Abstract
The role of T-lymphocyte subsets in recovery from foot-and-mouth disease virus (FMDV) infection in calves was investigated by administering subset-specific monoclonal antibodies. The depletion of circulating CD4+ or WC1+ γδ T cells was achieved for a period extending from before challenge to after resolution of viremia and peak clinical signs, whereas CD8+ cell depletion was only partial. The depletion of CD4+ cells was also confirmed by analysis of lymph node biopsy specimens 5 days postchallenge. Depletion with anti-WC1 and anti-CD8 antibodies had no effect on the kinetics of infection, clinical signs, and immune responses following FMDV infection. Three of the four CD4+ T-cell-depleted calves failed to generate an antibody response to the nonstructural polyprotein 3ABC but generated a neutralizing antibody response similar to that in the controls, including rapid isotype switching to immunoglobulin G antibody. We conclude that antibody responses to sites on the surface of the virus capsid are T cell independent, whereas those directed against the nonstructural proteins are T cell dependent. CD4 depletion was found to substantially inhibit antibody responses to the G-H peptide loop VP1135-156 on the viral capsid, indicating that responses to this particular site, which has a more mobile structure than other neutralizing sites on the virus capsid, are T cell dependent. The depletion of CD4+ T cells had no adverse effect on the magnitude or duration of clinical signs or clearance of virus from the circulation. Overall, we conclude that CD4+ T-cell-independent antibody responses play a major role in the resolution of foot-and-mouth disease in cattle.
Foot-and-mouth disease (FMD) is a highly contagious, clinically acute, cytopathic viral disease of wild and domestic cloven-hoofed animals. The causal agent is a member of the family Picornaviridae and consists of a single-stranded, positive-sense RNA genome enclosed within a nonglycosylated icosahedral capsid comprising 60 copies each of the four structural polypeptides VP1 to VP4 (1). The genome encodes a unique polyprotein from which the structural and nine nonstructural proteins are cleaved by viral proteases (61). FMD virus (FMDV) shows high genetic and antigenic variability such that infection with a virus of one of the seven serotypes does not confer protection against other serotypes (3). Experimental infection is characterized by a short incubation period of 1 to 3 days followed by pyrexia, the formation of vesicles, and a short viremic phase with clinical resolution and virus clearance coinciding closely with the emergence of serum neutralizing antibodies (3). However, ruminants exposed to virus, whether vaccinated or not, can carry FMDV in the oropharynx for years following the resolution of the acute infection (2).
In contrast to the well-defined role of humoral immune responses, the contribution of T-cell-mediated responses to immunity and their role in the induction of protective B-cell responses to FMDV in the natural host species are poorly understood. Observations of murine infection models indicate that acute cytopathic viral infections frequently induce T-cell-independent antibody responses, and it was previously proposed that such rapid responses are required to allow the control of virus spread through the circulation and to ensure host survival (5, 22, 38). Borca et al. previously reported that the protective immune response against FMDV in a murine experimental model was T cell independent (8). However, a role for T cells in the induction of antibody responses in ruminants has been suggested based on the demonstration of FMDV-specific CD4+ T-cell-proliferative responses following infection or vaccination with virus or peptide (7, 15, 27). Until recently, CD8+ T-cell responses to FMDV in livestock had been demonstrated only for infected animals, but the T-cell proliferation assays employed were unable to demonstrate whether or not the detected responses were class I major histocompatibility complex (MHC) restricted (12). Recently, Guzman et al. (28) used gamma interferon production to demonstrate virus-specific MHC class I-restricted CD8+ T-cell responses in cattle infected or vaccinated with FMDV, but the role of these CD8+ T cells in immunity to FMDV infection is still not known. There is an abundant γδ T-cell population in ruminants; however, there is no clear consensus on the role of these cells in immunity to infections (13, 52). FMDV vaccine antigen has been shown to induce proliferation and cytokine production in naïve pig γδ T cells, suggesting that these cells could contribute to the early immune response to FMD vaccination (67).
The three major subpopulations of bovine T lymphocytes identified in the circulation and secondary lymphoid organs of cattle can be effectively depleted in vivo by administering the appropriate mouse monoclonal antibody (MAb) (34, 46). In the present study, we used mouse MAbs to selectively deplete CD4+, CD8+, or WC1+ T-lymphocyte subpopulations to investigate the role of these T-cell subsets in the acute stage of FMDV infection in naïve cattle.
MATERIALS AND METHODS
Experimental design.
A total of 12 cattle, 2 to 4 months of age, were used in the studies. Animal experimentation was approved by the Institute for Animal Health (IAH) ethical review board under the authority of a Home Office project license in accordance with the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act of 1986 and associated guidelines. In an initial experiment, eight cattle were allocated into four pairs, each of which received either anti-CD4, anti-CD8, anti-WC1, or an isotype-matched control MAb over a period of 3 days, starting the day before virus challenge. Doses of 3 mg, 21.5 mg, and 21 mg were administered intravenously to each calf on days −1, 0 (challenge day), and 1, respectively, giving a total dose of approximately 0.76 mg of antibody per kg body weight. In a second experiment, four cattle were divided into pairs that received either anti-CD4 or a control MAb over a 4-day period starting 2 days before challenge. The animals were given 20 mg of MAb on day −2 and 45 mg on each of the following 3 days, giving a total dose of approximately 2.58 mg of antibody per kg body weight. Cattle were challenged with FMDV by subepidermolingual injection of 0.2 ml of 105 50% tissue culture infective doses into each of two sites with the cattle-adapted type O UKG/34/2001 strain of the virus. Clinical observations were conducted daily until the resolution of disease. Clinical signs of FMD and rectal temperatures were scored (see Table S1 in the supplemental material) using a modified subjective scoring system based on a method described previously by Quan et al. (53). The right prescapular lymph node was removed from animals in the second experiment 5 days postchallenge, under sedation and local anesthetic. Clotted blood and heparinized blood were collected at intervals throughout the study and at postmortem on day 30 for animals in experiment 1 and on day 29 for animals in experiment 2. Prescapular lymph node, mandibular lymph node, and probang samples were collected at postmortem examination.
MAbs used for depletion.
The MAbs used for depletion, which are described in the proceedings of the First International Workshop on Bovine, Sheep, and Goat Leukocyte Differentiation Antigens (31), were CC8 (anti-CD4), IL-A11 (anti-CD4), CC63 (anti-CD8), and CC15 (anti-WC1). MAb TRT3, raised against turkey rhinotracheitis virus, was administered to control animals (17). All MAbs were murine immunoglobulin G2a (IgG2a), and all of the hybridomas were produced at the IAH, except IL-A11, which was provided by the International Livestock Research Institute, Nairobi, Kenya.
Preparation of mononuclear cells from tissue and blood.
Mononuclear cells were prepared from samples of prescapular lymph nodes by slicing the tissue into small fragments, which were gently teased apart in phosphate-buffered saline (PBS) containing 5% fetal calf serum. The tissue fragments were then disrupted through sterile gauze with a syringe. Viable mononuclear cells were isolated from these lymph node suspensions and from heparinized peripheral blood by diluting them with an equal volume of PBS and underlying them with 13 ml Histopaque 1077 (Sigma, United Kingdom) before centrifugation at 1,000 × g for 30 min at 18°C. Cells at the interface were collected, washed three times with PBS, and counted, and their viability was assessed by trypan blue staining.
Flow cytometry.
Blood and lymph node mononuclear cells were analyzed by flow cytometry to evaluate the degree of lymphocyte depletion using the following MAbs: CC30 (anti-CD4), CC58 (anti-CD8), and CC39 (anti-WC1) (31). MAb CC37 (anti-CD21) was used as a positive control, and MAb TRT1, raised against turkey rhinotracheitis virus, was used as an isotype-matched negative control (17, 31). All MAbs were murine IgG1 produced at the IAH. Preliminary studies using blood and lymph node mononuclear cells from noninfected animals confirmed that the incubation of cells with the MAbs used for depletion for 20 h did not block the staining by IgG1 MAbs of the respective specificities used for subsequent staining of the cells. Cell suspensions were stained with MAbs as described previously (32, 34) except that a goat anti-mouse isotype-specific secondary antibody (Alexa Fluor; Molecular Probes, United Kingdom) was used. Cells were gated for viability based on their forward-scatter and side-scatter profiles. A minimum of 10,000 viable cells were analyzed in each sample; in addition, 100,000 viable peripheral blood mononuclear cells were analyzed on day 1 in experiment 1 and on days 0 and 4 in experiment 2 to assess CD4+ T-cell depletion. Cells were analyzed using a Becton Dickinson FACSCalibur apparatus with Cellquest software, and results were analyzed using FCS Express, version 3 (De Novo Software).
