Abstract
The olfactory system (OS) is involved in many infectious and neurodegenerative diseases, both human and animal, and it has recently been investigated in regard to transmissible spongiform encephalopathies. Previous assessments of nasal mucosa infection by prions following intracerebral challenge suggested a potential centrifugal spread along the olfactory nerve fibers of the pathological prion protein (PrPSc). Whether the nasal cavity may be a route for centripetal prion infection to the brain has also been experimentally studied. With the present study, we wanted to determine whether prion deposition in the OS occurs also under field conditions and what type of anatomical localization PrPSc might display there. We report here on detection by different techniques of PrPSc in the nasal mucosa and in the OS-related brain areas of sheep affected by natural scrapie. PrPSc was detected in the perineurium of the olfactory nerve bundles in the medial nasal concha and in nasal-associated lymphoid tissue. Olfactory receptor neurons did not show PrPSc immunostaining. PrPSc deposition was found in the brain areas of olfactory fiber projection, chiefly in the olfactory bulb and the olfactory cortex. The prevalent PrPSc deposition patterns were subependymal, perivascular, and submeningeal. This finding, together with the discovery of an intense PrPSc immunostaining in the meningeal layer of the olfactory nerve perineurium, at the border with the subdural space extension surrounding the nerve rootlets, strongly suggests a probable role of cerebrospinal fluid in conveying prion infectivity to the nasal submucosa.
Transmissible spongiform encephalopathies (TSEs) are a group of fatal neurodegenerative diseases caused by the conformational conversion of the normal host-encoded cellular prion protein (PrPC) into a pathological protease-resistant isoform, termed pathological prion protein (PrPSc) (40). TSEs include Creutzfeldt-Jacob disease (CJD) in humans, bovine spongiform encephalopathy (BSE) in cattle, scrapie in sheep and goats, and chronic wasting disease (CWD) in deer and elk (41).
Horizontal transmission, though its mechanism has not yet been fully clarified (26), may play a major role in the maintenance of prion infection in the field, particularly in scrapie and CWD (35). Contaminated environments and direct contact with infected animals are believed to be the two principal routes of horizontal transmission of prion diseases. In both instances, the spread of infection has been linked to the tissues and body fluids of diseased animals (45).
The contamination of pastures by the placentas (42) and decaying carcasses (6) of prion-infected animals is a well-known problem. Furthermore, the finding that prions adhere to soil minerals and remain infectious (25) confirms the role of soil as a reservoir for TSE infectivity.
Recently, prion infectivity has been detected in different body fluids, specifically in the urine of mice experimentally coinfected by scrapie and nephritis agents (48) and in the saliva and blood of cervids affected by CWD (34). In naturally occurring scrapie infection, PrPSc deposition has been demonstrated in the salivary glands (57), and sheep milk has been assessed as a route of transmission for the disease (31). Together, these findings point to a probable role of blood, saliva, urine, and milk in the horizontal spread of prion diseases, leading to the supposition that sensory organ involvement results from the transmission of infectivity through contact with such body fluids.
Peripheral routes involving the sensorial system have been assessed as a gate of entry of prions to the brain. Neuroinvasion was shown to occur in hamsters following experimental tongue infection (3); this route appeared to be even more efficient than transmission per os in causing the disease.
The olfactory system (OS), the focus of the present study, is highly developed in animals. Their sense of smell governs much of their ethological behavior, such as securing food, environment exploration, courtship, and offspring recognition. In these circumstances, contact of the nasal mucosa with prion-infected materials might well occur, thus allowing PrPSc to gain entry into the body and spread, as found experimentally following extranasal inoculation with hyper strain of transmissible mink encephalopathy (HY TME) (28) and with scrapie prions (18).
In human prion diseases a possible role of olfaction has also been hypothesized for the pathogenesis of variant CJD (36). In that context, the OS is thought to be a feasible route by which inhaled prion protein from the dust of BSE-contaminated feedstuffs is conveyed to the brain (46, 49, 51).
Besides the experimental assessment that prions centripetally propagate via the olfactory pathway, the centrifugal dissemination of prion infectivity from the brain to the olfactory mucosa has also been experimentally demonstrated. Hamsters intracerebrally inoculated with the TME agent showed PrPSc deposition in the olfactory receptor neurons (ORNs), the support cells of nasal mucosa, and the nasal-associated lymphoid tissue (NALT) (10). Recent results from pathogenetic studies have suggested OS involvement in experimental prion infection, but few have investigated it in regard to naturally occurring prion diseases. PrPSc deposition in the olfactory mucosa has been observed in sporadic CJD (sCJD)-affected patients (63), mainly in the cilia of the ORNs and faintly in the basal cells. According to one study, the presence of PrPSc in an olfactory biopsy from a sCJD patient was discovered 45 days after the onset of clinical symptoms, suggesting early involvement of the nasal mucosa in the spread of prion infection (55).
To date, no studies have reported on detection of PrPSc in the OS of animals with naturally occurring prion diseases. To fill this gap, the present study aimed to determine whether and to what extent the peripheral and central OS are affected in the course of natural sheep scrapie infection.
Specifically, the study's focus on investigating PrPSc deposition in nasal mucosa cells, which undergo rapid turnover, under conditions of natural infection was crucial to assessing the likelihood of prion release into the environment via nasal secretions, under the assumption that this route is a feasible one in the horizontal transmission of the disease. Within this perspective, samples from the nasal cavity and the OS-related brain areas of naturally scrapie-affected sheep were collected and examined by different techniques.
MATERIALS AND METHODS
Animals and tissue collection.
Twenty-four sheep of the Biellese breed, ranging from 3 to 8 years of age and coming from the same scrapie-affected Piedmontese flock, were killed within the framework of a selective culling to eradicate the disease. All sheep belonged to susceptible genotypes (ARQ/ARQ [n = 20] and ARQ/AHQ [n = 4]). All animals underwent accurate ante mortem neurological clinical examination before being humanely euthanized. Four out of the 24 sheep displayed clinical evidence of the disease (tremors, emaciation, or falling); the remaining sheep showed no clinical symptoms (Table 1). All 24 sheep initially tested scrapie positive by rapid testing (Bio-Rad TeSeE rapid assay; Bio-Rad Laboratories Inc., Hercules, CA); disease was confirmed by histology, immunohistochemistry (IHC), and Western blot (WB) analysis of the brain stem. The controls were 10 age-matched regularly slaughtered sheep that resulted negative at TSEs confirmatory tests.
TABLE 1.
