Abstract
Characterization of the immune responses induced in the initial stages of human immunodeficiency virus type 1 (HIV-1) infection is of critical importance for an understanding of early viral pathogenesis and prophylactic vaccine design. Here, we used sequential plasma samples collected during the eclipse and exponential viral expansion phases from subjects acquiring HIV-1 (or, for comparison, hepatitis B virus [HBV]or hepatitis C virus [HCV]) to determine the nature and kinetics of the earliest systemic elevations in cytokine and chemokine levels in each infection. Plasma viremia was quantitated over time, and levels of 30 cytokines and chemokines were measured using Luminex-based multiplex assays and enzyme-linked immunosorbent assays. The increase in plasma viremia in acute HIV-1 infection was found to be associated with elevations in plasma levels of multiple cytokines and chemokines, including rapid and transient elevations in alpha interferon (IFN-α) and interleukin-15 (IL-15) levels; a large increase in inducible protein 10 (IP-10) levels; rapid and more-sustained increases in tumor necrosis factor alpha and monocyte chemotactic protein 1 levels; more slowly initiated elevations in levels of additional proinflammatory factors including IL-6, IL-8, IL-18, and IFN-γ; and a late-peaking increase in levels of the immunoregulatory cytokine IL-10. Notably, there was comparatively little perturbation in plasma cytokine levels during the same phase of HBV infection and a delayed response of more intermediate magnitude in acute HCV infection, indicating that the rapid activation of a striking systemic cytokine cascade is not a prerequisite for viral clearance (which occurs in a majority of HBV-infected individuals). The intense early cytokine storm in acute HIV-1 infection may have immunopathological consequences, promoting immune activation, viral replication, and CD4+ T-cell loss.
Recent studies have highlighted the impact of events in acute human immunodeficiency virus type 1 (HIV-1) infection on subsequent disease pathogenesis (10). Following HIV transmission, there is an eclipse phase of approximately 10 days (18) during which virus is initially amplified at the transmission site and in local lymphoid tissues, and then systemic dissemination begins (23). Exponential virus expansion is associated with a massive depletion of memory CD4+ T cells, particularly from gut-associated lymphoid tissues (30, 32). Plasma viral titers subsequently decline, but immune activation and viral replication continue and are associated with a further T-cell loss (24, 33), eventually leading to the development of AIDS.
Appreciation of the role of acute-phase events in HIV pathogenesis emphasizes the need to understand the immune responses activated in the earliest stages of infection and the roles that they may be playing in the containment of viral replication or in fueling immune activation and viral spread (6). A previously reported study of simian immunodeficiency virus (SIV)-infected macaques suggested that the earliest immune response in the genital tract was dominated by the induction of proinflammatory cytokines, which may have helped to promote viral spread, with the induction of antiviral cytokines such as alpha interferon (IFN-α) and IFN-β occurring too late to prevent virus replication and dissemination (1). Further support for an immunopathological role for components of the immune response activated in the early stages of infection is provided by comparisons of pathogenic versus nonpathogenic SIV infections of nonhuman primates, where an immunosuppressive cytokine profile associated with low levels of immune activation has been identified as being a key correlate of good disease prognosis (27). This suggests that prophylactic HIV vaccines must induce immune responses that block transmission or blunt viral replication so that immunopathological cytokine production is reduced or down-modulate the cytokine response stimulated in the context of early viral replication.
To inform vaccine design and identify the window of time that a vaccine has to induce salutary secondary responses after transmission, it is critical to define the nature and timing of the early cytokine response in HIV-infected individuals. Several groups have attempted to characterize the cytokine response induced in acute HIV infection by analysis of plasma cytokine levels or cytokine mRNA expression levels in peripheral blood leukocytes. These studies have yielded somewhat conflicting results, with some reporting the upregulation of cytokines and chemokines including IFN-α (17, 47), tumor necrosis factor alpha (TNF-α) (3, 20, 36, 39, 47), IFN-γ (5, 20, 36, 42), interleukin-1β (IL-1β) (42), IL-10 (20, 36), inducible protein 10 (IP-10) (40), and regulated on activation, normal T-expressed and -secreted (RANTES) (31) and no change or a decrease in levels of cytokines including IL-2, IL-4, and IL-6 (5, 20, 42), while others have not observed these changes or have reported opposing findings, e.g., reduced TNF-α levels (5). The discrepancies may be due in part to differences in the assays used to measure cytokine responses in different studies but likely also reflect the dynamic nature of acute HIV infection and the variation between studies in the timing of sample collection. Many studies were cross-sectional, while others focused on time points relatively late in acute infection.
