture, 0 indicates normal and 1 indicates hunched. For appetite, 0 indicates normal and 1 indicates reduced. For activity, 0 indicates normal, 1 indicates calm, and 2 indicates depressed. For respiration, 0 indicates normal, 1 indicates some nose exudate and sneezing, and 2 indicates coughing and dyspnea. For diarrhea, 0 indicates none, 1 indicates soft stool or mild diarrhea, and 2 indicates severe bloody diarrhea. For neurological signs, 0 indicates none, 1 indicates paralysis or aggression, and 2 indicates paralysis and aggression. Animals were euthanized when the following predefined endpoints were reached: respiratory distress, more than 20% weight loss, hemorrhage, diarrhea leading to severe dehydration, seizures, paralysis, or animal found moribund. All animal studies were approved by the respective IACUC committees.
Western blot analysis.
Lung and nasal turbinate homogenates were prepared by grinding the tissue in a mortar, and trachea epithelial cells were scraped off the trachea with a scalpel. The homogenates were lysed in buffer containing 50 mM HEPES (pH 7.4), 125 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM dithiothreitol, 100 U/ml of benzonase, and protease inhibitors, as recommended by the manufacturer (Roche). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% gel and transferred onto polyvinylidene difluoride membranes (Millipore). The membranes were probed with monoclonal mouse antibody M143, which exhibits reactivity to Mx proteins from a broad range of mammals (7), and a monoclonal mouse antibody against β-tubulin (Sigma). Horseradish peroxidase-labeled secondary antibodies and the chemiluminescence detection system (Pierce) were used to visualize specific bands.
Statistics.
Tukey's Studentized range test and Student's paired t test were used for statistical analyses.
RESULTS
Recombinant ftIFN-α produced in 293T cells is active in vitro and in vivo.
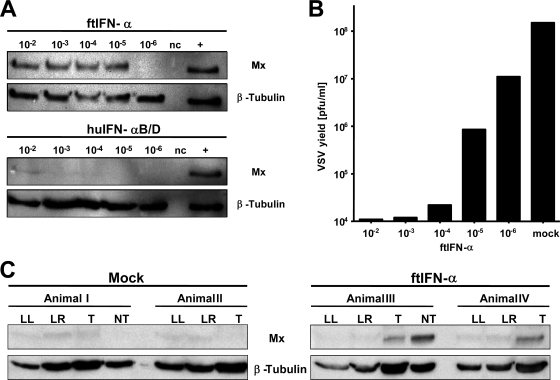
IFN-α subtypes exhibit a high degree of species specificity (9, 46), which complicates experiments in species for which reagents are not commercially available. To assess the functionality of ftIFN-α encoded by a newly developed expression construct, a ferret alveolar epithelial cell line (FtAEpC) was established. Supernatant of 293T cells transfected with the ftIFN-α expression construct efficiently activated the Mx gene in FtAEpCs up to a 105-fold dilution, whereas supernatant of mock-transfected 293T cells had no effect (Fig. 1A, upper panels). Surprisingly, similarly produced human hybrid IFN-αB/D, which is known to exhibit broad host specificity (18), was a comparatively poor Mx inducer in FtAEpCs (Fig. 1A, lower panels). Only if a very high dose (104 U/ml) of E. coli-produced purified huIFN-αB/D was used was the Mx gene of FtAEpCs induced strongly (positive control). Interestingly, 293T-produced huIFN-αB/D efficiently induced Mx expression in human T98G cells while ftIFN-α had no effect on these cells (data not shown). To confirm the potency of ftIFN-α in a second bioassay, we treated FtAEpCs with various dilutions for 16 h prior to infection with GFP-expressing VSV and quantified the virus titers in the FtAEpC supernatants 15 h later. Treatment with ftIFN-α at dilutions of up to 104-fold resulted in more than 1,000-fold inhibition, and a 105-fold dilution was still able to reduce the yield of VSV-GFP approximately 100-fold compared to supernatant of mock-transfected 293T cells (Fig. 1B). Since no international standard for ftIFN activity exists, the activity of our ftIFN-α preparation cannot be quantified easily. If our virus yield reduction assay were used as reference, the calculated activity of the preparation would be above 106 U/ml; if the Mx induction assay were used, this value would be slightly lower.