Epidemiological data for the examined sheep and PrPSc deposition in the peripheral and central OS
| Sheep | Age (yr) | Genotype | Clinical signs | PrPSc immunoreactivity in nasal cavity determined bya:
|
IHCb
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Obex | Olfactory system related brain areas
|
||||||||||
| WB | IHC | OB | OT | FC | PL | Hi | |||||
| 76462/1/1/05 | 4 | ARQ/ARQ | No | + | − | ++++ | NA | +++ | +++ | − | NA |
| 101354/1/2/05 | 3.5 | ARQ/AHQ | Yes | − | − | ++ | + | −/+ | −/+ | − | − |
| 3174/1/3/06 | 5 | ARQ/ARQ | No | + | − | NA | NA | +++ | +++ | NA | + |
| 52407/1/3/06 | 5 | ARQ/ARQ | No | − | − | NA | NA | NA | − | − | − |
| 52407/1/9/06 | 7 | ARQ/ARQ | No | + | − | NA | +++ | ++++ | ++ | + | ++ |
| 52407/1/10/06 | 4 | ARQ/ARQ | No | + | + | NA | + | ++ | ++ | +/++ | + |
| 79348/1/2/06 | 4 | ARQ/ARQ | No | + | − | +++ | − | − | ++ | − | − |
| 79348/1/3/06 | 5 | ARQ/ARQ | Yes | − | − | +++ | NA | +/++ | ++ | +/++ | + |
| 94938/1/1/06 | 3 | ARQ/ARQ | Yes | + | − | +++ | + | −/+ | + | NA | −/+ |
| 94938/2/2/06 | 5 | ARQ/ARQ | No | + | − | +++ | + | −/+ | ++ | NA | − |
| 94938/2/6/06 | 4 | ARQ/ARQ | Yes | + | + | ++++ | ++ | ++ | ++ | NA | − |
| 94938/2/8/06 | 5 | ARQ/ARQ | No | + | − | ++ | NA | −/+ | − | − | − |
| 125704/1/4/06 | 3 | ARQ/ARQ | No | + | + | ++++ | +++ | +++ | +/++ | − | ++ |
| 125704/1/6/06 | 5 | ARQ/ARQ | No | + | + | +++ | −/+ | +/++ | ++ | − | + |
| 1273/1/1/07 | 7 | ARQ/ARQ | No | + | + | +++ | ++ | +++ | +/++ | −/+ | −/+ |
| 20286/1/4/07 | 4 | ARQ/AHQ | No | + | + | +++ | + | −/+ | ++ | ++ | −/+ |
| 33927/1/3/07 | 8 | ARQ/ARQ | No | + | + | ++ | + | +/++ | + | − | − |
| 45231/1/2/07 | 7 | ARQ/ARQ | No | + | + | ++++ | +++ | +++ | ++ | −/+ | + |
| 45231/1/3/07 | 5 | ARQ/ARQ | No | + | + | ++++ | +++ | +/++ | +/++ | −/+ | ++ |
| 50619/1/2/07 | 4 | ARQ/ARQ | No | + | + | ++ | +/++ | + | + | − | −/+ |
| 58132/1/1/07 | 6 | ARQ/ARQ | No | + | + | +++ | + | − | + | − | NA |
| 63239/1/1/07 | 8 | ARQ/AHQ | No | + | + | +++ | +++ | +++ | +/++ | −/+ | NA |
| 71929/1/4/07 | 3.5 | ARQ/ARQ | No | + | + | ++ | + | NA | +/++ | NA | NA |
| 78979/1/1/07 | 4.5 | ARQ/AHQ | No | + | + | ++++ | + | −/+ | ++ | − | −/+ |
+, PrPSc immunoreactivity, −, no PrPSc immunoreactivity.
OB, olfactory bulb; OT, olfactory tract; FC, frontal cortex; PL, pyriform lobe; Hi, hippocampus; NA, not available. ++++, very marked; +++, marked; ++, moderate; +, apparent; +/++, apparent/moderate; −/+, barely apparent; −, absent.
At necropsy, the brain was removed and then longitudinally cut in two halves. One was frozen at −80°C until biochemical studies were performed, and the other was fixed in 10% buffered formaldehyde solution for IHC analysis. The skulls were cut longitudinally in two halves in order to study nasal cavity tissues. In sheep, the two nasal cavities, separated by the nasal septum (NS), each contain a dorsal nasal concha, a medial nasal concha (MNC) (a part of the endoturbinate system), and a ventral nasal concha (VNC). Samples were taken from each side of the NS, MNC, and VNC, as the concentration of ORNs is higher in these areas (30). In detail, the samples were taken at the most aboral portion of the MNC and NS, in close proximity to the ethmoidal lamina cribrosa, where the olfactory mucosa is easily recognizable macroscopically because of its natural yellowish color. Samples of the VNC were collected from the aboral portion, which is the only one presenting olfactory epithelium (OE). The samples from one side were fixed in 10% buffered formalin for IHC, immunofluorescence, and paraffin-embedded tissue (PET) blot analysis, and the corresponding tissues from the other side were frozen for WB analysis.
WB and PrPSc quantification. (i) Pretreatment of extraneural tissue.
The MNC, NS mucosa, and VNC samples were cut into small pieces by means of scalpels and incubated in a phosphate-buffered saline (PBS)-1% trypsin solution overnight at room temperature (RT) with gentle agitation.
(ii) WB technique.
At least 200 mg of different tissues was homogenized in 10% sarcosyl and clarified by centrifugation at 22,000 × g for 20 min. For the extraneural tissue samples, the supernatant was collected and incubated with benzonase nuclease (50 meq/ml) (Novagen, San Diego, CA) for 30 min at 37°C. Samples were clarified by centrifugation at 22,000 × g for 20 min at 10°C, and the supernatants were incubated with 40 μg/ml proteinase K for 1 h at 37°C with continuous shaking. After centrifugation at 215,000 × g for 1 h at 10°C (Optima TLX ultracentrifuge, rotor TLA 110; Beckman Coulter, Fullerton, CA), the pellet was dissolved in Laemmli buffer and boiled for 10 min at 99°C. The samples were separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis on a 12% minigel and then transferred to a polyvinylidene fluoride membrane (Immobilion P; Millipore, Billerica, MA) by wet blotting. PrPSc was detected using the P4 (0.1 μg/ml; R-Biopharm, Darmstadt, Germany) (19) monoclonal antibody and an anti-mouse antiserum conjugated with alkaline phosphatase (Zymed, Invitrogen, Carlsbad, CA). The reaction was revealed with a chemiluminescent substrate (ImmunoStar; Bio-Rad, Hercules, CA) and visualized on Hyperfilm ECL (GE-Healthcare Ltd., St. Giles, United Kingdom) or with the UVI Prochemi gel documentation and analysis system (Uvitec, Cambridge, United Kingdom).
(iii) PrPSc quantification.
In order to estimate the relative concentration of PrPSc in the MNC, NS mucosa, and VNC, we compared the intensities of the proteinase K-digested WB signals with calibration curves obtained by diluting the corresponding brain stem of the scrapie-positive sheep with a scrapie-negative nasal mucosa homogenate. This comparison was done with three of the scrapie-positive animals, two of which showed an ARQ/ARQ PrP genotype and one an ARQ/AHQ genotype. The extraction method and WB technique were identical to those described above. Quantification analyses were performed using the UVI Prochemi gel documentation and analysis system (Uvitec, Cambridge, United Kingdom).
IHC.
Following fixation, brain sections (5 mm thick) were coronally cut. The OS-related brain areas (i.e., the olfactory bulb, olfactory tract, frontal cortex at the level of the basal nuclei, pyriform lobe, and hippocampus) were sampled, processed according to routine methods, and embedded in paraffin wax (7).
Except for the NS mucosa, all nasal tissue samples were placed in a decalcifying solution (Carlo Erba, Rodano, Italy) at RT for 4 days (MNC) or 2 days (VNC). The samples were rinsed under running tap water, transversely cut into 0.5-cm-thick pieces, and then processed as described above for the brain sections.
For each sheep, 5-μm-thick sections of each OS-related brain area and each nasal cavity tissue were cut. The brain and nasal cavity tissues were then immunostained to detect PrPSc immunoreactivity. The slides were dewaxed and rehydrated by routine methods and then immersed in 98% formic acid for 25 min. After washing in distilled water, the sections were autoclaved for 30 min at 121°C in citrate buffer (pH 6.1). Endogenous peroxidase activity was blocked in 3% hydrogen peroxide for 20 min at RT. To block nonspecific tissue antigens, the sections were incubated with 5% horse blocking serum for 20 min at RT and then incubated for 1 h at RT with the primary monoclonal antibody F99/97.6.1 (52) (epitope QYQRES, amino acids 220 to 225 of the ovine PrP, 1:1,000 dilution; VMRD Inc., Pullman, WA). After rinsing, a biotinylated goat anti-mouse secondary antibody (1: 200 dilution; Vector Laboratories, Burlingame, CA) was applied to the tissue sections for 30 min at RT, followed by the avidin-biotin-peroxidase complex (Vectastain ABC kit; Vector Laboratories, Burlingame, CA), according to the manufacturer's protocol. PrPSc immunoreactivity was visualized using 3,3′-diaminobenzidine (Dakocytomation, Carpinteria, CA) as a chromogen; the sections were then counterstained with Meyer's hematoxylin. The usual specificity control tests were carried out.