In the current study, we constructed a comprehensive picture of the dynamics of systemic innate and initial adaptive immune activation in the earliest stages of HIV-1 infection by performing a kinetic analysis of 30 plasma cytokines and chemokines during the eclipse and viral expansion phases using sequential samples, typically collected 2 to 5 days apart, from plasma donors acquiring HIV-1 infection. For comparison, we studied similar plasma panels from donors who acquired either hepatitis B virus (HBV) or hepatitis C virus (HCV) infection. We found that in contrast to HBV and HCV, acute-phase HIV replication is associated with the activation of a dramatic cytokine cascade, with plasma levels of some of the most rapidly induced innate cytokines peaking 7 days after the first detection of plasma viremia and multiple other cytokines being upregulated as viral titers increase to their peak.
MATERIALS AND METHODS
Plasma panels and viral load analysis.
Sequential samples were obtained by plasmapheresis from 35 plasma donors with acute HIV infection, 10 with acute HBV infection, and 10 with acute HCV infection (Zeptometrix Corporation and SeraCare Life Sciences) and were cryopreserved. These panels were collected prior to the advent of FDA-approved viral load assays, so samples from each donor were screened by serological assays. Donors who tested positive in these assays were informed of their test results and counseled to follow up with local health care providers, and no further plasma units were collected. The sample time courses for each donor thus spanned time points from prior to plasma virus detection through seroconversion. HIV viral loads were retrospectively analyzed by Quest Diagnostics by using a Roche Amplicor HIV Ultra assay (sensitivity, 100 copies/ml). HBV viral loads were analyzed by Zeptometrix Corporation using a Roche Cobas AmpliPrep TaqMan 48 (CAP-G CTM) HBV assay (sensitivity, 70 copies/ml) or a Roche Monitor assay (sensitivity, 200 copies/ml). HCV viral loads were analyzed using a Roche Amplicor-HCV Monitor assay (sensitivity, 600 copies/ml).
Luminex cytokine assays.
Thirteen cytokines/chemokines were measured using a high-sensitivity human cytokine Lincoplex kit (Millipore): IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12(p70), IL-13, IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), and TNF-α. Thirteen additional factors were measured by a Bio-Plex cytokine assay (Bio-Rad): IL-1Rα, IL-9, IL-17, fibroblast growth factor (FGF) basic, eotaxin, granulocyte colony-stimulating factor (G-CSF), RANTES, IP-10, macrophage inflammatory protein 1α (MIP-1α), MIP-1β, monocyte chemotactic protein 1 (MCP-1), platelet-derived growth factor BB (PDGF-BB), and vascular endothelial growth factor (VEGF). Each sample was assayed in duplicate, and cytokine standards supplied by the manufacturer were run on each plate. Data were acquired using a Luminex-100 system and analyzed using Bio-Plex Manager software, v4.1 (Bio-Rad). Multiplex test kit results were validated using high-sensitivity enzyme-linked immunosorbent assay (ELISA) kits where the latter were available. In all cases tested, comparable elevations were observed using the Luminex-based multiplex assays and individual ELISA kits (data not shown).
ELISA.
Plasma levels of additional cytokines were determined by ELISA. IL-15 was quantitated using a high-sensitivity chemiluminescent assay (R&D Systems), while IFN-α, IL-22 (both R&D Systems), IFN-β, and IL-18 (both Invitrogen) were quantitated using colorimetric ELISAs. Undiluted plasma samples were run in duplicate, and concentrations were calculated from the kit's standard curve.
Type I IFN bioassay.
Type 1 IFN bioactivity was quantitated using a bioluminescence kit (nEUtekBio Ltd.). Briefly, plasma was incubated overnight with cells that are sensitive to human type I IFN, followed by lysis and the addition of substrate. The resulting bioluminescence is proportional to the amount of type I IFN activity in the plasma and was measured on a luminometer (Wallac). Samples were run in duplicate and compared to an IFN standard curve.
Statistical analysis. (i) Estimation of time origin and viral expansion rate.
The plasma donor panels were aligned to a time origin (T0), defined as the time point at which the viral load first reached 100 copies/ml, 200 copies/ml, or 600 copies/ml for the panels from HIV-, HBV-, and HCV-infected individuals, respectively. Linear mixed-effects models were used to estimate T0 and the viral expansion rate for each subject, accounting for data censoring due to the assays' limits of detection (44, 45).
(ii) Background estimation and definition of positive responses.
Transformation and statistical analysis of the data indicated that there were no significant elevations in plasma levels of any of the factors measured in the HIV- or HBV-infected subjects at time points prior to T0, although some fluctuation in TNF-α levels was observed between day −20 and T0 in the HCV-infected subjects. Data from all time points prior to T0 were used to calculate baseline levels of analytes in the HIV- and HBV-infected subjects, while only data from time points prior to day −20 were used for the HCV-infected subjects. Linear mixed-effects models were fit to these data to estimate the subject-specific baseline levels of each analyte in each subject (45). Post-T0 responses above the 95% upper prediction bound were termed positive (46).