FIG. 1.
ftIFN-α is active in alveolar epithelial cells and upper respiratory tract of ferrets. (A) Supernatant of transfected 293T cells containing ftIFN-α or huIFN-αB/D was diluted as indicated and incubated with FtAEpCs for 16 h before the cells were lysed and subjected to Western blot analysis using Mx-specific monoclonal antibody M143. The blots were reprobed with a β-tubulin-specific antibody to verify similar loading of all lanes. Representative results from three independent experiments are shown. +, FtAEpCs treated with 104 U of E. coli-produced, purified huIFN-αB/D served as a positive control; nc, FtAEpCs treated with a 10−2 dilution of supernatant of mock-transfected 293T cells served as a negative control. (B) FtAEpCs were treated with different dilutions of ftIFN-α for 16 h before infection with VSV-GFP at an MOI of 1. Mock-treated FtAEpCs served as a control. Viral titers in the supernatants were determined at 15 h postinfection. Representative results from two independent experiments are shown. (C) Ferrets were treated intranasally with 300 μl of undiluted supernatant of transfected 293T cells containing ftIFN-α or supernatant of mock-transfected 293T cells (mock) at 20 and 4 h before harvesting of the lung, nasal turbinates, and cells from the trachea. A Western blot analysis of protein extracts (∼50 μg/lane) was done as in panel A. LL, lung left lobe; LR, lung right lobe; T, tracheal epithelial cells; NT, nasal turbinate.
To determine the activity of ftIFN-α in vivo, groups of two ferrets were treated with either 300 μl ftIFN-α or mock supernatant at 20 and 4 h prior to harvesting nasal turbinates, tracheal cells, and lungs. The Mx protein content of tissue lysates was then determined by Western blotting. Tracheal cells of IFN-treated animals (animals III and IV) contained easily detectable levels of Mx protein, while those of mock-treated animals (animals I and II) did not (Fig. 1C). Nasal turbinates available from IFN-treated animal III were also strongly positive in this assay, whereas no Mx expression was detected in mock-treated animals (Fig. 1C). Interestingly, lung samples from all animals contained very low levels of Mx protein irrespective of treatment (Fig. 1C), suggesting that intranasal cytokine application efficiently reaches cells in the upper respiratory tract but not the lung.
Human IFN-αB/D is only partially active in ferrets.
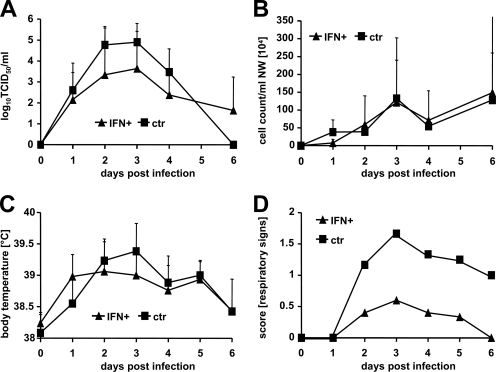
Since treatment of FtAEpCs with the E. coli-produced human hybrid IFN-αB/D resulted in strong Mx expression (Fig. 1A, top panel, positive control), and this product was readily available at high purity and concentration, we initially assessed its efficacy against the seasonal influenza virus strain PC/73 (H3N2) in ferrets. Toward this end, groups of six animals were treated intranasally with 107 U of IFN-αB/D at 20 and 4 h before infection and at 24 h postinfection or remained untreated. We observed no significant differences in virus titers and cell numbers of nasal washes and in the body temperature between the IFN-treated and the control groups (Fig. 2A to C). However, the IFN-treated animals developed only mild signs of respiratory disease compared to the controls (Fig. 2D).
FIG. 2.
HuIFN-αB/D reduces respiratory signs after influenza A virus challenge. Groups of six animals were treated intranasally with 107 U of E. coli-produced IFN-αB/D in a volume of 250 μl of supernatant at 20 and 4 h before infection and at 24 h post-intranasal infection with 105 TCID50 of the H3N2 strain PC/73. The control animals were not treated. (A) Virus titers in nasal washes; (B) nasal wash (NW) cell counts; (C) body temperature. Group averages at the indicated time points are shown. Error bars represent standard deviations. (D) Respiratory signs. Sneezing, nose exudates, and congestion were graded on a 0-1-2 scale, with 0 indicating minimal deviation of the physiologic state; 1 indicating moderate nasal discharge, congestion, and/or occasional sneezing; and 2 indicating severe nasal discharge and/or labored breathing, dyspnea, and frequent sneezing. Group averages are shown.
Treatment with ftIFN-α reduces clinical signs after influenza A virus infection.
