Abstract
Most adenoviruses bind directly to the coxsackie and adenovirus receptor (CAR) on target cells in vitro, but recent research has shown that adenoviruses can also use soluble components in body fluids for indirect binding to target cells. These mechanisms have been identified upon addressing the questions of how to de- and retarget adenovirus-based vectors for human gene and cancer therapy, but the newly identified mechanisms also suggest that the role of body fluids and their components may also be of importance for natural, primary infections. Here we demonstrate that plasma, saliva, and tear fluid promote binding and infection of adenovirus type 5 (Ad5) in respiratory and ocular epithelial cells, which corresponds to the natural tropism of most adenoviruses, and that plasma promotes infection by Ad31. By using a set of binding and infection experiments, we also found that Ad5 and Ad31 require coagulation factors IX (FIX) or X (FX) or just FIX, respectively, for efficient binding and infection. The concentrations of these factors that were required for maximum binding were 1/100th of the physiological concentrations. Preincubation of virions with heparin or pretreatment of cells with heparinase I indicated that the role of cell surface heparan sulfate during FIX- and FX-mediated adenovirus binding and infection is mechanistically serotype specific. We conclude that the use of coagulation factors by adenoviruses may be of importance not only for the liver tropism seen when administering adenovirus vectors to the circulation but also during primary infections by wild-type viruses of their natural target cell types.
The human adenoviruses (Ads) are divided into six different species (A to F) that between them contain more than 50 distinct serotypes. These cause infections in airways (species B, C, and E), the eyes (species B, C, D, and E), the tonsils (species C), the liver (species C), the urinary tract (species B), and the intestine (species A and F) (50). The tropisms of these viruses are complex, and they may either overlap or vary between or within species, or they can even be specific for serotypes within a certain species. For example, the severe ocular condition known as epidemic keratoconjunctivitis (EKC) is mainly caused by only three Ad serotypes (Ad8, Ad19, and Ad37) of the 31 Ad serotypes in total in species D, and cystitis is caused by some, but not all, species B Ads. Moreover, the prevalence of serum antibodies to species A Ads is relatively high (49), but they are not associated with disease to the same extent as they are with many other Ads. In otherwise healthy individuals, Ads of species A are mainly asymptomatic, but in immunocompromised individuals, these Ads (Ad31 in particular) are frequently associated with disease and morbidity (16).
The complexity of Ad tropism is matched by the complexity and large number of receptors identified for these viruses. Previously, it was assumed that all Ads attach to target cells directly through an interaction between the knob domain of the viral fiber protein and specific cell surface receptors. It was suggested that the coxsackie and adenovirus receptor (CAR) acted as a cellular receptor for selected Ads belonging to species A, C, D, E, and F (7, 36, 41). Heparan sulfate (13, 40), integrins (6, 24, 48), major histocompatibility complex class I α2 (17), vascular cell adhesion molecule 1 (11), and scavenger receptors (51) have been suggested as alternative receptors or coreceptors for species C Ads. Species B Ads and EKC-causing species D Ads do not use CAR as a receptor (2, 36). Instead, most species B Ads use CD46 as a cellular receptor (14, 26, 37) and the EKC-causing Ads use sialic acid (3, 4, 9).
Clearly, CAR is an efficient receptor for several Ads on nonpolarized cells that have been cultured in vitro. More recent research has, however, indicated that CAR is not expressed apically on polarized epithelial cells (46) that more closely mimic the in vivo situation. In addition, CAR is not expressed at all on primary T-lymphoid cells (10). Consequently, in the in vivo situation, it is likely that species C Ads can attach to and enter epithelial and T-lymphoid target cells independently of CAR; thus, the use of alternative receptors has been suggested (12).
Great efforts have been made to minimize the liver tropism obtained in vivo, by using gene therapy vectors based mainly on species C Ads. These efforts have attempted to disrupt binding to CAR, integrins, and/or heparan sulfate (8, 22, 23, 25, 28, 39, 40, 52); however, the results from these experiments have been inconsistent. Transduction of hepatocytes by species C-based vectors was recently suggested to depend on the binding of the most abundant structural protein of the virus particle, the hexon, to coagulation factor X (FX); the latter mediates indirect viral binding to cell surface heparan sulfate proteoglycans on hepatocytes (21, 42). Similar indirect binding of species C Ads to target cells can be achieved with dipalmitoyl phosphatidylcholine (DPPC) or lactoferrin (5, 19). DPPC is secreted by alveolar epithelial cells and lactoferrin is secreted by, for example, neutrophils into epithelial mucosa and tear fluid. Lactoferrin-mediated infection is specific for species C Ads and is associated with the ability of these Ads to cause tonsillitis, a feature that has not been observed for other Ads. As with FX, DPPC also binds to the hexon protein. Both coagulation factor IX (FIX) and lactoferrin have been suggested to interact with the fiber protein (19, 38), but in both of these cases, this still has to be confirmed.
Based on the above findings, we hypothesized that soluble components in various body fluids may be of more importance for the cellular binding and viral tropism of Ads than was previously recognized. Here we set out to investigate the effects of four different human body fluids—plasma, tear fluid, breast milk, and saliva—on Ad infection in cells that reflect the ocular and respiratory tropism of several Ads. We found that FIX and FX promote binding of species C Ads, and also species A Ads, to human epithelial cells. These coagulation factors are mainly present in blood. Various stimuli, however, including inflammatory responses, may trigger exudation of plasma components at the respiratory mucosa (34). Moreover, upon damage to tissue, various coagulation factors may be produced directly by respiratory epithelial cells (33), which suggests that coagulation factors may also be present outside the blood and, specifically, in tissues that are targeted by Ads. The data presented here indicate that coagulation factors may be of importance not only when Ads enter the circulation, i.e., when used as gene therapy vectors, but also during natural Ad infections in humans.
MATERIALS AND METHODS
Cells, viruses, antibodies, and body fluids.