(iii) Kaplan-Meier plots and mean curves.
Kaplan-Meier analysis was used to estimate the timing of initial elevation of each analyte, which was subject to censoring (12). Nonparametric regression was used to obtain the spline estimate of the mean curve for each analyte over time (21). Subject-specific curve estimates were predicted by functional mixed-effects models (22). Change curves were calculated to display the mean curve based on the change relative to the baseline level of each analyte. Proportional scaled change curves were also prepared to show the change curve scaled from 0 to 1, which was weighted by the fraction of positive responders for each analyte. All analyses were limited to 20 days post-T0 given the small number of plasma panels extending beyond day 20. Note that results obtained for the HBV- and HCV-infected cohorts have less precision due to the smaller sample sizes.
(iv) Binomial tests.
To compare the relative timings of initial elevation between pairs of analytes, a two-sided binomial test was conducted using a positive difference in timing as a success and the number of nonzero differences as the number of trials. The false-discovery-rate multiple-adjustment procedure was used to compute adjusted P values (43).
RESULTS
Exponential viral load expansion in plasma donors with acute HIV, HBV, or HCV infection.
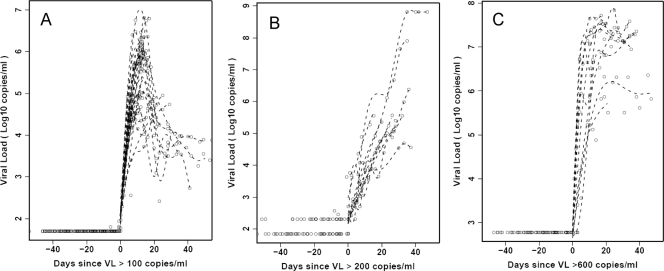
Sequential samples collected from plasma donors acquiring HIV, HBV, or HCV infections present a rare opportunity to analyze kinetic changes in systemic cytokine levels during the earliest stages of these infections. Plasma panels from 35 donors who acquired HIV, 10 donors who acquired HCV, and 10 donors who acquired HBV infections were studied; each donor was infected with just one of these viruses. All donors were initially seronegative for HIV, HBV, and HCV, with plasma viral nucleic acid levels below the limit of detection of commercial assays. Each one exhibited a dramatic elevation in plasma viral load during the time frame studied, which is indicative of acute HIV, HBV, or HCV infection (Fig. 1). Viral load data were aligned relative to a common T0, which is defined as the time point when viremia first reached detectable levels (100 RNA copies/ml for HIV, 200 DNA copies/ml for HBV, and 600 RNA copies/ml for HCV). The kinetics of HIV replication were the most rapid (mean upswing slope, 0.49), followed closely by HCV (mean slope, 0.36), while HBV amplification was considerably slower (mean slope, 0.13). The median times from T0 until the highest recorded viral load were 12 days for the HIV-infected donors, 17.5 days for the HCV-infected donors, and 30 days for the HBV-infected donors (most of whom did not reach a peak or plateau during the time frame studied). In HIV-infected subjects, plasma viral titers typically reached a peak and then declined sharply, whereas in HCV-infected subjects, plasma virus titers were frequently sustained at high levels after reaching their maximum.
FIG. 1.
Plasma viral titers in subjects acutely infected with HIV-1, HCV, and HBV. Longitudinal viral nucleic acid titers in plasma samples from donors acquiring HIV-1 infection (A), HBV infection (B), and HCV infection (C) are shown. The sample time courses are aligned relative to a T0, which is defined as the time point when the plasma viral load (VL) first reached detectable levels (100 RNA copies/ml for HIV, 200 DNA copies/ml for HBV, and 600 RNA copies/ml for HCV). The smooth lines are spline estimates.
Elevations in plasma levels of multiple cytokines and chemokines in acute HIV-1 infection.