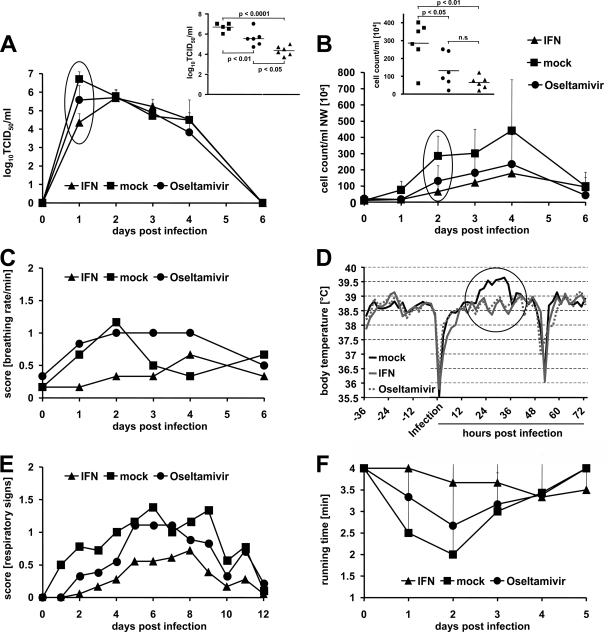
To truly assess the therapeutic potential of type I IFNs against influenza virus, homologous ftIFN-α was used for all further experiments. Groups of six ferrets were treated with 300 μl of ftIFN-α or mock supernatant at 20 and 4 h prior to infection with 105 TCID50 of USSR/77 (H1N1). This influenza A virus strain causes a more severe disease than PC/73, thereby allowing a better discrimination of therapeutic effects. An additional group of animals received 2.5 mg oral oseltamivir every 12 h for 5 days starting 4 h prior to infection. At 24 h postinfection, the virus titers in nasal washes of IFN-treated animals were about 10-fold lower than those in the oseltamivir group and at least 100-fold lower than those in the mock-treated controls (Fig. 3A). When evaluating the cellular content of the nasal wash fluid, we observed an approximately 10-fold increase in the cell number in the mock-treated ferrets compared to either the IFN- or the oseltamivir-treated groups at 48 h postinfection (Fig. 3B). IFN-treated ferrets showed fewer upper and lower respiratory symptoms than the controls (Fig. 3C and E), but no significant differences in body weight change were observed between the groups (data not shown). In addition, IFN- and oseltamivir-treated groups developed fewer general clinical signs, and the characteristic fever peak at day 2 postinfection was not observed (Fig. 3D). The decrease in body temperature observed on the day of infection and around 55 h postinfection was anesthesia related (Fig. 3D). No conclusive pathological changes were observed on chest X rays obtained for three animals of each group on days 2 and 4 postinfection (data not shown). To objectively assess the impact of the infection on endurance, we trained all animals to use a treadmill before the start of the experiment. Healthy animals voluntarily exercised for approximately 4 min. On day 2 postinfection, all control animals displayed strongly reduced running times, whereas the endurance of IFN-treated ferrets was only slightly affected (Fig. 3F). The oseltamivir-treated animals showed an intermediate phenotype. Overall, the efficacy of IFN was thus similar to or better than that of oseltamivir. IFN treatment resulted in a significant reduction of nasal wash titers, clinical signs, and loss of activity compared to the control group.
FIG. 3.
ftIFN-α protects against infection with a seasonal influenza A virus strain. Groups of six animals were treated with 300 μl of undiluted supernatant of transfected 293T cells containing ftIFN-α or 300 μl of supernatant of mock-transfected 293T cells at 20 and 4 h before infection with 105 TCID50 of H1N1 influenza A virus strain USSR/77. An additional group of animals received 2.5 mg of oseltamivir, corresponding to 2.2 to 2.5 mg/kg, twice daily for five days, starting 4 h before infection. (A) Viral titers in nasal washes at various times postinfection. Error bars represent standard deviations. (Inset) Results of statistical analysis of circled data points at 24 h postinfection. (B) Cell counts in nasal washes (NW) at various times postinfection. (Inset) Results of statistical analysis of circled data points at 48 h postinfection. (C) The breathing rate was scored using a 0-1-2 scale (score 0, <28; score 1, 28 to 36; score 2, >36). (D) Body temperature as measured by telemetry. Average values of groups of three animals are shown. The pronounced temperature drops resulted from anesthesia used for virus infection and X-ray examination of the animals, respectively. (E) Respiratory signs. Sneezing, nose exudates, and congestion were graded on a 0-1-2 scale, with 0 indicating minimal deviation of the physiologic state; 1 indicating moderate nasal discharge, congestion and/or occasional sneezing; and 2 indicating severe nasal discharge and/or labored breathing, dyspnea, and frequent sneezing. (F) Treadmill performance. Error bars indicate the standard deviation.
Repeated intranasal treatment increases the protective effect of ftIFN-α.
To determine if repeated dosing would increase the protective effect of IFN-α, groups of six animals were treated intranasally with either 300 μl ftIFN-α or mock supernatant at 20 and 4 h before infection and at 24 and 48 h after infection with 105 TCID50 of USSR/77. Like in the first experiment (Fig. 3), a 150-fold reduction of nasal wash titers was observed in the IFN-treated animals at 24 h postinfection compared to the noninfected controls (Fig. 4A). Furthermore, the IFN-treated group had 100-fold fewer cells in the nasal wash fluid (Fig. 4B). IFN-treated animals also lacked the characteristic fever peak during the acute disease phase (Fig. 4C) and displayed neither clinical respiratory signs (Fig. 4D) nor decreased treadmill activity (data not shown) over the course of the infection. In addition, no significant differences in body weight between the two groups were observed (data not shown). Thus, repeated intranasal application of ftIFN-α enhanced the protective effect.