Immunofluorescence confocal microscopy.
Prescapular lymph node samples were snap-frozen in OCT compound (Tissue-Tek) and stored at −80°C until being processed. Ten acetone-fixed cryosections from different regions of the prescapular lymph nodes of each animal were labeled with the following murine MAbs: CC30 (anti-CD4), MM1A (anti-CD3, IgG1), CC51 (anti-CD21, IgG2b) (31), and isotype-matched control MAbs TRT1 and AV29 (a MAb directed against chicken CD4 antigen, IgG2b) (37). Stack images were analyzed to detect CD4+ T-cell depletion. Preliminary studies of prescapular lymph node samples from noninfected animals showed that the activity of MAb CC30 was not blocked by the respective MAbs used for CD4+ cell depletion. Acetone-fixed cryosections of mandibular lymph nodes were labeled with IB11, a murine MAb shown to be specific for conformational, nonneutralizing epitopes of the FMDV capsid (35), in combination with CC51, dark-zone follicular dendritic cell marker D46 (anti-ovine fibrinogen, IgG2a) (39), and isotype-matched control MAbs TRT1, TRT3 (IgG2a) (17), and AV29. All MAbs used for confocal microscopy were produced at the IAH. Goat anti-mouse Molecular Probes Alexa Fluor-conjugated secondary antibodies (Invitrogen, United Kingdom) were used for detection. All data were collected sequentially using a Leica SP2 scanning laser confocal microscope.
Quantitative rRT-PCR.
A quantitative real-time reverse transcription (rRT)-PCR method was used to quantify the FMDV genome copies in serum and in probang samples (53, 54, 56). A standard RNA dilution series of the internal ribosomal entry site of FMDV O/UKG/34/2001 was included on the plate for reverse transcription, which was performed as previously described (53, 55). rRT-PCR was performed using an MX3005P sequence detection system (Stratagene, United Kingdom) with primers SA-UK-IRES-248F and SA-UK-IRES-308R and a UK-IRES-271T TaqMan minor groove binding probe (Applied Biosystems, United Kingdom) as previously described (53). Fifty PCR cycles were carried out, and samples that did not have a detectable signal above the threshold after 50 cycles were taken to be negative (53). Samples with threshold cycle values greater or equal to 39 were designated “borderline” and were subsequently retested to confirm their positive or negative status (56).
Virus isolation and antigen detection ELISA.
The presence of virus in serum and in probang samples was determined by inoculation of monolayers of primary bovine thyroid cells (63) and examination for a cytopathic effect (CPE) 24, 48, and 72 h postinoculation. An enzyme-linked immunosorbent assay (ELISA) was used to confirm the presence of FMDV in cultures showing CPE (23). Replicates of bovine thyroid cell culture supernatants from samples showing no sign of CPE after 72 h postinoculation were pooled and repassaged once to confirm their positive or negative status.
Virus-neutralizing antibody test.
Serum samples were examined for anti-FMDV neutralizing antibodies as described in the Office International des Epizooties Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (48a). Sera with titers greater than or equal to 1/45 were considered to be positive.
Isotype-specific ELISA for detection of anti-FMDV antibodies.
An anti-FMDV sandwich ELISA was used to measure specific IgG1, IgG2, and IgM levels in serum samples (45). Ninety-six-well Maxisorb Nunc Immunoplates (Sigma, United Kingdom) were coated overnight with a solution of rabbit anti-FMDV serotype-specific hyperimmune antiserum (1:5,000) in 0.1 M carbonate-bicarbonate buffer. Coated plates were incubated with pretitrated inactivated O1 Manisa FMDV whole viral antigen in excess. Duplicate threefold dilution series of each serum sample were added at a starting dilution of 1/50. Antibody isotypes were detected using MAbs to bovine IgG1 (B37), IgG2 (B192), and IgM (B67) obtained from the Department of Veterinary Medicine, Bristol University, followed by horseradish peroxidase-conjugated rabbit anti-mouse IgG (DakoCytomation, United Kingdom), which was followed by the addition of O-phenylenediamine dihydrochloride (Sigma, United Kingdom). To avoid competition between IgM and IgG, all samples destined for anti-IgM analysis were first absorbed on plates coated with goat anti-bovine IgG (1 mg/ml; Southern Biotech, United Kingdom) and then transferred onto the viral-antigen-coated plates. Optical densities (ODs) were read at 492 nm on a MRX Dynex Technologies reader. Wells were considered positive only if they were greater than 1.5 times the mean background OD for that dilution. Antibody titers were expressed as the reciprocal of the last positive dilution.
3ABC nonstructural protein ELISA.
Serum samples were examined for the presence of antibodies directed against the nonstructural 3ABC protein of FMDV by using the commercially available Ceditest FMDV-NS (Cedi-Diagnostic). Samples were considered to be positive if the percentage of inhibition was ≥50 (64).
Indirect peptide ELISA.
Serum samples from animals in both experiments receiving anti-CD4 or TRT3 MAbs were examined for the presence of antibodies directed against the VP1135-156 G-H loop on the surface of FMDV capsids. A peptide encompassing amino acid residues 135 to 156 of FMDV O UKG/34/2001 (KYGESPVTNVRGDLQVLAQKAA) was kindly produced by L. Hunt, IAH. A second peptide, kindly provided by V. Fowler, IAH, encompassing the same residues of FMDV O1BFS (RYSRNAVPNLRGDLQVLAQKVA) was used for analysis to confirm that our results were consistent with previously published data (24). The indirect peptide ELISA was performed as previously described (24), with modifications. Ninety-six-well Maxisorb Nunc Immunoplates (Sigma, United Kingdom) were coated overnight at 4°C with 100 μl/well peptide in PBS, washed in PBS-Tween 20, and blocked with PBS containing sodium casein (Sigma, United Kingdom) at 1 mg/ml. This step and all subsequent incubation steps were carried out at 37°C for 1 h. Sera were added in duplicate at 50 μl per well starting at a 1/50 dilution with tripling dilutions in PBS-sodium casein, incubated, washed, and detected with horseradish peroxidase-conjugated goat anti-bovine IgG (Southern Biotech, United Kingdom). Plates were washed and visualized with O-phenylenediamine dihydrochloride (Sigma, United Kingdom). Reactions were stopped by the addition of 1.84 M sulfuric acid to the medium, and the absorbance was read at 490 nm on a MRX Dynex Technologies reader. Wells were considered to be positive only if they were greater than 1.5 times the mean background OD for that dilution. Antibody titers were expressed as the reciprocal of the last positive dilution.
Statistical analysis.
To investigate the effect of immune cell depletion on the titers of FMDV-specific antibody measured by the Ig isotype-specific ELISA, a Gompertz function was used to describe the antibody titer, Y, as a function of time, log10(Y) = κexp{−exp[−β(t − δ)]}, where κ is the upper asymptote (i.e., maximum titer), β is the rate of increase in titer, and δ is a delay parameter. The parameters κ, β, and δ were estimated using least-squares regression. Parallel curve analysis (57) of the data from individual animals was used to identify significant (P < 0.05) differences in the parameters among treatment groups (i.e., TRT3, anti-WC1, anti-CD4, and anti-CD8 groups), starting from a model in which all parameters differed among animals.
RESULTS
Efficiency of T-cell subset depletion.
