Abstract
The bacillus Calmette-Guerin (BCG) strain of Mycobacterium bovis is used in many parts of the world as a vaccine against Mycobacterium tuberculosis. Some epidemiological evidence has suggested that BCG immunization may have unpredicted effects on resistance to other pathogens. We show here in a mouse model that BCG immunization followed by antibiotic treatment to clear the host of the pathogen rendered three strains of mice partially resistant to infection with vaccinia virus (VV) but not to lymphocytic choriomeningitis virus (LCMV). VV-challenged BCG-immune mice developed a striking splenomegaly and elevated CD4 and CD8 T-cell responses by 6 days postinfection (p.i.). However, resistance to VV infection could be seen as early as 1 to 2 days p.i. and was lost after antibody depletion of CD4 T-cell populations. BCG- but not LCMV-immune memory phenotype CD4 T cells preferentially produced gamma interferon (IFN-γ) in vivo after VV challenge. In contrast, LCMV-immune CD8 T cells preferentially produced IFN-γ in vivo in response to VV infection. In BCG-immune mice the resistance to VV infection and VV-induced CD4 T-cell IFN-γ production were ablated by cyclosporine A, which inhibits signaling through the T-cell receptor. This study therefore demonstrates CD4 T-cell-mediated heterologous immunity between a bacterium and virus. Further, it poses the question of whether BCG immunization of humans alters resistance to unrelated pathogens.
The immune system is molded by histories of infections, and prior exposure to one pathogen may influence resistance to a second unrelated pathogen. Congenitally infected lymphocytic choriomeningitis virus (LCMV) carrier mice lack T-cell responses to LCMV but resist infections by a wide variety of viruses, perhaps due to chronic production of low levels of type 1 interferon (IFN) (7). Hosts acutely infected with many viruses can generate very high levels of type 1 IFN that provide strong resistance to superinfection (54). Mice latently infected with murine cytomegalovirus (MCMV) or mouse gammaherpesvirus chronically reactivate these viruses, whose antigens stimulate IFN-γ production by memory T cells; this response provides resistance to Listeria monocytogenes and Yersinia pestis, which grow poorly in IFN-γ-activated macrophages (4).
One does not, however, need ongoing infections like the above to provide resistance to newly encountered pathogens. In a phenomenon known as heterologous immunity, the immune memory lymphocyte repertoire created in response to one pathogen may influence the immune response to another unrelated pathogen, particularly if antigens from the second pathogen can cross-reactively stimulate the memory pool (55). The degenerative nature of T-cell recognition, where an antigenic peptide is presented in the context of a major histocompatibility complex (MHC) molecule (45), and where the T-cell receptor (TCR) undergoes conformational shifts to recognize a peptide/MHC complex (5), enhances the probability of cross-reactivity between unrelated pathogens. Indeed, cross-reactive CD8 T cells have been reported between the antigens of influenza virus and Epstein-Barr virus (EBV) (11), hepatitis C virus (HCV) (52), and human immunodeficiency virus (2), between human immunodeficiency virus and Mycobacterium tuberculosis (17, 32), between human coronavirus and papillomavirus (32), and between a variety of viruses in murine model systems (6, 21, 55).
Heterologous immunity studies in mouse models of viral infections have revealed complex patterns of CD8 T-cell cross-reactivities, influenced in part by the private specificities of the TCR repertoire of individual hosts (21). The consequence of this cross-reactivity can be either protective immunity or altered immunopathology, usually involving a more dramatic infiltrate of CD8 T cells into infected areas (9, 10, 42). For example, LCMV-immune mice clear vaccinia virus (VV) more rapidly than do immunologically naïve mice, yet the immune mice have more dramatic immunopathology, presenting as bronchiolitis obliterans in the lung and severe panniculitis in the peritoneal cavity (10, 42, 58). In humans, severe CD8 T-cell responses during EBV-induced mononucleosis (11), HCV-induced hepatitis (48), and dengue virus-induced hemorrhagic fever (28) have all been proposed as examples of the consequences of cross-reactive CD8 T-cell-mediated heterologous immunity. Examples of heterologous immunity in humans may be easier to recognize when its consequences are harmful but may be more difficult to recognize when its consequences are beneficial, as little attention is paid to mild or subclinical viral infections.
Heterologous immunity does not necessarily need to be mediated by cross-reactive T cells, as cross-reactive antibody responses might likewise alter immune responses. Further, some have argued that memory T cells can sometimes be activated by cytokines such as interleukin-12 (IL-12) and IL-18 independently of TCR signaling (15, 35), and the significance of that mechanism in heterologous immunity needs to be determined.
An important question is how histories of exposures to pathogen-specific vaccines have influenced the outcome of infections with other pathogens. Two human diseases that have affected millions are tuberculosis and smallpox, and millions of individuals have also been vaccinated against those diseases. Vaccination against M. tuberculosis uses a closely related but less virulent strain of Mycobacterium bovis, referred to as bacillus Calmette-Guerin (BCG) (33). Several early reports indicated that a recent exposure of mice to BCG or to M. tuberculosis rendered them more resistant to infection to a variety of bacteria and to ectromelia virus (16, 40, 44), the mouse form of smallpox, and to VV (3, 56), which is used for vaccination of humans against smallpox. An unspecified IFN was suggested to be involved in the protection against ectromelia virus (40, 44). Given the importance of these vaccines and pathogens, we have examined the mechanism of this protection, using modern immunological approaches. We show here evidence for a CD4 T-cell-mediated heterologous immunity between a mycobacterium (BCG) and a virus, VV.
MATERIALS AND METHODS
Mice.
Six- to 8-week-old female C57BL/6 (B6) mice (H2b) were purchased from Taconic Farms (Hudson, NY), Jackson Laboratories (Bar Harbor, ME), or Charles River Breeding Laboratories (Wilmington, MA). Six- to 8-week-old female C57BL/10SNJ (B10) (H2b) and B10.D2-Hc1 (B10.D2) (H2d) H2-T18c/nSnJ mice were also purchased from Jackson Laboratories. All experiments were done in compliance with institutional guidelines as approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School.
Pathogens.
VV strain WR was propagated in NCTC 929 cells and purified over sucrose gradients (42). LCMV Armstrong strain clone 13 was propagated in BHK21 baby hamster kidney cells, and MCMV Smith strain was propagated in vivo in salivary glands of BALB/c mice (42). VV and LCMV PFU for individual mice were determined by plaque assay of 10% tissue homogenates titrated on Vero cell monolayers. Titers are expressed as geometric mean titers, that is, the arithmetic average of the log10 PFU per whole spleen, liver, or both abdominal fat pads plus or minus the standard deviation (SD). Live attenuated M. bovis BCG Pasteur strain was propagated in 7H9 broth supplemented with 10% oleic acid-albumin-dextrose-catalase (Difco, Becton Dickinson, Sparks, MD), 0.2% glycerol, and 0.05% Tween 80. BCG stocks for immunization were prepared from mid-log-phase cultures that were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 80. BCG CFU were determined by plating 10-fold dilutions on Middlebrook 7H11 agar (Difco). Vesicular stomatitis virus strain Indiana was used as a challenge virus in IFN assays (53).
Immunization and infection of mice.
For BCG vaccination, mice received 5 × 106 CFU of BCG in PBS containing 0.05% Tween 80 by subcutaneous injection at the base of the tail. Controls were injected with PBS-Tween alone. After 3 weeks, the BCG-immunized and control mice were treated with the antibiotics isoniazid (200 mg/ml) plus rifampin (100 mg/ml) for 10 to 15 days in drinking water, in order to clear any residual BCG from the hosts (26). Four weeks or longer after immunization, mice were inoculated intraperitoneally (i.p.) with 1 × 106 PFU VV, 1 × 105 PFU LCMV, or 2 × 105 PFU of MCMV.
Isolation of leukocytes.
Leukocytes were isolated from the spleen, peritoneal cavity, and abdominal fat pads of immunized and virus-challenged infected mice, as described previously (42). The infiltrating leukocytes were isolated from the fat pads by mincing and digesting abdominal fat pads with 0.5 mg/ml type II collagenase (Sigma-Aldrich, St. Louis, MO) and 100 U/ml type I DNase (Sigma-Aldrich) in Hanks balanced salt solution buffer plus 10% heat-inactivated fetal calf serum for 45 min at 37°C. Erythrocytes were removed from cell populations by a brief treatment with 0.87% NH4Cl and analyzed.
