Abstract
Although the virological features of serologically silent hepadnaviral primary occult infection (POI) have been relatively well recognized in the woodchuck model of hepatitis B virus infection, the characteristics of accompanying immune responses remain unknown. In this study, the kinetics of woodchuck hepatitis virus (WHV)-specific and generalized (mitogen-induced) T-cell proliferative responses and cytokine expression profiles in circulating lymphoid cells and the liver, along with WHV-specific antibody responses, were investigated during experimentally induced POI and subsequent challenge with a liver-pathogenic dose (>103 virions) or liver-nonpathogenic dose (50 virions) of the same virus. The data revealed that POI, which does not prompt WHV surface antigenemia, antiviral antibody response, and hepatitis or protect from challenge with a liver-pathogenic virus dose, was accompanied by the appearance of a strong WHV-specific T-cell response directed against multiple viral epitopes that intermittently persisted at low levels for up to 10-months during follow-up. Furthermore, immediately after exposure to a liver-nonpathogenic dose of WHV, lymphocytes acquired a heightened capacity to proliferate in response to mitogenic stimuli and displayed augmented expression of alpha interferon, interleukin-12 (IL-12), and IL-2, but not tumor necrosis factor alpha. Overall, the kinetics of WHV-specific and mitogen-induced T-cell proliferative and cytokine responses in POI were closely comparable to those seen in infection induced by liver-pathogenic viral doses. The data demonstrated that virus-specific T-cell proliferative reactivity is a very sensitive indicator of exposure to hepadnavirus, even to small amounts inducing serologically mute infection. They also showed that hepadnaviral POI is not only a molecularly but also an immunologically identifiable and distinctive entity.
Hepatitis B virus (HBV) is a noncytopathic virus causing an infection having several distinctive clinical profiles ranging from acute hepatitis (AH) or chronic hepatitis (CH) to a serologically undetectable, seemingly asymptomatic infection, called an occult HBV infection (OBI) (67). Following exposure to HBV, more than 90% of immunocompetent adults developing AH resolve liver inflammation (4, 17), but they fail to eradicate the virus completely and persistent occult infection seems to invariably follow (52, 57, 67, 68, 77). The remaining ∼10% of individuals develop CH, which is diagnosed when detection of hepatitis B surface antigen (HBsAg) in serum and biochemical and histological indicators of liver inflammation protract for more than 6 months. This form of hepatitis frequently advances to cirrhosis and hepatocellular carcinoma (HCC) (4, 9).
In the last decade, it became apparent that HBV replication commonly persists at low levels after resolution of AH in the context of apparent absence of clinical symptoms. It is also expected that this form of HBV infection could be a consequence of resolution of a clinically asymptomatic, but serologically transiently evident (i.e., serum HBsAg-reactive) exposure to virus. The main features of this residual infection, also called a secondary occult infection (SOI) (49, 50, 54, 57, 67), are as follows: (i) the lack of detectable serum HBsAg, (ii) the presence of antibodies to HBV core antigen (anti-HBc), (iii) the usual but not inevitable occurrence of antibodies to HBsAg (anti-HBs), (iv) the occurrence of HBV DNA in circulation at levels usually not exceeding 200 virus genome equivalents (vge) per ml, and (v) the presence of the viral genome and its replicative intermediates in the liver and circulating lymphoid cells (52, 58, 68). This OBI can be a source of infectious virus available for transmission to healthy individuals through blood and organ donations, as well as a potential cause of liver diseases of seemingly unknown etiology, including HCC (reviewed in references 28 and 57).
The infection of eastern North American woodchucks (Marmota monax) with woodchuck hepatitis virus (WHV), a member of the Hepadnaviridae family (44, 47), provides a natural and highly valuable laboratory model of HBV infection. The molecular, virological, and pathological events that follow WHV invasion are highly compatible to those induced by HBV in humans. Moreover, the understanding of the natural course, virological properties, requirements of transmission, and potential pathological consequences of OBI is owed to a large degree to studies in the woodchuck model of HBV infection (reviewed in reference 49). Among others, it was established that replication of hepadnavirus in SOI progresses not only in the liver but also in the immune system (10, 50, 53, 56; reviewed in reference 49). In woodchucks, this infection persists for life, and virus replicative intermediates, including WHV covalently closed circular DNA and mRNA, are detectable by highly sensitive assays employing PCR combined with identification of the resulting amplicons by nucleic acid hybridization (NAH), i.e., PCR/NAH (10, 53). Moreover, the virus assembled during SOI is infectious, can induce hepatitis and HCC, and is transmissible from mothers to offspring (10, 11, 26, 53). Interestingly, SOI can be reactivated following treatment with an immunosuppressive agent, cyclosporine A, leading to the reappearance of serum WHsAg-positive infection (46). It also is of importance to note that approximately 20% of woodchucks with SOI finally develop HCC (37, 53).
Investigating the woodchuck model of HBV infection, we have recently uncovered yet another form of occult hepadnaviral infection, i.e., primary occult infection (POI) (50; reviewed in references 49 and 54). This form has not yet been identified in humans; however, the existence of HBV DNA-positive and serum HBsAg- and anti-HBc-negative individuals may imply its existence (7, 48, 49, 79). Originally, POI was discovered by studying woodchuck dams with SOI which were found to transmit to their offspring a low-level WHV infection limited to the lymphatic system that occasionally may also engage the liver (10). Subsequently, the same form of occult persistence was experimentally induced by inoculation of immunocompetent, adult woodchucks with WHV doses equal to or lower than 1,000 virions (50). In this experimental situation, POI coincided with WHV replication restricted to the lymphatic system, which with time could spread in some animals to the liver. In general, POI is characterized by the presence of WHV DNA in serum at levels compatible with those occurring in SOI (≤200 vge/ml) and in peripheral blood mononuclear cells (PBMC) (≤103 vge/μg of total DNA), but usually not in the liver, the absence of serological (immunovirological) markers of infection, including WHV surface antigen (WHsAg), antibodies against WHsAg (anti-WHs) and WHV core antigen (anti-WHc), and by normal liver morphology (reviewed in references 49 and 54). The WHV nucleotide sequence in POI has been found to be the wild type (50, 56). Further, the virus persisting in POI remains biologically competent, since when WHV derived from serum or lymphoid cells of animals with POI was administered to naive woodchucks, symptomatic AH was induced (10, 50). Most interestingly, woodchucks with POI are not protected from reinfection with liver-pathogenic doses of WHV (i.e., >103 virions) (50) and develop classical AH after the challenge (10, 53). Although it has previously been documented that POI does not induce a WHV-specific humoral immune response (reviewed in reference 49), it was not clear whether the virus-specific T-cell responses are also lacking in this form of WHV infection.
In regard to T-cell responses accompanying symptomatic HBV infection, the resolution of AH was found to be associated with strong, polyclonal HBV-specific T helper type 1 (Th1) proliferative and cytotoxic T-cell (CTL) reactivities, while the reverse was demonstrated in CH type B (reviewed in references 4, 9, and 21). Hepatic injury in HBV infection is an immune-mediated process. It was established that the CTL response generated against HBV epitopes can inhibit replication of intrahepatic virus, both by directly killing infected cells (22, 41, 68, 74) and by producing antiviral cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (21, 22). In chimpanzees infected with HBV, injection of anti-CD8 antibodies during AH resulted in prolonged viremia and milder hepatic injury, suggesting the dual role of CD8+ T cells in inhibiting HBV replication and mediating liver pathology (74). Further, the Th1 cytokine profile, coinciding with a greater expression of IFN-γ and TNF-α in HBV-specific CD4+ and CD8+ T cells, was found to be associated with resolution of AH (4, 16, 21). On the other hand, the roles of the innate immune responses in the pathogenesis of hepadnaviral hepatitis and determining the outcome of hepadnaviral hepatitis remain uncertain. However, it has been shown that activated natural killer (NK) cells (35), NK T cells (31), and antigen-presenting cells (APCs) (34), as well as cytokines, such as alpha interferon (IFN-α) (76), interleukin-12 (IL-12) (8), and IL-18 (35), can independently inhibit virus replication in HBV transgenic mice, implying the role of innate immunity in controlling HBV infection in this model. Moreover, upregulated hepatic expression of IFN-γ and TNF-α was found to precede HBV-specific T-cell responses and was accompanied by a decrease in HBV replication in the liver in chimpanzees, suggesting that activation of the local innate immunity is, at least partially, successful in limiting virus propagation in acute infection (9, 74). Also, in our recent study analyzing the expression profiles of the genes affiliated with intrahepatic immune responses in woodchucks immediately after inoculation with a liver-pathogenic dose of WHV, evidence of very early augmented transcription of the genes indicative of activation of APCs, NK cells, and NK T cells were found (25). However, this early innate response was transient and unable to prompt a virus-specific T-cell response in a timely manner, conversely to infections induced by many other viral pathogens. In patients who resolved AH, vigorous HBV-specific T-cell proliferative and CTL responses were identified for many years after recovery, suggesting their pivotal role in controlling virus replication during OBI (4, 68).
In the present study, the kinetics of WHV-specific and mitogen-induced (generalized) T-cell proliferative responses, humoral responses, and profiles of cytokine gene expression in lymphoid cells and in the liver, along with immunovirological (serological) and molecular markers of WHV infection, were analyzed in animals with experimentally induced POI and after reexposure of the animals to low (liver-nonpathogenic) or high (liver-pathogenic) doses of the same inoculum. Our study shows that POI, despite the absence of serological, biochemical, and histological indicators of infection, is accompanied by strong T-cell proliferative responses directed toward multiple virus epitopes with kinetics compatible with those observed after infection with liver-pathogenic virus doses leading to classical AH. The appearance of WHV-specific T-cell responses was preceded by a heightened capacity of lymphocytes to proliferate in response to mitogenic stimuli, as we have previously observed in a symptomatic, self-limited AH (24). Additionally, there was an aberrant expression of cytokines known to be involved in the activation of APCs and NK cells. Furthermore, woodchucks with POI challenged with either a liver-nonpathogenic or liver-pathogenic dose of WHV demonstrated similar profiles of virus-specific and mitogen-induced T-cell proliferative responses as those occurring after primary infection. Interestingly, these relatively strong secondary T-cell proliferative responses were unable to provide sufficient help to prevent hepatitis.
MATERIALS AND METHODS
Animals.
Twelve healthy adult woodchucks (Marmota monax) investigated in this study were housed in the Woodchuck Hepatitis Research Facility at Memorial University, St. John's, Newfoundland, Canada. Their WHV-naive status was confirmed by the absence of WHV DNA in randomly selected liver, PBMC, and serum samples analyzed by specific nested PCR/NAH (sensitivity of ≤10 vge/ml) and by repeated serum negativity for WHsAg and anti-WHc. Experimental protocols were approved by the Institutional President's Committee on Animal Bioethics and Care.
Inoculation, challenge, and rechallenge with WHV.