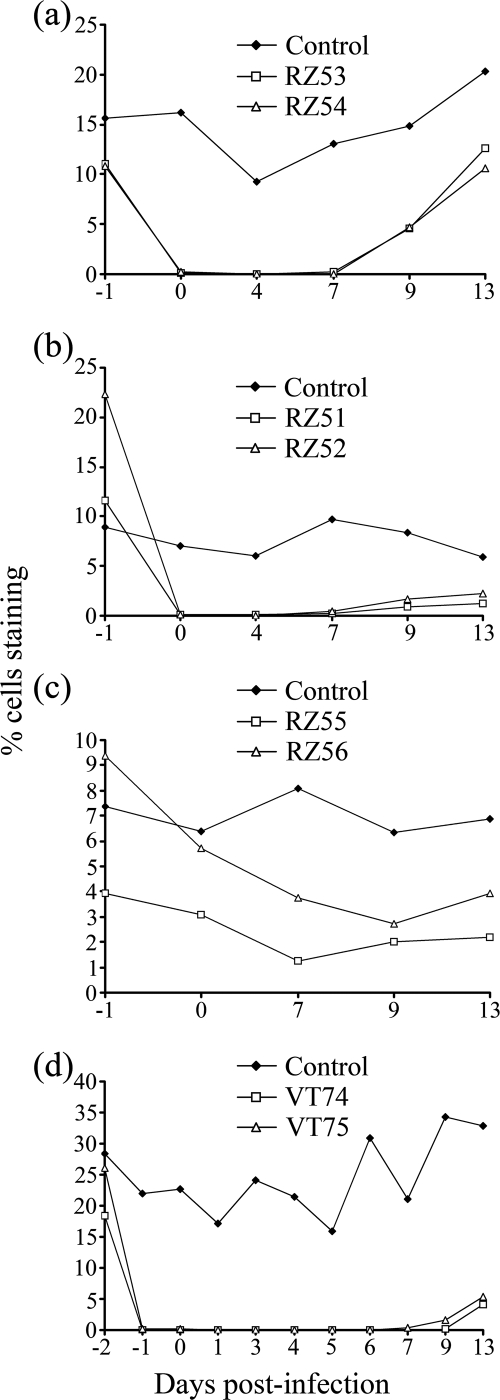
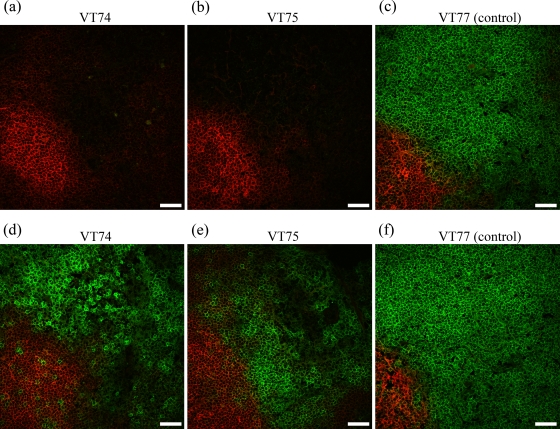
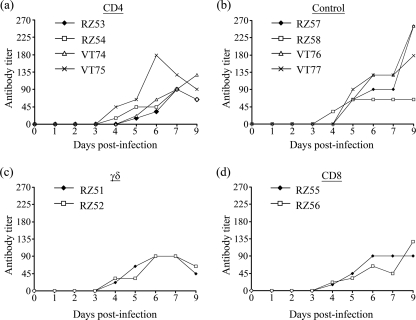
The administration of anti-CD4 MAb resulted in a rapid reduction in the percentage of circulating CD4+ cells within 24 h, from 11% and 10.8% to 0.15% and 0.17% for the two animals (animals RZ53 and RZ54, respectively) in experiment 1 and from 18.4% and 26% to 0.02% and 0.17% for the two animals (animals VT74 and VT75, respectively) in experiment 2. This depletion was confirmed by analysis of 100,000 viable cells in duplicate collected from experiment 1 animals on day 1 postinfection (0.04 and 0.05% CD4+ cells for animal RZ53 and 0.04 and 0.04% CD4+ cells for animal RZ54) and in triplicate for experiment 2 animals on days 0 and 4 postinfection (mean values [± standard deviations] of 0.05% [±0.02] CD4+ cells for animal VT74 and 0.04% [±0.01] CD4+ cells for animal VT75 on day 0 and 0.06% [±0.01] CD4+ cells for animal VT74 and 0.03% [±0.01] CD4+ cells for animal VT75 on day 4). Depletion was maintained for 7 days postinfection, with percentages of CD4+ cells consistently below or equal to background nonspecific binding detected with the isotype control MAbs, after which the numbers of CD4+ cells gradually increased (Fig. 1). A similar level and a similar duration of depletion were observed following treatment with anti-WC1 MAb: the numbers of circulating WC1+ cells in the two animals (animals RZ51 and RZ52) in experiment 1 decreased from 11.7% and 22.3% on day −1 to 0.06% and 0.06% on day 0, respectively, and maintained at these low levels until day 7 (Fig. 1). In contrast, treatment with anti-CD8+ MAb resulted in a more gradual and only partial depletion; the numbers of circulating CD8+ cells in the two treated animals (animals RZ55 and RZ56) decreased from 4.0% and 9.4% on day −1 to 3.1% and 5.7% on day 0 and to 1.3% and 4% on day 7, respectively (Fig. 1). There were no major changes in the proportions of the T-cell subsets (or CD21+ B cells) in animals receiving the control antibody, although some fluctuation in the percent representation of each subset was observed during the course of the studies (see Fig. S1 in the supplemental material). In addition, in the anti-CD4, anti-WC1, and anti-CD8 MAb-treated animals, there were no major changes in the proportion of the T-cell subsets not targeted for depletion or CD21+ B cells, consistent with the specificity of these MAbs (see Fig. S2 and S3 in the supplemental material) (31). Immunohistological examination of sections of prescapular lymph nodes surgically removed from experiment 2 animals on day 5 postinfection demonstrated the absence of CD4+ cells throughout the node, including the cortex and follicles (Fig. 2), paracortical area, and medullary cords and sinuses (data not shown) of both anti-CD4 MAb-treated animals. These analyses used both 10 separate sections and stacking of images from the confocal microscopy examinations to confirm that the CD4+ cell depletion was indeed found throughout the node. These findings were supported by flow cytometry analysis of lymph node cell suspensions in which the percentages of CD4+ T cells were comparable to that detected with the isotype control MAbs (data not shown). By 29 days postinfection, lymph node cell suspensions obtained following slaughter of these two animals contained 12.4% and 16.4% CD4+ cells compared to 38.7% and 32.4% CD4+ cells in lymph node samples from the two control animals.
FIG. 1.
Effect of MAb administration on the percentage of T-lymphocyte subpopulations in peripheral blood. (a to c) Experiment 1 animals and MAbs administered over 3 days starting the day before FMDV challenge. (a) Percentage of CD4+ cells in anti-CD4 MAb-treated animals (animals RZ53 and RZ54) and a control animal (animal RZ57). (b) Percentage of WC1+ cells in anti-WC1 MAb-treated animals (animals RZ51 and RZ52) and a control animal (animal RZ57). (c) Percentage of CD8+ cells in anti-CD8 MAb-treated animals (animals RZ55 and RZ56) and a control animal (animal RZ57). (d) Percentage of CD4+ cells of experiment 2, with anti-CD4 MAb-treated animals (animals VT74 and VT75) and a control animal (animal VT77). MAbs were administered over 4 days starting 2 days before FMDV challenge.
FIG. 2.
Effect of anti-CD4 MAb injection on the target cell population in lymphoid tissue. Shown are immunofluorescence confocal microscopy images of prescapular lymph node cortices from experiment 2 anti-CD4 MAb-injected animals (animals VT74 and VT75) and a TRT3 control MAb-injected animal (animal VT77) biopsied at 5 days postinfection. (a to c) CD4+ lymphocytes are green, and CD21+ cells are red. (d to f) CD3+ lymphocytes are green, and CD21+ cells are red. Scale bars, 40 μm.
Effect of lymphocyte depletion on development of clinical FMD.
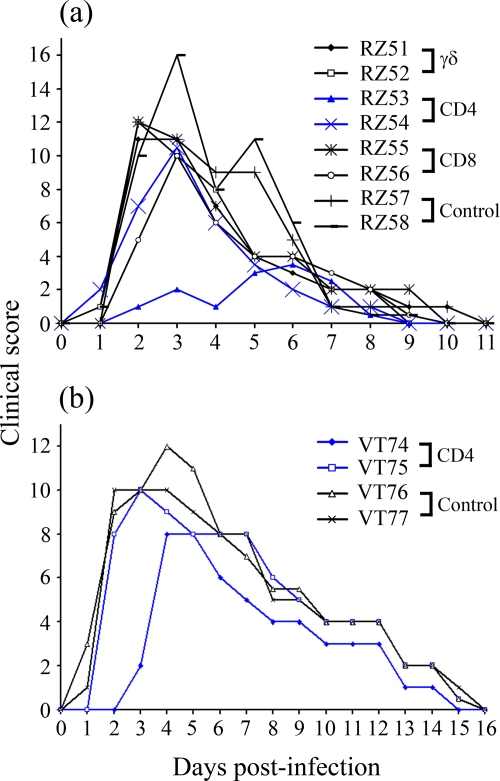
The clinical scores for all animals following FMDV infection, representing a measure of the induction, severity, and resolution of clinical signs, are displayed in Fig. 3. All cattle succumbed to disease within 1 to 3 days postchallenge. There was no adverse effect of the depletion of any of the T-cell subsets on the onset, magnitude, or resolution of clinical signs following infection.
FIG. 3.
Effect of lymphocyte depletion on development of clinical FMD. The clinical scores, consisting of rectal temperature and clinical signs of FMD (see Table S1 in the supplemental material), are displayed for experiment 1 animals (a) and experiment 2 animals (b). The data related to the anti-CD4 MAb-treated animals are highlighted in blue.