PrPSc deposition in the OS-related brain areas was evaluated by light microscopy and an intensity grade assigned to the different patterns detected: absent (−), barely apparent (−/+), apparent (+), moderate (++), marked (+++), or very marked (++++) (24).
Immunofluorescence.
Serial sections of the nasal cavity samples that initially stained positive for PrPSc by IHC were cut. Immunofluorescence staining was performed according to the two different protocols described below.
(i) PrPSc and carnosine detection.
Tissue sections were dewaxed, rehydrated, formic acid treated, and autoclaved as in the IHC protocol described above. After autoclaving, the sections were washed in distilled water and processed according to a dual-immunofluorescence protocol. To block nonspecific tissue antigens, the sections were incubated with 5% PBS-diluted normal goat serum for 20 min at RT. Primary monoclonal antibody F99/97.6.1 (VMRD Inc., Pullman, WA), diluted 1:200, was applied at RT for 1 h. The nasal tissue sections were then incubated at RT with a Cy3-conjugated goat anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), diluted 1:100, for 30 min. After rinsing in Tris-buffered saline, the nasal tissue sections were incubated at RT with primary polyclonal anticarnosine antibody, diluted 1:150 in Tris-buffered saline-Tween 20 (kindly provided by S. De Marchis, Dept. of Human and Animal Biology, University of Turin). The sections were then rinsed in Tris-buffered saline-Tween 20, and a Cy2-conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), diluted 1:100, was applied for 30 min at RT. The sections were mounted with PBS diluted 1:3 in glycerol and examined under a Nikon Eclipse 80i microscope equipped for fluorescence (Nikon Instruments, Florence, Italy). Dark-field fluorescence digital images were collected with a DS-5Mc camera (Nikon Instruments, Florence, Italy) using fluorescein isothiocyanate and tetramethyl rhodamine isocyanate filters. The specificity of the secondary antibodies was tested by applying these antisera without the primary antibodies. No PrPSc or carnosine immunolabeling signals were seen after omitting the primary antisera.
(ii) PrPSc and cytokeratin detection.
Dual immunofluorescence for PrPSc and cytokeratins was not feasible because the antigens need to be demasked by two different techniques in order to maximize the immunolabeling intensity. Therefore, the use of single immunofluorescence stainings for PrPSc and cytokeratins on serial sections was adopted. PrPSc detection was carried out according to the protocol described above. The cytokeratins were demasked in a water bath (95 to 99°C) in a pH 9 Tris-EDTA buffer for 20 min. The sections were then incubated for 1 h with a primary monoclonal anti-human cytokeratin antibody, clone AE1/AE3, (Dakocytomation, Carpinteria, CA), diluted 1:50, in 5% PBS-diluted normal goat serum. As secondary antibody, a Cy2-conjugated goat anti-mouse antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), diluted 1:100, was applied to the sections for 30 min at RT.
In order to study PrPSc and cytokeratin colocalization, images were taken at strictly corresponding areas of the serial tissue sections, and digital overlaying and manipulation of the images were performed using Photoshop 8.0 CS software.
PET blot analysis.
PET blotting was performed as described elsewhere (43) with slight modifications. Paraffin sections of 5 μm were mounted on nitrocellulose membranes (0.45-μm pore size) and dried flat at 60°C overnight. After washing, the sections were subjected to proteolysis with 25-μg/ml proteinase K at 55°C for 2 h. The sections were then denatured in 3 M guanidine isothiocyanate for 10 min, blocked in casein for 30 min, and incubated at 4°C overnight with monoclonal antibody F99/97.6.1 (1:2,000 dilution). Labeling was completed using a Vectastain ABC-AmP detection system (Vector Laboratories, Burlingame, CA). Labeling was visualized using 5-bromo-4-chloro-3 indolyl phosphate-nitroblue tetrazolium and observed using a stereomicroscope. For negative controls, the primary antibody was omitted and replaced with blocking serum.
RESULTS
PrPSc was detected by WB analysis in the nasal cavity tissues of 21 out of the 24 sheep examined (Table 1); 18 of the 21 sheep positive by WB belonged to the ARQ/ARQ genotype, and 3 were ARQ/AHQ. Two positive animals, both ARQ/ARQ, showed clinical symptoms of the disease. The three negative sheep carried two different genotypes; two were ARQ/ARQ, one of which showed clinical symptoms, and the third had the ARQ/AHQ genotype and was symptomatic.
By WB analysis, the main tissue involved in PrPSc deposition was the MNC (n = 19) (Table 2 and Fig. 1A). PrPSc was identified only in the MNC in five animals, in both the MNC and NS mucosa in five, in both the MNC and VNC mucosa in two, and in all three areas in seven. No PrPSc was detected by WB analysis in the MNC in two animals; in one of these it was found in both the NS and VNC mucosa and in the other only in the VNC mucosa (Table 2).
TABLE 2.
Localization of PrPSc deposition in sheep nasal cavities by WB and IHC analyses
| Sheep | PrPSc immunoreactivity determined bya:
|
|||||
|---|---|---|---|---|---|---|
| WB
|
IHC
|
|||||
| MNC | NS | VNC | OE | ONP | NALT | |
| 76462/1/1/05 | − | − | + | − | − | − |
| 101354/1/2/05 | NA | − | − | − | − | − |
| 3174/1/3/06 | + | + | − | − | − | − |
| 52407/1/3/06 | − | − | − | − | − | − |
| 52407/1/9/06 | + | + | − | − | − | − |
| 52407/1/10/06 | + | − | − | − | + | − |
| 79348/1/2/06 | + | − | − | − | − | − |
| 79348/1/3/06 | − | − | − | − | − | − |
| 94938/1/1/06 | + | − | − | − | − | − |
| 94938/2/2/06 | + | + | − | − | − | − |
| 94938/2/6/06 | + | − | + | − | − | + |
| 94938/2/8/06 | + | − | + | − | − | − |
| 125704/1/4/06 | + | + | + | − | + | − |
| 125704/1/6/06 | + | + | + | − | + | − |
| 1273/1/1/07 | + | + | − | − | + | + |
| 20286/1/4/07 | + | + | − | − | + | − |
| 33927/1/3/07 | + | + | + | − | + | + |
| 45231/1/2/07 | + | − | − | − | + | − |
| 45231/1/3/07 | + | + | + | − | + | − |
| 50619/1/2/07 | + | + | + | − | + | − |
| 58132/1/1/07 | + | − | − | − | + | − |
| 63239/1/1/07 | − | + | + | − | − | + |
| 71929/1/4/07 | + | + | + | − | + | − |
| 78979/1/1/07 | + | + | + | − | + | − |
+, PrPSc immunoreactivity; −, no PrPSc immunoreactivity; NA, not available.
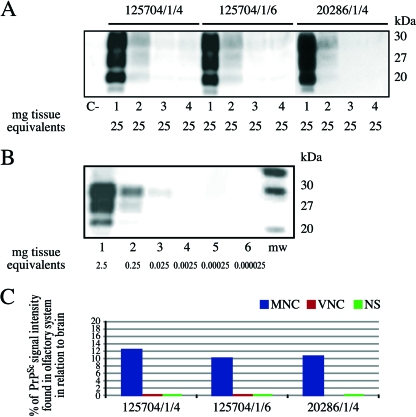
FIG. 1.
(A) Detection of PrPSc by highly sensitive WB analysis in the brain stems and nasal mucosae of three scrapie-positive sheep. Lanes 1 to 4, brain stem, MNC, VNC, and NS, respectively, of the three scrapie-positive sheep; lane C−, MNC of a scrapie-negative sample. The amount of tissue equivalents per lane is indicated in milligrams. All samples were proteinase K digested. (B) WB of a positive brain stem sample (identification number, 125704/1/4/06) serially diluted in negative MNC mucosa: 1 × 10−1 (lane 1), 1 × 10−2 (lane 2), 1 × 10−3 (lane 3), 1 × 10−4 (lane 4), 1 × 10−5 (lane 5), and 1 × 10−6 (lane 6). mw, molecular mass. The amount of tissue equivalents per lane is indicated in milligrams. All samples were proteinase K digested. (C) Graphical representation of the relative amounts of PrPSc signal intensity found in MNC, VNC, and NS in relation to brains of three scrapie-positive sheep.