Synthetic peptides.
Several previously defined CD8 T-cell epitopes encoded by VV were used in this study (12, 30). These included Kb-restricted epitopes from B8R protein, positions 20 to 27 (TSYKFESV), A11R protein, positions 198 to 206 (AIVNYANL), E7R protein, positions 130 to 137 (STLNFNNL), and A47L protein, positions 138 to 146 (AAFEFINSL). One Db- restricted epitope in the K3L protein, positions 6 to 15 (YSLPNAGDVI), was also used. Several recently defined CD4 T-cell epitopes encoded by VV were also used (29). These included the IAb-restricted epitopes from A18R protein, positions 49 to 63 (PKGFYASPSVKTSLV), A20R protein, positions 233 to 247 (DNIFIPSVITKSGKK), D13L protein, positions 486 to 500 (PKIFFRPTTITANVS), E9L protein, positions 179 to 193 (PSVFINPISHTSYCY), F15L protein, positions 55 to 69 (TPRYIPSTSISSSNI), H3L protein, positions 272 to 286 (PGVMYAFTTPLISFF), I1L protein, positions 7 to 21 (QLVFNSISARALKAY), and L4R protein, positions 176 to 190 (ISKYAGINILNVYSP).
In vivo depletions.
Mice were depleted of CD4 or CD8 T cells prior to infection at days −5 and −1. To deplete CD4 T cells, mice were injected with 100 μg of the monoclonal antibody (MAb) GK1.5 (anti-CD4) i.p. (57). To deplete CD8 T cells mice were injected with 50 μg of the MAb 2.43 (anti-CD8α) i.p. (41). Control mice were injected with a nondepleting immunoglobulin G2b isotype i.p. at the same time points (BioExpress, Lebanon, NH).
CsA.
To inhibit TCR signaling, mice were injected i.p. with cyclosporine A (CsA; Sigma-Aldrich, St. Louis, MO) diluted in olive oil (Sigma-Aldrich) at a dose of 40 mg/kg at days 0 and 1 of VV infection (19).
Intracellular IFN-γ staining.
Cytokine-producing T cells were detected using the Cytofix/Cytoperm Plus kit with GolgiPlug (BD Biosciences, Mountain View, CA), as previously described (34, 50). A total of 2 × 106 mouse leukocytes from spleen, peritoneal exudate cells (PEC), or fat pads were incubated in 96-well microtiter plates for 5 h at 37°C with 1 μM synthetic peptides or 0.1 μg/ml anti-CD3 antibody, 10 U/ml human recombinant IL-2 (BD Biosciences), and 0.2 μl of GolgiPlug. Cells were then washed in fluorescence-activated cell sorting buffer (Hanks balanced salt solution, 2% fetal calf serum, and 0.1% NaN3), blocked with anti-Fc (2.4G2), and incubated for 30 min at 4°C with a combination of fluorescently labeled MAbs specific for CD8, CD4, CD44, CD62L, CD69, CD11c, CD11b, CD25, CD3, and CD19 (BD Biosciences). Subsequent fixation and permeabilization of the cells was performed to allow intracellular access of MAb to IFN-γ (BD Biosciences). Freshly stained samples were analyzed either on an LSR II or FACSCalibur cytometer (BD Biosciences) and analyzed with either FlowJo (TreeStar) or CellQuest software (BD Biosciences).
In vivo cytokine staining.
Brefeldin A (BFA; catalog number B-6542; Sigma-Aldrich) was diluted to 20 mg/ml in sterile dimethyl sulfoxide and further diluted in PBS to 0.5 mg/ml. Mice were injected with 250 μg BFA intravenously in 500 μl, and 6 hours later spleen, PEC, and fat pad leukocytes were harvested and stained for intracellular cytokines without an in vitro stimulation with peptide or anti-CD3. With this assay the cytokine staining reflects cytokine production in vivo, rather than in vitro (24).
Type 1 IFN bioassay.
Dilutions (10%) of organ homogenates or peritoneal fluids were tested for IFN using a bioassay on L-929 cells. Briefly, L-929 cell monolayers in 96-well flat-bottom microtiter plates were exposed to twofold dilutions of sample for 24 h, challenged with vesicular stomatitis virus, and tested 1 day later for inhibition of cytopathic effect, as described previously (53).
Neutralizing antibody assay.
Serum was obtained from peripheral blood of BCG-immunized and control immune mice. The serum was heat inactivated at 56°C for 30 min, and then 1:10 serial dilutions were made across a 96-well plate in triplicate. A known amount of VV was added across the top row of the plate and titrated down at 1:10 dilutions. After 40 min of incubation at 37°C, samples were taken from the wells for plaque assay titration on Vero cell monolayers.
Statistics.
Most data are presented as the mean ± SD, and P values were derived using Student's t test.
RESULTS
Status of the BCG-induced resting memory state.
Experiments were designed to test whether immunity to BCG conferred protection against VV infection. In order to distinguish a resting immune state from an ongoing immune response to a persistent pathogen, mice infected with BCG for 3 weeks were initially treated for 10 days with the antibiotics isoniazid and rifampin, a regimen previously reported to clear BCG infection in mice (26). However, two of four mice in our study had a very small number of colony-forming bacilli in their draining inguinal lymph nodes near the site of immunization, with an average of 1.4 bacilli per lymph node (n = 4). We therefore extended antibiotic treatment to 15 days in subsequent experiments. Three weeks after a 15-day course of antibiotics was completed, no bacilli were found in whole-spleen homogenates (n = 2) or cell lysates prepared from half the splenocytes isolated from the spleen (n = 3). The majority of mice had no bacteria recovered from their draining lymph nodes, but three of seven of the mice had a very small number of bacilli in their draining lymph nodes, with an overall average of 1.7 bacilli per lymph node (n = 7).
To determine whether this extremely small number of residual bacteria was activating the immune system, BCG-immune and sham-immune mice, all treated with antibiotics, were examined for indicators of immune system activation. Although there seemed to be some variability in BCG-immune spleen size, there were no statistically significant differences in leukocyte numbers between BCG- and sham-immunized mice when tested at 8, 10 (Table 1), and 35 weeks postimmunization. Flow cytometric analysis of lymphocyte-gated samples at weeks 10 (Table 1) and 35 (not shown) showed no evidence of any state of T-cell activation, such as heightened expression of CD69 or reduced expression of CD62L. Expression of the memory T-cell marker CD44 was similar in both sets of mice. Expression of CD25 on CD4 T cells was comparable in both sets of mice (Table 1) and hardly any was expressed on CD8 T cells (not shown). No significant differences in the number of dendritic cells, as measured by staining with antibody to CD11c and CD11b, NK cells, as measured by antibody to NK1.1+, or granulocytes, as measured by antibody to GR-1, were found (data not shown). This indicates that these BCG-immune hosts were in a resting immune state without an overt ongoing immune response. This distinguishes this study from previous reports on resistance of BCG-infected or M. tuberculosis-infected hosts to heterologous pathogens at 7 to 21 days after exposure to the mycobacteria (3, 40, 44, 56).
TABLE 1.
Antigenic phenotype of lymphocytes prior to VV challengea
| Tissue sampled and immunization group | No. of leukocytes | No. of lymphocytes | % Cells displaying phenotype
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD19+ | CD8+ | CD8+ CD44+ | CD8+ CD69+ | CD8+ CD62L+ | CD4+ | CD4+ CD44+ | CD4+ CD69+ | CD4+ CD62L+ | CD4+ CD25+ | |||
| Spleen | ||||||||||||
| BCG | 135 ± 40 | 94 ± 26 | 56 ± 0.02 | 13 ± 0.01 | 29 ± 0.03 | 0.56 ± 0.00 | 96 ± 0.01 | 21 ± 0.01 | 31 ± 0.02 | 5.7 ± 0.01 | 75 ± 0.03 | 15 ± 0.01 |
| Sham | 99 ± 6.8 | 99 ± 6.8 | 56 ± 0.02 | 14 ± 0.00 | 30 ± 0.05 | 0.63 ± 0.00 | 96 ± 0.00 | 21 ± 0.01 | 30 ± 0.02 | 6 ± 0.01 | 74 ± 0.03 | 13 ± 0.00 |
| PEC | ||||||||||||
| BCG | 41 ± 5.1 | 15 ± 6.2 | 75 ± 0.05 | 7.3 ± 0.03 | 55 ± 0.07 | 0.27 ± 0.00 | 86 ± 0.07 | 14 ± 0.03 | 34 ± 0.09 | 0.14 ± 0.00 | 73 ± 0.09 | 12 ± 0.01 |
| Sham | 51 ± 18 | 23 ± 13 | 76 ± 0.04 | 6.9 ± 0.04 | 57 ± 0.03 | 0.15 ± 0.00 | 86 ± 0.07 | 14 ± 0.03 | 36 ± 0.06 | 0.11 ± 0.00 | 71 ± 0.06 | 8.7 ± 0.01 |
Spleen and PEC leukocytes were harvested from three B6 mice 10 weeks after BCG or sham immunization. Leukocyte counts reflect total counts made using a hemocytometer. Cells were stained with lymphocyte activation markers and analyzed by flow cytometry. Analysis of lymphocyte markers was based on a lymphocyte gate. Percentages of subsets under the CD8 or CD4 headings refer to their percentages within the respective CD8 or CD4 population.