The control group (study group A; Fig. 1) consisted of two healthy woodchucks which were intravenously (i.v.) infected with 1.1 × 1010 DNase digestion-protected vge of WHV/tm3 inoculum (GenBank accession number AY334075) (50) and then injected with 0.5 ml of sterile phosphate-buffered saline (PBS), pH 7.4, at 30 weeks post-primary infection (w.p.p.i.) (subgroup HN [n = 1]) or challenged with a low dose (50 DNase digestion-protected vge) of WHV/tm3 at 26 w.p.p.i. (subgroup HL [n = 1]) (Fig. 1). In addition, two other woodchucks were i.v. infected with a medium dose of WHV/tm3 (1.1 × 106 DNase digestion-protected vge) and challenged with the same dose of WHV/tm3 at 30 w.p.p.i. (subgroup MM [n = 1]) or injected with PBS at 30 w.p.p.i. (subgroup MN [n = 1]).
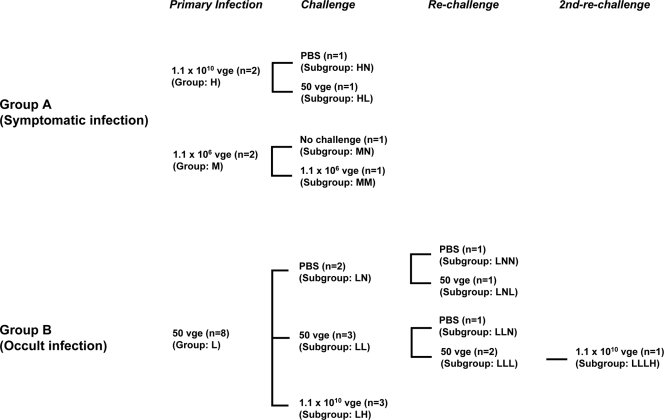
FIG. 1.
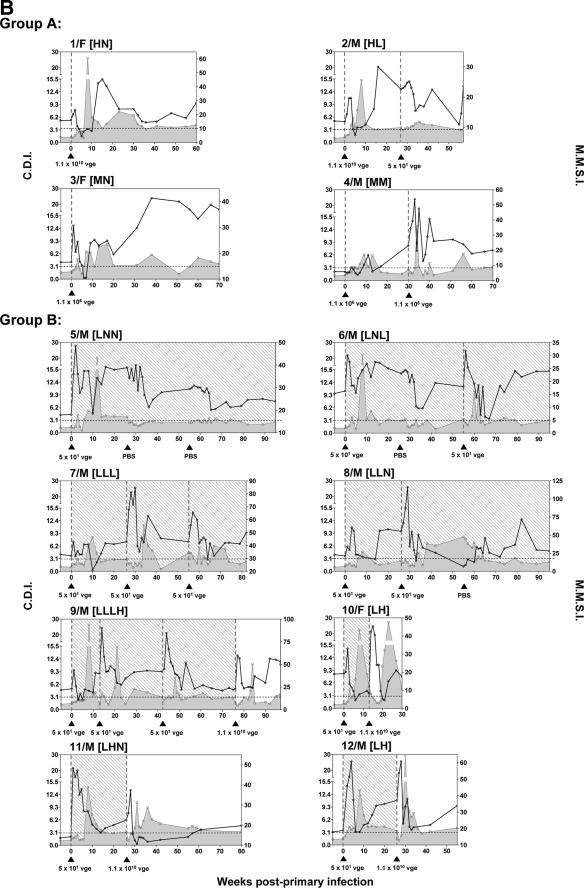
Schematic representation of the experimental protocol used to infect and challenge woodchucks with liver-pathogenic (group A) and liver-nonpathogenic (group B) doses of WHV. In total, 12 WHV-naive, healthy animals were i.v. injected with low (nonpathogenic; 50 vge), medium (liver-pathogenic; 1.1 × 106 vge), or high (liver-pathogenic; 1.1 × 1010 vge) doses of WHV/tm3 inoculum. (A) Animals in study group A (n = 4) were injected with either a high (1.1 × 1010 vge; n = 2) or medium (1.1 × 106 vge; n = 2) dose of WHV. Animals developed classical, serologically detectable WHV infection and were challenged with a low (50 vge; n = 1) or medium (1.1 × 106 vge; n = 1) dose of WHV/tm3 or injected with PBS. (B) Animals in study group B (n = 8) were initially injected with a 50-vge dose of WHV. All animals developed serum WHsAg and anti-WHc negative but WHV DNA reactive POI and were challenged or rechallenged with a low (50 vge; n = 5) or high (1.1 × 1010 vge; n = 4) dose of the same inoculum or injected with PBS. The animals from groups A and B were divided into subgroups according to the dose of WHV used for the challenge or rechallenge or injection with PBS, as described in detail in Materials and Methods. The groups and subgroups were named using the following designations: L, low virus dose (50 vge); M, medium virus dose (1.1 × 106 vge); H, high virus dose (1.1 × 1010 vge); N, no virus (injection with PBS).
In the experimental group (study group B; Fig. 1), eight woodchucks were i.v. injected with 50 DNase digestion-protected vge of WHV/tm3 inoculum. It was previously established that WHV doses below 1,000 virions of WHV/tm3 inoculum, as well as other WHV inocula, consistently induce POI in WHV-naive, immunologically competent woodchucks (50, 56). After 26 w.p.p.i., the animals were challenged with a high dose of WHV/tm3 (1.1 × 1010 vge; group LH [n = 3]) or a low dose of WHV/tm3 (50 vge; group LL [n = 3]) or injected with 0.5 ml of PBS (group LN; n = 2) (Fig. 1). Subsequently, the animals from groups LL and LN were rechallenged at 55 w.p.p.i. with a low dose of WHV (50 vge; subgroup LLL [n = 2] and subgroup LNL [n = 1]) or were injected with PBS (subgroup LLN [n = 1] and subgroup LNN [n = 1]). One animal (animal 9/M [animal 9, a male]) from subgroup LLL was rechallenged for the second time at 77 w.p.p.i. with a high dose of WHV (1.1 × 1010 vge; subgroup LLLH) (Fig. 1).
Sample collection and PBMC isolation.
Blood samples were collected on sodium-EDTA or without the addition of anticoagulant, weekly up to 10 weeks after each injection with virus, then biweekly up to 20 weeks, and then approximately monthly until challenge or autopsy. Serum was separated from untreated blood and stored at −20°C. PBMC were isolated from EDTA-treated samples on Ficoll-Paque Plus (Amersham Pharmacia Biotech, AB, Uppsala, Sweden) as described previously (23). Cell viability was measured by the trypan blue dye exclusion method and normally exceeded 98%.
Serological and molecular markers of WHV infection.
Serial serum samples were examined for the presence of WHsAg, anti-WHc, and anti-WHs using specific enzyme-linked immunosorbent assays as reported previously (50, 53). WHV DNA in serum and liver samples was detected by nested PCR/NAH assays with WHV core (C), surface (S), and X-gene-specific primers (sensitivity of <10 vge/ml or <10 vge/μg of total DNA) (10, 53). As a biochemical measure of liver injury, serum levels of sorbitol dehydrogenase (SDH) were evaluated by an appropriate spectrophotometric assay (3).
Liver biopsy samples and histopathology.
Up to seven liver tissue samples were collected from each animal by surgical laparatomy or at autopsy at the time points indicated in Fig. 2. The samples were routinely processed, embedded in paraffin, and stained (50). Hepatic lesions were graded on the numerical scale of 0 to 3 and reported as the degree of hepatitis as described previously (26, 51, 53).
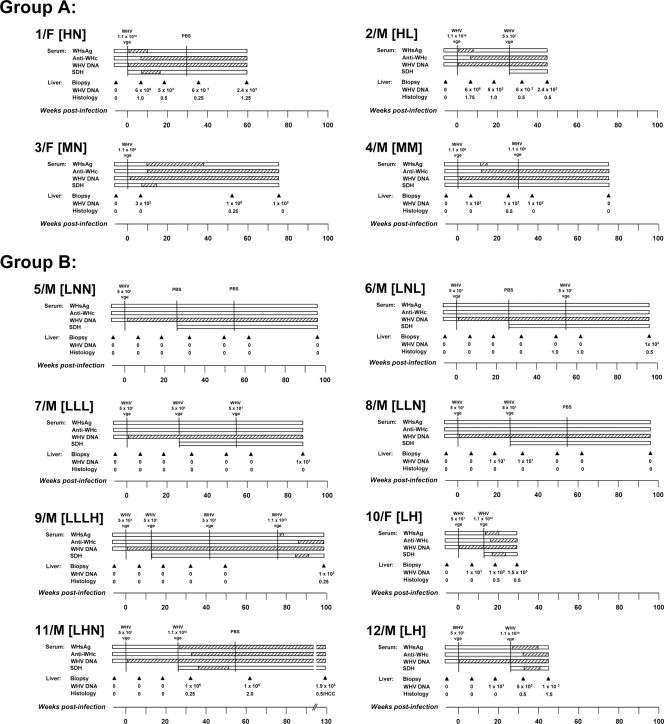
FIG. 2.
Profiles of serological markers of WHV infection and WHV DNA detection in sera and liver tissue samples in animals belonging to study groups A and B. Woodchucks were injected with 5 × 101, 1.1 × 106, or 1.1 × 1010 vge of WHV/tm3 inoculum or with PBS at the time points indicated in the figure by vertical solid black lines. For each animal, the appearance and duration of positivity of WHsAg, anti-WHc, WHV DNA, and SDH in sequential serum samples are represented by hatched horizontal bars. The liver samples were collected at the time points indicated by the solid black arrowheads, and estimated loads of WHV DNA presented in vge/μg of total liver DNA and histological degree of hepatitis graded on a scale from 0 to 3 in each liver sample are presented. HCC was diagnosed in liver tissue collected at autopsy from animal 11/M.
WHV antigens and T-cell mitogens.
Recombinant WHV core protein (rWHc), e protein (rWHe), and X protein (rWHx), used to measure WHV-specific T-cell proliferative responses, were produced in the pET41b(+) Escherichia coli expression system (Novagen, Darmstadt, Germany) and purified using histidine tag affinity chromatography (Qiagen, Mississauga, Canada) as described previously (24, 75). These antigens were tested extensively for nonspecific induction of T-cell proliferation by potential bacterial contamination using PBMC from WHV-naive animals, including woodchucks prior to injection with WHV investigated in this study, and were found entirely free of such impurities (24, 75). In addition, WHsAg was purified from pooled sera from a woodchuck with chronic WHV infection as reported previously (24, 50). Also, a synthetic peptide carrying the WHV T-cell immunodominant epitope located between amino acids 97 and 110 of WHc protein (WHc97-110) (45) was synthesized (Synprep Corporation, Dublin, CA). The recombinant WHV antigens and WHsAg were used at 1 and 2 μg/ml in the T-cell proliferative assays, while WHc97-110 peptide was used at 5 and 10 μg/ml.
The generalized (nonspecific) proliferative capacity of the woodchuck lymphocytes was measured by assessing PBMC proliferation in response to stimulation with mitogens as reported previously (24). For this purpose, concanavalin A (ConA) (Pharmacia Fine Chemicals, Uppsala, Sweden), pokeweed mitogen (PWM) (Phytolacca americana agglutinin; ICN Biochemicals Inc., Aurora, OH), and phytohemagglutinin (PHA) (ICN Biochemicals Inc.) were used in T-cell proliferation assays at five serial twofold concentrations ranging from 1.2 to 20 μg/ml for ConA and PHA and from 0.6 to 10 μg/ml for PWM.