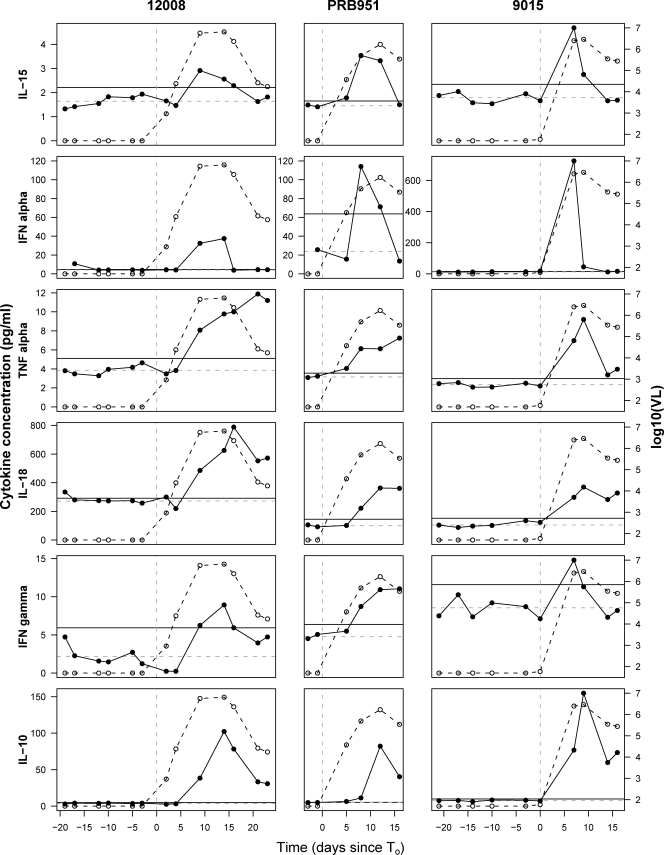
Levels of 30 cytokines and chemokines were quantitated in the plasma panels from the HIV-, HBV-, and HCV-infected donors. Baseline levels of each analyte were calculated for each subject, and analyte levels at post-T0 time points were deemed elevated if they were above the upper 95% prediction bound determined from the baseline data. Of the 30 factors measured, 15 became elevated as viremia increased in more than 50% of the HIV-infected subjects (Table 1). Figure 2 shows examples of the dynamic changes occurring in six analytes in three representative HIV-infected individuals. Different cytokines were elevated in plasma with distinct kinetics. IL-15 and IFN-α were among the first cytokines with elevated levels, but their increases were ephemeral, typically observed at only one or two time points. The activity of the IFN-α was confirmed using a bioassay; 26 of 27 panels with elevated IFN-α levels showed biological type I IFN activity at the same time point(s) (data not shown). No elevation in IFN-β was observed in any panel (data not shown). TNF-α was also among the first cytokines with levels that were found to be elevated in plasma but, unlike IL-15 and IFN-α, was sustained at elevated levels for longer, with its highest values typically not being recorded until around the time of peak viremia. The first elevations in IL-18, IFN-γ, and IL-10 typically occurred slightly later than for IL-15, IFN-α, and TNF-α. Here, too, differences were apparent in cytokine kinetics, with IL-18 levels frequently paralleling those of TNF-α, while IL-10 typically reached its highest recorded levels with relatively delayed kinetics. More rigorous analyses of the timing, frequency, magnitude, and duration of analyte elevations were performed as detailed below.
TABLE 1.
Percentages of HIV-, HBV-, and HCV-infected subjects who exhibited elevations in levels of each analyte and median day of initial analyte elevation in these subjects
| Analytea | HIV infection
|
HBV infection
|
HCV infection
|
|||
|---|---|---|---|---|---|---|
| % of subjects with elevation | Median day of initial elevation | % of subjects with elevation | Median day of initial elevation | % of subjects with elevation | Median day of initial elevation | |
| IP-10 | 100 | 6 | 30 | 7 | 70 | 10 |
| IL-15 | 91.4 | 6 | 50 | 12 | 40 | 22.5 |
| TNF-α | 80 | 6 | 60 | 10.5 | 80 | 11 |
| IFN-α | 77.1 | 6 | 40 | 10 | 50 | 4 |
| MCP-1 | 77.1 | 6 | 50 | 14 | 40 | 21.5 |
| IL-1Rα | 71.4 | 6 | 10 | 12 | 30 | 10 |
| MIP-1α | 22.9 | 6 | 10 | 7 | 20 | 25 |
| IL-10 | 91.4 | 7 | 60 | 10.5 | 70 | 12 |
| IL-8 | 71.4 | 7 | 60 | 17.5 | 50 | 20 |
| IL-17 | 17.1 | 7 | 10 | 1 | 30 | 6 |
| IL-6 | 60 | 8 | 50 | 8 | 60 | 21.5 |
| IFN-γ | 57.1 | 8 | 30 | 14 | 60 | 10 |
| Eotaxin | 54.3 | 8 | 20 | 10 | 10 | 23 |
| IL-18 | 74.3 | 8.5 | 50 | 8 | 80 | 19.5 |
| MIP-1β | 31.4 | 9 | 20 | 14.5 | 70 | 13 |
| VEGF | 31.4 | 9 | 20 | 13 | 60 | 11.5 |
| IL-4 | 40 | 9.5 | 30 | 21 | 40 | 11 |
| FGF basic | 22.9 | 9.5 | 20 | 8.5 | 30 | 34 |
| G-CSF | 31.4 | 10 | 10 | 22 | 10 | 27 |
| IL-22 | 36.4 | 11 | 20 | 21 | 66.7 | 27.5 |
| GM-CSF | 40 | 11.5 | 30 | 5 | 30 | 12 |
| IL-7 | 48.6 | 12 | 20 | 6 | 40 | 17.5 |
| IL-9 | 31.4 | 12 | 20 | 17.5 | 30 | 9 |
| IL-12(p70) | 51.4 | 12.5 | 40 | 9.5 | 50 | 12 |
| IL-2 | 51.4 | 12.5 | 40 | 13.5 | 50 | 13 |
| IL-5 | 45.7 | 12.5 | 30 | 5 | 40 | 12.5 |
| IL-13 | 28.6 | 12.5 | 40 | 12.5 | 40 | 14.5 |
| IL-1β | 51.4 | 13 | 40 | 2.5 | 40 | 17.5 |
| PDGF-BB | 37.1 | 14 | 30 | 12 | 40 | 19 |
| RANTES | 20 | 16 | 20 | 29 | 60 | 8 |
FIG. 2.