Effect of lymphocyte depletion on viral clearance.
All animals were confirmed to be viremic 24 h postinfection by virus isolation and rRT-PCR. The results of daily quantitative measurements of viral genome in serum using rRT-PCR are presented in Fig. 4. High levels of viral genome were detected in serum collected on days 1, 2, and 3 in all groups of animals, and levels subsequently declined in all groups. Viral genome was no longer detectable in all except two animals, one control and one CD8+ T-cell-depleted animal, by day 7 after infection. No serum samples were collected on day 8, but samples from the two remaining positive animals were negative for viral genome on day 9. There was no significant difference in the peak levels of viremia, as measured by rRT-PCR, between any of the different MAb-treated groups (P = 0.297 by analysis of variance with a general linear model). Live virus was isolated from serum samples of animals treated with anti-CD4 and anti-CD8 MAbs up to 4 days postinfection and from animals treated with anti-WC1 and control MAbs up to 3 days postinfection. No live virus or viral genome was detected in probang samples at postmortem examination by virus isolation and rRT-PCR (data not shown). FMDV capsid protein was detected by immunofluorescence confocal microscopy in germinal centers of mandibular lymph nodes harvested from all animals at postmortem examination (data not shown).
FIG. 4.
Effect of lymphocyte depletion on viremia. The viral genome was detected by rRT-PCR in serum samples collected from day 0 to 7 and day 9 postinfection. Genome copies per ml serum are displayed for anti-CD4 MAb treated-animals (a) and TRT3 control MAb-treated animals (b) from both experiments and for anti-WC1 MAb-treated (c) and anti-CD8 MAb-treated (d) animals from experiment 1.
Effect of lymphocyte depletion on virus-neutralizing antibody.
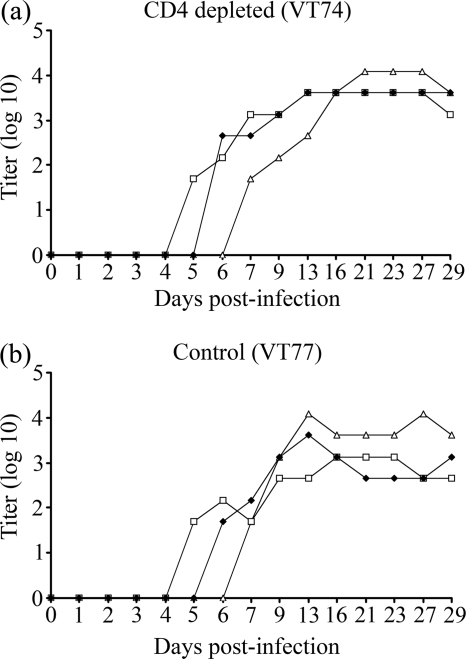
The results of virus-neutralizing antibody assays of serum samples are shown in Fig. 5. Titers of ≥45 (considered positive) were attained by 5 days postinfection in all four control animals, by 4 to 7 days in the animals treated with anti-CD4 MAb, and by 5 to 6 days in the animals treated with the anti-WC1 and anti-CD8 MAbs. There were no obvious differences in the onset of detectable neutralizing antibody in the calves receiving the different antibody treatments. In particular, the onset of detectable neutralizing antibody titers postinfection was not significantly different in the calves treated with anti-CD4 antibody and those treated with control antibody (P = 0.11 by Kruskal-Wallis test). The complete data set of virus-neutralizing antibody titers can be found in Table S2 in the supplemental material.
FIG. 5.
Effect of lymphocyte depletion on virus-neutralizing antibody. Virus-neutralizing antibody titers are displayed for anti-CD4 MAb-treated (a) and TRT3 control MAb-treated (b) animals from both experiments and for anti-WC1 MAb-treated (c) and anti-CD8 MAb-treated (d) animals from experiment 1. A titer of ≥45 is considered to be positive.
Effect of lymphocyte depletion on the isotype of FMDV-specific antibody responses.
Serum samples collected daily during the first 7 days of infection and at 2- to 5-day intervals up to day 29 (experiment 2) or day 30 (experiment 1) postinfection were analyzed using an ELISA with reagents specific for bovine IgM, IgG1, and IgG2 to determine the kinetics of the various isotypes generated by the FMDV-specific antibody response. Comparison of the kinetics of antibody titers over time by parallel curve analysis (see statistical analysis) did not reveal any statistically significant differences between the responses of animals in MAb-treated groups and those in the control MAb-treated groups (Fig. 6). IgG antibody isotypes were detected 5 to 7 days after infection, indicating rapid isotype switching in all animals. Indeed, in some cases, specific IgG2 antibodies were detected earlier than IgM antibodies. Antibody isotype class switching occurred during the phase of CD4+ T-cell depletion in animals that received anti-CD4 MAb.
FIG. 6.
Effect of lymphocyte depletion on the isotype of FMDV-specific antibody responses. Examples of the FMDV-specific antibody isotype profiles are displayed for an anti-CD4 MAb-treated animal (a) and for a TRT3 control MAb-treated animal (b) from experiment 2. ▵, IgG1; □, IgG2; ⧫, IgM. The CD4+ cell population was depleted from the lymphoid tissue of experiment 2 anti-CD4 MAb-treated animals on study day 5 postinfection, and circulating CD4+ lymphocytes were depleted from anti-CD4 MAb-treated animals in both experiments up to at least 7 days postinfection (9 days postinfection for animal VT74). Efficient antibody isotype class switching occurred during the period of CD4+ T-cell depletion.
Effect of lymphocyte depletion on the response to FMDV nonstructural proteins.
Serum samples collected at 3- to 6-day intervals, from day 0 to day 29 (experiment 2) or day 30 (experiment 1) postinfection, were analyzed for the presence of antibodies against the FMDV nonstructural protein 3ABC. The kinetics of the antibody response to 3ABC in animals receiving anti-CD8 or anti-WC1 MAb were similar to that of the control animals, with antibody initially being detected on days 6 to 16, and maximum titers were detected on day 29 or 30. In contrast, three out of the four anti-CD4 MAb-treated animals had no detectable antibodies against 3ABC throughout the 29 to 30 days, and the fourth animal (VT75) remained negative until day 29. Titers of anti-3ABC antibodies in serum samples obtained at the time of postmortem examination (day 29 or 30) are shown in Fig. 7. These results indicate that the depletion of CD4+ T cells during the phase of acute FMDV replication ablates the antibody response to nonstructural viral proteins.
FIG. 7.
Effect of lymphocyte depletion on the response to FMDV nonstructural protein 3ABC. By day 29 or 30 postinfection, three anti-CD4 MAb-treated animals had no detectable antibody response to FMDV nonstructural protein 3ABC. Samples were considered positive if the percentage of inhibition was ≥50 (64). Control, TRT3 MAb-treated animals from both experiments; CD4, anti-CD4 MAb-treated animals from both experiments; WC1, anti-WC1 MAb-treated experiment 1 animals; CD8, anti-CD8 MAb-treated experiment 1 animals.
Effect of lymphocyte depletion on the antibody response to G-H loop peptides.
Serum samples from animals receiving anti-CD4 MAbs and those receiving the control MAbs in both experiments were examined using an indirect ELISA for the presence of IgG antibodies to O UKG/34/2001 and O1BFS VP1135-156 peptide, which represent a superficial loop exposed on the surface of the viral capsid. No antibodies directed against the peptides were detected prechallenge. Titers of antibody specific for the O UKG/34/2001 peptide detected prior to the reappearance of circulating CD4+ T cells following depletion (day 7 postinfection for experiment 1 and day 9 postinfection for experiment 2) (Fig. 1) and following CD4+ T-cell repopulation (day 16) are displayed in Fig. 8. By the end of the period of CD4+ cell depletion, the four infected control animals all showed detectable antibody responses to the O UKG G-H loop peptide at day 7 (experiment 1) or day 9 (experiment 2). In contrast, antibody was undetectable in two of the CD4 T-cell-depleted animals and present at a very low titer in the other two depleted animals at these time points. By day 16, the titers of antibody in three of the depleted animals were still lower than those in the controls. These findings were corroborated by the data for the O1BFS peptide (data not shown), indicating that the antibody response to the G-H loop was inhibited by CD4+ T-cell depletion.
FIG. 8.
Effect of lymphocyte depletion on antibody response to FMDV O UKG/34/2001 G-H loop peptide. No antibodies directed against the peptide were detected prechallenge. (a) IgG antibody responses of experiment 1 CD4-depleted animals to FMDV O UKG/34/2001 VP1135-156 G-H loop peptide were completely absent or substantially less than those of the control animals by day 7 postinfection. By day 16, a stage when CD4 cells were repopulating (Fig. 1), the titers of antibody in the CD4-depleted animals were less than or equal to those of the controls. (b) The antibody response of experiment 2 CD4-depleted animals was similarly absent or substantially less than that of the control animals by day 9 postinfection. Although CD4 cells were repopulating by day 16 postinfection, the response of the experiment 2 CD4-depleted animals was still substantially less than that of the controls.