CFSE-based flow cytometry T-cell proliferation assay.
The WHV-specific T-cell proliferative response in woodchuck PBMC after stimulation with different WHV antigens or WHc97-110 peptide was assessed by CFSE-based flow cytometric assay, as described in detail elsewhere (23). Briefly, freshly isolated PBMC were labeled with 5- and 6-carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) at a final concentration of 1 μM and cultured at a density of 3 × 105 cells/well in 48-well tissue culture plates in triplicate wells with tested concentrations of WHV antigens or WHc97-110, as indicated above, or with medium alone as a control. The cells were incubated for 5 days at 37°C, harvested, and washed in PBS with 1 mM EDTA. The data were acquired by using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). The halving of CFSE fluorescence was deconvoluted using CellQuest Pro software (Becton Dickinson) (23). The cell division index (CDI) was defined by dividing the percentage of cells with halved CFSE fluorescence after stimulation with a test WHV antigen by the percentage of cells with halved CFSE fluorescence cultured in medium only. The CDI values of ≥3.1 for rWHc, rWHe, and rWHx and ≥ 2.1 for WHsAg and WHc97-110 peptide were considered a measure of WHV-specific T-cell proliferative response (23). However, for clarity of presentation, only the cutoff value of ≥3.1 was marked in the graphs showing WHV-specific T-cell response.
Adenine incorporation T-lymphocyte proliferation assay.
The generalized proliferative capacity of woodchuck lymphocytes in response to virus-nonspecific stimulation was evaluated using mitogens and [3H]adenine incorporation assay as described previously (23, 24, 39). Briefly, freshly isolated woodchuck PBMC were cultured at a density of 1 × 105 cells/well of a 96-well tissue culture plate in the presence of different concentrations of mitogens as indicated above or in medium alone in triplicate wells. After 5 days in culture, cells were pulsed with 0.1 μCi of [3H]adenine (Amersham Pharmacia Biotech, Uppsala, Sweden) for 12 to 18 h and harvested, and counts per minute (cpm) in each test well and control well were measured. The mean cpm values were calculated by averaging the cpm values from the respective triplicate wells. The stimulation index (SI) was calculated by dividing the mean cpm value obtained after a mitogenic stimulation by the mean cpm value observed without any stimulation (medium only) (24).
Real-time RT-PCR analysis of gene expression.
Sequential liver and PBMC samples collected prior to inoculation with WHV and after challenge with virus were evaluated for the expression of selected cytokine and immune cell marker genes by real-time reverse transcription-PCR (RT-PCR) using a LightCycler (Roche Diagnostics, Mannheim, Germany) (24, 25). In the case of PBMC, 5 × 105 to 1 × 106 freshly isolated cells were suspended in 1 ml of Trizol reagent (Invitrogen) and stored at −20°C. Liver samples collected by biopsy or autopsy were stored at −80°C until RNA isolation with Trizol reagent. After collection of all experimental samples, RNA was extracted simultaneously and reversely transcribed to cDNA as reported previously (24, 25). The expression of woodchuck IFN-α, IFN-γ, TNF-α, IL-2, IL-4, IL-10, and IL-12 was quantified using the equivalent of 50 ng of total RNA and woodchuck gene-specific primer pairs as reported in recent works from this laboratory (24, 25). Transcription of the genes was normalized against expression of woodchuck β-actin, and the expression levels detected after inoculation or challenge with WHV were compared with those determined for the same animal prior to infection to define the increase or decrease as previously described (24).
Statistical analysis.
The two-tailed, unpaired Student t test, with 95% confidence interval, was used to compare the means of the groups. P values of ≤0.05 were considered statistically significant. The values marked with one asterisk were statistically significantly different with a P value of <0.05, the values with two asterisks were significantly different with a P value of <0.005, and the values with three asterisks were significantly different with a P value of <0.0001.
RESULTS
Infection with a low WHV dose induces serologically silent but WHV DNA-positive infection.
Serial serum and liver tissue samples collected from woodchucks inoculated with low (50 vge), medium (1.1 × 106 vge), or high (1.1 × 1010 vge) doses of WHV were examined for serum WHsAg, anti-WHc, and anti-WHs (serological markers) and WHV DNA in serum and hepatic tissue (molecular markers). Animals injected with a high WHV dose (animals 1/F [animal 1, a female] and 2/M [animal 2, a male]) showed, as expected, the appearance of WHsAg at 3 to 4 w.p.p.i., which persisted until 8 to 10 w.p.p.i. (Fig. 2, group A). In animals injected with a medium dose of WHV (animals 3/F and 4/M), serum WHsAg became reactive at 10 and 12 w.p.p.i., and the antigen cleared from circulation at 16 w.p.p.i. in animal 4/M, while it persisted for up to 38 w.p.p.i. in animal 3/F. All four animals developed anti-WHc. Animals 1/F and 2/M developed anti-WHc beginning from 6 to 8 w.p.p.i., while animals 3/F and 4/M developed anti-WHc from 10 to 12 w.p.p.i. All animals remained antibody reactive until the end of follow-up (Fig. 2, group A). Anti-WHs appeared in woodchuck 1/F and was periodically detectable since 14 w.p.p.i., while the other three woodchucks remained antibody nonreactive (data not shown). There was no difference in the time of the appearance of WHV DNA in serum in animals injected with high or medium doses of WHV, and the genome became detectable starting from 1 w.p.p.i. and persisted throughout the entire observation period. The levels of WHV DNA in liver biopsy samples obtained at 7 w.p.p.i. from animals injected with a high dose were 6 × 106 vge/μg total DNA and between 1 × 102 and 3 × 102 vge/μg total DNA in animals injected with a medium WHV dose. After resolution of acute infection, hepatic load of WHV DNA usually ranged between 1 × 102 and 6 × 103 vge/μg DNA and persisted to the end of follow-up; however, it transiently rose up to 1 × 106 vge/μg DNA in the liver sample of animal 3/F acquired at 55 w.p.p.i. (Fig. 2, group A).
The histological features of AH were found in liver biopsy samples collected at 7 w.p.p.i. from woodchucks 1/F and 2/M inoculated with a high dose of WHV (Fig. 2, group A). These alterations coexisted with serum WHsAg positivity in both animals and elevated levels of SDH in woodchuck 1F (SDH was measured in woodchuck 2/M after challenge with WHV). In woodchucks 3/F and 4/M injected with a medium dose of WHV, liver biopsy samples obtained at 7 w.p.p.i. showed normal liver morphology at a time when serum WHsAg was negative and SDH levels remained normal (Fig. 2, group A), indicating that these samples were acquired prior to the acute phase of infection. Following clearance of WHsAg, minimal to moderate intermittent liver inflammation remained evident in all animals, similar to what has been observed in previous studies (11, 26, 53). Challenging animal 2/M with 50 vge and animal 4/M with 1.1 × 106 vge did not induce the reappearance of WHs antigenemia or increase WHV DNA load in serum or hepatic tissue (Fig. 2, group A).
In contrast to the woodchucks injected with high or medium doses of WHV, inoculation with 50 vge initiated POI in all eight animals (group B), as evidenced by the detection of WHV DNA in serum beginning 1 to 2 w.p.p.i. and its long-term persistence at levels of ≤100 vge/ml in the absence of detectable WHsAg and anti-WHc (Fig. 2, group B). This serologically mute infection was accompanied by the absence of WHV DNA in the liver and entirely normal liver morphology, as it was evident in animal 5/M followed for up to 96 w.p.p.i. (Fig. 2, group B). It is of note that although the liver remained virus genome negative, WHV DNA was detected in the bone marrow collected at autopsy of this animal (data not shown). The above findings were in full agreement with those reported previously for woodchucks with naturally acquired or experimentally induced POI (10, 50, 56; reviewed in reference 49).
The animals with POI challenged once or twice with a low, liver-nonpathogenic dose of the virus, i.e., animals 6/M (subgroup LNL), 7/M (subgroup LLL), 8/M (subgroup LLN), and 9/M prior to challenge with a high WHV dose (subgroup LLLH), remained WHsAg and anti-WHc nonreactive and did not show a measurable increase in the level of WHV DNA in serum, and their livers remained WHV DNA nonreactive and free of histologically evident alterations (Fig. 2, group B). The exception was animal 8/M (subgroup LLN) in which WHV DNA transiently appeared at levels of ∼100 vge/μg total DNA in two sequential liver biopsy samples, one collected at 17 w.p.p.i. and the second collected 6 weeks after the first challenge with WHV (Fig. 2, group B). This intermittent detection of the WHV genome was not accompanied by liver injury, as confirmed by histology examination and by normal SDH levels. Further, there was an unexplained appearance of mild liver inflammation in animal 6/M at 44 w.p.p.i. that protracted until the autopsy but was not accompanied by elevated SDH levels. As mentioned above, this inflammation also did not coincide with detection of WHV DNA in the liver (Fig. 2, group B).
As has been previously observed for either naturally acquired or experimentally induced POI, the challenge with high doses of WHV initiated serum WHsAg-positive infection and hepatitis (10, 50). Similarly, all animals with POI which were challenged with a high dose of WHV in the current study, i.e., animals 9/M (subgroup LLLH), 10/F and 12/M (subgroup LH), and 11/M (subgroup LHN) (Fig. 2, group B), developed WHs antigenemia and became anti-WHc reactive, as well as demonstrated transiently elevated levels of serum SDH, implying a self-limiting episode of acute liver injury. In animals 9/M, 10/F, and 12/M, WHsAg eventually vanished, while it persisted to the end of the observation period in animal 11/M, i.e., up to 104 weeks after administration of a high dose of virus (Fig. 2, group B). This animal developed CH and finally HCC. After challenge of animals with POI with a high dose of WHV, the liver tissue biopsy samples demonstrated histological features of hepatitis, except for the sample obtained 6 weeks postchallenge from animal 11/M, where only minimal inflammatory alterations were found (Fig. 2, group B). The same liver samples became WHV DNA reactive and remained virus genome positive until the autopsy. In summary, woodchucks infected, challenged, or rechallenged with 50 vge of otherwise pathogenic WHV acquired sustained, serologically, biochemically, and histologically silent but molecularly evident POI. The animals were not protected from infection with a high dose of the same virus and acquired acute hepatitis, as previously reported (10, 50, 56).
POI and serologically apparent WHV infection are accompanied by WHV-specific T-cell proliferative responses that were comparable in magnitude.