Kinetics of changes in plasma cytokine levels in three subjects acutely infected with HIV. Plasma levels of six cytokines (IL-15, IFN-α, TNF-α, IL-18, IFN-γ, and IL-10) in samples collected at sequential time points from three representative HIV-infected plasma donors (donors 12008, PRB951, and 9015) are shown (black circles and solid lines), together with the plasma HIV titers, expressed as log10 viral RNA copies/ml (white circles and dotted lines), for each of these individuals. For each subject, time is plotted relative to T0, the time point when plasma viremia first reached 100 RNA copies/ml. The vertical dashed line in each panel indicates T0. The horizontal dashed lines indicate the subject-specific baseline levels of each analyte, and the horizontal black lines indicate the 95% upper prediction bound, above which post-T0 responses were termed positive.
Successive waves of elevations in plasma cytokine and chemokine levels in acute HIV-1 infection.
First, we assessed the timing relative to T0 of the first elevation in plasma levels of different cytokines and chemokines in acute HIV infection in those subjects exhibiting a detectable elevation in the analyte (Table 1). The earliest median time of first elevation in plasma cytokine/chemokine levels was 6 days after the detection of viremia. The analytes elevated in this first wave included IFN-α, IL-15, TNF-α, IP-10, and MCP-1. A second group of analytes, including IL-6, IL-8, IL-10, IL-18, and IFN-γ, had levels that were first elevated at a median of 7 to 8.5 days post-T0. Other