IHC analysis for PrPSc confirmed the WB-positive results in the nasal cavity tissues of 14 animals but did not in those of 7 animals. This finding is attributable to the higher sensitivity of the WB method used here, which, being based on the sampling of a larger quantity of tissue with respect to IHC and on the concentration of the PrPSc by ultracentrifugation steps, increases the chances of detecting PrPSc if present.
Neither IHC nor WB analysis revealed PrPSc in any of the nasal tissues examined in three animals (Table 2). All 10 negative controls tested negative by WB and IHC analyses.
Quantification of PrPSc in the nasal cavity.
The signal intensity of the positive MNC was generally similar to that obtained when 5 mg of the corresponding positive brain stem homogenate was diluted with 50 mg of a negative nasal mucosa homogenate. From this we estimated that the levels of PrPSc in the three cases examined were lower than those found in the corresponding brain stem by a factor of approximately 1 × 10−1. The signal intensities of the positive NS and VNC mucosa were usually lower than those found in the corresponding brain stem by a factor of 1 × 10−5 to 1 × 10−6 (Fig. 1B). The graphical representation of the percentage of PrPSc signal intensity found in the OS in relation to the brain indicates that the largest amount of PrPSc detected in the MNC was approximately 12% of the PrPSc signal found in the brain (Fig. 1C).
PrPSc localization in the nasal cavity.
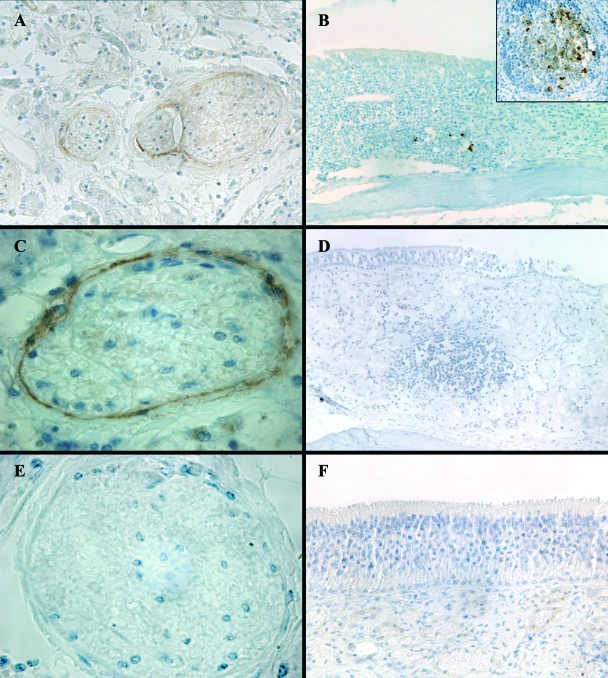
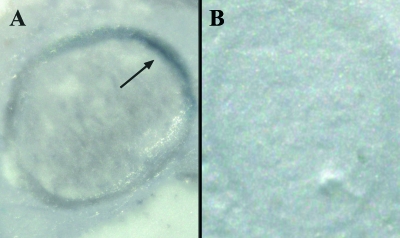
IHC analysis detected PrPSc only in the olfactory nerve perineurium (ONP) in 10 animals (Fig. 2A and C), where it appeared as fine intracellular granular deposits. Specifically, PrPSc deposition was localized in the ONP of fila olfactoria in the nasal cavity area adjacent to the foramina of the ethmoidal lamina cribrosa. In two cases, PrPSc was detected in both the ONP and the NALT (Fig. 2B). Two cases were positive in the NALT only. No PrPSc was ever detected in the sustentacular and basal cells of the OE or inside ORNs and their fibers (Fig. 2F). PET blot analysis consistently confirmed PrPSc deposition detected by IHC in the ONP (n = 12), thus excluding the presence of artifacts (Fig. 3).
FIG. 2.
PrPSc distribution in sheep nasal cavities. (A and C) ONP showing PrPSc immunostaining (magnifications, ×20 and ×100, respectively); (E) ONP of a negative control sheep (magnification, ×100); (B) PrPSc immunostaining in NALT (magnification, ×20). A higher-power view (magnification, ×40) of a NALT showing PrPSc deposition is given in the area outlined in the box at the top right of panel B. (D) NALT of a negative control sheep (magnification, ×20); (F) lack of PrPSc immunostaining in the olfactory mucosa of a scrapie-positive sheep (magnification, ×40).
FIG. 3.
PET blot analysis of olfactory nerve bundles from a scrapie-affected sheep (A) and a negative control (B). The arrow indicates the localization of PrPSc deposits.
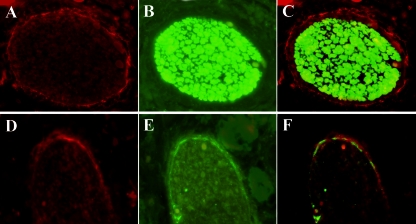
Nasal cavity innervation is provided by fibers of the first cranial nerve, which convey olfactory information from the nasal mucosa to the brain, and by the branches of the trigeminal nerve, specifically, its ophthalmic division, which conveys mechanical, thermal, chemical, and nociceptive stimuli (47). Using immunofluorescence for PrPSc and carnosine, a specific marker for olfactory fibers (4), we demonstrated that PrPSc deposition surrounded the olfactory nerves (Fig. 4A, B, and C).
FIG. 4.
Immunofluorescence labeling in the olfactory nerve of a scrapie-affected sheep. (A to C) Double immunofluorescence for PrPSc and carnosine (magnification, ×60). (A) Anti-PrP (Cy3, red); (B) anticarnosine (Cy2, green); (C) overlay of panels A and B. (D to F) Immunofluorescence for PrPSc and cytokeratins (magnification, ×60). (D) Anti-PrP (Cy3, red); (E) anticytokeratins (Cy2, green); (F) overlay of panels D and E.
The anatomical localization of PrPSc only in the ONP raised the question about which cell type was involved in PrPSc deposition. According to previous studies (12, 13, 23, 60), the perineurial cells abutting the olfactory fascicles at the level of ethmoidal lamina cribrosa are an extension of the meninges which pass through the foramina and surround the traversing fila olfactoria (Fig. 5). To distinguish between meningeal cells and the closely apposed fibroblasts, we wanted to identify a specific marker for meningeal cells. Unlike that of the fibroblasts (16, 32), the embryonic origin of the meninges can be partly traced back to neural ectoderm-derived cells (17, 20, 29, 38), which are known to express cytokeratins (15); this was the rationale for using an antibody against these markers to exclusively immunostain ONP meningeal cells. Immunofluorescence for PrPSc and cytokeratins on serial tissue sections demonstrated that cytokeratins and PrPSc colocalized inside the meningeal layer of the ONP (Fig. 4D, E, and F) and that no PrPSc deposition occurred in other cell types constituting the perineurium.
FIG. 5.
Schematic diagram of the transverse section of an olfactory nerve at the level of the ethmoidal lamina cribrosa, showing the ensheathments constituting its ONP at that level. Blue, fila olfactoria; magenta, olfactory ensheathing cells; green, endoneurium of fibroblasts and collagen fibers; cyan (pia mater), white (perineural space), and red (arachnoid membrane), components forming the perineural epithelium; yellow, epineurium. The cellular nature is common to all the above-mentioned layers; however, an acellular component of collagen fibers also takes part in the constitution of epineurium and endoneurium.