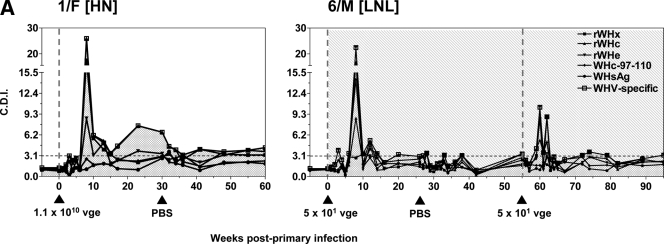
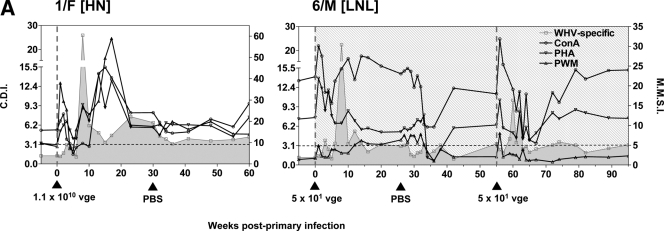
To determine the features of WHV-specific T-cell proliferative response in the course of POI and to compare them with those accompanying serologically evident infection associated with hepatitis, sequential PBMC samples were ex vivo exposed to WHV antigens or WHc97-110 peptide, and their proliferation was measured by a CFSE-based flow cytometric assay. As illustrated in Fig. 3A for animals 1/F and 6/M infected with 1.1 × 1010 vge and 50 vge of WHV, respectively, both virus doses induced strong, multispecific T-cell proliferative responses of comparable magnitudes between 8 and 15 w.p.p.i., which persisted at lower levels to the end of follow-up or until WHV challenge. In animal 6/M, which was challenged with 50 vge at 55 w.p.p.i., the augmented T-cell proliferation in response to WHV antigens reemerged between 59 to 62 w.p.p.i. (Fig. 3A).
FIG. 3.
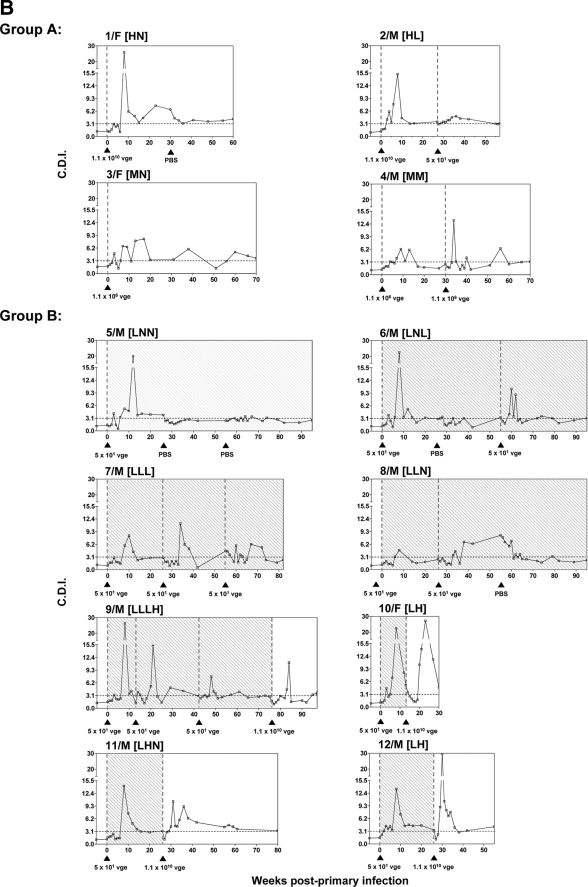
Kinetics of WHV-specific T-cell proliferative responses in woodchucks infected with liver-pathogenic and -nonpathogenic doses of WHV. The animals were infected with WHV, and serial PBMC samples were isolated, stimulated in vitro with four different WHV antigens or WHc97-110 peptide, and analyzed for resultant proliferation using CFSE-based proliferation assay to define the cell division index (CDI). The dashed vertical lines and solid black arrowheads show the time points at which animals were injected or challenged with WHV, while the dashed horizontal line represents the cutoff value for positive virus-specific T-cell response. The duration of POI induced in woodchucks belonging to study group B, identified by detection of WHV DNA in serum, is shown by the stippled background. (A) Representative profiles of WHV-specific T-cell proliferative responses in animal 1/F infected with a high, liver-pathogenic dose (1.1 × 1010 vge) and in animal 6/M inoculated with a low, liver-nonpathogenic dose (50 vge) of WHV after cell stimulation with rWHc, rWHe, rWHx, WHc97-110 peptide, or WHsAg, as determined by the CFSE assay. The results are represented as CDIs. The highest CDI value given by any of the WHV antigens tested or WHc97-110 peptide at a given time point was considered a measure of the WHV-specific T-cell response and is indicated by an open square and represented as a solid gray shaded area. (B) The profiles of WHV-specific T-cell responses identified in individual animals infected with a high or medium dose of WHV (group A) or with a low WHV dose (group B). The PBMC collected from these animals were stimulated with rWHc, rWHe, rWHx, WHc97-110 peptide, or WHsAg and the highest CDI value given by any of these antigens for each time point is represented as WHV-specific T-cell response as explained above. The subgroup designations are explained in the legend to Fig. 1.
In animals infected with a high or medium dose of WHV (Fig. 3B, group A), the peak of WHV-specific T-cell response occurred between 8 and 15 w.p.p.i., and the reactivity remained detectable, although at much lower levels up to the end of the observation period, as in woodchucks 1/F and 3/F, or until challenge with WHV, as in woodchucks 2/M (subgroup HL) and 4/M (subgroup MM). It is of note that challenging woodchuck 2/M with 50 vge did not induce a significant increase in the magnitude of WHV-specific response, while challenging woodchuck 4/M with another 1.1 × 106 vge dose caused strong, but delayed, secondary T-cell response directed against virus antigens (Fig. 3B, group A). These findings were closely compatible with those recently reported for woodchucks infected and challenged with high, liver-pathogenic doses of WHV (24).
Overall, the PBMC samples obtained from animals inoculated with 50 vge of WHV which established POI displayed virus-specific T-cell response profiles that were similar to those found in woodchucks injected with high or medium doses of the inoculum. Thus, all animals infected with low virus doses (Fig. 3B, group B) responded by generating WHV-specific lymphocyte proliferative reactivity with magnitudes comparable to those observed in the animals infected with liver-pathogenic doses between 8 and 15 w.p.p.i. Further, the challenge or rechallenge of the woodchucks with POI with a low dose of WHV, i.e., animals 6/M (subgroup LNL), 7/M (subgroup LLL), 8/M (subgroup LLN), and 9/M before challenge with a high dose (subgroup LLLH), induced WHV-specific T-cell responsiveness profiles similar to those of animals injected and then challenged with a high or medium dose; the T-cell responsiveness peaked between 6 and 12 weeks after each exposure to virus. In the animals with POI, including animal 5/M (subgroup LNN), the virus-specific reactivity was intermittently detectable throughout the entire observation period. Further, the virus-specific T-cell response profiles found in woodchucks infected in the first instance with a low dose and then challenged with a high dose, i.e., animals 9/M (subgroup LLLH), 10/F and 12/M (subgroup LH), and 11/M (subgroup LHN), were again similar to those observed during primary infection induced by a high, medium, or low dose of WHV and than challenged. Taken together, the results clearly showed that both primary and secondary virus-specific T-cell proliferative responses in POI are strong and multispecific despite the lack of serological evidence of infection or histological and biochemical signs of hepatitis and that their kinetics closely follow those occurring after infection with much greater (∼105- or 1010-fold) doses of the virus.
Heightened T-cell proliferation in response to mitogenic stimuli follows WHV inoculation independently of virus dose.
Following our previous findings that WHV invasion is almost immediately followed by augmented generalized, virus-induced but virus-nonspecific T-lymphocyte proliferation response (24), PBMC collected during POI and control symptomatic WHV infection were cultured in the presence of twofold serial dilutions of ConA, PHA, or PWM and assessed for their ability to proliferate in an adenine incorporation assay. As shown in Fig. 4A for animals 1/F and 6/M, both high and low doses of WHV induced heightened T-cell proliferation between 1 and 3 w.p.p.i. after stimulation with all mitogens tested. This increased responsiveness subsided after 4 to 6 w.p.p.i., at the time when the virus-specific T-cell response appeared, and then rebounded when the virus-specific response subsided. A similar pattern of mitogen-induced lymphocyte proliferation was also observed after reexposure of woodchucks with POI to a low dose of WHV (Fig. 4A, animal 6/M). Since the kinetics of proliferative responses following ConA, PHA, and PWM stimulation were comparable (Fig. 4A), only the ConA-induced profiles are shown for other animals in Fig. 4B.
FIG. 4.
Comparative kinetics of mitogen-induced (virus-nonspecific) and WHV-specific T-cell proliferative responses in woodchucks infected with high (liver-pathogenic) and low (liver-nonpathogenic) doses of WHV. Freshly isolated PBMC were stimulated with twofold serial dilutions of ConA, PHA, and PWM, and the resultant proliferation was measured by an adenine incorporation proliferation assay to determine the stimulation index (SI) for each concentration of mitogens tested. The SI values obtained for the five serial concentrations of a mitogen were averaged to define the mean mitogenic stimulation index (M.M.S.I.), which are presented by the solid black lines. The dashed vertical lines and solid black arrowheads show the time points at which animals were injected or challenged with WHV, while the dashed horizontal line represents the cutoff value for the virus-specific positive T-cell response. The WHV-specific T-cell response profile for each animal is shown with a shaded area under a solid gray line, and the duration of POI is shown by the stippled background. The CDI values for WHV-specific T-cell response and M.M.S.I. values after mitogenic stimulation are shown on the left and right y axes, respectively. (A) Representative profiles of lymphocyte proliferative responses induced by ConA, PHA, and PWM and WHV-specific T-cell response in animal 1/F infected with a liver-pathogenic dose (1.1 × 1010 vge) and in animal 6/M inoculated with a liver-nonpathogenic dose (50 vge) of WHV after cell stimulation with different mitogens, as explained above. (B) Comparative profiles of ConA-induced and WHV-specific lymphocyte proliferative responses in individual woodchucks infected with a high or medium dose of WHV (group A) or with a low dose of WHV (group B).
As shown in Fig. 4B and previously reported (24), PBMC collected from animals after injection with a high (animals 1/F and 2/M) or medium (animals 3/F and 4/M) dose of WHV displayed heightened capacity to proliferate in response to mitogenic stimuli between 1 and 3 w.p.p.i. This proliferative reactivity subsided between 4 and 6 w.p.p.i. when the virus-specific T-cell response appeared and then rose again when the virus-specific response declined. Further, challenge of animal 4/M infected with a medium dose with another medium dose of the same inoculum induced a T-cell proliferative response profile similar to that observed after primary exposure to WHV (Fig. 4B, group A). Interestingly, infection with a low, liver-nonpathogenic dose of WHV also induced immediate augmentation in lymphocyte proliferation in response to nonspecific stimuli, which declined when virus-specific T-cell reactivity appeared and subsequently increased again when the virus-specific response declined (Fig. 4B, group B). Similar transiently augmented mitogen-induced lymphocyte responsiveness was detected immediately after each challenge or rechallenge of the animals with POI with either a low or high dose of WHV. Taken together, the results showed that WHV invasion leading to establishment of either POI or serologically evident infection accompanied by hepatitis coincides with the highly compatible profiles of virus-specific and generalized (virus-nonspecific) T-cell proliferative responses.
Induction of POI and subsequent challenge with WHV are accompanied by aberrant expression of cytokines in peripheral lymphoid cells.