DISCUSSION
Our results confirm that the depletion of CD4+ lymphocytes from the blood circulation and superficial lymph nodes can be achieved in cattle by administering specific mouse MAbs. The application of different CD4 depletion protocols in calves during the early stages of infection with FMDV was found to result in a similar, substantial ablation of IgG antibody responses to nonstructural viral proteins but had little impact on the antibody responses to sites on the surface of the virus particles that induce neutralizing antibodies. The depletion of CD4 T cells also had no significant effect on the course of viremia or the clinical severity of disease associated with FMDV infection. Milder clinical scores were recorded for one of the CD4-depleted animals (animal RZ53); however, it is unlikely that this observation is significant considering the spectrum of clinical signs seen after FMDV challenge (3). There was no CD4 T-cell depletion in control animals following FMDV infection (see Fig. S1 in the supplemental material), which contrasts with the significant lymphopenia reported for swine following FMDV infection (6). We have also shown in separate studies that there is no generalized immunosuppression during the acute phase of FMDV infection in cattle or a loss of specific T-cell populations (data not shown). Capsid-induced T-cell recall responses are detectable after FMDV infection (12, 28). However, we have also shown that an FMDV-specific CD4 T-cell response is not detectable for at least 12 days after FMDV infection (data not shown), making the analysis of FMDV-specific T-cell responses during the period of depletion of little value in the present study. The animals in the high-dose antibody treatment group were immunized with commercial bovine herpesvirus vaccine (Tracherine; Schering-Plough, United Kingdom) approximately 1 month prior to the start of these studies. Unsurprisingly, the specific proliferative response to bovine herpesvirus antigen was not detectable in animals during the treatment with anti-CD4 MAbs.
Although the administration of anti-WC1 antibody was also found to result in a profound depletion of circulating WC1+ γδ T cells, such a depletion did not have any measurable effects on the course of infection with FMDV or specific antibody responses to the virus. The role of these cells in protection against infectious agents in ruminants is unclear. They are found extensively in the epithelium (34) and have been proposed to play a role in intracellular infection, promoting a Th1-biased immune response (52) and non-MHC-restricted NK-like cytotoxicity (11, 20). Previous reports of WC1+ T-cell depletion studies with cattle have shown an enhanced antibody response to nonreplicating antigen and an enhanced peripheral blood mononuclear cell-proliferative response to nonspecific mitogens in animals depleted of this population (34). These results were supported further by the detection of enhanced local and systemic antibody responses following respiratory syncytial virus infection in WC1+-depleted calves (68). By inspection, one cannot rule out a minor influence of WC1+ cell depletion on the duration of viremia as measured by rRT-PCR (Fig. 4), although it was not possible to assess the significance of this observation due to the small group size. Overall, our findings suggest that WC1+ γδ T cells do not play a major role in the resolution of clinical signs and control of viremia after acute FMDV infection in cattle.
The application of the same protocol to deplete CD8+ T cells was less successful, resulting in only a partial depletion of the circulating population, which had no discernible effect on the response to FMDV. This result is consistent with previous evidence that the MAb-mediated depletion of bovine CD8+ T cells is more difficult to achieve than for other T-cell subsets (46, 49, 68, 69). Therefore, it was not possible to conclusively evaluate the influence of CD8+ T cells on the course of infection with FMDV or early responses to the virus. However, a partial depletion of CD8+ T cells did not affect the resolution of acute FMDV infection.
Antiviral antibody responses may be classified as T dependent (T-D) or T independent (T-I) based on the requirement for T-cell help for antibody production. T-I type I antigens are mitogenic agents that activate Toll-like receptors to elicit polyclonal B-cell activation. Type II T-I antigens are complex structures, typically rigid two-dimensional arrays comprising epitopes displayed at 5- to 10-nm intervals, that engage and cross-link the immunoglobulin receptors on the surface of B cells, generating strong activation signals. These stimulatory activities result in antibody production in the absence of specific T-cell help but may depend upon accessory signals from antigen-presenting cells or T cells for B-cell activation (5, 30, 43, 44). Some viral capsids fall into this category. However, nonoligomerized viral proteins released from dying cells or disrupted virus particles generally act as T-D antigens.
The T dependency of antibody responses of cattle to a number of defined antigens and viral pathogens has been confirmed in several previous studies (33, 46, 68). CD4+ lymphocyte depletion with MAb doses as low as 0.3 mg/kg has been shown to result in a significant reduction in the antibody response of calves to human red blood cells and ovalbumin (34, 46). The same dose of MAb administered to calves subsequently infected with respiratory syncytial virus resulted in a marked suppressive effect on the antibody response and increased viral pathology (46, 68). Similar results have been reported after infection with noncytopathic bovine viral diarrhea virus, where incomplete circulating CD4+ lymphocyte depletion resulted in a delayed antibody response and longer duration and higher titer of circulating virus (33). Furthermore, the depletion of CD4+ lymphocytes in cattle previously vaccinated with commercial FMDV vaccine has been shown to ablate T-cell-proliferative responses to FMDV antigen, indicating a depletion of memory T cells (46). While Naessens et al. (46) previously depleted blood and splenic CD4+ cells with 0.2 mg/kg MAb, they needed 2 mg/kg to deplete CD4+ cells from peripheral lymph nodes. It is therefore likely that our own work with 2.58 mg/kg was effective at depleting the cells from peripheral lymph nodes, which was confirmed in our analyses of prescapular lymph nodes. Moreover, the present work clearly demonstrated that such a depletion had a strong influence on the anti-FMDV immune response, but this was prejudiced dependent on the antigenic determinants against which the humoral response was mounted. A particularly significant feature of the present study was the finding that CD4+ T-cell depletion resulted in an ablation of antibody responses to nonstructural proteins in three of the four animals examined while leaving intact the antibody responses to sites on the surface of the viral capsid. This is consistent with the notion that antibody responses to these antigenic components are T-D and T-I, respectively. The development of a delayed antibody response to the nonstructural proteins in one of the CD4-depleted calves may be the result of low-level replicating virus still being present in this animal when CD4+ T-cell function was restored.
Our findings are consistent with previously published results using the FMDV murine experimental model, where the protective immune response was shown to be T-I (8, 40). Early T-I protection and the production of antibody have been described for a number of other cytopathic viruses including vesicular stomatitis virus and influenza virus infection in mice (22, 38). A number of other picornaviruses have also been shown to act as T-I antigens (4). The T-I nature of these viral antigens is thought to be a result of their rigid, highly repetitive, and highly organized structure (5). Also, the magnitude of the T-I immune response and augmentation of isotype switching has been shown to correlate with the degree of antigen organization and the dose of antigen reaching the secondary lymphoid organs (4, 41, 47, 72). One of the key protective mechanisms to prevent the dissemination in the host of acute cytopathic viruses is the rapid induction of neutralizing antibodies (5). It has also been proposed that the surface antigenic structure of acute cytopathic viruses has evolved to stimulate early T-I antibody responses in order to limit the extent of viral infection and avoid rapid death of the host. Conversely, B-cell responses may have evolved to deal with such threats. The dynamics of infection with FMDV in cattle is consistent with the model described above, with infection being rapidly controlled and animals usually showing clinical signs for only a few days. Clearly, T-D antibody responses are also stimulated by these acute cytopathic viruses and are likely to be responsible for the production of affinity-maturated IgG isotype antibodies and long-term memory (30).
Although FMDV shares structural features with other picornaviruses, there is one unique feature that distinguishes aphthoviruses including FMDV from other picornaviruses: the absence of a canyon or pit which places the integrin cell attachment site in the protruding, fully exposed, highly disordered, and mobile immunogenic G-H loop (1). Previously reported studies with virus-specific MAbs, coupled with structural analyses of FMDV particles, have identified five antigenic sites on the FMDV capsid, including the G-H loop, which are involved in virus neutralization (18). The G-H loop is considered highly immunogenic, and immunization of cattle with synthetic peptides representing the loop has been shown to induce neutralizing antibody and, in some cases, protection against viral challenge (66). However, recent data describing VP1 G-H loop-substituted chimeric vaccines indicates that the G-H loop may not be required for producing a strong neutralizing antibody response or a protective immune response following vaccination of cattle (24). In the present study, although CD4+ T-cell depletion had no discernible effect on the overall neutralizing antibody response, it substantially inhibited the IgG antibody response against the G-H loop peptide. The neutralizing antibody in these animals was presumably directed against the other sites on the viral capsid. Our data suggest that the high degree of mobility of the G-H loop may result in it being less effective as a T-I type II antigen than the other antigenic sites, which have a more stable conformational structure. Antibodies directed against the G-H loop were detected in all CD4+ T-cell-depleted cattle after the phase of depletion albeit at lower levels than in infected control animals. Although circulating virus was no longer detectable at this time, the detection of FMDV capsid antigen in mandibular lymph node germinal centers at postmortem examination indicates that there remained a source of antigen for induction of G-H loop-specific antibody when CD4+ T-cell function was restored.