Susceptibility of BCG-immune mice to virus infection.
To test whether immunity to BCG rendered mice resistant to viral infections, the BCG-immune, antibiotic-treated B6 mice were challenged with VV (Table 2) or LCMV (Table 2) and tested for viral titers in different organs at 1 to 3 days postinfection. Table 2 shows that BCG-immune B6 mice had normal susceptibility to LCMV but had dramatic 10- to 100-fold reductions in titers to VV. Mycobacteria-induced resistance to VV has been reported before, but those studies examined VV infection during ongoing immune responses early after immunization and most likely before the mycobacteria were cleared (3, 56). Under those conditions there would be high levels of macrophage activation and cytokine production at the time of challenge. In contrast, we used experimental conditions designed to examine heterologous immunity in a resting immune state by using antibiotic-treated mice. The resistance to VV infection was long-lasting, as mice were still protected against VV 28 weeks after the initial BCG immunization (Table 2). The immunity manifested itself quickly, as greater than 10-fold reductions in VV titers were seen as early as day 1 after VV challenge. Similar studies with VV were done in BCG-immune B10 mice and BCG-immune MHC-congenic B10.D2 (H2d) mice. B10 mice are closely related to B6 mice and have the same H2b haplotype. BCG-immune B10 mice were as resistant to VV as their B6 counterparts. The protective effect of BCG immunization to VV infection also occurred in B10.D2 mice but seemed less pronounced than that in B10 mice (Table 2). This was not further addressed, but it does suggest that this heterologous immunity can occur with different MHC.
TABLE 2.
Effects of BCG immunization on PFU titers of challenge virusesa
| Challenge virus and mouse strain | Immunization (n) | Time of virus challenge (wks after BCG or sham immunization) | Day postchallenge that titers were determined | Titer (log10 PFU/ml) inb:
|
||
|---|---|---|---|---|---|---|
| Spleen | Liver | Fat pads | ||||
| VV | ||||||
| C57BL/6 | BCG (3) | 11 | 1 | 2.5 ± 0.31 | 3.1 ± 0.43 | 3.5 ± 0.28 |
| Sham (3) | 4.0 ± 0.23** | 4.3 ± 0.26* | 4.5 ± 0.16** | |||
| C57BL/6 | BCG (3) | 10 | 3 | 1.3 ± 0.45 | <2.0 ± 0.00 | 3.0 ± 1.6 |
| Sham (3) | 2.8 ± 0.78* | 2.8 ± 0.43* | 5.7 ± 0.29* | |||
| C57BL/6 | BCG (5) | 28 | 3 | 1.5 ± 0.55 | 2.3 ± 0.30 | 4.2 ± 0.85 |
| Sham (5) | 3.1 ± 0.25** | 3.9 ± 0.59** | 5.4 ± 0.42* | |||
| B10 | BCG (4) | 13 | 2 | <1.0 ± 0.00 | <2.0 ± 0.00 | 2.3 ± 1.2 |
| Sham (4) | 4.1 ± 0.10** | 4.4 ± 0.20** | 4.4 ± 0.32* | |||
| B10.D2 | BCG (4) | 13 | 2 | <1.0 ± 0.00 | <2.0 ± 0.00 | 3.3 ± 1.3 |
| Sham (4) | <1.0 ± 0.00 | 3.3 ± 0.25** | 4.4 ± 0.28 | |||
| LCMV clone 13 | ||||||
| C57BL/6 | BCG (3) | 12 | 3 | 5.3 ± 0.65 | 4.3 ± 0.30 | |
| Sham (3) | 5.3 ± 0.63 | 4.0 ± 0.30 | ||||
| C57BL/6 | BCG (3) | 17 | 3 | 5.5 ± 0.20 | 5.0 ± 0.28 | |
| Sham (3) | 5.4 ± 0.44 | 5.1 ± 0.22 | ||||
Mice of the designated strains were immunized with BCG or sham immunized, and at the designated time postimmunization they were challenged with either VV or LCMV, as described in Materials and Methods.
On the designated day postchallenge, mice were sacrificed and viral titers were assessed in tissue homogenates by plaque assay on Vero cells. Spleen and liver titers (both challenge groups) and fat pad titers (only the VV challenge group) are expressed as the log10 PFU/ml. Statistically significant differences between BCG- and sham-immunized mice: *, P < 0.05; **, P < 0.01 (Student's t test).
Magnitude of the VV-induced T-cell response.
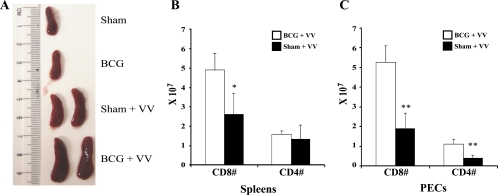
BCG immunization dramatically augmented the T-cell response to VV. Typically, the peak of the VV-induced T-cell response in B6 mice occurs 6 days after i.p. infection. Although the spleen size and T-cell number were not statistically different between BCG- and sham-immunized mice prior to VV infection (Table 1 and Fig. 1A), remarkable differences were seen at day 6 after infection. Spleen sizes became substantially larger in the VV-infected BCG-immune mice (Fig. 1A), reflecting larger numbers of T cells in both spleens and peritoneal cavities, where the inflammatory infiltrate was quite extensive. Expansions in the CD8 T-cell populations were most profound, as shown in the representative experiment in Fig. 1B and C. Averages of CD8 numbers from three experiments (n = 8) were as follows: BCG spleen, 6.7 × 107 ± 2.5 × 107, versus sham spleen, 3.2 × 107 ± 1.4 × 107 (P = 0.004); BCG PEC, 4.2 × 107 ± 1.6 × 107 versus sham PEC, 1.8 × 107 ± 0.7 × 107 (P = 0.002). At day 6, most of the CD8 (>85%) and CD4 (>65%) T cells expressed the activation marker CD44.
FIG. 1.
Increased spleen size and spleen T-cell numbers 6 days after VV infection of BCG-immunized mice. (A) Sizes of spleens before and after VV challenge. (B and C) CD8 and CD4 cell numbers in spleens and PEC, respectively, as determined by flow cytometry. There were four mice per group in this representative experiment. Statistically significant differences between BCG- and sham-immunized mice: *, P < 0.05; **, P < 0.01.
Mechanism of protective heterologous immunity.
Despite the heightened immune response at day 6, Table 2 shows that substantial differences in viral titers in spleen, visceral fat pads (lining the peritoneal cavity), and liver were detected as early as 1 or 2 days postinfection of BCG-immunized mice challenged with VV. This timing could be consistent with an early innate immune response. We thus tested the peritoneal fluid for type 1 IFNs in a bioassay and found none (titer, <1.2) prior to VV challenge (n = 7) or at 6 (n = 2) or 24 (n = 6) h after i.p. infection. Sera from BCG-immune mice were tested in neutralization plaque assays against VV, and no detectable neutralizing antibody was found (titer, <1:3) (n = 4).