FIG. 4.
Repeated intranasal treatment increases protective effect of ftIFN-α. Groups of six animals were treated with 300 μl of undiluted supernatant of transfected 293T cells containing ftIFN-α or 300 μl of supernatant of mock-transfected 293T cells at 20 and 4 h before infection with 105 TCID50 of H1N1 influenza A virus strain USSR/77. Two more treatments were done at 24 and 48 h postinfection. (A) Viral titers in nasal washes. (Inset) Results of statistical analysis of circled data points. (B) Cell counts in nasal washes (NW). (Inset) Results of statistical analysis of circled data points. Error bars represent the standard deviation. (C) Body temperature as measured by telemetry. Average values of groups of three animals are shown. (D) Respiratory signs. The scoring criteria outlined in the legend to Fig. 2 were used.
ftIFN-α pretreatment reduces virus shedding but not mortality after H5N1 infection.
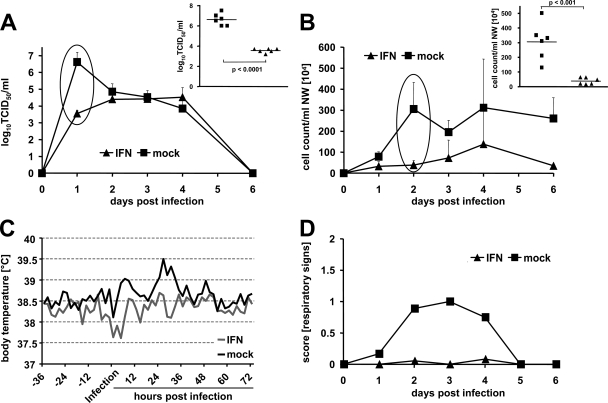
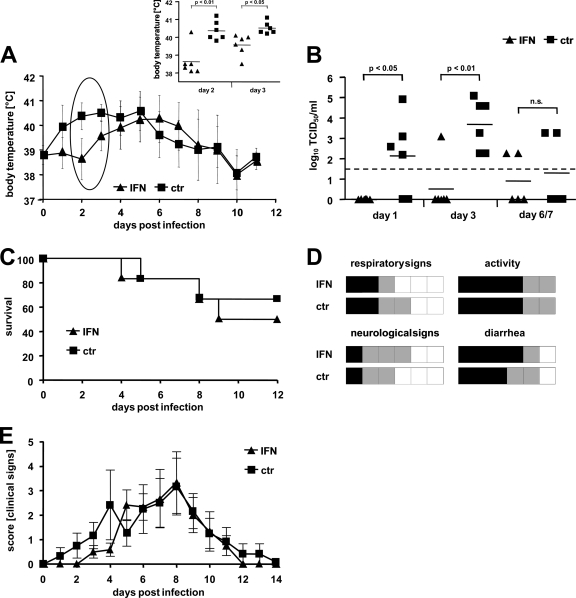
To assess the efficacy of IFN pretreatment against a highly pathogenic avian influenza A virus, groups of six ferrets were treated as outlined previously with ftIFN-α or mock supernatant at 20 and 4 h prior to infection with 104 PFU of the H5N1 strain Vietnam/04. In the untreated group, most animals developed fever within 24 to 48 h postinfection (Fig. 5A), and virus was readily detectable in nasal washes at 48 h postinfection (Fig. 5B). In contrast, the body temperature of IFN-treated ferrets only started to rise on day 3, and only one animal had detectable nasal wash titers at 48 h postinfection (Fig. 5A and B). However, no significant differences in mortality were observed (Fig. 5C). IFN-treated animals and untreated controls fared similarly with respect to the overall clinical course and individual clinical scores, with the IFN-treated animals experiencing overall slightly more frequent and severe rectal bleeding (Fig. 5D and E). Thus, IFN pretreatment alone is insufficient to influence the outcome of the H5N1 infection.
FIG. 5.