Human lung carcinoma A549 cells and human corneal epithelial (HCE) cells were grown as described previously (29). Human intestinal epithelial FHs74Int cells were purchased from LGC Promochem (Teddington, United Kingdom) and grown according to the instructions. The prototype strains of species A serotype Ad31 (strain 1315/63), species B serotypes Ad3 (GB) and Ad35 (Holden), species C serotype Ad5 (Ad75), species D serotype Ad37 (1477), species E serotype Ad4 (RI-67), and species F serotype Ad41 (Tak) were produced with or without 35S labeling in A549 cells, as described previously (20). To avoid the possibility that virions might be copropagated with traces of coagulation factors from bovine calf serum present in the cell culture medium, virions were purified only from nonlysed cells, thus preventing contact between virions and coagulation factors before removal of the medium. The absence of coagulation factors associated with purified virions was confirmed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). Serotype-specific rabbit polyclonal antisera to Ads were prepared as described previously (45). Body fluids (saliva, plasma, breast milk, and tear fluid) were collected from volunteers.
Fluorescent focus assay.
The effects of various body fluids and body fluid components on Ad infection of A549 and HCE cells were examined essentially as described previously (20). Virions were preincubated on ice with human body fluids (breast milk, tear fluid, plasma, or parotid saliva [all diluted 1:100]), FIX (Calbiochem, Darmstadt, Germany), FX or protein C (both from Haematologic Technologies Inc., Essex Junction, VT), FVII (Innovative Research Inc., Novi, MI), or complement components C3b, iC3b, C3dg, C4b, and C4dg (kindly provided by David Isenman, Department of Biochemistry, University of Toronto, Canada) for one hour before infection of cells. All coagulation factors were used at physiological concentrations: 5 μg/ml (FIX), 10 μg/ml (FX), and 0.5 μg/ml (FVII) (30, 35). The complement components were used at the following concentrations: 3 μg/ml (C3b), 10 μg/ml (iC3b), 5 μg/ml (C3dg), 4 μg/ml (C4dg), 2 μg/ml (C4b), and 4 μg/ml (protein C). In some cases, 10 mM EDTA (Merck, Darmstadt, Germany) was included in the incubation mixture. Other exceptions to the method from reference 20 included the following: (i) serum-free medium was used throughout; (ii) prior to the addition of virions, the cells were washed three times with serum-free medium to remove traces of coagulation factors from the cell culture medium; (iii) the number of virions added to the wells was adapted to optimize quantification of each serotype; (iv) after incubating the cells with virions, the cells were washed three times with serum-free medium; (v) in some cases, cells were treated with heparinase I or II (Sigma) prior to incubation with virions; (vi) an extra wash step with water was included before mounting; (vii) the fluorescein isothiocyanate-conjugated swine anti-rabbit immunoglobulin G antibody was diluted 1:50 with one exception (Table 1; 1:100); and (viii) 20× magnification instead of 10× was used when analyzing samples by fluorescence microscopy.
TABLE 1.
Infection levels detected with dilutions of various body fluidsa
| Serotype (species) | Infected cells with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Plasma
|
Tear fluid
|
Saliva
|
Breast milk
|
|||||
| HCE | A549 | HCE | A549 | HCE | A549 | HCE | A549 | |
| Ad31 (A) | ++ | + | — | — | — | — | — | — |
| Ad3 (B1) | — | — | — | — | — | — | — | — |
| Ad35 (B2) | — | — | — | — | — | — | — | — |
| Ad5 (C) | ++ | ++ | ++ | ++ | — | ++ | — | — |
| Ad37 (D) | — | — | — | — | — | — | — | — |
| Ad4 (E) | — | — | — | — | — | — | — | — |
| Ad41 (F) | — | — | — | — | — | — | — | — |
Virions were preincubated with 1:100 dilutions of various body fluids and then allowed to infect epithelial cells. Forty-four hours postinfection, the cells were fixed with methanol and stained as described in Materials and Methods, and the number of infected cells was determined by fluorescence microscopy. “+” and “++” denote a 0 to 300% and a >300% increase in the number of infected cells, respectively, whereas a dash denotes no increase or a small decrease (<50%).
Binding assay.
Study of the effects of plasma components on the binding of Ad5 and Ad31 to target cells was performed essentially as described previously (20). In some experiments, virions were preincubated with increasing concentrations of FIX or FX, with EDTA (10 mM), and/or with increasing concentrations of heparin (Sigma) before incubation with cells. In some experiments, cells were preincubated with increasing concentrations of heparinase I (Sigma) before incubation with virions.
Gene delivery assay.
Ad5CMVeGFP vectors (Baylor College of Medicine, Houston, TX) were incubated on ice for one hour with increasing concentrations of FIX or FX. Virion mixtures were added to subconfluent cultures of A549 or HCE cells in 24-well plates and incubated in Dulbecco's modified (high-glucose) essential medium [containing penicillin-streptomycin (PEST) and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), but no fetal calf serum] for one hour on ice. Unbound virions were removed by washing. The numbers of virions added to each cell type were adapted to optimize quantification by flow cytometry (104 virions per HCE cell, and 5 × 103 virions per A549 cell). After 44 h of incubation in supplemented hormone epithelial medium (containing PEST, HEPES, and 1% fetal calf serum) (1), the cells were harvested, washed, and resuspended in phosphate-buffered saline. The samples were analyzed using a FACScan flow cytometer and LYSIS II software (Becton Dickinson).
Statistical analysis.
All experiments were performed at least three times (except the experiments presented in Table 1, which were performed twice) with duplicate samples in each experiment. Results are expressed as means ± standard deviations (SD), and statistical significance was evaluated by paired Student t tests. All P values of <0.05 were considered statistically significant.
RESULTS
Tear fluid, plasma, and saliva promote infection of Ad5 in human epithelial cells.
Specific components in tear fluid, blood, and respiratory mucosa can promote species C Ad infection of target cells, mainly by serving as a bridge between virions and cells, which enhances viral binding to the target cell surface (5, 19, 38). These findings led us to investigate the effects of different body fluids on the infectivity of Ads in a broader sense. Purified virions representing each species (A, Ad31; B1, Ad3; B2, Ad35; C, Ad5; D, Ad37; E, Ad4; and F, Ad41) were preincubated with 100-fold-diluted tear fluid, plasma, breast milk, or saliva on ice for one hour and then allowed to infect cells representing the respiratory (A549) and ocular (HCE) tropism of many Ads. With few exceptions, Ads from species B and D to F were weakly inhibited by all body fluids (Table 1), possibly as a result of the presence of neutralizing antibodies. However, tear fluid and plasma efficiently promoted infection of Ad5 in both A549 and HCE cells, and saliva promoted infection of Ad5 in A549 cells but not in HCE cells. Ad31 infection in A549 and HCE cells was also promoted, but only by plasma.