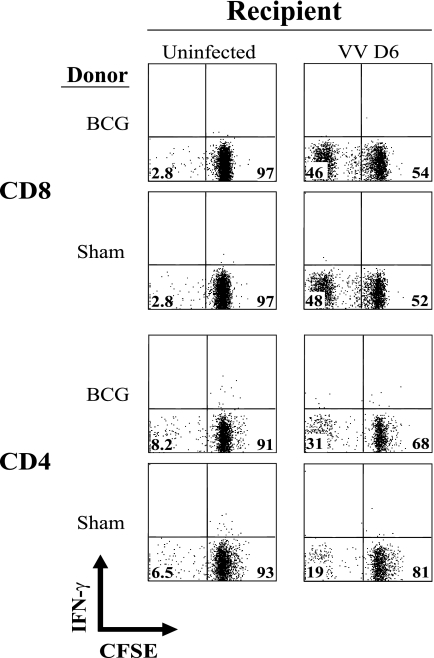
Heterologous immunity between LCMV and VV has been shown to be mediated by memory CD8 and CD4 T cells appearing early, with innate-like kinetics (42). In that system, when carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled splenocytes from LCMV-immune mice were adoptively transferred into naïve recipient mice challenged with VV, there was a dramatic increase in the number of CFSElo donor CD8 T cells and a much less dramatic response of CD4 T cells, in comparison to naïve donor cells (20). Generally, when CD8 T cells see antigen they undergo many rounds of clonal expansion and greatly increase in frequency (18, 27, 49). Expansion of CD4 T cells under similar conditions is usually much more limited (14). Nevertheless, we analyzed how well BCG-immune CD4 and CD8 T cells would proliferate after transfer into naïve mice that were subsequently challenged with VV. A total of 3 × 107 CFSE-labeled BCG-immune or sham-immune splenocytes (Ly5.2) were transferred intravenously into Ly5.1 congenic B6 mice, which were inoculated with VV 4 h later, and at day 5 or 6 postinfection the CSFE loss and total numbers of CD4 and CD8 cells were monitored (very little CFSE loss was seen at day 4 postinfection). There was some loss of CFSE, indicative of division, in the CD8 populations, but in five experiments (n = a total of 14 mice per group) there were no significant differences in CFSE loss between BCG-immune and sham-immune CD8 donor cells; representative CD8 plots shown in Fig. 2 reflect the similarities in CD8 responses. This is consistent with the CFSE loss in CD8 cells being a primary response to infection with either group of cells. In contrast, in these five experiments 12 out of 14 mice receiving BCG-immune donor cells showed a modestly enhanced CFSE loss in BCG-immune CD4 cells compared to the average (per experiment) in sham-immune CD4 donor cells, as depicted here for one of the responders in Fig. 2 (in this experiment three sham-immune mice averaged 19% CFSElow CD4 cells, whereas three BCG-immune mice averaged 30% CFSElow CD4 cells; P = 0.036). These CD4 responses were relatively weak but point to a possible role for CD4 but not CD8 T cells in the BCG-induced resistance to VV.
FIG. 2.
Moderate loss of CFSE in adoptively transferred BCG-immune CD4 T-cell populations. A total of 3 × 107 BCG- or sham-immune spleen leukocytes were transferred intravenously into naïve B6 female mice, which were challenged 4 h later with VV. At 6 days postinfection splenocytes were harvested and analyzed for CD4, CD8, and expression of CFSE. The cells were gated on CD4 or CD8 and plotted against IFN-γ (y axis) and CFSE (x axis). This was part of a larger experiment that included stimulation of intracellular IFN-γ production in vitro, but these plots are intended to show just cell number and are of the nonstimulated controls, so they all are IFN-γ-negative.
We therefore depleted BCG- or sham-immune mice with anti-CD4 (Table 3) or anti-CD8α antibodies (Table 3) and challenged them with VV. Depletion of CD8 T cells (and likely other CD8α+ cells) had no effect on the differential protective immunity in this and in another experiment. However, depletion of CD4 T cells eliminated the differential in titers between the BCG-immune and sham-immune hosts. Anti-CD4 treatment had no effect on the day 2 VV titers in sham-immune mice, indicating that any impact of naïve CD4 T cells in hosts by day 2 postinfection was minimal; however, anti-CD4 increased titers in some organs by over 10-fold in BCG-immune mice. We interpret this experiment to indicate that the protective heterologous immunity was dependent on memory CD4 cells, since depletion of CD4 cells enhanced viral titers at day 2 only in BCG-immune and not in sham-immune mice. This demonstration of heterologous immunity mediated by CD4 but not CD8 T cells is unique.
TABLE 3.
Effects of T-cell depletion and CsA on VV titers in BCG-immune micea
| Experiment | Immunization (n) | Time of challenge (wks after BCG or sham immunization) | Treatment | Day postchallenge that titers were determined | Titer (log10 PFU/ml) inb:
|
||
|---|---|---|---|---|---|---|---|
| Spleen | Fat pads | Liver | |||||
| A | BCG (3) | 13 | Isotype | 2 | 1.8 ± 1.3 | 3.7 ± 0.89 | 3.3 ± 1.4 |
| BCG (4) | 13 | Anti-CD4 | 2 | 4.9 ± 1.5* | 4.9 ± 0.24* | 4.7 ± 0.16 | |
| Sham (3) | 13 | Isotype | 2 | 4.5 ± 0.38 | 4.9 ± 0.21 | 4.9 ± 0.48 | |
| Sham (4) | 13 | Anti-CD4 | 2 | 4.3 ± 1.1 | 4.6 ± 0.21 | 4.8 ± 0.62 | |
| B | BCG (4) | 9 | Isotype | 2 | 1.5 ± 0.95 | 3.9 ± 0.62 | 2.6 ± 1.2 |
| BCG (4) | 9 | Anti-CD4 | 2 | 3.3 ± 0.90* | 5.2 ± 0.40* | 3.6 ± 0.73 | |
| Sham (4) | 9 | Isotype | 2 | 3.9 ± 0.31 | 4.9 ± 0.37 | 4.9 ± 0.33 | |
| Sham (4) | 9 | Anti-CD4 | 2 | 3.8 ± 1.9 | 5.0 ± 0.21 | 4.6 ± 0.41 | |
| C | BCG (4) | 9 | Isotype | 3 | 1.9 ± 0.63 | 4.2 ± 0.54 | NT |
| BCG (4) | 9 | Anti-CD4 | 3 | 3.0 ± 0.53* | 6.3 ± 0.44** | NT | |
| Sham (4) | 9 | Isotype | 3 | 2.9 ± 1.3 | 5.2 ± 0.50 | NT | |
| Sham (4) | 9 | Anti-CD4 | 3 | 3.2 ± 0.39 | 5.3 ± 0.66 | NT | |
| BCG (4) | 9 | Isotype | 3 | 1.9 ± 0.63 | 4.2 ± 0.54 | NT | |
| BCG (4) | 9 | Anti-CD8 | 3 | 1.8 ± 0.56 | 4.7 ± 0.41 | NT | |
| Sham (4) | 9 | Isotype | 3 | 2.9 ± 1.3 | 5.2 ± 0.50 | NT | |
| Sham (4) | 9 | Anti-CD8 | 3 | 2.6 ± 0.46 | 5.2 ± 0.41 | NT | |
| D | BCG (3) | 12 | No treatment | 1 | 4.0 ± 0.64 | 4.5 ± 0.48 | 4.6 ± 0.24 |
| BCG (4) | 12 | CsA | 1 | 5.2 ± 0.56 | 5.2 ± 0.69 | 5.5 ± 0.64 | |
| Sham (3) | 12 | No treatment | 1 | 4.3 ± 0.31 | 5.4 ± 0.46 | 5.1 ± 0.56 | |
| Sham (3) | 12 | CsA | 1 | 5.0 ± 0.66 | 5.7 ± 0.19 | 5.5 ± 1.4 | |
BCG- or sham-immunized B6 mice were injected in vivo with MAb to CD4 or CD8 or with the drug CsA, and then mice were challenged with VV, as described in Materials and Methods. The weeks after immunization and the days after challenge are listed in the table.
On the designated day postchallenge, mice were sacrificed and viral titers were assessed in tissue homogenates by plaque assay on Vero cells. Spleen, fat pad, and liver titers are expressed as the log10 PFU/ml. Statistically significant differences between isotype and anti-CD4 or anti-CD8 groups (or no treatment and CsA groups): *, P < 0.05; **, P < 0.01 (Student's t test). NT, not tested.
CD4 T cells produce high levels of IFN-γ early in infection.