ftIFN reduces infection of upper respiratory tract with H5N1 influenza A virus but does not prevent disease. Groups of six animals were treated with 300 μl of undiluted supernatant of transfected 293T cells containing ftIFN-α or 300 μl of supernatant of mock-transfected 293T cells at 20 and 4 h before infection with 104 PFU of H5N1 strain A/Vietnam/1203/2004 and observed for 12 days. (A) Body temperature. (Inset) Results of statistical analysis of circled data points. Error bars represent the standard deviation. ctr, control. (B) Viral titers in nasal washes. The detection level of the assay is indicated by a dotted line. (C) Survival. (D) Clinical signs. Each animal is represented by one square. Severity of disease is shown by color code (white, low; gray, moderate; black, severe). Clinical signs were scored daily. For respiratory signs, gray represents moderate nasal discharge and/or occasional sneezing, while black indicates severe nasal discharge and/or labored breathing and frequent sneezing. Reduced activity is represented by gray and loss of activity by black. Neurological signs were scored as moderate if either paralysis or aggressive behavior was observed and severe if both signs were present. For diarrhea, gray represents soft stool to mild diarrhea, while black indicates severe bloody diarrhea. (E) Composite score of clinical signs over the course of the disease. Posture, appetite, activity, respiration, diarrhea, and neurological signs were assessed daily and scored as outlined in Materials and Methods. The composite score represents the mean of the sum of all individual scores of each animal in the respective group. Error bars indicate standard error.
DISCUSSION
IFNs are key players in early host responses to viral infections. The exploration of the therapeutic potential of type I IFN began soon after its potent antiviral activity had been discovered (3, 15, 34, 44). Today, IFN-α is established as an essential component of hepatitis B and C treatment regimens (9, 17, 31). Even though clinical studies demonstrated that intranansal IFN-α treatment can also prevent rhinovirus infection and spread (6, 14, 15), side effects such as irritation of the nasal mucosa and occasional nose bleeding after long-time and repeated administration prevented further in-depth evaluation of the potential therapeutic use of IFN-α to combat respiratory diseases such as influenza. The current interest in drugs against influenza is fueled by the threat that circulating avian H5N1 viruses or another subtype may adapt to humans and cause a devastating pandemic. In the context of such an influenza pandemic, the known mild side effects of IFN may be acceptable if the treatment reduced virus spread or even decreased morbidity and mortality. Our study thus aimed at investigating the antiviral potential of intranasally applied IFN-α as influenza prophylaxis in the ferret model.
Previous studies have shown that intranasal or oral administration of IFN-α protects mice from lethal influenza virus challenge (1, 11, 45). We report here that in ferrets intranasal IFN-α was at least as effective against influenza viruses as oseltamivir at a dose of 2.5 mg/kg twice daily. We demonstrate that IFN-α treatment lowered viral titers in nasal washes of ferrets and reduced clinical signs of different seasonal influenza A virus subtypes. We further show that repeated treatment of the animals with IFN-α shortly before and after virus infection enhanced the protective effect. Since resistance to established anti-influenza drugs increases worldwide (22), IFN-α thus represent an attractive end-of-the-line treatment option for individuals at high risk of experiencing severe disease and complications. Different human IFN-α and IFN-β preparations are already approved by regulatory agencies for other applications (9), which will greatly facilitate their off-label use as influenza treatment in case of an emergency.
The kinetics of virus secretion in the presence and absence of IFN showed remarkable differences (Fig. 3A and 4A). As expected, if IFN was antivirally active against seasonal influenza viruses, viral titers in nasal washes of IFN-treated animals were significantly reduced at 24 h postinfection. However, these differences had disappeared by 48 h postinfection. We assume that the sharp drop in virus secretion by the control group between days 1 and 2 is due to endogenous virus-induced IFN. Since IFN treatment strongly reduced initial virus replication, its de novo synthesis by virus-infected cells of the respiratory tract is presumably far less vigorous and robust. As a consequence, the innate immune defense in the upper respiratory tract of untreated animals may actually be stimulated more strongly on day 2 postinfection than in IFN-treated animals. Although effective in the long run, this delayed activation of the innate immune system in untreated animals cannot prevent the initial high-level replication of the incoming virus.
Interestingly, intranasally applied IFN-α did not prevent disease following infection with a highly pathogenic H5N1 influenza virus strain, although it reduced viral titers in nasal washes as efficiently as it did in the case of the seasonal influenza virus. This unexpected finding may be explained by the fact that seasonal influenza virus strains primarily target the upper respiratory tract, whereas highly pathogenic H5N1 viruses replicate mainly in the lower respiratory tract (35, 49). These differences most likely result from a different distribution of the receptors: α2,6-linked sialic acids, which serve as receptor for seasonal human influenza virus strains, are mostly found in the upper respiratory tract of ferrets, whereas α2,3-linked sialic acids, which are preferentially used by avian influenza virus strains, are only present on cells lining small bronchi and alveoli (50). Using Mx gene expression as a marker for IFN action, we found that intranasally applied IFN reached the upper respiratory tract but failed to reach the lower respiratory tract (Fig. 1C). Most likely, the volume used for the treatment (300 μl) was too small for efficient delivery to the lower respiratory tract. It is of interest to note that IFN-α was highly effective against a highly pathogenic H5N1 influenza virus strain in mice (45). In this mouse model, IFN was applied in a volume of 50 μl, which corresponds to an eightfold higher weight-adjusted volume for ferrets than that used in our study. Therefore, additional studies with ferrets, in which the IFN is delivered more efficiently to the lower respiratory tract, are needed.