FIX and FX promote Ad5 infection of epithelial cells.
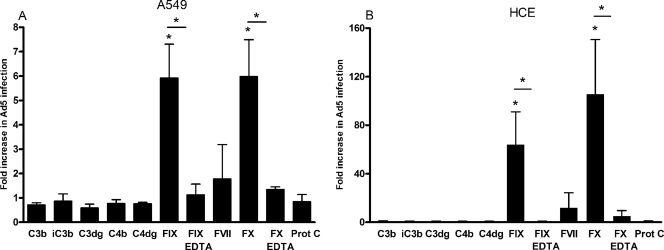
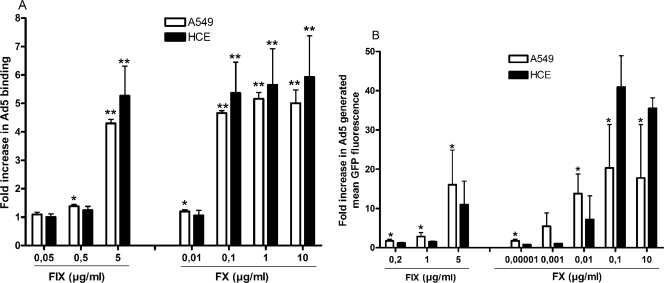
Several coagulation factors and/or complement components have been suggested to mediate transduction of Ad5 in hepatocytes (32, 38). We found that preincubation of Ad5 virions with complement components C3b, iC3b, C3dg, C4b, and C4dg (at physiological concentrations) did not promote infection of A549 cells (Fig. 1A) or HCE cells (Fig. 1B). However, at physiological concentrations, both FIX and FX promoted efficient infection of Ad5 in both A549 cells (fivefold) and HCE cells (60- to 100-fold). Coincubation with EDTA efficiently reduced the promoting effects of FIX and FX, indicating that divalent cations are of importance. Preincubation of Ad5 virions with FIX or FX promoted efficient virus binding to epithelial cells in a dose-dependent manner (Fig. 2A). The physiological concentration of FIX (5 μg/ml in blood) was required for efficient enhancement of Ad5 binding to A549 and HCE cells, whereas only 0.1 μg/ml of FX (corresponding to 1% of the physiological concentration of FX in blood) promoted Ad5 binding as efficiently as the physiological concentration (10 μg/ml in blood). Also, FIX and FX were both found to promote efficient transduction with green fluorescent protein (GFP)-expressing, Ad5-based vectors in A549 and HCE cells in a dose-dependent manner (Fig. 2B). Thus, physiological concentrations of FIX and only 1% of the physiological concentration of FX were necessary or sufficient, respectively, to mediate an efficient enhancement in gene delivery. This suggested that the mechanism behind plasma-mediated binding of Ad5 to and infection of human respiratory or ocular epithelial cells could possibly involve FIX or, more likely, FX. We also tested whether the effect of coagulation factors was due to proteolytic cleavage of Ad5 virions by analyzing virions treated with or without coagulation factors with sodium dodecyl sulfate-polyacrylamide gel electrophoresis, but we found no evidence of proteolytic degradation (data not shown), thus supporting the previous findings of Parker et al. that this phenomenon is mediated by direct binding between the virus and FX or FIX, instead of being a result of the enzymatic modification of virions (32).
FIG. 1.
Physiological concentrations of FIX and FX promote efficient Ad5 infection of A549 cells (A) and HCE cells (B). Purified Ad5 virions were preincubated with or without physiological concentrations of coagulation factors, complement components, and/or 10 mM EDTA, as indicated, and then allowed to infect adherent cells. Forty-four hours postinfection, the cells were stained and quantified in a fluorescence microscope. The control infection level corresponds to the y axis value of 1. Values are means ± SD. *, P of <0.05 versus control. The bars indicate comparisons between FIX and FIX-EDTA or between FX and FX-EDTA.
FIG. 2.
Coagulation factors promote efficient Ad5 binding and gene delivery to epithelial cells in a dose-dependent manner. (A) 35S-labeled CsCl-purified Ad5 virions were preincubated with or without increasing concentrations of FIX or FX and then incubated with A549 or HCE cells in suspension on ice for one hour. Unbound virions were removed by washing, and the cell-associated radioactivity was measured in a scintillation counter. (B) GFP-expressing, CsCl-purified Ad5 viruses were preincubated with or without increasing concentrations of FIX or FX and then incubated with adherent A549 or HCE cells on ice for one hour. Unbound viruses were removed by washing. The cells were incubated at 37°C for another forty-four hours. Next, GFP-expressing cells were quantified by flow cytometry. The control binding level corresponds to the y axis value of 1. Values are means ± SD. *, P of <0.05 versus control; **, P of <0.01 versus control.
Heparan sulfate is required for FIX- and FX-dependent binding of Ad5 to and infection of epithelial cells.