We questioned whether the dependence on CD4 cells for protective immunity was due to CD4 T-cell helper or effector mechanisms. The early manifestation of protection against VV in BCG-immune mice 1 to 2 days postinfection is inconsistent with the concept that BCG-immune CD4 T cells were protecting by augmenting antibody responses. Further, the fact that CD8 depletion had no effect on the differential of sham versus BCG-immunized mice on VV titers argues that the mechanism of protection was not due to CD4 T-cell help for CD8 T cells, even though there may have been enough CD4 T cells in the immune host (but not in the adoptively reconstituted host) to augment CD8 T-cell proliferation.
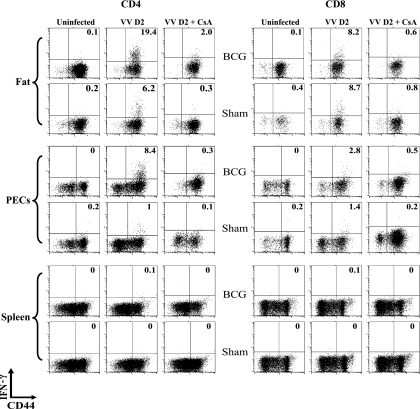
Control of VV infection in mice has been linked to the ability of T cells to produce IFN-γ, which is made by VV-specific CD4 and CD8 T cells on contact with MHC-displayed viral peptides (31, 42, 51). To test directly for T-cell IFN-γ-producing effector function in vivo, we employed an in vivo cytokine assay, where infected mice were inoculated with brefeldin A to prevent release of intracellular cytokines (24). Six hours later leukocytes were harvested and directly stained for intracellular IFN-γ and antigenic phenotype, without additional stimulation in vitro. Figure 3 shows that very little IFN-γ was made by CD4 or CD8 T cells isolated from spleen, peritoneum, and fat pads from unchallenged sham-immune or BCG-immune mice. This is further evidence that the immune system is at rest prior to VV challenge. Very little IFN-γ was made in the spleens of VV-infected mice (Fig. 3), perhaps because that was not the site of viral challenge and because the viral antigen load therein was low (Table 2). Further, at this time point most of the memory cells may have been recruited to peripheral sites like the fat and peritoneal cavity. Some low levels of IFN-γ staining were seen in fat and PEC CD8 T cells at day 2 after VV infection, but there were no significant differences in CD8 T-cell IFN-γ production between sham-immune and BCG-immune VV-infected mice. For example, in six experiments BCG-immune mice had on average a statistically insignificant 20% increase in the number of IFN-γ-producing CD8 T cells in the fat, in comparison to sham-immune mice (P = 0.84). In contrast, CD4 cells expressed much higher levels of IFN-γ in the BCG-immune versus the sham-immune mice. Figure 3 shows that as many as 20% of CD4 cells infiltrating visceral fat 2 days after VV infection were producing IFN-γ in vivo, and that there were 8 times more IFN-γ-producing CD4 T cells in the peritoneal exudates of BCG-immune versus sham-immune mice. In six experiments the VV-infected BCG-immune mice had on average 7.6 times the number of IFN-γ-producing CD4 T cells in the peritoneal cavity (P = 0.0001) and 4.2 times the number of IFN-γ-producing CD4 T cells in the fat (P = 0.0001) than VV-infected sham-immune mice. Thus, this heterologous immunity was associated with much higher CD4 T-cell production of IFN-γ, which is known to control VV replication in this model.
FIG. 3.
In vivo IFN-γ production by T cells infiltrating the visceral fat and the peritoneal cavity of day 2 (D2) VV-challenged BCG-or sham-immunized mice. As described in Materials and Methods, immunized mice were challenged with VV for 2 days and injected with BFA for 6 h, and isolated leukocytes were stained for antigenic phenotype and cytoplasmic IFN-γ. Some mice were treated with CsA on day 0 and day 1 before the day 2 harvest.
Blockade of heterologous immunity and IFN-γ production by CsA.
We questioned whether the protective effect of CD4 T cells and their production of IFN-γ was a TCR-independent event associated with the reported tendency of memory T cells to produce IFN-γ after exposure to cytokines in the absence of cognate antigen (15, 35). Blockade of TCR signaling by CsA should prevent IFN-γ production if antigen recognition by the TCR were required (36). Mice were therefore treated with CsA, which elevated VV titers overall and eliminated the differences in VV titers normally seen between BCG-immune and sham-immune mice. This is shown for day 1 postinfection in Table 3, where there were no statistically significant differences between CsA-treated BCG-immune or sham-immune mice challenged with VV and in another experiment at day 3 postinfection. CsA also prevented both the CD4 and CD8 T cells from synthesizing IFN-γ (Fig. 3). These results are consistent with the concept that a TCR-dependent event regulated both cytokine production and protective immunity and that the protection was mediated by IFN-γ. Treatment of mice with MAb to IFN-γ, not surprisingly, elevated viral titers in all groups (data not shown), as has been shown in similar studies of heterologous immunity between LCMV and VV (42).
BCG-induced memory T-cell populations respond to VV differently than do LCMV-induced memory T-cell populations.
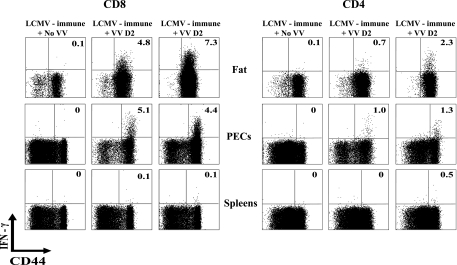
The selective proliferation and probable TCR dependence of the CD4 T cells is consistent with the concept of CD4 T-cell-mediated cross-reactivity in this system, but some evidence indicates that memory T-cell populations can be readily activated by cytokines, independent of TCR signaling (15, 35). We questioned, therefore, whether other memory T-cell populations would respond to VV in a similar manner to BCG-induced memory populations. In BCG-induced immune T-cell populations, the CD4 T cells preferentially made IFN-γ in vivo, with little selective production by CD8 T cells on VV challenge (Fig. 3), but the opposite was true in LCMV-induced immune cell populations, where it was the CD8 T cells which preferentially synthesized the IFN-γ in vivo after VV challenge, with much less production by the CD4 T cells (Fig. 4). This indicates that VV-induced cytokine production is not a function of just any heterologous memory T-cell population and is more consistent with the concept of an antigen-specific response.
FIG. 4.
In vivo IFN-γ production by T cells infiltrating the visceral fat and the peritoneal cavity of day 2 (D2) VV-challenged LCMV-immune mice. Immune mice were challenged with VV and at 2 days postinfection their lymphocytes were tested for IFN-γ production, as described in Materials and Methods and in the legend to Fig. 2. The same three mice (two infected and one uninfected) were used for the CD4 and CD8 data.
Search for cross-reactive class II epitopes between BCG and VV.
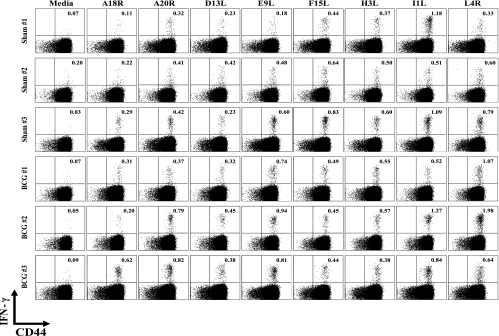
When heterologous immunity is mediated by CD8 T cells, the multicycled amplification of CD8 T cells that occurs after antigen recognition allows for the resolution of cross-reactive T-cell responses between unrelated viruses (20, 21). Identifying cross-reactive CD4 responses between unrelated pathogens has been more difficult to achieve, possibly because the amplification of CD4 T-cell clones is much more limited (14). Despite the demonstration that CD4 T cells mediated the heterologous immunity in this BCG-VV system, the selective division (CFSE loss) of adoptively transferred CD4 T cells was very modest and unlikely to reflect a major proliferative expansion of a cross-reactive population. Nevertheless, we attempted to define cross-reactivity between BCG and VV. Sette and coworkers have recently identified a number of class II IAb-restricted epitopes for VV that induce IFN-γ at very modest frequencies (usually <1%) from VV-specific CD4 T cells (29). We hypothesized that, if BCG immunization were inducing heterologous immunity to VV by cross-reactive CD4 T-cell responses, the hierarchy of CD4 T-cell-specific epitope usage might be altered between BCG-immune and sham-immune mice challenged with VV, if there were a substantial stimulus to cause expansion of clones of CD4 T cells. After an initial screen of all of those published epitopes, we tested the eight most VV-reactive epitopes at days 2, 6, and 9 post-VV infection for inducing IFN-γ production from CD4 T cells. Responses were undetectable at day 2 and low at day 6, but easily observed at day 9. Figure 5 shows representative day 9 data that confirm that these class II peptides elicit IFN-γ production from VV-induced CD4 T cells, but the small variations in responses between BCG-immune versus sham-immune mice seemed no different than the variations occurring within either of the groups. We therefore could not identify a putative cross-reactive peptide between these two viruses. In addition, staining of CD4 splenocytes or PEC with the panel of antibodies specific to mouse Vβ1 to Vβ14 TCR (Ebioscience) revealed no substantial alterations in CD4 Vβ repertoires at day 8 postinfection between BCG-immune and sham-immune groups (data not shown). This result was not surprising, given the limited selective proliferation of CD4 T cells seen during our adoptive transfer studies (Fig. 2).