analytes became elevated later still; e.g., the median first elevations in IL-4, IL-5, IL-12(p70), and IL-22 occurred 9.5 to 12.5 days post-T0. The use of binomial tests to compare the timing of initial elevations of analytes indicated that all pairwise comparisons between analytes elevated in the first wave and those in the last group were significant after multiplicity adjustment (P values of <0.0001 to 0.03).
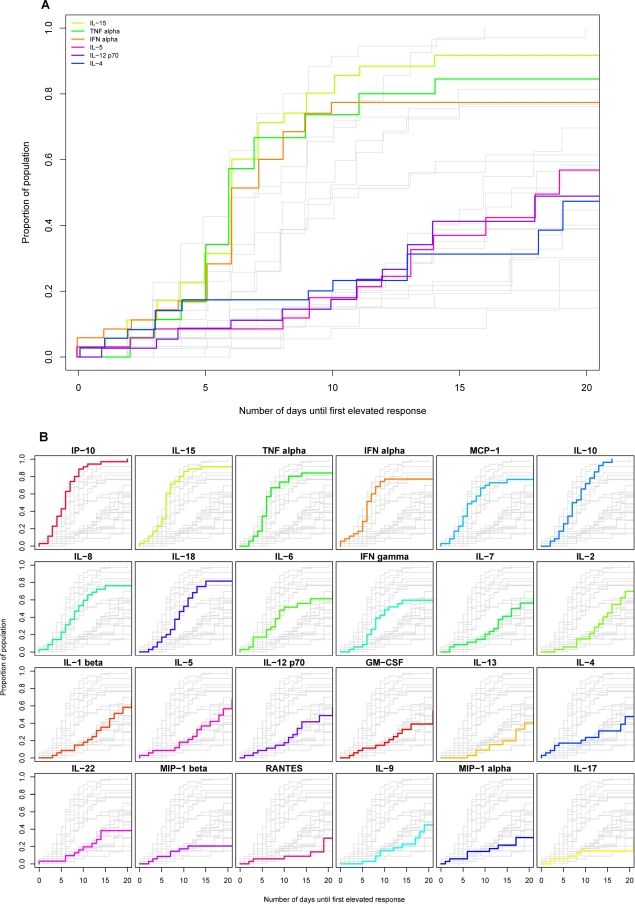
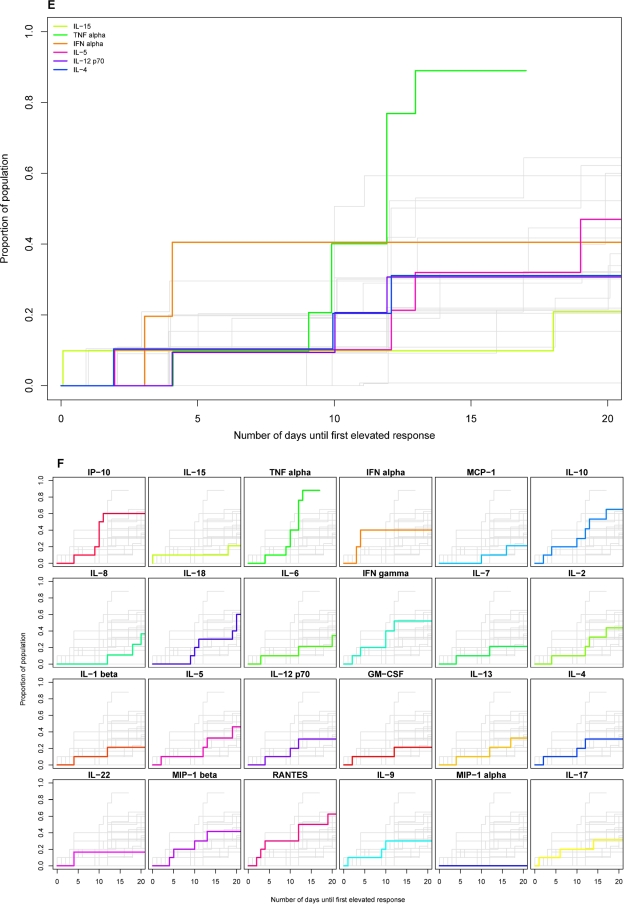
Kaplan-Meier analysis was used to estimate the time of initial elevation of each analyte in the entire group of subjects (Fig. 3A and B). Those analytes elevated earliest were typically elevated in a high proportion of subjects; e.g., levels of IFN-α, IL-15, TNF-α, IP-10, and MCP-1 were all elevated in >75% of subjects. Conversely, the majority of analytes upregulated later were upregulated in a lower proportion of subjects. Analytes elevated with delayed kinetics and only in approximately 50% or fewer of the HIV-infected subjects included both the Th1-biasing cytokine IL-12 and the Th2 cytokines IL-4, IL-5, and IL-13. Fisher's exact test showed high degrees of association between elevations in IL-4 and IL-5 (P = 8.997 × 10−5) and IL-4 and IL-12(p70) (P = 1.513 × 10−6), suggesting that these analytes were all reaching detectable levels in a common subset of subjects mounting a more rapid or exaggerated cytokine response (rather than different individuals making qualitatively different responses).
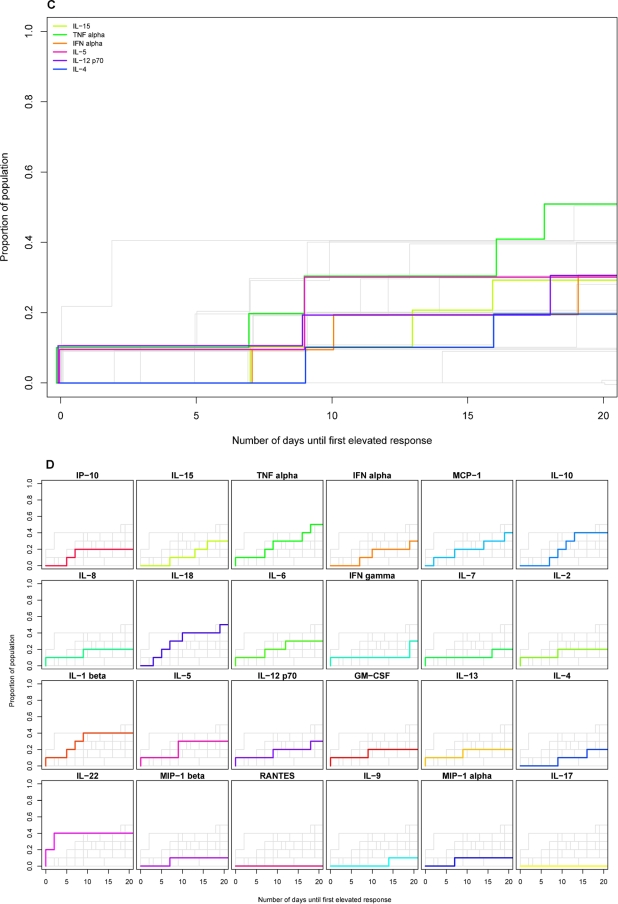
FIG. 3.