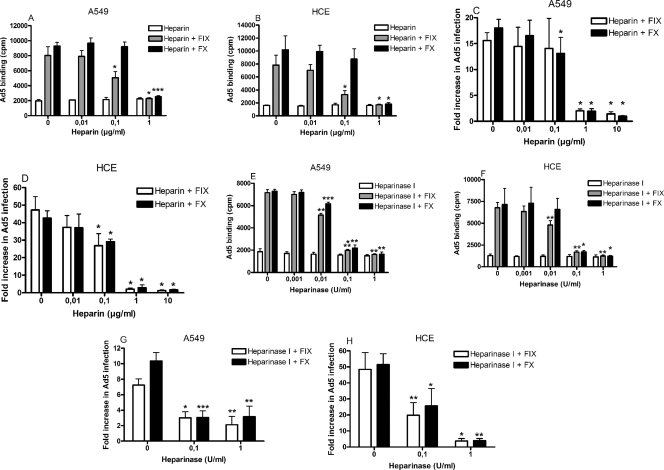
Cell surface heparan sulfate has been suggested to mediate FIX- and FX-dependent binding of Ad5 to target cells (31, 38). To determine whether heparan sulfate is also required for FIX- and/or FX-dependent binding of Ad5 to epithelial cells, we first coincubated Ad5 virions with physiological concentrations of FIX or FX in the presence of increasing concentrations of heparin, which is a somewhat more sulfated analog of heparan sulfate. At the concentrations used, heparin efficiently outcompeted both FIX- and FX-dependent binding of Ad5, but not basal level binding (i.e., in the absence of FIX and FX) to A549 cells (Fig. 3A) and HCE cells (Fig. 3B). Similarly, the effect of FIX and FX on Ad5 infection was efficiently reduced in both A549 cells (Fig. 3C) and HCE cells (Fig. 3D), indicating that cell surface heparan sulfate is required for FIX- and FX-dependent infection of Ad5 in epithelial cells. To study the involvement of heparan sulfate during FIX/FX-mediated Ad5 binding in greater depth, we pretreated epithelial cells with increasing concentrations of heparinase I, thus cleaving cell surface heparan sulfate from the cell surface (18), before incubation with virion-FIX/FX mixtures. As expected, heparinase I treatment efficiently blocked FIX- and FX-dependent binding of Ad5 to both A549 cells (Fig. 3E) and HCE cells (Fig. 3F), but not basal level binding. Also, similar results were obtained in the infectivity assay (Fig. 3G and H). Together, these experiments confirmed the role of cell surface heparan sulfate in FIX- and FX-dependent binding of Ad5 to epithelial cells.
FIG. 3.
Cell surface heparan sulfate is required for FIX- and FX-mediated binding of Ad5 to and infection of epithelial cells. Ad5 virions were preincubated with or without physiological concentrations of FIX or FX and increasing concentrations of heparin and then allowed to bind to A549 cells (A) or HCE cells (B) or to infect A549 cells (C) or HCE cells (D). Alternatively, Ad5 virions were preincubated with or without physiological concentrations of FIX or FX and then allowed to bind to A549 or HCE cells (E and G) or to infect A549 or HCE cells (F and H) pretreated with increasing concentrations of heparinase I. In binding assays, cell-associated radioactivity was measured with a scintillation counter, whereas in infection assays, infectivity was quantified by fluorescence microscopy. Values are means ± SD. *, P of <0.05 versus control; **, P of <0.01 versus control; ***, P of <0.001 versus control.
Ad31 infection of epithelial cells is promoted by FIX, but not by FX.
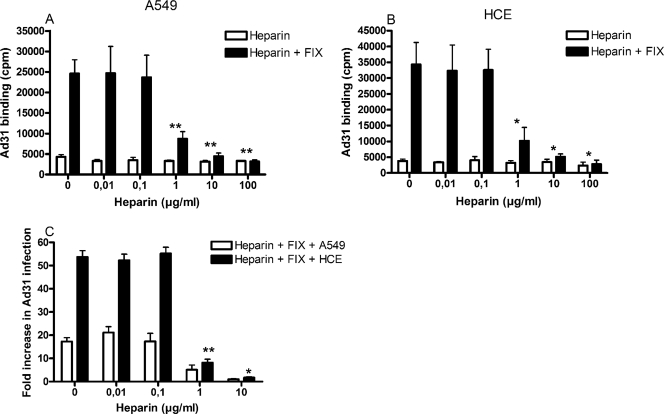
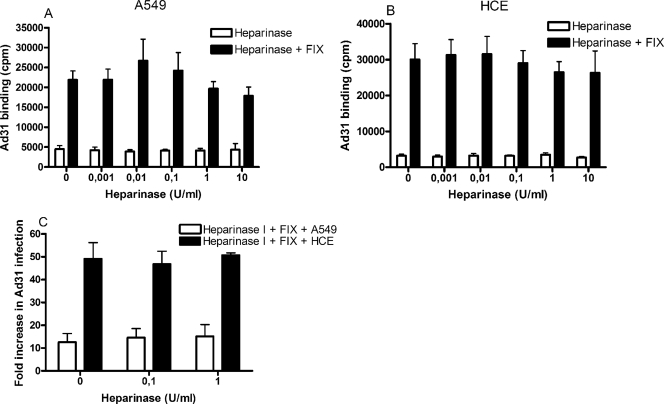
In addition to Ad5, the only Ad serotype that was found to infect A549 and HCE cells more efficiently in the presence of plasma was Ad31. Selected coagulation factors and complement components were investigated for their possible effects in promoting Ad31 infection of A549 cells (Fig. 4A) and HCE cells (Fig. 4B). As for Ad5, none of the complement components used had any substantial effects. Unlike the situation with Ad5, however, only FIX—but surprisingly not FX—promoted efficient Ad31 infection of both A549 and HCE cells. Physiological concentrations of FIX promoted Ad31 infection more than 10-fold in A549 cells and more than 50-fold in HCE cells, compared to the levels in the corresponding controls (without FIX). Also, as with Ad5, addition of EDTA to the incubation mixtures inhibited the promoting effects of FIX on Ad31 binding. Binding experiments gave a similar pattern: binding of Ad31 to A549 and HCE cells increased 5- to 15-fold with as little as 0.05 μg/ml FIX (Fig. 4C), i.e., 100-fold less than the physiological concentration, which was similar to the concentration of FX that was sufficient to promote efficient binding of Ad5. FIX-mediated binding of Ad31 to A549 and HCE cells was reduced to basal levels when virions were preincubated with heparin (Fig. 5A and B). However, basal binding (in the absence of FIX) was not affected by heparin. Similar results were obtained in infection assays: 1 μg/ml heparin reduced, and 10 μg/ml completely blocked, FIX-mediated Ad5 infection of A549 and HCE cells (Fig. 5C). The concentrations of heparin required to completely block the promoting effect of FIX on Ad31 binding and infection were higher than those required to block the promoting effect of FIX and FX on Ad5 binding and infection (compare Fig. 3A and B with Fig. 5A and B, and Fig. 3C and D with Fig. 5C). Moreover, heparinase I pretreatment of A549 and HCE cells had little or no effect on FIX-mediated binding of Ad31 to and infection of A549 and HCE cells (Fig. 6). Prolonging the incubation time, elevating the temperature (incubation at 37°C instead of room temperature), or replacing heparinase I with heparinase II, which cleaves heparan sulfate more efficiently than heparinase I (18), did not lead to reduced binding (data not shown). Together, these results suggested that FIX, but not FX, efficiently promotes efficient Ad31 infection of human epithelial cells and that the involvement of heparan sulfate is not as pronounced for FIX-mediated Ad31 infection as it is for FIX- and FX-mediated Ad5 infection of epithelial cells.