FIG. 5.
Hierarchies of VV-encoded class II epitope-specific CD4 T cells in BCG- or sham-immunized mice 9 days after VV challenge, as determined by peptide-induced intracellular IFN-γ production in vitro.
Specificity of the CD8 T-cell response.
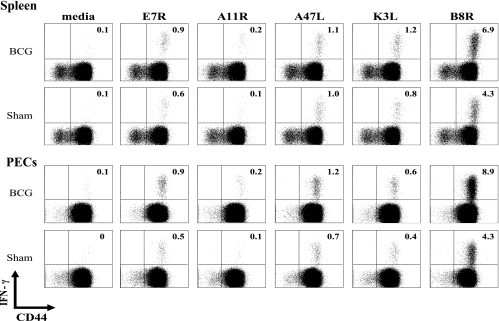
Although we found no role for CD8 T cells in the heterologous immunity and could not show selective proliferation of CD8 T cells under conditions of T-cell adoptive transfers, there remained the phenomenon that VV infection of BCG-immune mice induced splenomegaly accompanied by high frequencies of CD8 T cells. To clarify whether the augmented CD8 T-cell number represented a selective proliferation of T cells with a given specificity, an event that would be consistent with a cross-reactive CD8 cell expansion, the specificity of VV-specific T cells was evaluated in a peptide-induced intracellular IFN-γ assay. CD8 T-cell responses to five VV-specific class I epitopes were examined, but no evidence of any change in the hierarchy of epitope-specific responses between BCG-immune and sham-immune mice was found; representative data are shown in Fig. 6. In contrast, changes in such hierarchies have been observed in LCMV-immune mice challenged with VV, where protective immunity is partially mediated by CD8 T cells (21). CD8 T cells specific to the major immunodominant VV-encoded B8R epitope in some VV-challenged BCG-immune mice were about double the frequency seen in VV-challenged sham-immune mice (Fig. 6) but, as a percentage of the response in comparison to four other tested epitopes in five experiments encompassing 13 mice, B8R+ cell percentages were 61% in nonimmune and 65% in BCG-immune mice. Because this was only a slight amplification of the response to the immunodominant epitope, it was unclear whether this represented cross-reactivity or simply a nonspecific augmentation of a dominant high-affinity response. In addition, staining of CD8 splenocytes or PEC with a panel of antibodies specific to mouse Vβ1 to Vβ14 TCR revealed no substantial alterations in Vβ repertoires (data not shown). This lack of any selective expansion is consistent with our data showing no role for CD8 T cells in the protective immunity. The general increase in CD8 T-cell number may have either been due to enhanced help from CD4 T cells or to the fact that high levels of VV can be suppressive to T-cell responses, and the VV loads were much lower in BCG-immune mice (Fig. 1; Table 2).
FIG. 6.
Hierarchies of VV-encoded class I epitope-specific CD8 T cells in BCG- or sham-immunized mice 6 days after VV challenge, as determined by peptide-induced intracellular IFN-γ production in vitro.
DISCUSSION
Two novel conclusions can be made from this work. The first is that heterologous immunity can be demonstrated between mycobacteria and viruses. Earlier reports had indicated that BCG or M. tuberculosis infection provided resistance in mice to VV or ectromelia virus, but in those studies the virus challenge was early after immunization, when immune responses to the mycobacteria were likely ongoing (3, 16, 40, 44, 56). In this present report, mice were treated with antibiotics to clear residual mycobacteria to minimum levels, the immune system appeared at rest (Table 1; Fig. 3), and resistance to VV challenge was still observed even more than 6 months after BCG immunization (Table 2). This indicates that this is true heterologous immunity and not just a resistance to infection caused by an activated innate immune system. The second novel observation was that the heterologous immunity was mediated primarily by CD4 T cells, which were shown to be activated in vivo to make IFN-γ. The mechanism of heterologous immunity is not clarified in most systems, but a CD8 T-cell mechanism has been described or suggested for heterologous immunity between influenza virus and HCV (48), between influenza virus and EBV (11), between different strains of dengue virus (28), and between LCMV and Pichinde virus (6, 42). Cross-reactive CD8 T cells have been directly shown to mediate heterologous immunity between LCMV and VV in mice (12), although adoptive transfer studies of LCMV-immune splenocytes into naïve mice have also shown that CD4 T cells were necessary, along with the CD8 T cells, to mediate protective immunity against VV (42). This is the first study demonstrating an exclusive role for CD4 cells in protective heterologous immunity, apparently independent of antibody or CD8 T cells. While successful with our immune depletion studies shown in Table 3, we were unable to use adoptive transfer techniques in this system to show a protective role for memory CD4 T cells (data not shown); this is not surprising, considering the relatively poor ability of CD4 T cells to proliferate after transfer, as shown in Fig. 2.
The importance of developing an understanding of this process is attested to by epidemiological studies that have shown that vaccination with BCG, as well as other vaccines, such as measles, can sometimes provide unexpected health benefits, including greater resistance to unrelated pathogens and overall lower mortality in vaccinated populations in developing countries (1, 22, 38, 39). Alternatively, there is considerable debate over whether too many inactivated vaccines may alter or inhibit proper development of the immune system in industrialized nations. This hygiene hypothesis argues that living in too clean an environment with exposure to inactivated vaccines rather than normal infections may promote allergic or autoimmune responses (37). Of interest is that the incidence of atopy in human populations is reported to be reduced by vaccination with BCG, which is an attenuated live replicating vaccine rather than an inactivated pathogen (43).
Heterologous immunity has been implicated in increased resistance as well as enhancement of infections, with altered immunopathology in either case (9, 42, 48, 55). Some viruses, such as EBV, cause greater symptomatology in young adults than in children, and the characteristic pathology of EBV-associated monononucleosis has recently been linked to the activation of influenza virus-specific CD8 T cells in some patients (48). It is noteworthy that the VV-challenged BCG-immune mice in this study had greater splenomegaly and total T-cell numbers than the sham-immune controls, much like that seen in EBV infections of young adults.
It is clear from our studies that this heterologous immunity was not a consequence of an already ongoing immune response at the time of initial infection and that it was dependent on the effector function of memory phenotype CD4 T cells (Tables 1 and 3). The question is whether this resistance was due to some cytokine-mediated nonspecific activation of memory cells or due to cross-reactive memory cells activated through their TCR, and arguments can be made for both sides of this issue. Some have argued that memory cells are “twitchy” and can be activated by cytokines such as IL-12 and IL-18 independently of TCR signaling (15, 35). The high frequency of IFN-γ-synthesizing CD4 T cells infiltrating infected tissues as early as 2 days after VV challenge may be consistent with that concept. Our failure thus far to define cross-reactive epitopes or to show convincing changes in epitope-specific T-cell hierarchies would also be consistent with a nonspecific process (Fig. 5 and 6). Indeed, our demonstration that the heterologous immunity occurred in congenic mice bearing different MHC molecules is also consistent with the “nonspecific” hypothesis, as this would require additional cross-reactive epitopes to be presented by disparate class II MHC molecules.