In summary, our results indicate that IFN treatment by the nasal route may protect high-risk groups against seasonal influenza, and a systematic efficacy assessment of this approach is certainly warranted. In the scenario of pandemic influenza, IFN might also be advantageous as the treatment reduces virus transmission by lowering the amount of virus produced in the upper respiratory tract of diseased individuals. However, this beneficial effect will need to be evaluated against a possible negative impact of IFN treatment on the course of disease. Similar conclusions were drawn from experiments in which the guinea pig model was used to assess the efficacy of IFN-α in preventing influenza A virus infections (48).
Acknowledgments
We thank J. Schulte Mönting at the Institute for Medical Biometry, University of Freiburg, for help with the statistical analysis of our data; Rosita Frank for excellent technical assistance; and Michael Gray, Jason Gren, Shane Jones, and Tracy Taylor for technical support with the containment experiments at the National Microbiology Laboratory.
Financial support was provided by the German Ministry of Education and Research (FluResearchNet program to G.K., O.H., and P.S.), the Canadian Institutes of Health Research (grant OPC-167324 to V.V.M., O.H., G.K., and P.S. and grant NIP-79931 to V.V.M.), the Canadian Foundation for Innovation (grant 9488 to V.V.M.), and the Public Health Agency of Canada (financial support to G.P.K. and D.K.).
Footnotes
Published ahead of print on 4 February 2009.
REFERENCES
- 1.Beilharz, M. W., J. M. Cummins, and A. L. Bennett. 2007. Protection from lethal influenza virus challenge by oral type 1 interferon. Biochem. Biophys. Res. Commun. 355740-744. [DOI] [PubMed] [Google Scholar]
- 2.Boltz, D. A., J. E. Rehg, J. McClaren, R. G. Webster, and E. A. Govorkova. 2008. Oseltamivir prophylactic regimens prevent H5N1 influenza morbidity and mortality in a ferret model. J. Infect. Dis. 1971315-1323. [DOI] [PubMed] [Google Scholar]
- 3.Borecky, L., and N. Fuchsberger. 1983. Interferon as therapeutic agent. Acta Virol. 27359-370. [PubMed] [Google Scholar]
- 4.Boritz, E., J. Gerlach, J. E. Johnson, and J. K. Rose. 1999. Replication-competent rhabdoviruses with human immunodeficiency virus type 1 coats and green fluorescent protein: entry by a pH-independent pathway. J. Virol. 736937-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enserink, M. 2004. Influenza. W. H. O. adds more “1918” to pandemic predictions. Science 3062025. [DOI] [PubMed] [Google Scholar]
- 6.Finter, N. B., S. Chapman, P. Dowd, J. M. Johnston, V. Manna, N. Sarantis, N. Sheron, G. Scott, S. Phua, and P. B. Tatum. 1991. The use of interferon-alpha in virus infections. Drugs 42749-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flohr, F., S. Schneider-Schaulies, O. Haller, and G. Kochs. 1999. The central interactive region of human MxA GTPase is involved in GTPase activation and interaction with viral target structures. FEBS Lett. 46324-28. [DOI] [PubMed] [Google Scholar]
- 8.Frese, M., G. Kochs, H. Feldmann, C. Hertkorn, and O. Haller. 1996. Inhibition of bunyaviruses, phleboviruses, and hantaviruses by human MxA protein. J. Virol. 70915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman, R. M. 2008. Clinical uses of interferons. Br. J. Clin. Pharmacol. 65158-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs, S., M. Gumbleton, U. F. Schaefer, and C.-M. Lehr. 2002. Models of the alveolar epithelium, p. 189-210. In C.-M. Lehr (ed.), Cell culture models of biological barriers: in vitro test systems for drug absorption and delivery. CRC Press, Boca Raton, FL.