The induction of IgG after FMDV immunization has been shown to be T-D in a murine experimental model (16). These results have been confirmed in vitro in a mouse model in which FMDV-infected dendritic cells could directly stimulate B lymphocytes to secrete FMDV-specific IgM, but T-cell help was required to induce class switching toward IgG (50). Comparison of the kinetics of the FMDV-specific antibody response of experiment 1 and 2 animals over time did not reveal any statistically significant differences between the depleted groups and the control MAb-treated groups. Specific IgM was detected from 4 days postinfection, and specific IgG1 and IgG2 were detected from 5 days postinfection, consistent with reports by other investigators (14, 21, 58). Our results show that in vivo, in a natural ruminant host, FMDV infection can induce not only a specific and rapid IgM response but also efficient and rapid isotype class switching in the absence of CD4+ T cells. The ability of T-I viral antigens to induce efficient class switching in the absence of T-cell help is thought to be related to the repetitiveness of the viral antigens (5) and the formation of antigen-specific germinal centers by a T-I process in the absence of T-cell-derived CD40 ligand (26). T-I B-cell proliferation and isotype class switching in mice following exposure to type II T-I antigens have been shown to be dependent on an intact follicular dendritic cell network and signaling through CD40 on the surface of B cells and follicular dendritic cells. The signaling through CD40 is dependent on complement, specifically through C4b binding protein in the absence of the T-cell-derived CD40 ligand CD154 (9, 26, 48, 60, 65). We have shown previously with cattle that FMDV localizes to germinal centers as early as 3 to 4 days postchallenge (35), a process that may provide the signals required for T-I isotype class switching and an early FMDV-specific IgG response (26, 47). The tumor necrosis factor family ligands BAFF and APRIL have also been shown to contribute to CD154-independent antibody isotype switching, germinal center maintenance, and T-I antibody responses (59). In addition to these potential mechanisms in the CD4+ T-cell-depleted cattle exposed to FMDV, gamma interferon produced by γδ T cells (41), NK cells (36, 65), or activated B cells (51, 71) may also provide alternative but less efficient support for CD154/CD4+ T-cell-independent isotype switching by acting directly on B cells potentially in the absence of specific germinal center formation (62).
In conclusion, the results of this study indicate that functional CD4+ T cells are not required, either to provide help for antibody production or as antiviral effector cells, for the effective control of primary infection with FMDV in cattle. Isotype switching of the antibody response was also found to be independent of CD4+ T cells. The current studies do not identify whether CD4+ T cells play a role in the development or duration of a memory response or contribute to the efficacy of immunity to subsequent viral challenge. Further studies are required to address these questions, possibly using similar depletion protocols in vaccinated animals.
A number of molecular approaches to FMD vaccine development have been used since the mid-1970s, including the use of viral subunit proteins, protein fragments, and peptides, isolated from viral particles or produced in bacteria, baculovirus, and transgenic plants or as synthetic peptides (10, 29, 66). A general problem with most subunit vaccines is that they do not elicit a protective immune response comparable with that induced by live virus or killed whole-virus vaccines (66). Peptide vaccines based on the G-H loop (70) do not appear to fully mimic the conformation of the native B-cell epitopes and stimulate antibody of rather narrow specificity, which appears to be T-D (19, 25, 42). In contrast, studies of responses to traditional FMDV vaccines, which utilize intact inactivated virus, have shown that they stimulate rapid antibody responses that can provide protection against disease within 4 to 5 days. The results of the current study, together with other findings, indicate that the preservation of the complex three-dimensional structure of the FMDV capsid is critical for inducing rapid and effective antibody responses. This is consistent with current thinking on the development of safer and more effective vaccines based on the use of empty viral capsids produced using recombinant DNA constructs.
Supplementary Material
Acknowledgments
We thank B. Bankowski, P. Barnett, S. Cox, D. Gibson, and J. F. Valarcher for helping to devise the clinical scoring system. We also thank C. Randall, L. Fitzpatrick, and M. Jenkins for their care of the experimental animals.
The work was funded by the Biotechnology and Biological Sciences Research Council, United Kingdom. B.C. and P.C.L.B. are Jenner investigators.
Footnotes
Published ahead of print on 28 January 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Acharya, R., E. Fry, D. Stuart, G. Fox, D. Rowlands, and F. Brown. 1989. The three-dimensional structure of foot-and-mouth disease virus at 2.9 Å resolution. Nature 337709-716. [DOI] [PubMed] [Google Scholar]
- 2.Alexandersen, S., Z. Zhang, and A. I. Donaldson. 2002. Aspects of the persistence of foot-and-mouth disease virus in animals—the carrier problem. Microbes Infect. 41099-1110. [DOI] [PubMed] [Google Scholar]
- 3.Alexandersen, S., Z. Zhang, A. I. Donaldson, and A. J. M. Garland. 2003. The pathogenesis and diagnosis of foot-and-mouth disease. J. Comp. Pathol. 1291-36. [DOI] [PubMed] [Google Scholar]
- 4.Bachmann, M. F., and R. M. Zinkernagel. 1996. The influence of virus structure on antibody responses and virus serotype formation. Immunol. Today 17553-558. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann, M. F., and R. M. Zinkernagel. 1997. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 15235-270. [DOI] [PubMed] [Google Scholar]
- 6.Bautista, E. M., G. S. Ferman, and W. T. Golde. 2003. Induction of lymphopenia and inhibition of T cell function during acute infection of swine with foot and mouth disease virus (FMDV). Vet. Immunol. Immunopathol. 9261-73. [DOI] [PubMed] [Google Scholar]
- 7.Blanco, E., M. Garcia-Briones, A. Sanz-Parra, P. Gomes, E. De Oliveira, M. L. Valero, D. Andreu, V. Ley, and F. Sobrino. 2001. Identification of T-cell epitopes in nonstructural proteins of foot-and-mouth disease virus. J. Virol. 753164-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borca, M. V., F. M. Fernandez, A. M. Sadir, M. Braun, and A. A. Schudel. 1986. Immune response to foot-and-mouth disease virus in a murine experimental model: effective thymus-independent primary and secondary reaction. Immunology 59261-267. [PMC free article] [PubMed] [Google Scholar]
- 9.Brodeur, S. R., F. Angelini, L. B. Bacharier, A. M. Blom, E. Mizoguchi, H. Fujiwara, A. Plebani, L. D. Notarangelo, B. Dahlback, E. Tsitsikov, and R. S. Geha. 2003. C4b-binding protein (C4BP) activates B cells through the CD40 receptor. Immunity 18837-848. [DOI] [PubMed] [Google Scholar]
- 10.Brown, F. 1999. Foot-and-mouth disease and beyond: vaccine design, past, present and future. Arch. Virol. Suppl. 15179-188. [DOI] [PubMed] [Google Scholar]
- 11.Brown, W. C., W. C. Davis, S. H. Choi, D. A. E. Dobbelaere, and G. A. Splitter. 1994. Functional and phenotypic characterization of WC1+ γ/δ T cells isolated from Babesia bovis-stimulated T cell lines. Cell. Immunol. 1539-27. [DOI] [PubMed] [Google Scholar]
- 12.Childerstone, A. J., L. Cedillo-Baron, M. Foster-Cuevas, and R. M. Parkhouse. 1999. Demonstration of bovine CD8+ T-cell responses to foot-and-mouth disease virus. J. Gen. Virol. 80663-669. [DOI] [PubMed] [Google Scholar]
- 13.Clevers, H., N. D. MacHugh, A. Bensaid, S. Dunlap, C. L. Baldwin, A. Kaushal, K. Iams, C. J. Howard, and W. I. Morrison. 1990. Identification of a bovine surface antigen uniquely expressed on CD4− CD8− T cell receptor γ/δ+ T lymphocytes. Eur. J. Immunol. 20809-817. [DOI] [PubMed] [Google Scholar]
- 14.Collen, T. 1994. Foot and mouth disease (aphthovirus): viral T cell epitopes, p. 173-197. In B. M. L. Goddeeris and W. I. Morrison (ed.), Cell-mediated immunity in ruminants. CRC Press, Boca Raton, FL.