FIG. 4.
Low concentrations of FIX promote efficient binding of Ad31 to and infection of epithelial cells. Purified Ad31 virions were preincubated with or without physiological concentrations of complement components, or coagulation factors, with or without EDTA as indicated, and then allowed to infect adherent A549 cells (A) or HCE cells (B). Forty-four hours postinfection, infected cells were stained and quantified by fluorescence microscopy. (C) 35S-labeled, CsCl-purified Ad31 virions were preincubated with or without increasing concentrations of FIX and then incubated with A549 or HCE cells in suspension on ice for one hour. Unbound virions were removed by washing, and cell-associated radioactivity was measured with a scintillation counter. The control binding level corresponds to the y axis value of 1. Values are means ± SD. *, P of <0.05 versus control; **, P of <0.01 versus control. The bars in panels A and B indicate comparisons between FIX and FIX-EDTA or between FX and FX-EDTA.
FIG. 5.
Preincubation of Ad31 with heparin inhibits FIX-mediated binding to and infection of epithelial cells. Ad31 virions were preincubated with or without physiological concentrations of FIX and increasing concentrations of heparin and then allowed to bind to A549 cells (A) or HCE cells (B) or to infect A549 cells or HCE cells (C). Cell-associated radioactivity (i.e., the extent of 35S-labeled Ad31 virions) was measured with a scintillation counter, and the infectivity was quantified by fluorescence microscopy. Values are means ± SD. *, P of <0.05 versus control; **, P of <0.01 versus control.
FIG. 6.
Treatment of epithelial cells with heparinase I has no effect on FIX-mediated Ad31 binding and infection. Ad31 virions were preincubated with or without physiological concentrations of FIX and then allowed to bind to A549 cells (A) or HCE cells (B) or to infect A549 or HCE cells (C) pretreated with increasing concentrations of heparinase I. In binding assays, cell-associated radioactivity was measured with a scintillation counter, whereas in infection assays, infectivity was quantified by fluorescence microscopy.
FIX promotes binding of Ad31 to and infection of intestinal epithelial cells.
Species A Ads are rarely isolated from adults, but the seroprevalence is relatively high (44, 49), indicating that these infections are often asymptomatic. Whenever symptomatic, however, the clinical manifestations are usually gastrointestinal (44). To determine whether coagulation factors are used by Ad31 for infection of intestinal cells, we first preincubated Ad31 virions with coagulation factors and then analyzed infection of intestinal FHs74Int cells. As with A549 and HCE cells, FIX promoted infection of FHs74Int cells almost 15-fold but FVII and FX had no detectable effect (Fig. 7A) compared to the control. Coincubation with EDTA abolished the promoting effect of FIX, again indicating a role of divalent cations. Preincubation of Ad31 with as little as 0.025 to 0.05 μg/ml FIX resulted in an approximately fivefold increase in Ad31 binding to FHs74Int cells (Fig. 7B), which was similar to the concentrations required for FIX-mediated binding of Ad31 to A549 and HCE cells. Heparin pretreatment of Ad31 virions reduced the promoting effect of FIX on Ad31 infection of FHs74Int cells (Fig. 7C), but heparinase I pretreatment of FHsInt74 cells did not have any inhibitory effect (Fig. 7D); the results thus corresponded to those with A549 and HCE cells. However, heparinase I treatment of FHs74Int cells efficiently reduced both FIX- and FX-mediated Ad5 binding (data not shown), indicating that the difference in sensitivity to heparinase I was virus specific rather than cell type specific. Thus, the involvement of heparan sulfate in FIX-mediated binding of Ad31 to and infection of epithelial cells is different from that of the FIX- and FX-mediated binding and infection of Ad5.
FIG. 7.
FIX promotes efficient binding of Ad31 to and infection of intestinal cells, independently of cell surface heparan sulfate. (A) Ad31 virions were preincubated with coagulation factors, with or without EDTA as indicated, and allowed to infect adherent intestinal FHs74Int cells for forty-four hours. Infected cells were stained and quantified as described in Materials and Methods. (B to D) 35S-labeled, CsCl-purified Ad31 virions were preincubated with increasing concentrations of FIX (B) or with/without fixed (physiological) concentrations of FIX and increasing concentrations of heparin (C), or with/without fixed concentrations of FIX, and then mixed with cells pretreated with increasing concentrations of heparinase I (D) for one hour on ice. Unbound virions were washed, and cell-associated radioactivity was measured in a scintillation counter. Values are means ± SD. *, P of <0.05 versus control; **, P of <0.01 versus control. Bars in panel A indicate comparisons between FIX and FIX-EDTA or between FX and FX-EDTA.
DISCUSSION
It has been shown previously that three types of soluble components in human body fluids promote binding of Ads to human target cells. Coagulation factors, and FX in particular, mediate binding of Ads to hepatocytes (21, 31, 32, 38, 42, 43), whereas DPPC (5) and lactoferrin (19) mediate binding of Ads to and infection of respiratory and other epithelial cells as well as T cells. Here we have shown that two serotypes that commonly infect humans, Ad5 (of species C) and Ad31 (of species A), infect human epithelial cells—cells that represent the respiratory and ocular tropisms of several Ads—more efficiently in the presence of plasma, tear fluid, or saliva. Ad5 was found to use FIX or FX, whereas Ad31 was found to use FIX only, for efficient binding to and infection of epithelial cells. These effects are dose dependent and require divalent cations. FIX- and FX-mediated binding of Ad5 to and infection of epithelial cells appears to require the presence of cell surface heparan sulfate, but the role of heparan sulfate during FIX-mediated binding and infection of Ad31 is still unclear.