Nevertheless, demonstration of cross-reactivity can be quite difficult, and an argument can be made in support of cross-reactivity in this model. It is becoming apparent that there may be hundreds of class II epitopes encoded by these pathogens. An increasing number of VV epitopes are now being defined (12, 29, 30, 47), and, while less is known about the epitope spectrum for BCG and M. tuberculosis, it is likely to be quite large, considering that these organisms encode about 4,000 proteins, or about 20 times the number encoded by VV (8). Thus, the probability of there being cross-reactive CD4 epitopes between BCG and VV is high, but the probability of our detecting cross-reactivity within the small subset of potential peptides tested was low. In addition, the programmed proliferative expansion of CD4 T cells is substantially less than that of CD8 T cells and requires exposure to antigen to continue (14, 23). VV is rapidly cleared by the CD4 T cells in BCG-immune mice (Table 2), so the CD4 T cells may not get that second antigen signal to continue their expansion. It is the dramatic proliferative expansion of CD8 T cells that enabled us to define cross-reactive CD8 epitopes between LCMV and VV (21).
The arguments that this heterologous immunity may be due to cross-reactivity are several. First, the protective immunity to VV was specific in that there was no protection against LCMV (Table 2). In addition, LCMV, unlike VV (Fig. 2), did not induce IFN-γ from BCG-immune T-cell populations in vivo (data not shown). In two experiments (not shown) we could find no BCG-induced protective immunity against a third virus, MCMV; however, MCMV infection did induce elevated levels of IFN-γ in BCG-immune memory CD4-cell populations, though not as high as VV did. MCMV, unlike LCMV, which encodes only four proteins, is a large complex pathogen likely to encode some epitopes cross-reactive with BCG and is being separately studied. Second, the theory that memory cells are getting activated only by cytokines (15, 35) has been challenged by experiments where we compared BCG-immune to LCMV-immune mice, both challenged with VV (Fig. 3 and 4). Although both BCG and LCMV infections elicit CD4 and CD8 memory cell populations, VV selectively induced IFN-γ from the BCG-immune CD4 populations, whereas it selectively induced IFN-γ from the LCMV-immune CD8 populations. This argues for a qualitative difference in these immune T-cell populations generated in response to two different pathogens, and that difference could be accounted for by differences in their TCR. Finally, TCR signaling is blocked by CsA (36), and this drug blocked the CD4 T-cell IFN-γ production and the protective immunity to VV in the BCG-immune mice (Fig. 3; Table 3). We acknowledge that CsA can at times act in a TCR-independent manner, but collectively, these experiments support a cross-reactive, TCR-dependent mechanism.
Because high doses of IL-12 have been reported to nonspecifically activate memory T cells (35), an experiment to determine the likelihood of nonspecific activation in this system might be to inhibit IL-12 production in VV-infected BCG-immune mice with MAb to IL-12. However, we chose not to do that experiment, because our studies with LCMV-induced memory cell populations showed that the presence of IL-12 greatly augmented IFN-γ production by peptide-stimulated LCMV-specific memory cells (H. Chen, R. M. Welsh, and L. K. Selin, unpublished data). Therefore, such an experiment would not discriminate between specific versus nonspecific mechanisms.
Whether due to cross-reactivity or not, this study shows how immunization against a mycobacterium may influence a subsequent immunization or infection with a virus. One hundred million doses of BCG are given to children every year in most of the world, although not in North America (46). One can speculate how this influences the quality of immune responses to other vaccines in these different areas. Indeed, epidemiological studies have suggested widespread pleiotropic effects of BCG vaccination, and these effects may be related to heterologous immunity (22, 38, 39, 43). Given the enormous number of persons vaccinated with BCG worldwide (more than 3 billion) (13), understanding how memory to BCG influences the host response to viruses is an important issue for clinical research and practice. The significance of such heterologous immunity might be particularly relevant to interpreting clinical trials of poxvirus-based tuberculosis vaccines administered to BCG-vaccinated populations (25).
Acknowledgments
This study was supported by NIH research grants U19-AI-057330 to R.W., RO1-AR-035506 to R.W., R01 CA34461 to R.W., R21-AI-78509 to H.K., and NIH training grant T32 AI07349 to G.M.
The opinions expressed are those of the authors and do not represent the official opinions of the NIH.
We thank Keith Daniels for technical support.
Footnotes
Published ahead of print on 4 February 2009.
REFERENCES
- 1.Aaby, P., B. Samb, F. Simondon, A. M. Seck, K. Knudsen, and H. Whittle. 1995. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. Br. Med. J. 311481-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acierno, P. M., D. A. Newton, E. A. Brown, L. A. Maes, J. E. Baatz, and S. Gattoni-Celli. 2003. Cross-reactivity between HLA-A2-restricted FLU-M1:58-66 and HIV p17 GAG:77-85 epitopes in HIV-infected and uninfected individuals. J. Transl. Med. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, E. G., and S. Mudd. 1973. Protection of mice against vaccinia virus by bacterial infection and sustained stimulation with specific bacterial antigens. Infect. Immun. 762-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton, E. S., D. W. White, J. S. Cathelyn, K. A. Brett-McClellan, M. Engle, M. S. Diamond, V. L. Miller, and H. W. Virgin. 2007. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447326-329. [DOI] [PubMed] [Google Scholar]
- 5.Boniface, J. J., Z. Reich, D. S. Lyons, and M. M. Davis. 1999. Thermodynamics of T cell receptor binding to peptide-MHC: evidence for a general mechanism of molecular scanning. Proc. Natl. Acad. Sci. USA 9611446-11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brehm, M. A., A. K. Pinto, K. A. Daniels, J. P. Schneck, R. M. Welsh, and L. K. Selin. 2002. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat. Immunol. 3627-634. [DOI] [PubMed] [Google Scholar]
- 7.Bukowski, J. F., C. A. Biron, and R. M. Welsh. 1983. Elevated natural killer cell-mediated cytotoxicity, plasma interferon, and tumor cell rejection in mice persistently infected with lymphocytic choriomeningitis virus. J. Immunol. 131991-996. [PubMed] [Google Scholar]
- 8.Camus, J. C., M. J. Pryor, C. Medigue, and S. T. Cole. 2002. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology 1482967-2973. [DOI] [PubMed] [Google Scholar]
- 9.Chen, H. D., A. E. Fraire, I. Joris, R. M. Welsh, and L. K. Selin. 2003. Specific history of heterologous virus infections determines antiviral immunity and immunopathology in the lung. Am. J. Pathol. 1631341-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, H. D., A. E. Fraire, I. Joris, M. A. Brehm, R. M. Welsh, and L. K. Selin. 2001. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat. Immunol. 21067-1076. [DOI] [PubMed] [Google Scholar]
- 11.Clute, S. C., L. B. Watkin, M. Cornberg, Y. N. Naumov, J. L. Sullivan, K. Luzuriaga, R. M. Welsh, and L. K. Selin. 2005. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J. Clin. Investig. 1153602-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornberg, M., B. S. Sheridan, F. M. Saccoccio, M. A. Brehm, and L. K. Selin. 2007. Protection against vaccinia virus challenge by CD8 memory T cells resolved by molecular mimicry. J. Virol. 81934-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine, P. E. 1988. BCG vaccination against tuberculosis and leprosy. Br. Med. Bull. 44691-703. [DOI] [PubMed] [Google Scholar]
- 14.Foulds, K. E., L. A. Zenewicz, D. J. Shedlock, J. Jiang, A. E. Troy, and H. Shen. 2002. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J. Immunol. 1681528-1532. [DOI] [PubMed] [Google Scholar]
- 15.Gilbertson, B., S. Germano, P. Steele, S. Turner, B. Fazekas de St. Groth, and C. Cheers. 2004. Bystander activation of CD8+ T lymphocytes during experimental mycobacterial infection. Infect. Immun. 726884-6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gledhill, A. W., and R. J. Rees. 1960. Effect of a primary tuberculous infection on the resistance of male and female mice to Ectromelia. Nature 187703-704. [DOI] [PubMed] [Google Scholar]