- 15.Collen, T., and T. R. Doel. 1990. Heterotypic recognition of foot-and-mouth disease virus by cattle lymphocytes. J. Gen. Virol. 71309-315. [DOI] [PubMed] [Google Scholar]
- 16.Collen, T., L. Pullen, and T. R. Doel. 1989. T cell-dependent induction of antibody against foot-and-mouth disease virus in a mouse model. J. Gen. Virol. 70395-403. [DOI] [PubMed] [Google Scholar]
- 17.Cook, J. K. A., B. V. Jones, M. M. Ellis, L. Jing, and D. Cavanagh. 1993. Antigenic differentiation of strains of turkey rhinotracheitis virus using monoclonal antibodies. Avian Pathol. 22257-273. [DOI] [PubMed] [Google Scholar]
- 18.Crowther, J. R., S. Farias, W. C. Carpenter, and A. R. Samuel. 1993. Identification of a fifth neutralizable site on type O foot-and-mouth disease virus following characterization of single and quintuple monoclonal antibody escape mutants. J. Gen. Virol. 741547-1553. [DOI] [PubMed] [Google Scholar]
- 19.Cubillos, C., B. G. de la Torre, A. Jakab, G. Clementi, E. Borras, J. Barcena, D. Andreu, F. Sobrino, and E. Blanco. 2008. Enhanced mucosal immunoglobulin A response and solid protection against foot-and-mouth disease virus challenge induced by a novel dendrimeric peptide. J. Virol. 827223-7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daubenberger, C. A., E. L. N. Taracha, L. Gaidulis, W. C. Davis, and D. J. McKeever. 1999. Bovine γδ T-cell responses to the intracellular protozoan parasite Theileria parva. Infect. Immun. 672241-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doel, T. R. 2005. Foot-and-mouth disease virus. Curr. Top. Microbiol. Immunol. 288103-132. [DOI] [PubMed] [Google Scholar]
- 22.Fehr, T., M. F. Bachmann, H. Bluethmann, H. Kikutani, H. Hengartner, and R. M. Zinkernagel. 1996. T-independent activation of B cells by vesicular stomatitis virus: no evidence for the need of a second signal. Cell. Immunol. 168184-192. [DOI] [PubMed] [Google Scholar]
- 23.Ferris, N. P., and M. Dawson. 1988. Routine application of enzyme-linked immunosorbent assay in comparison with complement fixation for the diagnosis of foot-and-mouth and swine vesicular diseases. Vet. Microbiol. 16201-209. [DOI] [PubMed] [Google Scholar]
- 24.Fowler, V. L., D. J. Paton, E. Reider, and P. V. Barnett. 2008. Chimeric foot-and-mouth disease viruses: evaluation of their efficacy as potential marker vaccines in cattle. Vaccine 261982-1989. [DOI] [PubMed] [Google Scholar]
- 25.Francis, M. J., G. Z. Hastings, A. D. Syred, B. McGinn, F. Brown, and D. J. Rowlands. 1987. Non-responsiveness to a foot-and-mouth disease virus peptide overcome by addition of foreign helper T-cell determinants. Nature 330168-170. [DOI] [PubMed] [Google Scholar]
- 26.Gaspal, F. M. C., F. M. McConnell, M.-Y. Kim, D. Gray, M. H. Kosco-Vilbois, C. R. Raykundalia, M. Botto, and P. J. L. Lane. 2006. The generation of thymus-independent germinal centers depends on CD40 but not on CD154, the T cell-derived CD40-ligand. Eur. J. Immunol. 361665-1673. [DOI] [PubMed] [Google Scholar]
- 27.Gerner, W., B. V. Carr, K. H. Wiesmüller, E. Pfaff, A. Saalmüller, and B. Charleston. 2007. Identification of a novel foot-and-mouth disease virus specific T-cell epitope with immunodominant characteristics in cattle with MHC serotype A31. Vet. Res. 38565-572. [DOI] [PubMed] [Google Scholar]
- 28.Guzman, E., G. Taylor, B. Charleston, M. A. Skinner, and S. A. Ellis. 2008. An MHC-restricted CD8+ T-cell response is induced in cattle by foot-and-mouth disease virus (FMDV) infection and also following vaccination with inactivated FMDV. J. Gen. Virol. 89667-675. [DOI] [PubMed] [Google Scholar]
- 29.Grubman, M. J., and P. W. Mason. 2002. Prospects, including time-frames, for improved foot and mouth disease vaccines. Rev. Sci. Tech. 21589-600. [DOI] [PubMed] [Google Scholar]
- 30.Hangartner, L., R. M. Zinkernagel, and H. Hengartner. 2006. Antiviral antibody responses: the two extremes of a wide spectrum. Nat. Rev. Immunol. 6231-243. [DOI] [PubMed] [Google Scholar]
- 31.Howard, C. J., and W. I. Morrison. 1991. Leukocyte antigens of cattle, sheep and goats. Vet. Immunol. Immunopathol. 271-94. [PubMed] [Google Scholar]
- 32.Howard, C. J., K. R. Parsons, B. V. Jones, P. Sopp, and D. H. Pocock. 1988. Two monoclonal antibodies (CC17, CC29) recognizing an antigen (Bo5) on bovine T lymphocytes, analogous to human CD5. Vet. Immunol. Immunopathol. 19127-139. [DOI] [PubMed] [Google Scholar]
- 33.Howard, C. J., M. C. Clarke, P. Sopp, and J. Brownlie. 1992. Immunity to bovine virus diarrhoea virus in calves: the role of different T-cell subpopulations analysed by specific depletion in vivo with monoclonal antibodies. Vet. Immunol. Immunopathol. 32303-314. [DOI] [PubMed] [Google Scholar]
- 34.Howard, C. J., P. Sopp, K. R. Parsons, and J. Finch. 1989. In vivo depletion of BoT4 (CD4) and of non-T4/T8 lymphocyte subsets in cattle with monoclonal antibodies. Eur. J. Immunol. 19757-764. [DOI] [PubMed] [Google Scholar]
- 35.Juleff, N., M. Windsor, E. Reid, J. Seago, Z. Zhang, P. Monaghan, I. W. Morrison, and B. Charleston. 2008. Foot-and-mouth disease virus persists in the light zone of germinal centres. PLoS ONE 3e3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh, C. Y., and D. Yuan. 1997. The effect of NK cell activation by tumor cells on antigen-specific antibody responses. J. Immunol. 1594745-4752. [PubMed] [Google Scholar]
- 37.Kwong, L. S., J. C. Hope, M. L. Thom, P. Sopp, S. Duggan, G. P. Bembridge, and C. J. Howard. 2002. Development of an ELISA for bovine IL-10. Vet. Immunol. Immunopathol. 85213-223. [DOI] [PubMed] [Google Scholar]
- 38.Lee, B. O., J. Rangel-Moreno, J. E. Moyron-Quiroz, L. Hartson, M. Makris, F. Sprague, F. E. Lund, and T. D. Randall. 2005. CD4 T cell-independent antibody response promotes resolution of primary influenza infection and helps to prevent reinfection. J. Immunol. 1755827-5838. [DOI] [PubMed] [Google Scholar]
- 39.Lefevre, E. A., W. R. Hein, Z. Stamataki, L. S. Brackenbury, E. A. Supple, L. G. Hunt, P. Monaghan, G. Borhis, Y. Richard, and B. Charleston. 2007. Fibrinogen is localized on dark zone follicular dendritic cells in vivo and enhances the proliferation and survival of a centroblastic cell line in vitro. J. Leukoc. Biol. 82666-677. [DOI] [PubMed] [Google Scholar]
- 40.Lopez, O. J., A. M. Sadir, M. V. Borca, F. M. Fernandez, M. Braun, and A. A. Schudel. 1990. Immune response to foot-and-mouth disease virus in an experimental murine model. II. Basis of persistent antibody reaction. Vet. Immunol. Immunopathol. 24313-321. [DOI] [PubMed] [Google Scholar]
- 41.Maloy, K. J., B. Odermatt, H. Hengartner, and R. M. Zinkernagel. 1998. Interferon γ-producing γδ T cell-dependent antibody isotype switching in the absence of germinal center formation during virus infection. Proc. Natl. Acad. Sci. USA 951160-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCullough, K. C., F. De Simone, E. Brocchi, L. Capucci, J. R. Crowther, and U. Kihm. 1992. Protective immune response against foot-and-mouth disease. J. Virol. 661835-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mond, J. J., A. Lees, and C. M. Snapper. 1995. T cell-independent antigens type 2. Annu. Rev. Immunol. 13655-692. [DOI] [PubMed] [Google Scholar]
- 44.Morrissey, P. J., H. S. Boswell, I. Scher, and A. Singer. 1981. Role of accessory cells in B cell activation. IV. Ia+ accessory cells are required for the in vitro generation of thymic independent type 2 antibody responses to polysaccharide antigens. J. Immunol. 1271345-1347. [PubMed] [Google Scholar]
- 45.Mulcahy, G., C. Gale, P. Robertson, S. Iyisan, R. D. DiMarchi, and T. R. Doel. 1990. Isotype responses of infected, virus-vaccinated and peptide-vaccinated cattle to foot-and mouth disease virus. Vaccine 8249-256. [DOI] [PubMed] [Google Scholar]
- 46.Naessens, J., J.-P. Scheerlinck, E. V. De Buysscher, D. Kennedy, and M. Sileghem. 1998. Effective in vivo depletion of T cell subpopulations and loss of memory cells in cattle using mouse monoclonal antibodies. Vet. Immunol. Immunopathol. 64219-234. [DOI] [PubMed] [Google Scholar]
- 47.Ochsenbein, A. F., D. D. Pinschewer, B. Odermatt, A. Ciurea, H. Hengartner, and R. M. Zinkernagel. 2000. Correlation of T cell independence of antibody responses with antigen dose reaching secondary lymphoid organs: implications for splenectomized patients and vaccine design. J. Immunol. 1646296-6302. [DOI] [PubMed] [Google Scholar]
- 48.Ochsenbein, A. F., D. D. Pinschewer, B. Odermatt, M. C. Carroll, H. Hengartner, and R. M. Zinkernagel. 1999. Protective T cell-independent antiviral antibody responses are dependent on complement. J. Exp. Med. 1901165-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48a.Office International des Epizooties. 2004. Manual of diagnostic tests and vaccines for terrestrial animals, 5th ed. Office International des Epizooties, Paris, France.