In the nasal cavities of sheep, NALT consists of individual lymphoid nodules distributed throughout the lamina propria of the olfactory mucosa. The general composition of the lymphoid nodules is similar in structure to that of the secondary lymphoid follicles observed in ovine jejunal Peyer's patches (54). By IHC, PrPSc accumulation in NALT was evidenced as granular depositions localized in the cytoplasm or on the surface of large cells, which, according to their morphology, appeared to be macrophages and follicular dendritic cells (Fig. 2B).
PrPSc in the OS-related brain areas.
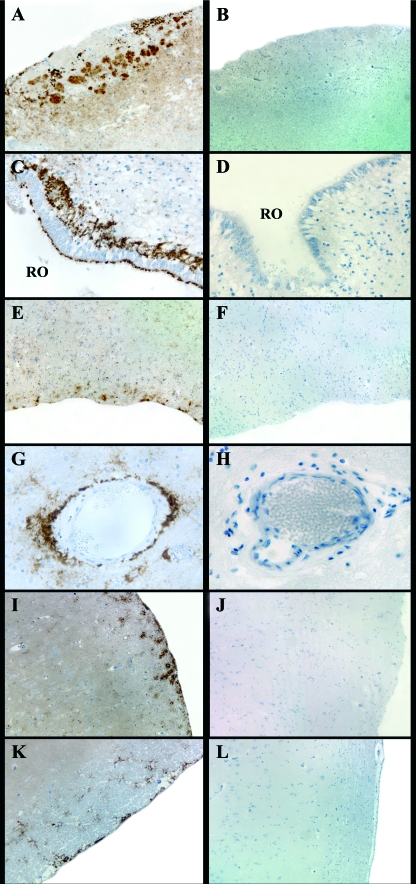
In the OS-related brain areas of the sheep examined, PrPSc deposition was most abundant at the level of the olfactory bulbs and showed an overall marked grade of intensity. Submeningeal (Fig. 6A) and subependymal (Fig. 6C) patterns, together with a fine punctate staining inside the olfactory glomeruli, were consistently detected in the olfactory bulbs. In the samples where the olfactory nerve layer was evaluable, PrPSc was found as granular deposits along the course of the olfactory nerve fibers entering the olfactory bulb (data not shown). The internal plexiform layer, mitral cell layer, and external plexiform layer disclosed mainly a glial PrPSc deposition pattern. The accessory olfactory bulb, when examined, always displayed a fine punctate staining inside the glomeruli (Fig. 6A).
FIG. 6.
Main PrPSc deposition patterns detected in the OS-related brain areas of the scrapie-positive sheep (A, C, E, G, I, and K) and negative control sheep (B, D, F, H, J, and L) examined. (A) Marked fine punctate staining inside the glomeruli of the accessory olfactory bulb (magnification, ×4); (C) recessus olfactorius (RO) of the main olfactory bulb showing a marked subependymal pattern (magnification, ×40); (E) olfactory tract displaying moderate submeningeal and glial PrPSc deposits (magnification, ×10); (G) marked perivascular PrPSc deposition pattern in the frontal cortex at the level of the basal nuclei (magnification, ×40); (I) moderate submeningeal and glial PrPSc deposits in the pyriform lobe (magnification, ×10); (K) apparent subependymal pattern in the hippocampus (magnification, ×10). (B) tissue sections of negative control sheep showing lack of PrPSc immunostaining at the level of the accessory olfactory bulb (magnification, ×4); (D) recessus olfactorius of the main olfactory bulb (magnification, ×40); (F) olfactory tract (magnification, ×10); (H) frontal cortex (magnification, ×40); (J) pyriform lobe (magnification, ×10); (L) hippocampus (magnification, ×10).
At the level of the olfactory tract, immunostaining was moderate to marked, and the PrPSc deposition patterns were mainly of the submeningeal and glial types (Fig. 6E). In the frontal cortex there was a moderate submeningeal and glial type pattern and a marked perivascular PrPSc deposition (Fig. 6G).
In the olfactory-related areas of the limbic system (i.e., the pyriform lobe and hippocampus), the amount of PrPSc immunolabeling appeared minimal or even absent, being characterized mainly by submeningeal (Fig. 6I), subependymal (Fig. 6K), and perivascular deposits. These patterns were the prevalent ones in all the olfactory-related brain areas examined but were not exclusive to them, as they were identified all over the brain.
DISCUSSION
Involvement of the OS in the course of animal prion diseases has not been widely investigated to date. Except for three previous experimental studies on rodent models (10, 21, 28), the only report describing PrPSc deposition in the OS under conditions of natural infection was a poster presentation by Vidal et al. in 2003 (59), reporting data on 19 BSE-affected cows. In all the OS-related brain areas examined, WB and IHC detected the presence of PrPSc more intensively in the cows at more advanced stages of the disease. However, no PrPSc was detected by either WB or IHC in the ORNs or in the supporting and basal cells of the OE.
To our knowledge, no previous studies have investigated PrPSc deposition in the OS of naturally scrapie-affected sheep. Our data confirm not only the involvement of the central OS in natural scrapie disease, as reported by Vidal et al. (59) for natural BSE, but also that PrPSc deposition occurs in the nasal cavity tissues. At this level, WB analysis detected PrPSc deposition in 21 out of the 24 scrapie-affected sheep examined, including animals with both clinical and preclinical disease, demonstrating that nasal cavity tissues are involved in scrapie disease, even in its asymptomatic stages. On the other hand the peripheral OS, consisting only of ORNs and just evaluable by IHC, in our study appeared to be completely free of PrPSc deposition.
In regard to the anatomical distribution of PrPSc in the nasal mucosa, both the respiratory epithelium and the OE appeared to be completely spared. This observation is also in line with previous reports describing PrPSc distribution in hamsters extranasally infected with the TME agent (28). These results might be due to the small amount of PrPC at the level of OE as reported in humans (63) or to the high turnover of the ORNs, which undergo continuous regeneration throughout life (9). The short lifetime of the ORNs (6 to 10 weeks) (56) is thought to be unfavorable for PrPSc accumulation in the OE (10). Nevertheless, the presence of PrPSc has been selectively detected in the OE of hamsters intracerebrally challenged with the TME agent (10) and of sCJD patients (63); however, in the latter instance the estimated concentration of PrPSc in the OE was particularly low (about 3% of that in the olfactory bulb).
As reported elsewhere, the olfactory nerve fibers, unlike all other fiber pathways, are known to display high levels of PrPC (44). Contrary to what one might expect, and according previous reports (28), IHC analysis, as applied in our study, detected no PrPSc inside the olfactory nerve fibers in any of the animals examined; instead, an immunoreaction, never described before, appeared at the level of the ONP.
The detection of PrPSc in the ONP, almost exclusively at the level of the lamina cribrosa, needs to be explained by considering the ultrastructural organization of the ensheathments of the olfactory fascicules in that area (Fig. 5). Studies of ONP ultrastructure at the level of the lamina cribrosa in sheep in particular are lacking, but such descriptions have been made for rabbits (13) and rats (12, 60). According to these reports, the olfactory nerves are enveloped along their entire length by olfactory ensheathing cells (OECs) that accompany the olfactory fascicules' route toward the olfactory bulb (12), constituting the innermost layer of the ONP. The OECs are separated by a basal lamina from an outermost directly apposed connective tissue covering of collagen fibers and fibroblasts, which also closely surrounds the olfactory bundles along their course.
At the transitional zone between the peripheral and the central nervous systems, where the fila olfactoria cross the cribriform plate, an additional meningeal component joins the perineurium, composed of the pia mater and the arachnoid membrane. The two meninges extend through the foramina of the lamina cribrosa and remain closely apposed to the olfactory nerves, thus delimiting a virtual perineurial space which is a direct prolongation of the subarachnoid space (SAS) of the brain. Confined between the pia mater and the arachnoid membrane, this space is filled with cerebrospinal fluid (CSF) and extends along the olfactory nerves until the two meninges merge together with the perineurium (12, 13, 60). The olfactory route turns out to be, besides the arachnoid villi and Pacchioni's granulations in the brain, an additional drainage route of CSF at the nasal submucosal level, as previously documented in many species and in sheep as well (27, 62).