Freshly isolated, unmanipulated PBMC (i.e., not subjected to ex vivo stimulation with either mitogens or WHV-specific antigens) collected from woodchucks after infection with a low dose of WHV and subsequent challenge with either a high or low dose of the same virus were quantified for the expression of selected cytokines by real-time RT-PCR. Considering the opposing profiles of WHV-specific and mitogen-induced lymphocyte responses observed in this study and taking under consideration previous findings for woodchucks infected and challenged with high doses (≥1.1 × 1010 vge) of WHV (24), the progression of POI was divided into four phases: (i) the phase before infection (phase A), (ii) the phase between 1 and 3 w.p.p.i. characterized by the heightened mitogen-inducible T-cell proliferative reactivity and essentially absent WHV-specific T-cell response (phase B), (iii) the phase between 4 and 6 w.p.p.i. accompanied by decreasing nonspecific and undetectable virus-specific T-cell proliferative responses (phase C), and (iv) the phase between 7 and 15 w.p.p.i. with essentially absent nonspecific T-cell proliferative reactivity and arising and then peaking WHV-specific T-cell response (phase D). Similarly, because of the overall opposing kinetics of the specific and nonspecific T-cell responses identified after challenge of animals with POI with WHV, the observation period after challenge was also divided into phases B to D (Fig. 5).
FIG. 5.
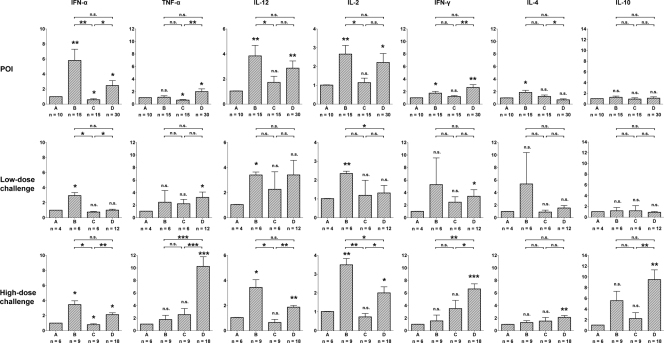
Quantitative analysis of cytokine gene expression in circulating lymphoid cells in woodchucks during different phases of WHV POI and the animals' subsequent challenge with either a low (liver-nonpathogenic) or high (liver-pathogenic) dose of WHV. The expression of IFN-α, TNF-α, IL-12, IL-2, IFN-γ, IL-4, and IL-10 was analyzed in freshly isolated PBMC collected from animals with POI (n = 6) and after challenge with either a low (low-dose challenge; n = 3) or high (high-dose challenge; n = 3) dose of the virus. After each primary or subsequent exposure to WHV, the time line of infection was divided into four phases (phases A, B, C, and D) according to the distinct profiles of WHV-specific and mitogen-induced T-cell responses as described in Results. The number (n) under each bar shows the number of PBMC samples examined. The level of expression of each cytokine was normalized against the level of β-actin and then compared to the level observed prior to primary infection (phase A) to calculate the increase or decrease in expression. The P values above each bar represented by asterisks were obtained by comparing the mean expression level of a given gene during phase B, C, or D with the mean level of expression determined for the gene in phase A, while the P values shown at the top of each panel were obtained by comparing with each other the mean expression level of a given gene determined for phases B, C, and D. Values marked with one asterisk were significantly different at a P value of ≤0.05, values with two asterisks were significantly different at a P value of ≤0.005, and values with three asterisks were significantly different at a P value of ≤0.0001. n.s., not significantly different.
As shown in the top row of Fig. 5, inoculation with a low, liver-nonpathogenic dose of WHV induced significantly higher expression of IFN-α (P = 0.0092), IL-12 (P = 0.0089), and IL-2 (P = 0.005) along with elevated transcription of IFN-γ (P = 0.0196) and IL-4 (P = 0.0358) during phase B than expression levels before infection (phase A). Thus, increased mRNA levels of these genes were detected in intact PBMC at the time when these cells displayed heightened proliferation in response to mitogenic but not to virus-specific stimuli (Fig. 4). Subsequently, during phase C, when the mitogen-induced T-cell proliferative responsiveness subsided, the expression of the cytokines mentioned above, except IFN-γ and IL-4, significantly decreased. Most interestingly, the level of transcription of TNF-α, which remained at approximately the same level in phases A and B, significantly (P = 0.021) declined in phase C (Fig. 5, top row). Finally, the expression of IFN-α, TNF-α, IL-12, IL-2, and IFN-γ, but not that of IL-4 and IL-10, was synchronously upregulated during phase D, coinciding with the appearance and peaking of the WHV-specific T-cell response. Following challenge of the animals with POI with a low dose (i.e., 50 vge) of WHV (Fig. 5, middle row), overall, the patterns of expression of the cytokines detected during phases B to D were similar to those identified after primary injection with the same low dose, although the differences in transcription levels between different phases after challenge were less pronounced for some cytokines.
When the woodchucks with POI were challenged with a high dose (i.e., 1.1 × 1010 vge) of WHV (Fig. 5, bottom row), the expression of IFN-α (P = 0.0167), IL-12 (P = 0.0296), and IL-2 (P = 0.0051) was again elevated during phase B but then subsided in phase C, while transcription of TNF-α remained unchanged until phase D. Similarly, during phase D, which corresponded to serum WHsAg-positive infection accompanied by histologically evident hepatitis (Fig. 2, group B) and the peak WHV-specific T-cell response, the expression of TNF-α (P = 0.001) and IFN-γ (P = 0.0063) became significantly augmented (Fig. 5, bottom row). Overall, the increases in the mRNA levels of the cytokines were much greater than those detected in phase D of POI (Fig. 5, bottom row) or in animals with POI which were challenged with low virus dose (Fig. 5, middle row). After challenge with a high dose, the synchronously upregulated expression of IFN-α, TNF-α, IL-12, IL-2, and IFN-γ was also accompanied by the significantly greater levels of transcription of IL-4 (P = 0.009) and IL-10 (P = 0.0097) than those accompanying phase D after primary infection or challenge with a low dose of virus (Fig. 5, top row and middle row, respectively). Collectively, quantitative analysis of cytokine expression in circulating lymphoid cells serially acquired during POI revealed that the infection with low, liver-nonpathogenic dose of hepadnavirus not only almost immediately enhanced T-cell responsiveness to mitogenic stimuli but also upregulated transcription of a number of functionally important cytokines in unmanipulated cells, although, as in serologically evident infection caused by liver-pathogenic doses of the virus (24), POI did not induce TNF-α expression in circulating lymphoid cells that are known to be essential for activation of APCs and NK cells.
Hepatic expression of IFN-α but not IFN-γ or TNF-α is upregulated during POI.
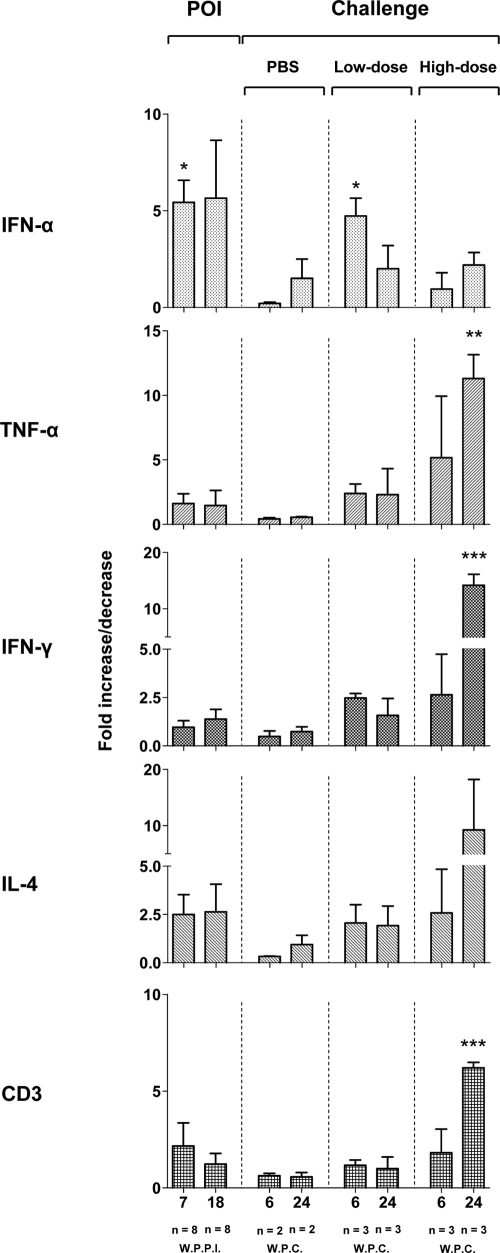
The liver biopsy samples obtained at 7 and 18 w.p.p.i. with a low dose and after challenge of the animals with POI with either a low or high dose of WHV or after injection with PBS for a control, which were collected at 6 and 24 weeks postchallenge (w.p.c.), were evaluated for expression of IFN-α, TNF-α, IFN-γ, IL-4, and CD3. The results were compared with the transcription levels detected for the same genes in the liver samples obtained from the same animals prior to the first injection with virus. As shown in Fig. 6, the liver samples collected at 7 w.p.p.i. displayed significantly higher mRNA levels of IFN-α (P = 0.0001), in the absence of significantly elevated TNF-α, IFN-γ, IL-4, or CD3 expression, than the biopsy samples collected prior to infection (Fig. 6). Furthermore, the intrahepatic expression of the genes examined, including IFN-α, was not significantly upregulated at 18 w.p.p.i. as well as after control injection with PBS, compared to the levels detected in healthy animals prior to injection with WHV. Comparable patterns of cytokine and CD3 expression were also observed in the liver biopsy samples from the animals with POI after challenge with a low dose of WHV (Fig. 6). As anticipated, liver samples collected at 24 w.p.c., but not those challenged with a high dose that were obtained at 6 w.p.c., showed strong expression of TNF-α (P = 0.0032), IFN-γ (P = 0.0001), and CD3 (P = 0.0003), but not IFN-α. Collectively, these results showed that POI is associated with elevated expression of IFN-α in the absence of TNF-α and IFN-γ response and, most intriguingly, in the absence of detectable WHV in the liver.
FIG. 6.
Hepatic expression of selected cytokines and CD3 during POI and after challenge with either a high or low dose of WHV or injection with PBS. The levels of IFN-α, TNF-α, IFN-γ, IL-4, and CD3 mRNA were measured by real-time RT-PCR in liver samples collected from woodchucks during POI (n = 8) and from woodchucks after challenge with a low (n = 3) or high (n = 3) dose of WHV or after control injection with PBS (n = 2). The numbers (n) under each bar at the bottom of the figure indicate, respectively, the number of liver biopsy samples analyzed and the time of their collection presented in weeks after primary infection (W.P.P.I.) or in weeks postchallenge (W.P.C.). The mean expression level of a particular gene during POI or after challenge was normalized against β-actin expression and then compared with the mean determined in the liver samples collected prior to infection. Values marked with one asterisk were significantly different at a P value of ≤0.05, values with two asterisks were significantly different at a P value of ≤0.005, and values with three asterisks were significantly different at a P value of ≤0.0001.