- 17.Hohn, H., C. Kortsik, G. Tully, K. Nilges, A. Necker, K. Freitag, C. Neukirch, P. Galle, H. Lohr, and M. J. Maeurer. 2003. Longitudinal analysis of Mycobacterium tuberculosis 19-kDa antigen-specific T cells in patients with pulmonary tuberculosis: association with disease activity and cross-reactivity to a peptide from HIVenv gp120. Eur. J. Immunol. 331613-1623. [DOI] [PubMed] [Google Scholar]
- 18.Kaech, S. M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kasaian, M. T., and C. A. Biron. 1990. Cyclosporin A inhibition of interleukin 2 gene expression, but not natural killer cell proliferation, after interferon induction in vivo. J. Exp. Med. 171745-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, S. K., M. A. Brehm, R. M. Welsh, and L. K. Selin. 2002. Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J. Immunol. 16990-98. [DOI] [PubMed] [Google Scholar]
- 21.Kim, S. K., M. Cornberg, X. Z. Wang, H. D. Chen, L. K. Selin, and R. M. Welsh. 2005. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J. Exp. Med. 201523-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristensen, I., P. Aaby, and H. Jensen. 2000. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ 3211435-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, W. T., G. Pasos, L. Cecchini, and J. N. Mittler. 2002. Continued antigen stimulation is not required during CD4+ T cell clonal expansion. J. Immunol. 1681682-1689. [DOI] [PubMed] [Google Scholar]
- 24.Liu, F., and J. L. Whitton. 2005. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J. Immunol. 1745936-5940. [DOI] [PubMed] [Google Scholar]
- 25.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 101240-1244. [DOI] [PubMed] [Google Scholar]
- 26.Medina, E., and R. J. North. 1999. Genetically susceptible mice remain proportionally more susceptible to tuberculosis after vaccination. Immunology 9616-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 1656833-6839. [DOI] [PubMed] [Google Scholar]
- 28.Mongkolsapaya, J., W. Dejnirattisai, X. N. Xu, S. Vasanawathana, N. Tangthawornchaikul, A. Chairunsri, S. Sawasdivorn, T. Duangchinda, T. Dong, S. Rowland-Jones, P. T. Yenchitsomanus, A. McMichael, P. Malasit, and G. Screaton. 2003. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 9921-927. [DOI] [PubMed] [Google Scholar]
- 29.Moutaftsi, M., H. H. Bui, B. Peters, J. Sidney, S. Salek-Ardakani, C. Oseroff, V. Pasquetto, S. Crotty, M. Croft, E. J. Lefkowitz, H. Grey, and A. Sette. 2007. Vaccinia virus-specific CD4+ T cell responses target a set of antigens largely distinct from those targeted by CD8+ T cell responses. J. Immunol. 1786814-6820. [DOI] [PubMed] [Google Scholar]
- 30.Moutaftsi, M., B. Peters, V. Pasquetto, D. C. Tscharke, J. Sidney, H. H. Bui, H. Grey, and A. Sette. 2006. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat. Biotechnol. 24817-819. [DOI] [PubMed] [Google Scholar]
- 31.Muller, U., U. Steinhoff, L. F. L. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Auger. 1994. Functional role of type I and type II interferons in antiviral defense. Science 2641918-1921. [DOI] [PubMed] [Google Scholar]
- 32.Nilges, K., H. Hohn, H. Pilch, C. Neukirch, K. Freitag, P. J. Talbot, and M. J. Maeurer. 2003. Human papillomavirus type 16 E7 peptide-directed CD8+ T cells from patients with cervical cancer are cross-reactive with the coronavirus NS2 protein. J. Virol. 775464-5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oettinger, T., M. Jorgensen, A. Ladefoged, K. Haslov, and P. Andersen. 1999. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber. Lung Dis. 79243-250. [DOI] [PubMed] [Google Scholar]
- 34.Openshaw, P., E. E. Murphy, N. A. Hosken, V. Maino, K. Davis, K. Murphy, and A. O'Garra. 1995. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 an T helper 2 populations. J. Exp. Med. 1821357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raue, H. P., J. D. Brien, E. Hammarlund, and M. K. Slifka. 2004. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J. Immunol. 1736873-6881. [DOI] [PubMed] [Google Scholar]
- 36.Reem, G. H., L. A. Cook, and J. Vilcek. 1983. Gamma interferon synthesis by human thymocytes and T lymphocytes inhibited by cyclosporin A. Science 22163-65. [DOI] [PubMed] [Google Scholar]
- 37.Rook, G. A., and J. L. Stanford. 1998. Give us this day our daily germs. Immunol. Today 19113-116. [DOI] [PubMed] [Google Scholar]
- 38.Roth, A., P. Gustafson, A. Nhaga, Q. Djana, A. Poulsen, M. L. Garly, H. Jensen, M. Sodemann, A. Rodriques, and P. Aaby. 2005. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int. J. Epidemiol. 34540-547. [DOI] [PubMed] [Google Scholar]
- 39.Roth, A. E., L. G. Stensballe, M. L. Garly, and P. Aaby. 2006. Beneficial non-targeted effects of BCG: ethical implications for the coming introduction of new TB vaccines. Tuberculosis (Edinburgh) 86397-403. [DOI] [PubMed] [Google Scholar]
- 40.Sakuma, T., T. Suenaga, I. Yoshida, and M. Azuma. 1983. Mechanisms of enhanced resistance of Mycobacterium bovis BCG-treated mice to ectromelia virus infection. Infect. Immun. 42567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarmiento, M., A. L. Glasebrook, and F. W. Fitch. 1980. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J. Immunol. 1252665-2672. [PubMed] [Google Scholar]
- 42.Selin, L. K., S. M. Varga, I. C. Wong, and R. M. Welsh. 1998. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J. Exp. Med. 1881705-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirakawa, T., T. Enomoto, S. Shimazu, and J. M. Hopkin. 1997. The inverse association between tuberculin responses and atopic disorder. Science 27577-79. [DOI] [PubMed] [Google Scholar]
- 44.Suenaga, T., T. Okuyama, I. Yoshida, and M. Azuma. 1978. Effect of Mycobacterium tuberculosis BCG infection on the resistance of mice to ectromelia virus infection: participation of interferon in enhanced resistance. Infect. Immun. 20312-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Townsend, A. R. M., J. Rothband, F. Gotch, G. Bahadur, D. C. Wraith, and A. J. McMichael. 1986. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 44959-968. [DOI] [PubMed] [Google Scholar]
- 46.Trunz, B. B., P. Fine, and C. Dye. 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 3671173-1180. [DOI] [PubMed] [Google Scholar]
- 47.Tscharke, D. C., W. P. Woo, I. G. Sakala, J. Sidney, A. Sette, D. J. Moss, J. R. Bennink, G. Karupiah, and J. W. Yewdell. 2006. Poxvirus CD8+ T-cell determinants and cross-reactivity in BALB/c mice. J. Virol. 806318-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urbani, S., B. Amadei, P. Fisicaro, M. Pilli, G. Missale, A. Bertoletti, and C. Ferrari. 2005. Heterologous T cell immunity in severe hepatitis C virus infection. J. Exp. Med. 201675-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Stipdonk, M. J., E. E. Lemmens, and S. P. Schoenberger. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2423-429. [DOI] [PubMed] [Google Scholar]
- 50.Varga, S. M., and R. M. Welsh. 1998. Cutting edge: detection of a high frequency of virus-specific CD4+ T cells during acute infection with lymphocytic choriomeningitis virus. J. Immunol. 1613215-3218. [PubMed] [Google Scholar]
- 51.Waldrop, S. L., C. J. Pitcher, D. M. Peterson, V. C. Maino, and L. J. Picker. 1997. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J. Clin. Investig. 991739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wedemeyer, H., E. Mizukoshi, A. R. Davis, J. R. Bennink, and B. Rehermann. 2001. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J. Virol. 7511392-11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welsh, R. M. 1978. Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J. Exp. Med. 148163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welsh, R. M. 1984. Natural killer cells and interferon. Crit. Rev. Immunol. 555-93. [PubMed] [Google Scholar]
- 55.Welsh, R. M., and L. K. Selin. 2002. No one is naive: the significance of heterologous T-cell immunity. Nat. Rev. Immunol. 2417-426. [DOI] [PubMed] [Google Scholar]
- 56.Werner, G. T. 1979. The effect of BCG vaccination on vaccinia virus infections in mice. Experientia 351514-1515. [DOI] [PubMed] [Google Scholar]
- 57.Wilde, D. B., P. Marrack, J. Kappler, D. P. Dialynas, and F. W. Fitch. 1983. Evidence implicating L3T4 in class I MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J. Immunol. 1312178-2183. [PubMed] [Google Scholar]
- 58.Yang, H., I. Joris, G. Majno, and R. M. Welsh. 1985. Necrosis of adipose tissue induced by sequential infections with unrelated viruses. Am. J. Pathol. 120173-177. [PMC free article] [PubMed] [Google Scholar]