In the present study, PrPSc immunostaining in the ONP appeared only in the region of the cribriform plate and in the adjacent portion of the MNC where it inserts. In detail, using a double-immunofluorescence study, we demonstrated that PrPSc colocalized with cytokeratins, thus determining the ONP meningeal component as the actual PrPSc deposition site. This particular anatomical localization most likely points to a probable role of CSF in conveying PrPSc to the meningeal cells abutting the SAS extension that surrounds the nerve rootlets. To date, only PrPC has been identified in the CSF of sheep (39, 58); even so, there is good evidence that PrPSc may be present there too, as demonstrated by high-performance liquid chromatography (2) and protein misfolding cyclic amplification (1) of the CSF of mice and hamsters, respectively, experimentally infected with scrapie agent.
Our findings are very similar to those described in a tracer study by Walter et al. (60), in which ink was injected into the SAS of experimental rats. The ink was detected by electron microscopy inside the arachnoidal cells of the ONP but not in the dura mater cells or in the fibroblasts of the perineurium. The sole involvement of the arachnoid cells in both PrPSc and ink deposition, with sparing of other contiguous cells, may be linked to the type of intercellular junctions existing between them. The junctions between the fibroblasts of the perineurium are spaced widely apart but are of the tight junction type (13), which is known to restrict the passage of molecules through the spaces between cells. Moreover, the presence of a basal lamina around the OECs abutting the olfactory nerves is an additional feature that may contribute to preventing the uptake of molecules, even PrPSc, by the underlying ORNs.
While the olfactory neurons and most of the cells constituting the ONP do not appear to allow PrPSc deposition, the lymphatic capillaries of the nasal submucosa and the NALT are well known routes for drainage of CSF and the molecules it may convey. In sheep, it has been calculated that a considerable fraction of CSF (40% to 48%) drains into the extracranial lymphatics of the NALT and from there to the downstream retropharyngeal and cervical lymph nodes (5). Although these lymph nodes were not examined in the present study, PrPSc was detected in NALT in 4 out of 24 animals. It could be argued, therefore, that the CSF has a probable role in determining NALT involvement during scrapie infection. Furthermore, the detection of nasal mucosa inflammation associated with the presence of Oestrus ovis larvae in two out of the four sheep positive in NALT (data not shown) supports the hypothesis that an inflammatory state associated with scrapie infection may lead to the spread of PrPSc to unusual deposition sites (22). Following this line of argument, the presence of PrPSc in NALT may also be related to an inflammatory condition in nasal tissues, as occurs in myiasis. Lastly, as previously reported for naturally occurring CWD (53) and experimental TME (3, 10), our findings confirm the involvement of NALT in the course of sheep natural scrapie.
The probable role of CSF in scrapie agent neuroinvasion is also supported by the findings from IHC analysis of the brains of the sheep examined in this study. At the level of the olfactory-related brain structures, PrPSc deposition consistently occurred in those areas in direct contact with CSF and presented chiefly as subependymal, perivascular, and submeningeal patterns. It is noteworthy that such patterns were not specific for olfactory structures, as they were detected throughout the brain, in animals with both clinical and preclinical disease. These findings seem to strengthen the hypothesis of a probable role of CSF in conveying PrPSc to the whole brain.
Other authors have speculated on the possible role of the CSF pathway as an alternative route to the autonomic nervous system for PrPSc neuroinvasion (2, 33, 50). In agreement with previous reports (14), we observed PrPSc deposits in the sub- and supraependymal regions; this indicates that the ependymocytes are susceptible to the scrapie agent and play a possible role in the interchange of PrPSc between brain parenchyma and CSF.
Evidence for a possible correlation between CSF and PrPSc deposition pattern is given by the perivascular pattern consistently identified in the olfactory areas we examined. The particular ultrastructure of the brain arteries is such that the perivascular space around the intracerebral arteries is in direct continuity with the perivascular space around the subarachnoid arteries (64). This anatomical structure permits the brain interstitial fluid to drain into the perivascular space or even possibly leak into the CSF of the SAS, the amount of which remains to be quantified. As observed in animal experiments (37, 61, 65), the communication between interstitial fluid and CSF might provide a pathway for the spread of different tracer and molecules, and so most likely PrPSc as well, into the brain.
The consistent identification of a submeningeal pattern, with sparing of the underlying cortical brain areas, may be attributed to a possible transport of PrPSc by the CSF into the brain. This hypothesis has not yet been completely elucidated; however, previous studies indicate that the pia mater is permeable to solutes and larger molecules and enzymes such as peroxidase (64).
In regard to the involvement of the OS-related brain areas, PrPSc deposition appeared mainly in the more frontal olfactory structures in all the sheep examined (i.e., the olfactory bulb, tract, and frontal cortex), which constitute the primary afferent areas of olfactory axons. In contrast, the second and third olfactory fiber-receiving systems, the pyriform lobe and the hippocampus, respectively, showed only weak PrPSc positivity. This finding does not preclude a possible anterograde spread of PrPSc via the olfactory axons, even if PrPSc deposition was never detected inside either the OE or the olfactory fibers. It should be emphasized that the lack of PrPSc staining in the olfactory nerves might be due to their high physiological turnover, which, however, may not necessarily prevent the transport of PrPSc toward the brain. Moreover, the PrPSc immunoreactivity observed in the fibers of the olfactory nerve layer of the bulb seems to corroborate this hypothesis.
In the sheep examined for the study, the PrPSc immunolabeling intensity grade in the olfactory structures always appeared, in animals with both clinical and preclinical disease, from slightly to much weaker than that evidenced in the corresponding obex tissue at level of the dorsal motor nucleus of the vagus nerve. By comparing the PrPSc staining in olfactory structures and the respective obex sections, the hypothesis that neuroinvasion may have occurred from the gastrointestinal tract via the well-known route of the vagus nerve should be considered.
It is still unclear which of the three supposed routes, i.e., (i) CSF mediated, (ii) lymphatic system mediated, or (iii) nerve associated (or an interaction between them), is responsible for PrPSc deposition in the OS in the course of natural TSEs. However, the relatively large amount of PrPSc (1 × 10−1 of that present in the brain) quantified in the nasal cavity tissues of the sheep examined suggests their major involvement in scrapie pathogenesis.
The presence of PrPSc in nasal mucosa is likely evidence for the possible diffusion of PrPSc in the environment through nasal mucus and parasites such Oestrus ovis larvae (8). The likelihood of horizontal transmission of scrapie in sheep is also supported by the experimental finding that the nasal route offers an efficient pathway for scrapie neuroinvasion in this species (18).
Further investigations to establish possible genotype-related, scrapie strain-related differences and the onset of PrPSc deposition in sheep nasal cavities tissues are conceivable. In view of the present findings, there is an increasing body of evidence indicating that the OS is precociously involved in scrapie pathogenesis, even in the early stages of the disease when the animals are still asymptomatic. Our results seem to correlate with findings for different human neurodegenerative diseases, particularly Alzheimer's and Parkinson's diseases, in which OS involvement during pathogenesis has been clearly established in several cases (11).
Acknowledgments
We thank Danilo Muratore and Patrizia Davico for their technical assistance during animal sampling.
This study was funded by Italian Ministry of Health grants IZSPLV 2003RF and IZSPLV20/04.
Footnotes
Published ahead of print on 21 January 2009.