In immunocompetent individuals, Ad5 viremia is uncommon and systemic Ad5 infections, including hepatitis, are also rare. Despite this, Ad5 has evolved to interact efficiently with FX via the hexon protein, which results in efficient transduction of hepatocytes after administration of Ad5-based vectors into the circulation. The KD (equilibrium dissociation constant) values reported for Ad5 virions binding to FX range from 0.229 nM (21) to 1.83 nM (42), depending on the experimental approach. The high affinity suggests that this interaction, during natural infections, may have functions other than mediating liver tropism. Blood is obviously the natural environment for coagulation factors, but they may also—via exudation—be transferred to the respiratory mucosa (34). In addition, coagulation factors may also be produced directly by bronchial cells and secreted into the mucosa (33). The concentrations of coagulation factors in body fluids other than blood are unknown. We found that as little as 1% of the blood concentrations of FX was sufficient to mediate efficient binding and infection of Ad5 and that as little as 1% of the blood concentration of FIX was sufficient to mediate efficient binding and infection of Ad31, in both cases using cells that correspond to the natural tropisms of these (and other) Ads. Consequently, these results suggest that even at low concentrations, these factors may promote natural infections by Ads at locations other than the liver, such as the respiratory tract, the eyes, or the intestine.
The interactions of FX with Ad serotypes of species A to F have been studied with surface plasmon resonance (42). Interestingly, unlike Ad5, Ad31 was not found to interact with FX, which is in agreement with our observation that FIX, but not FX, promoted binding of Ad31 to and infection of epithelial cells. Another difference between Ad5 and Ad31 was that heparinase I efficiently blocked both FIX- and FX-mediated binding of Ad5, but not FIX-mediated binding of Ad31. We found this to be rather puzzling, since heparin inhibited FIX-mediated Ad31 binding, albeit at higher concentrations than were required for inhibition of Ad5 binding. The effects of heparin indicate that the cellular receptor binding sites on FX and FIX that mediate indirect binding of Ad5 and Ad31 (respectively) to target cells are related to each other in amino acid sequence and are probably located in similar regions of FIX and FX. However, the results from the heparinase I experiments indicate that whereas FIX and FX mediate binding of Ad5 specifically to cell surface heparan sulfate, this glycosaminoglycan does not appear to be involved in FIX-mediated binding of Ad31 to target cells. Thus, FIX-mediated binding of Ad31 to epithelial cells is likely to involve a heparin binding site on FIX but most likely does not involve cell surface heparan sulfate. Alternatively, FIX-mediated binding of Ad31 to target cells may involve a glycosaminoglycan other than heparan sulfate that is not sensitive to the heparinases used here. Moreover, the concentration of FIX that was found to be required for efficient Ad31 binding was much lower than the concentrations of FIX found to be required for efficient Ad5 binding, which further indicates that the mechanisms of FIX-mediated binding of these two viruses may be fundamentally different. It is, however, too early to exclude alternative explanations to those suggested above.
It has been demonstrated previously that lactoferrin in tear fluid, DPPC in the respiratory mucosa, and blood coagulation factors promote binding to, or transduction of, target cells by species C Ads. Here we have shown that saliva also promotes Ad5 infection of A549 cells, but not of HCE cells. The reason for this difference is unclear. However, the concentrations of DPPC, lactoferrin, and coagulation factors would be expected to be very low in saliva; if this is true, it is likely that saliva contains components, other than those mentioned above, that promote binding of Ad5 to cells in a way similar to that seen for the other soluble components. In our system, breast milk was the only body fluid that did not promote infection of any Ads. The only effect of milk was a small and general inhibition of infection. The reasons for this could be a lack of specific components, such as coagulation factors, in milk. However, the high concentrations of lactoferrin in milk indicate that the slightly inhibitory effect of milk may be due to the presence of inhibitory components, such as antibodies or antiviral peptides, rather than the absence of promoting components.
The effect of coagulation factors on infection of HCE cells by Ads was generally greater than the effect in A549 cells. This is probably because HCE cells express less CAR than do A549 cells (29). Thus, the basal, coagulation factor-independent level of infection is therefore likely to be higher in A549 cells, and consequently, the effect of coagulation factors becomes less obvious. In vivo, however, where CAR is absent on the apical sides of epithelial cells (47), the relative effect of coagulation factors would be expected to be even higher. There is an accumulating amount of data suggesting that whereas CAR is an efficient receptor for Ads on nonpolarized target cells in vitro, it may not be the major receptor for Ads in vivo. In addition to the lack of apically located CAR on polarized target epithelial cells, CAR is not expressed at all on T cells (10), which are secondary target cells for species C Ads (15). Unlike CAR, heparan sulfate is indeed expressed apically on polarized epithelial cells (27), which suggests that viral binding to cell surface heparan sulfate on polarized epithelial cells via coagulation factors is likely to be relevant in vivo. Moreover, a major role of the fiber-CAR interaction may be to facilitate escape from the site of infection rather than cellular entry (46). Based on this, it is reasonable to believe that other components and mechanisms (including coagulation factors, DPPC, and lactoferrin) mediate CAR-independent Ad infections of natural target cells in vivo. When Ads (or Ad-based vectors) enter the circulation, however, it appears that FX is a key determinant of liver transduction. Outside the circulation, the situation is more complex and the relative importance of soluble components requires further examination. It is, however, clear that soluble components in various body fluids have important roles in Ad tropism and that additional components and mechanisms that are used by Ads and perhaps other, unrelated viruses remain to be discovered.
Acknowledgments
We thank David Isenman, University of Toronto, for the generous gift of complement components.
This work was supported by the Swedish Scientific Research Council (Medicine) (grants no. 2003-6008, 2004-6174, and 2007-3402), the Kempe Foundation (grant no. JCK-2818), the Swedish Foundation for Strategic Research (grant no. F06-0011), the Jeansson Foundations, and the Swedish Society of Medicine and was performed within the Umeå Centre for Microbial Research (UCMR).