DISCUSSION
In this report, POI experimentally induced by exposure of woodchucks to a low, liver-nonpathogenic dose of WHV was found to be accompanied by the following: (i) induction of virus-specific T-cell proliferative response against multiple epitopes of structural and nonstructural proteins of WHV in the absence of virus-specific humoral response; (ii) induction of a heightened capacity of circulating lymphoid cells to proliferate in response to mitogenic stimuli soon after exposure to WHV, similar to that seen for infection induced by liver-pathogenic virus doses (24), and (iii) aberrant expression in the peripheral immune cells of cytokines mediating early innate immune responses. In addition, the kinetics of secondary T-cell proliferative responses, induced in woodchucks with POI by challenge with either liver-nonpathogenic or -pathogenic WHV doses, were highly comparable to those seen after primary WHV infection. However, this secondary T-cell response failed to protect animals with POI against development of AH following exposure to a liver-pathogenic dose of the virus.
Findings from studies of other viral infections, such as hepatitis C virus (HCV) (32, 38), human immunodeficiency virus (HIV) (66), and simian immunodeficiency virus (42) infections, parallel the results from our study in that inoculation with a subpathogenic quantity of WHV induced a virus-specific T-cell response without stimulating a virus-specific antibody response. In this context, the sexual partners of patients with acute HCV or HIV infections (32, 40), individuals seronegative for HCV but with a history of a HCV-contaminated needle stick injury (38), health care workers with occupational exposure to the bodily fluids of HIV-infected individuals (65), or infants born to HIV-positive mothers (14), all of whom remain persistently seronegative for antiviral antibodies display virus multiepitope-specific CD8+ CTL and CD4+ T-cell proliferative responses. Similarly, in our study, all eight animals exposed to a low virus dose established persistent POI in the face of strong WHV-specific T-cell proliferative reactivity, the absence of detectable anti-WHc or anti-WHs antibodies, and no liver injury. It has been previously hypothesized that following exposure to low doses of hepadnavirus, although the T-cell response is induced, it is suboptimal and unable to prompt liver injury as well as to protect from challenge with a pathogenic dose of the virus (10, 38). Further, in woodchucks infected with WHV, virus-specific T-cell response during POI was lingeringly detectable, albeit intermittently and at a magnitude lower than that detected in SOI continuing after resolution of AH. This suggests protracted stimulation of this response, probably by low-level WHV replication, as observed after recovery from experimental AH in woodchucks (53) and in patients convalescing from acute HBV infection (68).
Overall, the roles of hepadnavirus-specific T-cell responses in the pathogenesis and control of HBV infection are well recognized. Studies of humans, chimpanzees, and woodchucks have also uniformly revealed that T-cell responsiveness to hepadnavirus is delayed until 6 to 12 weeks postinfection (w.p.i.) compared to the responses observed following invasions with other viral pathogens, such as lymphocytic choriomeningitis virus (LCMV) (19), murine cytomegalovirus (MCMV) (69), and influenza virus (18; reviewed in reference 70). This delay in the appearance of hepadnavirus-specific T-cell reactivity was believed to be a consequence of the long incubation period of the virus or an inappropriate innate cytokine microenvironment during the preacute phase of infection (4). In our most recent study (24), we have shown that this delay in the appearance of virus-specific T-cell proliferative response in WHV infection is preceded by virus-induced but nonspecific, aberrant T-cell activation occurring between 1 and 6 w.p.i., which is associated with the impaired expression of cytokines, especially that of TNF-α and IFN-γ, and followed by apoptotic death of activated T cells. In other viral infections, the early and synchronous expression of the above cytokines, together with IFN-α, IL-2, and IL-12, initiate the innate responses leading to the differentiation and maturation of dendritic cells to professional APCs, as well as to the activation of NK cells to produce IFN-γ and acquire cytotoxic capacity (30, 33, 62, 63). The proper activation of this innate response guides the rise of adaptive immunity by stimulating generation of antigen-specific T and B lymphocytes (2, 30, 33). On the other hand, APCs and NK cells generated in the absence of TNF-α or IFN-α have impaired T-cell stimulatory capacities and display reduced antiviral activities (13, 29, 43). Thus, similar to our observations made during the period preceding development of acute WHV infection (24), the data from animals with experimentally induced POI suggest that the induction of a strong, nonspecific T-cell proliferative response and the aberrant expression of innate cytokines soon after exposure to a low, liver-nonpathogenic dose of hepadnavirus also delay the appearance of a virus-specific T-cell immune response. It is noteworthy that irrespective of the WHV dose, strong T-cell proliferation directed against multiple antigenic WHV epitopes occurred only after the synchronous expression of IFN-α, TNF-α, IL-12, IFN-γ, and IL-2 detected from 7 w.p.i. onwards. This provides indirect validation that the initiation of antiviral T-cell response in WHV infection has to be accompanied by coordinated expression of appropriate cytokines in immune cells, albeit the magnitudes of IFN-γ and TNF-α transcription in the PBMC from animals with POI were significantly lower (P = 0.024 and P = 0.0075, respectively) than those detected during acute hepatitis.
Despite the fact that hepadnaviral core protein is recognized as a strong T-cell-independent antigen (55), the absence of anti-WHc antibody responses in the presence of detectable virus-specific T-cell reactivity after infection with a low dose of WHV, either following primary exposure or challenge, is difficult to explain. Nonetheless, similar observations have been made for seronegative HCV- and HIV-infected individuals (32, 38). Furthermore, infection with a low dose of LCMV, resulting in the inability to detect virus antigens in secondary lymphoid organs, was suggested to hamper the development of the T-cell-independent antibody response (61), implying that similarly minute quantities of WHcAg might fail to induce the specific antibody response in woodchucks during POI. Additionally, the initiation of T-cell-dependent B-cell activity requires the presence of properly activated CD4+ T cells in the context of the appropriate cytokine milieu enriched with IL-10 and IL-4 (27). The analysis of expression of cytokines in circulating lymphoid cells obtained during POI in our study revealed that after initial exposure to or challenge with a low dose of WHV, expression of IL-4 and IL-10 remains unchanged, even during phase C (Fig. 5), equivalent to the acute phase of infection caused by liver-pathogenic dose of WHV (24), when expression of IFN-α and TNF-α rebounds. This may imply that the absence of anti-WHc response could also stem from the inappropriate cytokine milieu. This line of reasoning is indirectly supported by the fact that woodchucks with POI challenged with a high dose of WHV, which led to increased expression of IL-4 and IL-10 in the PBMC during the acute phase of infection, showed anti-WHc antibody response.
The capacity of lymphoid cells to proliferate in response to ex vivo stimulation with nonspecific mitogens provided valuable insight into the generalized immune competency of the cells during infections with a variety of microbial pathogens, including HIV (64), measles virus (60), MCMV (1), Schistosoma japonica (20), and recently, WHV (24). Similar evaluations of PBMC from patients with CH type B have shown a decreased proliferative capacity of lymphocytes after stimulation with mitogens (15, 72). In our study, irrespective of the quantity of invading virus, each primary or subsequent exposure to WHV immediately induced a heightened capacity of lymphocytes to proliferate in response to mitogen stimulation, which was also accompanied by elevated transcription of genes encoding IFN-α, IL-12, and IL-2 in unmanipulated circulating lymphoid cells. The roles of IL-2 in T-cell and NK cell growth and survival are well acknowledged, and the absence of this cytokine is associated with decreased proliferative responsiveness of these cells (36, 59, 71). However, it is of note that 5 to 10% of PBMC is comprised of NK cells, which can undergo blastogenesis and incorporate greater amounts of radioactive thymidine in vitro in the presence of increased IFN-α expression, as is the case in LCMV and MCMV infections (5, 6, 62, 63, 73). Further, NK cells cultured in the presence of both IFN-α and IL-12 demonstrate greater rates of blastogenesis than those cultured with IFN-α alone and can promote mitogen-induced proliferation of peripheral T cells, probably due to their increased ability to produce IL-2 (62, 63). From this study and from the data on the mitogen-induced lymphocyte hyperresponsiveness and concurrent cytokine expression observed immediately after exposure to WHV, it could be inferred that the elevated expression of cytokines is behind the early, heightened proliferative capacity of T cells in our study.
Although the appearance and magnitude of T-cell responses during POI are similar to those observed during symptomatic WHV infection, these responses do not protect against hepatitis after challenge with large amounts of the virus. It has been postulated that the phenotypic heterogeneity of the cells mediating cellular and humoral immune responses can be dictated by the dose of antigen, with small doses usually favoring the development of cellular immune responses (27, 78). In this context, it is of note that in other studies it has been shown that CD4+ T-cell responses generated during vesicular stomatitis virus infection induced by low doses tend to inhibit the virus-specific CD8+ T-cell response (12). This may suggest that infection with a low dose of WHV could induce a defective CD8+ T-cell response incapable of protecting the host against challenge with liver-pathogenic doses of WHV. Nonetheless, patients who recovered from AH type B and developed anti-HBs are protected from development of hepatitis after reexposure to HBV, as those immunized with HBV vaccine, implying the important protective role of anti-HBs response. As we have established before (10, 50) and confirmed in the course of the current study, woodchucks infected with low doses of WHV do not develop detectable anti-WHs or anti-WHc. Taken together, this implies that not only improper activation of T-cell responses but also the absence of induction of specific antibodies may contribute to the failure of the immune system to provide protective immunity in POI.
The analysis of cytokine gene expression in liver biopsy samples collected during POI and after challenge with a low dose of WHV showed that at 6 to 7 w.p.p.i., transcription of IFN-α, but not IFN-γ or TNF-α, was significantly increased. The evidence of IFN-α expression in the liver, along with the absence of lymphocytic infiltrations, as evidenced by histological examination at multiple time points after primary infection or challenge with a low WHV dose, indicates that administration of such a dose, although unable to prompt immunological responses leading to hepatic injury, was not entirely immunologically inconsequential to the liver. Further, elevated transcription of this important cytokine in the context of the absence of detectable WHV replication and immune cell infiltrations suggests that the event might be due to activation of the cells constitutively residing within the liver or circulating throughout the organ after encountering small quantities of virus. The mechanism of this activation and identification of the phenotype of the cells involved will require further investigations. Nonetheless, the present finding needs to be interpreted with caution, due to the relatively small number of liver biopsy samples analyzed. On the other hand, the absence of intrahepatic expression of IFN-γ and TNF-α, as well as CD3 at 7 and 18 w.p.i. and following challenge with a low dose of WHV (Fig. 6) is consistent with the protracted absence of hepadnaviral replication in the liver during POI, as observed in the current study (Fig. 1) and also reported previously (10, 50; reviewed in reference 49). Further, serologically evident infection induced by high, liver-pathogenic doses of WHV (25), as well as by challenge of animals with POI with similar high doses in the current study, has been shown to be associated with significantly upregulated intrahepatic expression of IFN-γ, TNF-α, and CD3, suggesting the successful generation and recruitment of virus-specific CD8+ CTLs and CD4+ Th1 cells to the liver. Taken together, the analysis of gene expression in the hepatic tissue samples performed in our study implies that a low dose of WHV, although it does not initiate infection in the liver, induces a local type I interferon response by an as yet unknown mechanism.