REFERENCES
- 1.Atarashi, R., R. A. Moore, V. L. Sim, A. G. Hughson, D. W. Dorward, H. A. Onwubiko, S. A. Priola, and B. Caughey. 2007. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat. Methods 4645-650. [DOI] [PubMed] [Google Scholar]
- 2.Banks, W. A., M. L. Niehoff, C. Adessi, and C. Soto. 2004. Passage of murine scrapie prion protein across the mouse vascular blood-brain barrier. Biochem. Biophys. Res. Commun. 318125-130. [DOI] [PubMed] [Google Scholar]
- 3.Bartz, J. C., A. E. Kincaid, and R. A. Bessen. 2003. Rapid prion neuroinvasion following tongue infection. J. Virol. 77583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonfanti, L., P. Peretto, S. De Marchis, and A. Fasolo. 1999. Carnosine-related dipeptides in the mammalian brain. Prog. Neurobiol. 59333-353. [DOI] [PubMed] [Google Scholar]
- 5.Boulton, M., M. Flessner, D. Armstrong, J. Hay, and M. Johnston. 1998. Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am. J. Physiol. 274R88-R96. [DOI] [PubMed] [Google Scholar]
- 6.Brown, P., and D. C. Gajdusek. 1991. Survival of scrapie virus after 3 years' internment. Lancet 337269-270. [DOI] [PubMed] [Google Scholar]
- 7.Casalone, C., G. Zanusso, P. Acutis, S. Ferrari, L. Capucci, F. Tagliavini, S. Monaco, and M. Caramelli. 2004. Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jacob disease. Proc. Natl. Acad. Sci. USA 1013065-3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corona, C., F. Martucci, B. Iulini, M. Mazza, P. L. Acutis, C. Porcario, M. Pezzolato, B. Manea, A. Maroni, S. Barocci, E. Bozzetta, M. Caramelli, and C. Casalone. 2006. Could Oestrus ovis act as vector for scrapie?, abstr. PA-10, p. 239. Abstr. Prion 2006: strategies, advances and trends towards protection of society, Turin, Italy.
- 9.Cowan, C. M., and A. J. Roskams. 2002. Apoptosis in the mature and developing olfactory neuroepithelium. Microsc. Res. Tech. 58204-215. [DOI] [PubMed] [Google Scholar]
- 10.DeJoia, C., B. Moreaux, K. O' Connell, and R. A. Bessen. 2006. Prion infection of oral and nasal mucosa. J. Virol. 804546-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doty, R. L. 2008. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann. Neurol. 637-15. [DOI] [PubMed] [Google Scholar]
- 12.Doucette, R. 1991. PNS-CNS transitional zone of the first cranial nerve. J. Comp. Neurol. 312451-466. [DOI] [PubMed] [Google Scholar]
- 13.Erlich, S. S., J. G. McComb, S. Hyman, and M. H. Weiss. 1986. Ultrastructural morphology of the olfactory pathway for cerebrospinal fluid drainage in the rabbit. J. Neurosurg. 64466-473. [DOI] [PubMed] [Google Scholar]
- 14.Fournier, J. G., K. Adjou, V. Grigoriev, and J. P. Deslys. 2008. Ultrastructural evidence that ependymal cells are infected in experimental scrapie. Acta Neuropathol. 115643-650. [DOI] [PubMed] [Google Scholar]
- 15.Frank, E. H., B. W. Burge, B. H. Liwnicz, L. J. Lotspeich, J. C. White, S. L. Wechsler, F. H. Mayfield, and J. T. Keller. 1983. Cytokeratin provides a specific marker for human arachnoid cells grown in vitro. Exp. Cell Res. 146371-376. [DOI] [PubMed] [Google Scholar]
- 16.Franke, W. W., D. Mayer, E. Schmid, H. Denk, and E. Borenfreund. 1981. Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp. Cell Res. 134345-365. [DOI] [PubMed] [Google Scholar]
- 17.Gil, D. R., and G. D. Ratto. 1973. Contribution to the study of the origin of leptomeninges in the human embryo. Acta Anat. 85620-623. [DOI] [PubMed] [Google Scholar]
- 18.Hamir, A. N., R. A. Kunkle, J. A. Richt, J. M. Miller, and J. J. Greenlee. 2008. Experimental transmission of US scrapie agent by nasal, peritoneal, and conjunctival routes to genetically susceptible sheep. Vet. Pathol. 457-11. [DOI] [PubMed] [Google Scholar]
- 19.Harmeyer, S., E. Pfaff, and M. H. Groschup. 1998. Synthetic peptide vaccines yield monoclonal antibodies to cellular and pathological prion proteins of ruminants. J. Gen. Virol. 79937-945. [DOI] [PubMed] [Google Scholar]
- 20.Harvey, S. C., and H. S. Burr. 1926. The development of the meninges. Arch. Neurol. Psychiat. 15545-567. [Google Scholar]
- 21.Haybaeck, J., M. Heikenwalder, I. Margalith, N. Zeller, C. Bridel, P. Schwarz, K. Merz, L. Stitz, and A. Aguzzi. 2007. Intranasal and aereosolic prion transmission, abstr. P03.103, p. 72. Abstr. Prion 2007, Edinburgh, Scotland, United Kingdom.
- 22.Heikenwalder, M., N. Zeller, H. Seeger, M. Prinz, P. C. Klohn, P. Schwarz, N. H. Ruddle, C. Weissman, and A. Aguzzi. 2005. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science 3071107-1110. [DOI] [PubMed] [Google Scholar]
- 23.Jackson, R. T., J. Tigges, and W. Arnold. 1979. Subarachnoid space of the CNS, nasal mucosa, and lymphatic system. Arch. Otolaryngol. 105180-184. [DOI] [PubMed] [Google Scholar]
- 24.Jeffrey, M., S. Martin, L. González, S. J. Ryder, S. J. Bellworthy, and R. Jackman. 2001. Differential diagnosis of infections with the bovine spongiform encephalopathy (BSE) and scrapie agents in sheep. J. Comp. Pathol. 125271-284. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, C. J., K. E. Phillips, P. T. Schramm, D. McKenzie, J. M. Aiken, and J. A. Pedersen. 2006. Prions adhere to soil minerals and remain infectious. PloS Pathog. 2296-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, R. T. 2005. Prion diseases. Lancet Neurol. 4635-642. [DOI] [PubMed] [Google Scholar]
- 27.Johnston, M., A. Zakharov, C. Papaiconomou, G. Salmasi, and D. Armstrong. 2004. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kincaid, A. E., and J. C. Bartz. 2007. The nasal cavity is a route for prion infection in hamsters. J. Virol. 814482-4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klika, E. 1968. Ultrastructure of the meninges during human ontogeny. Z. Mikrosk. Anat. Forsch. 79209-222. [PubMed] [Google Scholar]
- 30.Kolb, A. 1979. Lichtmikroskopische Untersuchungen an der Riechschleimhaut des Hausschafes (Ovis aries L.). Anat. Anz. 146444-455. [PubMed] [Google Scholar]
- 31.Konold, T., S. J. Moore, S. J. Bellworthy, and H. A. Simmons. 2008. Evidence of scrapie transmission via milk. BMC Vet. Res. 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazarides, E. 1980. Intermediate filaments as mechanical integrators of cellular space. Nature 283249-256. [DOI] [PubMed] [Google Scholar]
- 33.Marsh, R. F., and R. H. Kimberlin. 1975. Comparison of scrapie and transmissible mink encephalopathy in hamsters. II. Clinical signs, pathology and pathogenesis. J. Infect. Dis. 131104-110. [DOI] [PubMed] [Google Scholar]
- 34.Mathiason, C. K., J. G. Powers, S. J. Dahmes, D. A. Osborn, K. V. Miller, R. J. Warren, G. L. Mason, S. A. Hays, J. Hayes-Klug, D. M. Seelig, M. A. Wild, L. L. Wolfe, T. R. Spraker, M. W. Miller, C. J. Sigurdson, G. C. Telling, and E. A. Hoover. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314133-136. [DOI] [PubMed] [Google Scholar]
- 35.Miller, M. W., and E. S. Williams. 2003. Prion disease: horizontal prion transmission in mule deer. Nature 42535-36. [DOI] [PubMed] [Google Scholar]