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Araki-Sasaki, K., K. Y. Ohasi, T. Sasabe, K. Hayashi, H. Watanabe, Y. Tano, and H. Handa. 1995. An SV-40-immortalized human corneal epithelial cell line and its characterization. Investig. Ophthalmol. 36614-621. [PubMed] [Google Scholar]
- 2.Arnberg, N., K. Edlund, A. H. Kidd, and G. Wadell. 2000. Adenovirus type 37 uses sialic acid as a cellular receptor. J. Virol. 7442-48. [PMC free article] [PubMed] [Google Scholar]
- 3.Arnberg, N., A. H. Kidd, K. Edlund, F. Olfat, and G. Wadell. 2000. Initial interactions of subgenus D adenoviruses with A549 cellular receptors: sialic acid versus αv integrins. J. Virol. 747691-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnberg, N., P. Pring-Akerblom, and G. Wadell. 2002. Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J. Virol. 768834-8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balakireva, L., G. Schoehn, E. Thouvenin, and J. Chroboczek. 2003. Binding of adenovirus capsid to dipalmitoyl phosphatidylcholine provides a novel pathway for virus entry. J. Virol. 774858-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belin, M. T., and P. Boulanger. 1993. Involvement of cellular adhesion sequences in the attachment of adenovirus to the HeLa cell surface. J. Gen. Virol. 741485-1497. [DOI] [PubMed] [Google Scholar]
- 7.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 2751320-1323. [DOI] [PubMed] [Google Scholar]
- 8.Breidenbach, M., D. T. Rein, M. Wang, D. M. Nettelbeck, A. Hemminki, I. Ulasov, A. R. Rivera, M. Everts, R. D. Alvarez, J. T. Douglas, and D. T. Curiel. 2004. Genetic replacement of the adenovirus shaft fiber reduces liver tropism in ovarian cancer gene therapy. Hum. Gene Ther. 15509-518. [DOI] [PubMed] [Google Scholar]
- 9.Burmeister, W. P., D. Guilligay, S. Cusack, G. Wadell, and N. Arnberg. 2004. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J. Virol. 787727-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Z., M. Ahonen, H. Hamalainen, J. M. Bergelson, V. M. Kahari, and R. Lahesmaa. 2002. High-efficiency gene transfer to primary T lymphocytes by recombinant adenovirus vectors. J. Immunol. Methods 26079-89. [DOI] [PubMed] [Google Scholar]
- 11.Chu, Y., D. Heistad, M. I. Cybulsky, and B. L. Davidson. 2001. Vascular cell adhesion molecule-1 augments adenovirus-mediated gene transfer. Arterioscler. Thromb. Vasc. Biol. 21238-242. [DOI] [PubMed] [Google Scholar]
- 12.Coyne, C. B., and J. M. Bergelson. 2005. CAR: a virus receptor within the tight junction. Adv. Drug Deliv. Rev. 57869-882. [DOI] [PubMed] [Google Scholar]
- 13.Dechecchi, M. C., P. Melotti, A. Bonizzato, M. Santacatterina, M. Chilosi, and G. Cabrini. 2001. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate initial binding of adenovirus types 2 and 5. J. Virol. 758772-8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 91408-1412. [DOI] [PubMed] [Google Scholar]
- 15.Garnett, C. T., D. Erdman, W. Xu, and L. R. Gooding. 2002. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J. Virol. 7610608-10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hierholzer, J. C. 1992. Adenoviruses in the immunocompromised host. Clin. Microbiol. Rev. 5262-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong, S. S., L. Karayan, J. Tournier, D. T. Curiel, and P. A. Boulanger. 1997. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 162294-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jandik, K. A., K. Gu, and R. J. Linhardt. 1994. Action pattern of polysaccharide lyases on glycosaminoglycans. Glycobiology 4289-296. [DOI] [PubMed] [Google Scholar]
- 19.Johansson, C., M. Jonsson, M. Marttila, D. Persson, X. L. Fan, J. Skog, L. Frangsmyr, G. Wadell, and N. Arnberg. 2007. Adenoviruses use lactoferrin as a bridge for CAR-independent binding to and infection of epithelial cells. J. Virol. 81954-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansson, S. M., E. C. Nilsson, M. Elofsson, N. Ahlskog, J. Kihlberg, and N. Arnberg. 2007. Multivalent sialic acid conjugates inhibit adenovirus type 37 from binding to and infecting human corneal epithelial cells. Antivir. Res. 7392-100. [DOI] [PubMed] [Google Scholar]
- 21.Kalyuzhniy, O., N. C. Di Paolo, M. Silvestry, S. E. Hofherr, M. A. Barry, P. L. Stewart, and D. M. Shayakhmetov. 2008. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 1055483-5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koizumi, N., K. Kawabata, F. Sakurai, Y. Watanabe, T. Hayakawa, and H. Mizuguchi. 2006. Modified adenoviral vectors ablated for coxsackievirus-adenovirus receptor, alphav integrin, and heparan sulfate binding reduce in vivo tissue transduction and toxicity. Hum. Gene Ther. 17264-279. [DOI] [PubMed] [Google Scholar]
- 23.Kritz, A. B., C. G. Nicol, K. L. Dishart, R. Nelson, S. Holbeck, D. J. Von Seggern, L. M. Work, J. H. McVey, S. A. Nicklin, and A. H. Baker. 2007. Adenovirus 5 fibers mutated at the putative HSPG-binding site show restricted retargeting with targeting peptides in the HI loop. Mol. Ther. 15741-749. [DOI] [PubMed] [Google Scholar]
- 24.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 755405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, K., A. Brie, P. Saulnier, M. Perricaudet, P. Yeh, and E. Vigne. 2003. Simultaneous CAR- and alpha V integrin-binding ablation fails to reduce Ad5 liver tropism. Mol. Ther. 8485-494. [DOI] [PubMed] [Google Scholar]