Our study demonstrates that a low dose of hepadnavirus, causing an asymptomatic infection progressing in the absence of liver infection and virus-specific humoral immune response, induces multispecific antiviral T-cell proliferative responses and that overall, their kinetics are compatible to that accompanying symptomatic infection. Although this virus-specific T-cell response does not coincide with protection against hepatitis after challenge with a liver-pathogenic dose of the same virus, this response appears to be a reliable immunological indicator of the initiation and progression of occult infection. Similar to our observations, a recently published study has demonstrated that patients with HBV OBI progressing in the absence of virus-specific antibodies carry circulating HBV-specific CD8+ T cells, as visualized by staining with class I major histocompatibility complex tetramers (79). The assessment of such a cellular response can aid the diagnosis of low-dose HBV infection in high-risk groups, such as health care workers experiencing contaminated needle stick injuries, family members of infected individuals or intravenous drug users, which otherwise could be underestimated due to the lack of virus-specific antibody responses and sufficiently sensitive HBV DNA detection assays. Comparative analysis of virus-specific and mitogen-induced T-cell proliferative capacities along with cytokine responses during POI and symptomatic infection accomplished in the current study should increase our understanding of immunological processes underlying the pathogenesis of HBV infection caused by different doses of invading virus.
Acknowledgments
We thank Norma D. Churchill and Colleen L. Trelegan for expert technical assistance and Luke Grenning for assistance during woodchuck laparotomies.
The study was supported by operating grant MOP-14818 from the Canadian Institutes of Health Research. T.I.M. is the Canada Research Chair (Tier 1) in Viral Hepatitis/Immunology sponsored by the Canada Research Chair Program and funds from the Canadian Institutes of Health Research and the Canada Foundation for Innovation.
Footnotes
Published ahead of print on 4 February 2009.
REFERENCES
- 1.Allan, J. E., G. R. Shellam, and J. E. Grundy. 1982. Effect of murine cytomegalovirus infection on mitogen responses in genetically resistant and susceptible mice. Infect. Immun. 36235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andoniou, C. E., S. L. van Dommelen, V. Voigt, D. M. Andrews, G. Brizard, C. Asselin-Paturel, T. Delale, K. J. Stacey, G. Trinchieri, and M. A. Degli-Esposti. 2005. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 61011-1019. [DOI] [PubMed] [Google Scholar]
- 3.Asada, M., and J. T. Galambos. 1963. Sorbitol dehydrogenase and hepatocellular injury: An experimental and clinical study. Gastroenterology 44578-587. [Google Scholar]
- 4.Bertoletti, A., and A. J. Gehring. 2006. The immune response during hepatitis B virus infection. J. Gen. Virol. 871439-1449. [DOI] [PubMed] [Google Scholar]
- 5.Biron, C. A., and R. M. Welsh. 1982. Blastogenesis of natural killer cells during viral infection in vivo. J. Immunol. 1292788-2795. [PubMed] [Google Scholar]
- 6.Biron, C. A., G. Sonnenfeld, and R. M. Welsh. 1984. Interferon induces natural killer cell blastogenesis in vivo. J. Leukoc. Biol. 3531-37. [DOI] [PubMed] [Google Scholar]
- 7.Brechot, C., V. Thiers, D. Kremsdorf, B. Nalpas, S. Pol, and P. Paterlini-Brechot. 2001. Persistent hepatitis B virus infection in subjects without hepatitis B surface antigen: clinically significant or purely “occult”? Hepatology 34194-203. [DOI] [PubMed] [Google Scholar]
- 8.Cavanaugh, V. J., L. G. Guidotti, and F. V. Chisari. 1997. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J. Virol. 713236-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chisari, F. V. 2000. Viruses, immunity, and cancer: lessons from hepatitis B. Am. J. Pathol. 1561117-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffin, C. S., and T. I. Michalak. 1999. Persistence of infectious hepadnavirus in the offspring of woodchuck mothers recovered from viral hepatitis. J. Clin. Investig. 104203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coffin, C. S., T. N. Q. Pham, P. M. Mulrooney, N. D. Churchill, and T. I. Michalak. 2004. Persistence of isolated antibodies to woodchuck hepatitis virus core antigen is indicative of occult infection. Hepatology 401053-1061. [DOI] [PubMed] [Google Scholar]
- 12.Cose, S., C. Brammer, D. J. Zammit, D. A. Blair, and L. Lefrançois. 2006. CD4 T cells inhibit the CD8 T cell response during low-dose virus infection. Int. Immunol. 181285-1293. [DOI] [PubMed] [Google Scholar]
- 13.Dauer, M., K. Pohl, B. Obermaier, T. Meskendahl, J. Röbe, M. Schnurr, S. Endres, and A. Eigler. 2003. Interferon-alpha disables dendritic cell precursors: dendritic cells derived from interferon-alpha-treated monocytes are defective in maturation and T-cell stimulation. Immunology 11038-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Maria, A., C. Cirillo, and L. Moretta. 1994. Occurrence of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T cell activity in apparently uninfected children born to HIV-1-infected mothers. J. Infect. Dis. 1701296-1299. [DOI] [PubMed] [Google Scholar]
- 15.Feighery, C., J. F. Greally, and D. G. Weir. 1980. Mitogen responsiveness in viral hepatitis and chronic active hepatitis: the role of reversible suppressive influences. Gut 9738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari, C., A. Penna, A. Bertoletti, A. Valli, A. D. Antoni, T. Giuberti, A. Cavalli, M. A. Petit, and F. Fiaccadori. 1990. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 1453442-3449. [PubMed] [Google Scholar]
- 17.Ferrari, C., A. Bertoletti, A. Penna, A. Cavalli, A. Valli, G. Missale, M. Pilli, P. Fowler, T. Giuberti, and F. V. Chisari. 1991. Identification of immunodominant T cell epitopes of the hepatitis B virus nucleocapsid antigen. J. Clin. Investig. 88214-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn, K. J., G. T. Belz, J. D. Altman, R. Ahmed, D. L. Woodland, and P. C. Doherty. 1998. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity 8683-691. [DOI] [PubMed] [Google Scholar]
- 19.Gallimore, A., A. Glithero, A. Godkin, A. C. Tissot, A. C. Plückthun, T. Elliott, H. Hengartner, and R. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 1871383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garb, K. S., A. B. Stavitsky, and A. A. Mahmoud. 1981. Dynamics of antigen and mitogen-induced responses in murine schistosomiasis japonica: in vitro comparison between hepatic granulomas and splenic cells. J. Immunol. 127115-120. [PubMed] [Google Scholar]
- 21.Guidotti, L. G., and F. V. Chisari. 2006. Immunobiology and pathogenesis of viral hepatitis. Annu. Rev. Pathol. 123-61. [DOI] [PubMed] [Google Scholar]
- 22.Guidotti, L. G., R. Rochford, J. Chung, M. Shapiro, R. Purcell, and F. V. Chisari. 1999. Viral clearance without destruction of infected cells during acute HBV infection. Science 284825-829. [DOI] [PubMed] [Google Scholar]
- 23.Gujar, S. A., and T. I. Michalak. 2005. Flow cytometric quantification of T cell proliferation and division kinetics in woodchuck model of hepatitis B. Immunol. Investig. 34215-236. [PubMed] [Google Scholar]
- 24.Gujar, S. A., A. K. Jenkins, C. S. Guy, J. Wang, and T. I. Michalak. 2008. Aberrant lymphocyte activation precedes delayed virus-specific T-cell response after both primary infection and secondary exposure to hepadnavirus in the woodchuck model of hepatitis B virus infection. J. Virol. 826992-7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guy, C. S., P. M. Mulrooney-Cousins, N. D. Churchill, and T. I. Michalak. 2008. Intrahepatic expression of genes affiliated with innate and adaptive immune responses immediately after invasion and during acute infection with woodchuck hepadnavirus. J. Virol. 828579-8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodgson, P. D., and T. I. Michalak. 2001. Augmented hepatic interferon gamma expression and T-cell influx characterize acute hepatitis progressing to recovery and residual lifelong virus persistence in experimental adult woodchuck hepatitis virus infection. Hepatology 341049-1059. [DOI] [PubMed] [Google Scholar]
- 27.Hosken, N. A., K. Shibuya, A. W. Heath, K. M. Murphy, and A. O'Garra. 1995. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J. Exp. Med. 1821579-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda, K., H. Marusawa, Y. Osaki, T. Nakamura, N. Kitajima, Y. Yamashita, M. Kudo, T. Sato, and T. Chiba. 2007. Antibody to hepatitis B core antigen and risk for hepatitis C-related hepatocellular carcinoma: a prospective study. Ann. Intern. Med. 146649-656. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto, S., S. Iwai, K. Tsujiyama, C. Kurahashi, K. Takeshita, M. Naoe, A. Masunaga, Y. Ogawa, K. Oguchi, and A. Miyazaki. 2007. TNF-alpha drives human CD14+ monocytes to differentiate into CD70+ dendritic cells evoking Th1 and Th17 responses. J. Immunol. 1791449-1457. [DOI] [PubMed] [Google Scholar]
- 30.Kadowaki, N., S. Antonenko, J. Y. Lau, and Y. J. Liu. 2000. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J. Exp. Med. 192219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakimi, K., L. G. Guidotti, Y. Koezuka, and F. V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamal, S. M., A. Amin, M. Madwar, C. S. Graham, Q. He, A. Al Tawil, J. Rasenack, T. Nakano, B. Robertson, A. Ismail, and M. J. Koziel. 2004. Cellular immune responses in seronegative sexual contacts of acute hepatitis C patients. J. Virol. 7812252-12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7131-137. [DOI] [PubMed] [Google Scholar]
- 34.Kimura, K., K. Kakimi, S. Wieland, L. G. Guidotti, and F. V. Chisari. 2002. Activated intrahepatic antigen-presenting cells inhibit hepatitis B virus replication in the liver of transgenic mice. J. Immunol. 1695188-5195. [DOI] [PubMed] [Google Scholar]
- 35.Kimura, K., K. Kakimi, S. Wieland, L. G. Guidotti, and F. V. Chisari. 2002. Interleukin-18 inhibits hepatitis B virus replication in the livers of transgenic mice. J. Virol. 7610702-10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi, M., L. Fitz, M. Ryan, R. M. Hewick, S. C. Clark, S. Chan, R. Loudon, F. Sherman, B. Perussia, and G. Trinchieri. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korba, B. E., F. V. Wells, B. Baldwin, P. J. Cote, B. C. Tennant, H. Popper, and J. L. Gerin. 1989. Hepatocellular carcinoma in woodchuck hepatitis virus-infected woodchucks: presence of viral DNA in tumor tissue from chronic carriers and animals serologically recovered from acute infections. Hepatology 9461-470. [DOI] [PubMed] [Google Scholar]