- 36.Mori, I., Y. Nishiyama, T. Yokochi, and Y. Rimura. 2005. Olfactory transmission of neurotropic viruses. J. Neurovirol. 11129-137. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson, C., and E. Syková. 1998. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 21207-215. [DOI] [PubMed] [Google Scholar]
- 38.Pease, D. C., and R. L. Schultz. 1958. Electron microscopy of rat cranial meninges. Am. J. Anat. 102301-321. [DOI] [PubMed] [Google Scholar]
- 39.Picard-Hagen, N., V. Gayrard, C. Viguie, M. Moudjou, C. Imbs, and P. L. Toutain. 2006. Prion protein in the cerebrospinal fluid of healthy and naturally scrapie-affected sheep. J. Gen. Virol. 873723-3727. [DOI] [PubMed] [Google Scholar]
- 40.Prusiner, S. B. 1982. Novel proteinaceus infectious particles cause scrapie. Science 216136-144. [DOI] [PubMed] [Google Scholar]
- 41.Prusiner, S. B. 1998. The prion diseases. Brain Pathol. 8499-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Race, R., A. Jenny, and D. Sutton. 1998. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen and lymph node: implications for transmission and antemortem diagnosis. J. Infect. Dis. 178949-953. [DOI] [PubMed] [Google Scholar]
- 43.Ritchie, D. L., M. W. Head, and J. W. Ironside. 2004. Advances in the detection of prion protein in peripheral tissues of variant Creutzfeldt-Jakob disease patients using paraffin-embedded tissue blotting. Neuropathol. Appl. Neurobiol. 30360-368. [DOI] [PubMed] [Google Scholar]
- 44.Salès, N., K. Rodolfo, R. Hässig, B. Faucheux, L. Di Giamberardino, and K. L. Moya. 1998. Cellular prion protein localization in rodent and primate brain. Eur. J. Neurosci. 102464-2471. [DOI] [PubMed] [Google Scholar]
- 45.Salman, M. D. 2003. Chronic wasting disease in deer and elk: scientific facts and findings. J. Vet. Med. Sci. 65761-768. [DOI] [PubMed] [Google Scholar]
- 46.Sawcer, S. J., G. M. Yuill, T. F. G. Esmonde, P. Estibeiro, J. W. Ironside, J. E. Bell, and R. G. Will. 1993. Creutzfeldt-Jacob disease in an individual occupationally exposed to BSE. Lancet 341642. [DOI] [PubMed] [Google Scholar]
- 47.Schaefer, M. L., B. Böttger, W. L. Silver, and T. E. Finger. 2002. Trigeminal collaterals in the nasal epithelium and olfactory bulb: a potential route for direct modulation of olfactory information by trigeminal stimuli. J. Comp. Neurol. 444221-226. [DOI] [PubMed] [Google Scholar]
- 48.Seeger, H., M. Heikenwalder, N. Zeller, J. Kranich, P. Schwarz, A. Gaspert, B. Seifert, G. Miele, and A. Aguzzi. 2005. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science 310324-326. [DOI] [PubMed] [Google Scholar]
- 49.Shaw, I. C. 1995. BSE and farmworkers. Lancet 3461365. [DOI] [PubMed] [Google Scholar]
- 50.Sisó, S., M. Jeffrey, H. Baird, S. Martin, S. Bellworthy, F. Chianini, and L. Gonzalez. 2006. The topographical distribution of prion protein in the brains of sheep challenges the current hypothesis of neuroinvasion, abstr. PA-52, p. 281. Abstr. Prion 2006: Strategies, advances and trends towards protection of society, Turin, Italy.
- 51.Smith, P. E. M., M. Zeidler, J. W. Ironside, P. Estibeiro, and T. H. Moss. 1995. Creutzfeldt-Jacob disease in a dairy farmer. Lancet 346898. [DOI] [PubMed] [Google Scholar]
- 52.Spraker, T. R., K. I. O' Rourke, A. Balachandran, R. R. Zink, B. A. Cummings, M. W. Miller, and B. E. Powers. 2002. Validation of monoclonal antibody F99/97.6.1 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with chronic wasting disease. J. Vet. Diagn. Investig. 143-7. [DOI] [PubMed] [Google Scholar]
- 53.Spraker, T. R., R. R. Zink, B. A. Cummings, M. A. Wild, M. W. Miller, and K. I. O' Rourke. 2002. Comparison of histological lesions and immunohistochemical staining of proteinase-resistant prion protein in a naturally occurring spongiform encephalopathy of free-ranging mule deer (Odocoileus hemionus) with those of chronic wasting disease of captive mule deer. Vet. Pathol. 39110-119. [DOI] [PubMed] [Google Scholar]
- 54.Stanley, A. C., J. F. Huntley, M. Jeffrey, and D. Buxton. 2001. Characterization of ovine nasal-associated lymphoid tissue and identification of M cells in the overlying follicle-associated epithelium. J. Comp. Pathol. 125262-270. [DOI] [PubMed] [Google Scholar]
- 55.Tabaton, M., S. Monaco, M. P. Cordone, M. Colucci, G. Giaccone, F. Tagliavini, and G. Zanusso. 2004. Prion deposition in olfactory biopsy of sporadic Creutzfeldt-Jacob disease. Ann. Neurol. 55294-296. [DOI] [PubMed] [Google Scholar]
- 56.Treloar, H. B., J. C. Bartolomei, B. W. Lipscomb, and C. A. Greer. 2001. Mechanisms of axonal plasticity: lessons from the olfactory pathway. Neuroscientist 755-63. [DOI] [PubMed] [Google Scholar]
- 57.Vascellari, M., R. Nonno, F. Mulinelli, M. Bagolaro, M. A. Di Bari, E. Melchiotti, S. Marcon, C. D'Agostino, G. Vaccai, M. Conte, L. De Grossi, F. Rosone, F. Giordani, and U. Agrimi. 2007. PrPsc in salivary glands of scrapie-affected sheep. J. Virol. 814872-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vella, L. J., D. L. V. Greenwood, R. Cappai, J. P. Y. Scheerlinck, and A. F. Hill. 2008. Enrichment of prion protein in exosomes derived from ovine cerebral spinal fluid. Vet. Immunol. Immunopathol. 124385-393. [DOI] [PubMed] [Google Scholar]
- 59.Vidal, E., M. Ordonez, J. M. Vela, and M. Pumarola. 2003. Study of the olfactory pathway and related structures of 19 BSE naturally affected cows, abstr. PG-26, p. 203. Abstr. Prion 2003: prion diseases: from basic research to intervention concepts, Gasteig, Munich, Germany.
- 60.Walter, B. A., V. A. Valera, S. Takahashi, and T. Ushiki. 2006. The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol. Appl. Neurobiol. 32388-396. [DOI] [PubMed] [Google Scholar]
- 61.Weller, R. O. 1998. Pathology of cerebrospinal fluid and interstitial fluid of the CNS: significance for Alzheimer disease, prion disorders and multiple sclerosis. J. Neuropathol. Exp. Neurol. 57885-894. [DOI] [PubMed] [Google Scholar]
- 62.Zakharov, A., C. Papaiconomou, J. Djenic, R. Midha, and M. Johnston. 2003. Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of Microfil. Neuropathol. Appl. Neurobiol. 29563-573. [DOI] [PubMed] [Google Scholar]
- 63.Zanusso, G., S. Ferrari, F. Cardone, P. Zampieri, M. Gelati, M. Fiorini, Farinazzo, A., M. Gardiman, T. Cavallaro, M. Bentivoglio, P. G. Rigetti, M. Pocchiari, N. Rizzato, and S. Monaco. 2003. Detection of pathologic prion protein in the olfactory epithelium in sporadic Creutzfeldt-Jacob disease. N. Engl. J. Med. 348711-719. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, E. T., C. B. E. Inman, and R. O. Weller. 1990. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J. Anat. 170111-123. [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang, E. T., H. K. Richards, S. Kida, and R. O. Weller. 1992. Directional and compartimentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta Neuropathol. 83233-239. [DOI] [PubMed] [Google Scholar]