- 26.Marttila, M., D. Persson, D. Gustafsson, M. K. Liszewski, J. P. Atkinson, G. Wadell, and N. Arnberg. 2005. CD46 is a cellular receptor for all species B adenoviruses except types 3 and 7. J. Virol. 7914429-14436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mertens, G., B. Van der Schueren, H. van den Berghe, and G. David. 1996. Heparan sulfate expression in polarized epithelial cells: the apical sorting of glypican (GPI-anchored proteoglycan) is inversely related to its heparan sulfate content. J. Cell Biol. 132487-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicol, C. G., D. Graham, W. H. Miller, S. J. White, T. A. Smith, S. A. Nicklin, S. C. Stevenson, and A. H. Baker. 2004. Effect of adenovirus serotype 5 fiber and penton modifications on in vivo tropism in rats. Mol. Ther. 10344-354. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson, E. C., F. Jamshidi, S. M. Johansson, M. S. Oberste, and N. Arnberg. 2008. Sialic acid is a cellular receptor for coxsackievirus a24 variant, an emerging virus with pandemic potential. J. Virol. 823061-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osterud, B., B. N. Bouma, and J. H. Griffin. 1978. Human blood coagulation factor IX. Purification, properties, and mechanism of activation by activated factor XI. J. Biol. Chem. 2535946-5951. [PubMed] [Google Scholar]
- 31.Parker, A. L., J. H. McVey, J. H. Doctor, O. Lopez-Franco, S. N. Waddington, M. J. Havenga, S. A. Nicklin, and A. H. Baker. 2007. Influence of coagulation factor zymogens on the infectivity of adenoviruses pseudotyped with fibers from subgroup D. J. Virol. 813627-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker, A. L., S. N. Waddington, C. G. Nicol, D. M. Shayakhmetov, S. M. Buckley, L. Denby, G. Kemball-Cook, S. Ni, A. Lieber, J. H. McVey, S. A. Nicklin, and A. H. Baker. 2006. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 1082554-2561. [DOI] [PubMed] [Google Scholar]
- 33.Perrio, M. J., D. Ewen, M. A. Trevethick, G. P. Salmon, and J. K. Shute. 2007. Fibrin formation by wounded bronchial epithelial cell layers in vitro is essential for normal epithelial repair and independent of plasma proteins. Clin. Exp. Allergy 371688-1700. [DOI] [PubMed] [Google Scholar]
- 34.Persson, C. G., I. Erjefält, U. Alkner, C. Baumgarten, L. Greiff, B. Gustafsson, A. P. Luts, U. Pipkorn, F. Sundler, C. Svensson, and P. Wollmer. 1991. Plasma exudation as a first line respiratory mucosal defence. Clin. Exp. Allergy 2117-24. [DOI] [PubMed] [Google Scholar]
- 35.Rao, L. V., and S. I. Rapaport. 1988. Activation of factor VII bound to tissue factor: a key early step in the tissue factor pathway of blood coagulation. Proc. Natl. Acad. Sci. USA 856687-6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roelvink, P. W., A. Lizonova, J. G. Lee, Y. Li, J. M. Bergelson, R. W. Finberg, D. E. Brough, I. Kovesdi, and T. J. Wickham. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 727909-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 779183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shayakhmetov, D. M., A. Gaggar, S. Ni, Z.-Y. Li, and A. Lieber. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 797478-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith, T., N. Idamakanti, H. Kylefjord, M. Rollence, L. King, M. Kaloss, M. Kaleko, and S. C. Stevenson. 2002. In vivo hepatic adenoviral gene delivery occurs independently of the coxsackievirus-adenovirus receptor. Mol. Ther. 5770-779. [DOI] [PubMed] [Google Scholar]
- 40.Smith, T. A., N. Idamakanti, M. L. Rollence, J. Marshall-Neff, J. Kim, K. Mulgrew, G. R. Nemerow, M. Kaleko, and S. C. Stevenson. 2003. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 14777-787. [DOI] [PubMed] [Google Scholar]
- 41.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 943352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waddington, S. N., J. H. McVey, D. Bhella, A. L. Parker, K. Barker, H. Atoda, R. Pink, S. M. Buckley, J. A. Greig, L. Denby, J. Custers, T. Morita, I. M. Francischetti, R. Q. Monteiro, D. H. Barouch, N. van Rooijen, C. Napoli, M. J. Havenga, S. A. Nicklin, and A. H. Baker. 2008. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 132397-409. [DOI] [PubMed] [Google Scholar]
- 43.Waddington, S. N., A. L. Parker, M. Havenga, S. A. Nicklin, S. M. Buckley, J. H. McVey, and A. H. Baker. 2007. Targeting of adenovirus serotype 5 (Ad5) and 5/47 pseudotyped vectors in vivo: a fundamental involvement of coagulation factors and redundancy of CAR binding by Ad5. J. Virol. 819568-9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wadell, G. 2000. Adenoviruses, p. 308-327. In A. J. Zuckerman, J. E. Banatvala, and J. R. Pattison (ed.), Principles and practice of clinical virology, 4 ed. John Wiley & Sons, New York, NY.
- 45.Wadell, G., A. Allard, and J. C. Hierholzer. 1999. Adenoviruses, p. 970-982. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, DC.
- 46.Walters, R. W., P. Freimuth, T. O. Moninger, I. Ganske, J. Zabner, and M. J. Welsh. 2002. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 110789-799. [DOI] [PubMed] [Google Scholar]
- 47.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 27410219-10226. [DOI] [PubMed] [Google Scholar]
- 48.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73309-319. [DOI] [PubMed] [Google Scholar]
- 49.Vogels, R., D. Zuijdgeest, R. van Rijnsoever, E. Hartkoorn, I. Damen, M. P. de Bethune, S. Kostense, G. Penders, N. Helmus, W. Koudstaal, M. Cecchini, A. Wetterwald, M. Sprangers, A. Lemckert, O. Ophorst, B. Koel, M. van Meerendonk, P. Quax, L. Panitti, J. Grimbergen, A. Bout, J. Goudsmit, and M. Havenga. 2003. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 778263-8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wold, W. S. M., and M. S. Horwitz. 2007. Adenoviruses, p. 2395-2436. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 51.Xu, Z., J. Tian, J. S. Smith, and A. P. Byrnes. 2008. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J. Virol. 8211705-11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yun, C. O., A. R. Yoon, J. Y. Yoo, H. Kim, M. Kim, T. Ha, G. E. Kim, H. Kim, and J. H. Kim. 2005. Coxsackie and adenovirus receptor binding ablation reduces adenovirus liver tropism and toxicity. Hum. Gene Ther. 16248-261. [DOI] [PubMed] [Google Scholar]