- 38.Koziel, M. J., D. K. Wong, D. Dudley, M. Houghton, and B. D. Walker. 1997. Hepatitis C virus-specific cytolytic T lymphocyte and T helper cell responses in seronegative persons. J. Infect. Dis. 176859-866. [DOI] [PubMed] [Google Scholar]
- 39.Kreuzfelder, E., S. Menne, S. Ferencik, M. Roggendorf, and H. Grosse-Wilde. 1996. Assessment of peripheral blood mononuclear cell proliferation by [2-3H]adenine uptake in the woodchuck model. Clin. Immunol. Immunopathol. 78223-227. [DOI] [PubMed] [Google Scholar]
- 40.Langlade-Demoyen, P., N. Ngo-Giang-Huong, F. Ferchal, and E. Oksenhendler. 1994. Human immunodeficiency virus (HIV) nef-specific cytotoxic T lymphocytes in noninfected heterosexual contact of HIV-infected patients. J. Clin. Investig. 931293-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maini, M. K., C. Boni, G. S. Ogg, A. S. King, S. Reignat, C. K. Lee, J. R. Larrubia, G. J. Webster, A. J. McMichael, C. Ferrari, R. Williams, D. Vergani, and A. Bertoletti. 1999. Direct ex vivo analysis of hepatitis B virus-specific CD8+ T cells associated with the control of infection. Gastroentrology 1171386-1396. [DOI] [PubMed] [Google Scholar]
- 42.McChesney, M. B., J. R. Collins, D. Lu, X. Lu, J. Torten, R. L. Ashley, M. W. Cloyd, and C. J. Miller. 1998. Occult systemic infection and persistent simian immunodeficiency virus (SIV)-specific CD4+-T-cell proliferative responses in rhesus macaques that were transiently viremic after intravaginal inoculation of SIV. J. Virol. 7210029-10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McRae, B. L., T. Nagai, R. T. Semnani, J. M. van Seventer, and G. A. van Seventer. 2000. Interferon-alpha and -beta inhibit the in vitro differentiation of immunocompetent human dendritic cells from CD14+ precursors. Blood 96210-217. [PubMed] [Google Scholar]
- 44.Menne, S., and P. J. Cote. 2007. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J. Gastroenterol. 13104-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menne, S., J. Maschke, T. K. Tolle, M. Lu, and M. Roggendorf. 1997. Characterization of T-cell response to woodchuck hepatitis virus core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J. Virol. 7165-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menne, S., P. J. Cote, S. D. Butler, I. A. Toshkov, J. L. Gerin, and B. C. Tennant. 2007. Immunosuppression reactivates viral replication long after resolution of woodchuck hepatitis virus infection. Hepatology 45614-622. [DOI] [PubMed] [Google Scholar]
- 47.Michalak, T. I. 1998. The woodchuck animal model of hepatitis B. Viral Hepatitis Rev. 4139-165. [Google Scholar]
- 48.Michalak, T. I. 2000. Occult persistence and lymphotropism of hepadnaviral infection: insights from the woodchuck viral hepatitis model. Immunol. Rev. 17498-111. [DOI] [PubMed] [Google Scholar]
- 49.Michalak, T. I. 2007. Characteristics and consequences of experimental occult hepatitis B virus infection in the woodchuck model of hepatitis B. Curr. Top. Virol. 61-13. [Google Scholar]
- 50.Michalak, T. I., P. M. Mulrooney, and C. S. Coffin. 2004. Low doses of hepadnavirus induce infection of the lymphatic system that does not engage the liver. J. Virol. 781730-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michalak, T. I., B. Lin, N. D. Churchill, P. Dzwonkowski, and J. R. Desousa. 1990. Hepadnavirus nucleocapsid and surface antigens and the antigen-specific antibodies associated with hepatocyte plasma membranes in experimental woodchuck acute hepatitis. Lab. Investig. 62680-689. [PubMed] [Google Scholar]
- 52.Michalak, T. I., C. Pasquinelli, S. Guilhot, and F. V. Chisari. 1994. Hepatitis B virus persistence after recovery from acute viral hepatitis. J. Clin. Investig. 93230-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michalak, T. I., I. U. Pardoe, C. S. Coffin, N. D. Churchill, D. S. Freake, P. Smith, and C. L. Trelegan. 1999. Occult lifelong persistence of infectious hepadnavirus and residual liver inflammation in woodchucks convalescent from acute viral hepatitis. Hepatology 29928-938. [DOI] [PubMed] [Google Scholar]
- 54.Michalak, T. I., T. N. Q. Pham, and P. M. Mulrooney-Cousins. 2007. Molecular diagnosis of occult HCV and HBV infections. Future Virol. 2451-465. [Google Scholar]
- 55.Milich, D. R., and A. McLachlan. 1986. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science 2341398-1401. [DOI] [PubMed] [Google Scholar]
- 56.Mulrooney-Cousins, P. M., and T. I. Michalak. 2008. Repeated passage of wild-type woodchuck hepatitis virus in lymphoid cells does not generate cell type-specific variants or alters virus infectivity. J. Virol. 827540-7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mulrooney-Cousins, P. M., and T. I. Michalak. 2007. Persistent occult hepatitis B virus infection: experimental findings and clinical implications. World J. Gastroenterol. 135682-5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murakami, Y., M. Minami, Y. Daimon, and T. Okanoue. 2004. Hepatitis B virus DNA in liver, serum, and peripheral blood mononuclear cells after the clearance of serum hepatitis B virus surface antigen. J. Med. Virol. 72203-214. [DOI] [PubMed] [Google Scholar]
- 59.Murphy, W. J., J. R. Keller, C. L. Harrison, H. A. Young, and D. L. Longo. 1992. Interleukin-2-activated natural killer cells can support hematopoiesis in vitro and promote marrow engraftment in vivo. Blood 80670-677. [PubMed] [Google Scholar]
- 60.Niewiesk, S., M. Gotzelmann, and V. ter Meulen. 2000. Selective in vivo suppression of T lymphocyte responses in experimental measles virus infection. Proc. Natl. Acad. Sci. USA 974251-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ochsenbein, A. F., D. D. Pinschewer, B. Odermatt, A. Ciurea, H. Hengartner, and R. M. Zinkernagel. 2000. Correlation of T cell independence of antibody responses with antigen dose reaching secondary lymphoid organs: implications for splenectomized patients and vaccine design 1. J. Immunol. 1646296-6302. [DOI] [PubMed] [Google Scholar]
- 62.Orange, J. S., and C. A. Biron. 1996. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J. Immunol. 1564746-4756. [PubMed] [Google Scholar]
- 63.Orange, J. S., and C. A. Biron. 1996. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J. Immunol. 1561138-1142. [PubMed] [Google Scholar]
- 64.Pedersen, C., E. Dickmeiss, J. Gaub, L. P. Ryder, P. Platz, B. O. Lindhardt, and J. D. Lundgren. 1990. T-cell subset alterations and lymphocyte responsiveness to mitogens and antigen during severe primary infection with HIV: a case series of seven consecutive HIV seroconverters. AIDS 4523-526. [DOI] [PubMed] [Google Scholar]
- 65.Pinto, L. A., J. Sullivan, J. A. Berzofsky, M. Clerici, H. A. Kessler, A. L. Landay, and G. M. Shearer. 1995. ENV-specific cytotoxic T lymphocyte responses in HIV seronegative health care workers occupationally exposed to HIV-contaminated body fluids. J. Clin. Investig. 96867-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Promadej, N., C. Costello, M. M. Wernett, P. S. Kulkarni, V. A. Robison, K. E. Nelson, T. W. Hodge, V. Suriyanon, A. Duerr, and J. M. McNicholl. 2003. Broad human immunodeficiency virus (HIV)-specific T cell responses to conserved HIV proteins in HIV-seronegative women highly exposed to a single HIV-infected partner. J. Infect. Dis. 1871053-1063. [DOI] [PubMed] [Google Scholar]
- 67.Raimondo, G., J. P. Allain, M. R. Brunetto, M. A. Buendia, D. S. Chen, M. Colombo, A. Craxì, F. Donato, C. Ferrari, G. B. Gaeta, W. H. Gerlich, M. Levrero, S. Locarnini, T. Michalak, M. U. Mondelli, J. M. Pawlotsky, T. Pollicino, D. Prati, M. Puoti, D. Samuel, D. Shouval, A. Smedile, G. Squadrito, C. Trépo, E. Villa, H. Will, A. R. Zanetti, and F. Zoulim. 2008. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J. Hepatol. 49652-657. [DOI] [PubMed] [Google Scholar]
- 68.Rehermann, B., C. Ferrari, C. Pasquinelli, and F. V. Chisari. 1996. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat. Med. 21104-1108. [DOI] [PubMed] [Google Scholar]
- 69.Rentenaar, R. J., L. E. Gamadia, N. van DerHoek, F. N. van Diepen, R. Boom, J. F. Weel, P. M. Wertheim-van Dillen, R. A. van Lier, and I. J. ten Berge. 2000. Development of virus-specific CD4+ T cells during primary cytomegalovirus infection. J. Clin. Investig. 105541-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rocha, B., and C. Tanchot. 2006. The Tower of Babel of CD8+ T-cell memory: known facts, deserted roads, muddy waters, and possible dead ends. Immunol. Rev. 211182-196. [DOI] [PubMed] [Google Scholar]
- 71.Siefer, A. K., D. L. Longo, C. L. Harrison, C. W. Reynolds, and W. J. Murphy. 1993. Activated natural killer cells and interleukin-2 promote granulocytic and megakaryocytic reconstitution after syngeneic bone marrow transplantation in mice. Blood 822577-2584. [PubMed] [Google Scholar]
- 72.Sodomann, C. P., M. Rother, K. Havemann, and G. A. Martini. 1979. Lymphocyte proliferation to phytohemagglutinin (PHA) in hepatitis B antigen-positive and -negative hepatitis. Res. Exp. Med. (Berlin) 17595-107. [DOI] [PubMed] [Google Scholar]
- 73.Su, H. C., K. B. Nguyen, T. P. Salazar-Mather, M. C. Ruzek, M. Y. Dalod, and C. A. Biron. 2001. NK cell functions restrain T cell responses during viral infections. Eur. J. Immunol. 313048-3055. [DOI] [PubMed] [Google Scholar]
- 74.Thimme, R., S. Wieland, C. Steiger, J. Ghrayeb, K. A. Reimann, R. H. Purcell, and F. V. Chisari. 2003. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 7768-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang, J., S. A. Gujar, L. Cova, and T. I. Michalak. 2007. Bicistronic woodchuck hepatitis virus core and gamma interferon DNA vaccine can protect from hepatitis but does not elicit sterilizing antiviral immunity. J. Virol. 81903-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wieland, S. F., L. G. Guidotti, and F. V. Chisari. 2000. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J. Virol. 744165-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yuki, N., T. Nagaoka, M. Yamashiro, K. Mochizuki, A. Kaneko, K. Yamamoto, M. Omura, K. Hikiji, and M. Kato. 2003. Long-term histologic and virologic outcomes of acute self-limited hepatitis B. Hepatology 371172-1179. [DOI] [PubMed] [Google Scholar]
- 78.Zanetti, M., and G. Franchini. 2006. T cell memory and protective immunity by vaccination: is more better? Trends Immunol. 27511-517. [DOI] [PubMed] [Google Scholar]
- 79.Zerbini, A., M. Pilli, C. Boni, P. Fisicaro, A. Penna, P. Di Vincenzo, T. Giuberti, A. Orlandini, G. Raffa, T. Pollicino, G. Raimondo, C. Ferrari, and G. Missale. 2008. The characteristics of the cell-mediated immune response identify different profiles of occult hepatitis B virus infection. Gastroenterology 1341470-1481. [DOI] [PubMed] [Google Scholar]