- 49.Oldham, G., J. C. Bridger, C. J. Howard, and K. R. Parsons. 1993. In vivo role of lymphocyte subpopulations in the control of virus excretion and mucosal antibody responses of cattle infected with rotavirus. J. Virol. 675012-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ostrowski, M., M. Vermeulen, O. Zabal, P. I. Zamorano, A. M. Sadir, J. R. Geffner, and O. J. Lopez. 2007. The early protective thymus-independent antibody response to foot-and-mouth disease virus is mediated by splenic CD9+ B lymphocytes. J. Virol. 819357-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pang, Y., Y. Norihisa, D. Benjamin, R. R. Kantor, and H. A. Young. 1992. Interferon-gamma gene expression in human B-cell lines: induction by interleukin-2, protein kinase C activators, and possible effect of hypomethylation on gene regulation. Blood 80724-732. [PubMed] [Google Scholar]
- 52.Pollock, J. M., and M. D. Walsh. 2002. The WC1+ γδ T-cell population in cattle: a possible role in resistance to intracellular infection. Vet. Immunol. Immunopathol. 89105-114. [DOI] [PubMed] [Google Scholar]
- 53.Quan, M., C. M. Murphy, Z. Zhang, and S. Alexandersen. 2004. Determinants of early foot-and-mouth disease virus dynamics in pigs. J. Comp. Pathol. 131294-307. [DOI] [PubMed] [Google Scholar]
- 54.Reid, S. M., N. P. Ferris, G. H. Hutchings, Z. Zhang, G. J. Belsham, and S. Alexandersen. 2002. Detection of all seven serotypes of foot-and-mouth disease virus by real-time, fluorogenic reverse transcription polymerase chain reaction assay. J. Virol. Methods 10567-80. [DOI] [PubMed] [Google Scholar]
- 55.Reid, S. M., N. P. Ferris, G. H. Hutchings, Z. Zhang, G. J. Belsham, and S. Alexandersen. 2001. Diagnosis of foot-and-mouth disease by real-time fluorogenic PCR assay. Vet. Rec. 149621-623. [DOI] [PubMed] [Google Scholar]
- 56.Reid, S. M., S. S. Grierson, N. P. Ferris, G. H. Hutchings, and S. Alexandersen. 2003. Evaluation of automated RT-PCR to accelerate the laboratory diagnosis of foot-and-mouth disease virus. J. Virol. Methods 107129-139. [DOI] [PubMed] [Google Scholar]
- 57.Ross, G. J. S. 1990. Nonlinear estimation. Springer series in statistics. Springer-Verlag, Heidelberg, Germany.
- 58.Salt, J. S., G. Mulcahy, and R. P. Kitching. 1996. Isotype-specific antibody responses to foot-and-mouth disease virus in sera and secretion of ‘carrier’ and ‘non-carrier’ cattle. Epidemiol. Infect. 117349-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider, P. 2005. The role of APRIL and BAFF in lymphocyte activation. Curr. Opin. Immunol. 17282-289. [DOI] [PubMed] [Google Scholar]
- 60.Schriever, F., A. S. Freedman, G. Freeman, E. Messner, G. Lee, J. Daley, and L. M. Nadler. 1989. Isolated human follicular dendritic cells display a unique antigenic phenotype. J. Exp. Med. 1692043-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seipelt, J., A. Guarné, E. Bergmann, M. James, W. Sommergruber, I. Fita, and T. Skern. 1999. The structures of picornaviral proteinases. Virus Res. 62159-168. [DOI] [PubMed] [Google Scholar]
- 62.Snapper, C. M., T. M. McIntyre, R. Mandler, L. M. T. Pecanha, F. D. Finkelman, A. Lees, and J. J. Mond. 1992. Induction of IgG3 secretion by interferon γ: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J. Exp. Med. 1751367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snowdon, W. A. 1966. Growth of foot-and-mouth disease virus in monolayer cultures of calf thyroid cells. Nature 2101079-1080. [DOI] [PubMed] [Google Scholar]
- 64.Sorensen, K. J., K. G. Madsen, E. S. Madsen, J. S. Salt, J. Nqindi, and D. K. Mackay. 1998. Differentiation of infection from vaccination in foot-and-mouth disease by the detection of antibodies to the non-structural proteins 3D, 3AB and 3ABC in ELISA using antigens expressed in baculovirus. Arch. Virol. 1431461-1476. [DOI] [PubMed] [Google Scholar]
- 65.Szomolanyi-Tsuda, E., J. D. Brien, J. E. Dorgan, R. L. Garcea, R. T. Woodland, and R. M. Welsh. 2001. Antiviral T-cell-independent type 2 antibody responses induced in vivo in the absence of T and NK cells. Virology 280160-168. [DOI] [PubMed] [Google Scholar]
- 66.Taboga, O., C. Tami, E. Carrillo, J. I. Nunez, A. Rodriguez, J. C. Saiz, E. Blanco, M. L. Valero, X. Roig, J. A. Camarero, D. Andreu, M. G. Mateu, E. Giralt, E. Domingo, F. Sobrino, and E. L. Palma. 1997. A large-scale evaluation of peptide vaccines against foot-and-mouth disease: lack of solid protection in cattle and isolation of escape mutants. J. Virol. 712606-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takamatsu, H. H., M. S. Denyar, C. Stirling, S. Cox, N. Aggarwal, P. Dash, T. E. Wileman, and P. V. Barnett. 2006. Porcine γδ T cells: possible roles on the innate and adaptive immune responses following virus infection. Vet. Immunol. Immunopathol. 11249-61. [DOI] [PubMed] [Google Scholar]
- 68.Taylor, G., L. H. Thomas, S. G. Wyld, J. Furze, P. Sopp, and C. J. Howard. 1995. Role of T-lymphocyte subsets in recovery from respiratory syncytial virus infection in calves. J. Virol. 696658-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Villarreal-Ramos, B., M. McAulay, V. Chance, M. Martin, J. Morgan, and C. J. Howard. 2003. Investigation of the role of CD8+ T cells in bovine tuberculosis in vivo. Infect. Immun. 714297-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang, C. Y., T. Y. Chang, A. M. Walfield, J. Ye, M. Shen, S. P. Chen, M. C. Li, Y. L. Lin, M. H. Jong, P. C. Yang, N. Chyr, E. Kramer, and F. Brown. 2002. Effective synthetic peptide vaccine for foot-and-mouth disease in swine. Vaccine 202603-2610. [DOI] [PubMed] [Google Scholar]
- 71.Yoshimoto, T., H. Okamura, Y.-I. Tagawa, Y. Iwakura, and K. Nakanishi. 1997. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proc. Natl. Acad. Sci. USA 943948-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zinkernagel, R. M. 2000. Localization dose and time of antigens determine immune reactivity. Semin. Immunol. 12163-171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.