Timing of initial elevation in plasma levels of different cytokines and chemokines in groups of subjects acutely infected with HIV, HBV, or HCV. Kaplan-Meier estimation was used to represent the distribution of time (relative to T0) of the initial elevation in levels of different analytes in groups of subjects infected with HIV (A and B), HBV (C and D), and HCV (E and F). Each panel shows a composite of the results for 24 different analytes plotted as the cumulative proportion of the study population who had exhibited an elevation in plasma analyte levels over time (days from T0). In A, C, and E, results for six cytokines are highlighted in color [lime green, IL-15; emerald green, TNF-α; orange, IFN-α; pink, IL-5; purple, IL-12(p70); dark blue, IL-4] in a single panel, while results for other analytes are in gray. In B, D, and F, there are 24 small panels, each of which shows the results for a single analyte highlighted in color, while results for other analytes are in gray. The panels in B, D, and F are ordered (left to right across four rows) according to the timing of initial analyte elevation in the HIV-infected subject group, with the analyte most rapidly elevated in 50% of subjects shown first.
Relative magnitude and duration of elevations in cytokine and chemokine levels in acute HIV infection.
The median highest recorded level of each cytokine/chemokine in the HIV-infected subjects exhibiting elevations in the analyte was calculated (Table 2). As samples were obtained a median of 4 days apart, peak analyte levels may have been higher, particularly for those analytes with rapid kinetics, such as IFN-α. These data, together with the results shown in Fig. 2, illustrate that levels of some analytes (e.g., IFN-α and IP-10) increased markedly in acute HIV infection, while increases in levels of other analytes (e.g., IL-15 and TNF-α) were typically more modest, often only two- to threefold. Comparison of the median time of initial elevation (Table 1) and the median time of maximal elevation (Table 2) illustrates that whereas some analytes, e.g., IFN-α, increased to their highest levels very rapidly (within 1 day), others took much longer to do so; e.g., the time from median initial to median peak elevation was 5.5 days for TNF-α and 5 days for IL-10.
TABLE 2.
Median baseline and highest recorded level of each analyte and median day at which the highest analyte level was recorded in HIV-infected subjects with detectable elevations in levels of analyte
| Analyte | Lower limit of assay detection (pg/ml)a | Median baseline level (pg/ml) | Median highest recorded level (pg/ml) | Median day of highest recorded level |
|---|---|---|---|---|
| IP-10 | 6.5 | 296.9 | 2204.9 | 9 |
| IL-15 | 0.19 | 1.9 | 3.6 | 6 |
| TNF-α | 0.13 | 3.4 | 7.7 | 11.5 |
| IFN-α | 12.5 | 4.6 | 37.5 | 7 |
| MCP-1 | 6.7 | 59.5 | 135.3 | 7 |
| IL-1Rα | 1.4 | 140.4 | 310.5 | 9 |
| MIP-1α | 2.4 | 15.2 | 18.8 | 10.5 |
| IL-10 | 0.13 | 10.3 | 54.4 | 12 |
| IL-8 | 0.13 | 4.4 | 11.4 | 10 |
| IL-17 | 0.2 | 3.9 | 16.6 | 8.5 |
| IL-6 | 0.13 | 4.7 | 10 | 12 |
| IFN-γ | 0.13 | 6 | 17.4 | 11 |
| Eotaxin | 14.60 | 97.1 | 149.6 | 9 |
| IL-18 | 0.026 | 196.1 | 439.3 | 11.5 |
| MIP-1b | 1.1 | 66.5 | 92.2 | 9 |
| VEGF | 0.5 | 11.7 | 27.4 | 16 |
| IL-4 | 0.13 | 10.1 | 22.6 | 15.5 |
| FGF basic | 6.8 | 72.8 | 108.7 | 10.5 |
| G-CSF | 1.1 | 59.6 | 71.2 | 13 |
| IL-22 | 15.6 | 14.1 | 29 | 12 |
| GM-CSF | 0.13 | 2.3 | 4.8 | 18 |
| IL-7 | 0.13 | 2.3 | 4.6 | 13 |
| IL-9 | 0.7 | 84.3 | 129.4 | 14 |
| IL-12(p70) | 0.13 | 2.3 | 5.3 | 13.5 |
| IL-2 | 0.13 | 2.2 | 9.2 | 16 |
| IL-5 | 0.13 | 0.7 | 1.4 | 16 |
| IL-13 | 0.13 | 2.9 | 14.9 | 18 |
| IL-1β | 0.13 | 0.6 | 2.3 | 16 |
| PDGF-BB | 1.00 | 636.6 | 1,395.2 | 14 |
| RANTES | 1.2 | 5,966.3 | 9,849.4 | 16 |
Lower limit of analyte detection in Luminex assays as determined by the assay manufacturer or bottom point on the standard curve for analytes measured by ELISA.