- 11.Grimm, D., P. Staeheli, M. Hufbauer, I. Koerner, L. Martinez-Sobrido, A. Solorzano, A. Garcia-Sastre, O. Haller, and G. Kochs. 2007. Replication fitness determines high virulence of influenza A virus in mice carrying functional Mx1 resistance gene. Proc. Natl. Acad. Sci. USA 1046806-6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haller, O., P. Staeheli, and G. Kochs. 2007. Interferon-induced Mx proteins in antiviral host defense. Biochimie 89812-818. [DOI] [PubMed] [Google Scholar]
- 14.Hayden, F. G., D. J. Innes, Jr., S. E. Mills, and P. A. Levine. 1988. Intranasal tolerance and histopathologic effects of a novel synthetic interferon, rIFN-alpha Con1. Antivir. Res. 10225-234. [DOI] [PubMed] [Google Scholar]
- 15.Herzog, C., R. Berger, M. Fernex, K. Friesecke, L. Havas, M. Just, and U. C. Dubach. 1986. Intranasal interferon (rIFN-alpha A, Ro 22-8181) for contact prophylaxis against common cold: a randomized, double-blind and placebo-controlled field study. Antivir. Res. 6171-176. [DOI] [PubMed] [Google Scholar]
- 16.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 7751-59. [DOI] [PubMed] [Google Scholar]
- 17.Hoofnagle, J. H., and L. B. Seeff. 2006. Peginterferon and ribavirin for chronic hepatitis C. N. Engl. J. Med. 3552444-2451. [DOI] [PubMed] [Google Scholar]
- 18.Horisberger, M. A., and K. de Staritzky. 1987. A recombinant human interferon-alpha B/D hybrid with a broad host-range. J. Gen. Virol. 68945-948. [DOI] [PubMed] [Google Scholar]
- 19.Isomura, S., T. Ichikawa, M. Miyazu, H. Naruse, M. Shibata, J. Imanishi, A. Matsuo, T. Kishida, and T. Karaki. 1982. The preventive effect of human interferon-alpha on influenza infection: modification of clinical manifestations of influenza in children in a closed community. Biken J. 25131-137. [PubMed] [Google Scholar]
- 20.Jefferson, T., V. Demicheli, D. Rivetti, M. Jones, C. Di Pietrantonj, and A. Rivetti. 2006. Antivirals for influenza in healthy adults: systematic review. Lancet 367303-313. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser, L., R. S. Fritz, S. E. Straus, L. Gubareva, and F. G. Hayden. 2001. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J. Med. Virol. 64262-268. [DOI] [PubMed] [Google Scholar]
- 22.Lackenby, A., O. Hungnes, S. G. Dudman, A. Meijer, W. J. Paget, A. J. Hay, and M. C. Zambon. 2008. Emergence of resistance to oseltamivir among influenza A(H1N1) viruses in Europe. Euro Surveill. 138026. [DOI] [PubMed] [Google Scholar]
- 23.Le, Q. M., M. Kiso, K. Someya, Y. T. Sakai, T. H. Nguyen, K. H. Nguyen, N. D. Pham, H. H. Ngyen, S. Yamada, Y. Muramoto, T. Horimoto, A. Takada, H. Goto, T. Suzuki, Y. Suzuki, and Y. Kawaoka. 2005. Avian flu: isolation of drug-resistant H5N1 virus. Nature 4371108. [DOI] [PubMed] [Google Scholar]
- 24.Maher, J. A., and J. DeStefano. 2004. The ferret: an animal model to study influenza virus. Lab. Anim. 3350-53. [DOI] [PubMed] [Google Scholar]
- 25.McKinlay, M. A. 2001. Recent advances in the treatment of rhinovirus infections. Curr. Opin. Pharmacol. 1477-481. [DOI] [PubMed] [Google Scholar]
- 26.Meister, A., G. Uze, K. E. Mogensen, I. Gresser, M. G. Tovey, M. Grutter, and F. Meyer. 1986. Biological activities and receptor binding of two human recombinant interferons and their hybrids. J. Gen. Virol. 671633-1643. [DOI] [PubMed] [Google Scholar]
- 27.Monto, A. S., and R. J. Whitley. 2008. Seasonal and pandemic influenza: a 2007 update on challenges and solutions. Clin. Infect. Dis. 461024-1031. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson, K. G., J. M. Wood, and M. Zambon. 2003. Influenza. Lancet 3621733-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohmit, S. E., J. C. Victor, E. R. Teich, R. K. Truscon, J. R. Rotthoff, D. W. Newton, S. A. Campbell, M. L. Boulton, and A. S. Monto. 2008. Prevention of symptomatic seasonal influenza in 2005-2006 by inactivated and live attenuated vaccines. J. Infect. Dis. 198312-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavlovic, J., H. A. Arzet, H. P. Hefti, M. Frese, D. Rost, B. Ernst, E. Kolb, P. Staeheli, and O. Haller. 1995. Enhanced virus resistance of transgenic mice expressing the human MxA protein. J. Virol. 694506-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrillo, R. P. 2005. Current treatment of chronic hepatitis B: benefits and limitations. Semin. Liver Dis. 25(Suppl. 1)20-28. [DOI] [PubMed] [Google Scholar]
- 32.Rothberg, M. B., S. D. Haessler, and R. B. Brown. 2008. Complications of viral influenza. Am. J. Med. 121258-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadler, A. J., and B. R. Williams. 2008. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8559-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samuel, C. E., and G. S. Knutson. 1982. Mechanism of interferon action. Kinetics of decay of the antiviral state and protein phosphorylation in mouse fibroblasts treated with natural and cloned interferons. J. Biol. Chem. 25711796-11801. [PubMed] [Google Scholar]
- 35.Shinya, K., M. Ebina, S. Yamada, M. Ono, N. Kasai, and Y. Kawaoka. 2006. Avian flu: influenza virus receptors in the human airway. Nature 440435-436. [DOI] [PubMed] [Google Scholar]
- 36.Sock, E., M. Wegner, E. A. Fortunato, and F. Grummt. 1993. Large T-antigen and sequences within the regulatory region of JC virus both contribute to the features of JC virus DNA replication. Virology 197537-548. [DOI] [PubMed] [Google Scholar]
- 37.Sun, K., and D. W. Metzger. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med. 14558-564. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki, H., R. Saito, H. Masuda, H. Oshitani, M. Sato, and I. Sato. 2003. Emergence of amantadine-resistant influenza A viruses: epidemiological study. J. Infect. Chemother. 9195-200. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki, Y., T. Ito, T. Suzuki, R. E. Holland, Jr., T. M. Chambers, M. Kiso, H. Ishida, and Y. Kawaoka. 2000. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 7411825-11831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svitek, N., P. A. Rudd, K. Obojes, S. Pillet, and V. von Messling. 2008. Severe seasonal influenza in ferrets correlates with reduced interferon and increased IL-6 induction. Virology 37653-59. [DOI] [PubMed] [Google Scholar]
- 41.Svitek, N., and V. von Messling. 2007. Early cytokine mRNA expression profiles predict Morbillivirus disease outcome in ferrets. Virology 362404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takaoka, A., Z. Wang, M. K. Choi, H. Yanai, H. Negishi, T. Ban, Y. Lu, M. Miyagishi, T. Kodama, K. Honda, Y. Ohba, and T. Taniguchi. 2007. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448501-505. [DOI] [PubMed] [Google Scholar]
- 43.Tambyah, P. A. 2008. Update on influenza vaccines. Respirology 13(Suppl. 1)S41-S43. [DOI] [PubMed] [Google Scholar]
- 44.Tomita, Y., and T. Kuwata. 1981. Suppressive effects of interferon on cell fusion by Sendai virus. J. Gen. Virol. 55289-295. [DOI] [PubMed] [Google Scholar]
- 45.Tumpey, T. M., K. J. Szretter, N. Van Hoeven, J. M. Katz, G. Kochs, O. Haller, A. Garcia-Sastre, and P. Staeheli. 2007. The Mx1 gene protects mice against the pandemic 1918 and highly lethal human H5N1 influenza viruses. J. Virol. 8110818-10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tyrrell, D. A. 1959. Interferon produced by cultures of calf kidney cells. Nature 184(Suppl. 7)452-453. [DOI] [PubMed] [Google Scholar]
- 47.van der Laan, J. W., C. Herberts, R. Lambkin-Williams, A. Boyers, A. J. Mann, and J. Oxford. 2008. Animal models in influenza vaccine testing. Expert Rev. Vaccines 7783-793. [DOI] [PubMed] [Google Scholar]
- 48.van Hoeven, N., J. A. Belser, K. J. Szretter, H. Zeng, P. Staeheli, D. E. Swayne, J. M. Katz, and T. M. Tumpey. 14 January 2009. Pathogenesis of the 1918 pandemic and H5N1 influenza virus infection in a guinea pig model: the antiviral potential of exogenous alpha-interferon to reduce virus shedding. J. Virol. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 49.van Riel, D., V. J. Munster, E. de Wit, G. F. Rimmelzwaan, R. A. Fouchier, A. D. Osterhaus, and T. Kuiken. 2006. H5N1 virus attachment to lower respiratory tract. Science 312399. [DOI] [PubMed] [Google Scholar]
- 50.van Riel, D., V. J. Munster, E. de Wit, G. F. Rimmelzwaan, R. A. Fouchier, A. D. Osterhaus, and T. Kuiken. 2007. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am. J. Pathol. 1711215-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoneyama, M., and T. Fujita. 2007. Function of RIG-I-like receptors in antiviral innate immunity. J. Biol. Chem. 28215315-15318. [DOI] [PubMed] [Google Scholar]
- 52.Yu, H., Z. Gao, Z. Feng, Y. Shu, N. Xiang, L. Zhou, Y. Huai, L. Feng, Z. Peng, Z. Li, C. Xu, J. Li, C. Hu, Q. Li, X. Xu, X. Liu, Z. Liu, L. Xu, Y. Chen, H. Luo, L. Wei, X. Zhang, J. Xin, J. Guo, Q. Wang, Z. Yuan, L. Zhou, K. Zhang, W. Zhang, J. Yang, X. Zhong, S. Xia, L. Li, J. Cheng, E. Ma, P. He, S. S. Lee, Y. Wang, T. M. Uyeki, and W. Yang. 2008. Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS ONE 3e2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zurcher, T., J. Pavlovic, and P. Staeheli. 1992. Mechanism of human MxA protein action: variants with changed antiviral properties. EMBO J. 111657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]