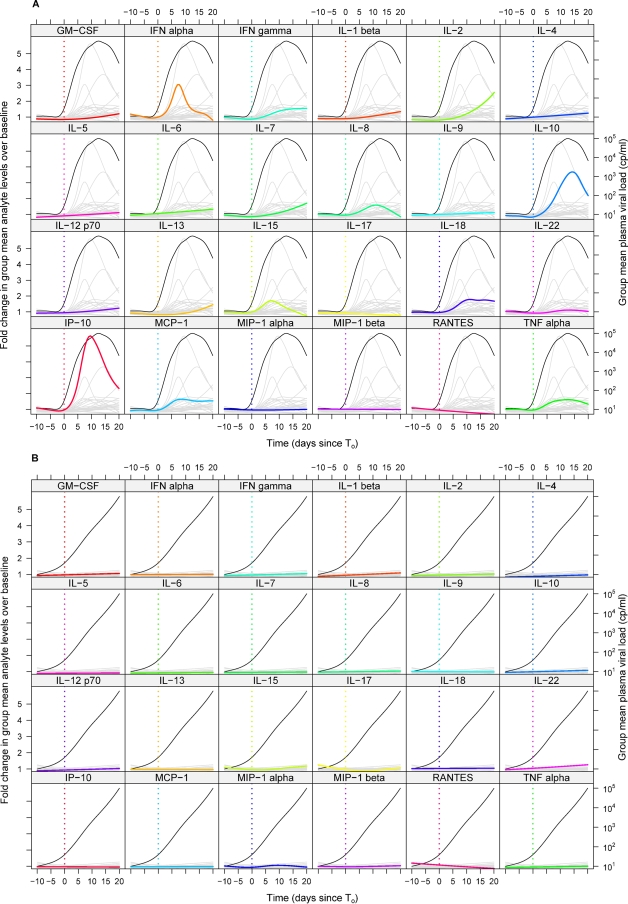
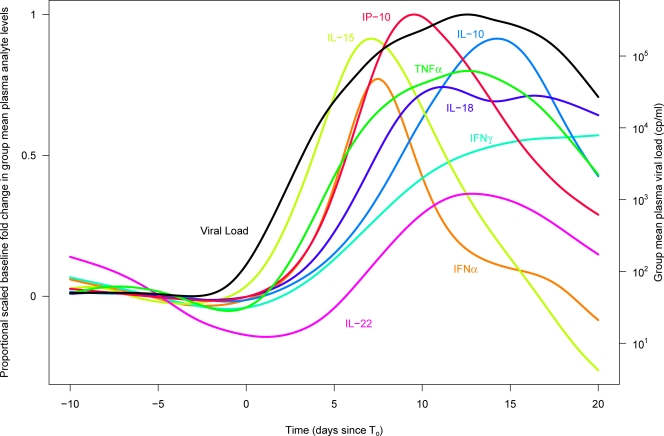
Figure 4A illustrates the average change (relative to baseline) in each analyte over time in the entire group of HIV-infected subjects. Whereas the data in Fig. 2 and Tables 1 and 2 show subject-specific changes in plasma analyte levels, Fig. 4 depicts the group-specific changes in analyte levels, illustrating the average dynamics with which plasma levels of each analyte increased and decreased during acute HIV infection. The peak group mean changes in analyte levels in Fig. 4A seem low given the median highest recorded analyte elevations shown in Table 2, but this is because the latter displays cross-sectional data from only those subjects with detectable elevations in each analyte. In addition, for a few analytes, most notably IFN-γ, there was considerable variation among subjects in the timing of initial cytokine elevation, resulting in the group mean IFN-γ level undergoing an initially rapid increase followed by a gradual rise over the time frame studied rather than reaching a peak and decreasing again, as seen in individual subjects (Fig. 2). To summarize the kinetics and prevalence of elevations of cytokine and chemokine levels in acute HIV infection in a single graph, the proportional increase in plasma levels of a subset of analytes was also plotted together (Fig. 5). In Fig. 5, the peak heights for different analytes are scaled according to the percentage of HIV-infected donors who exhibited an elevation in levels of the analyte concerned. Together, Fig. 4A and 5 illustrate group-specific features of elevations of plasma cytokine levels in acute HIV infection, including the rapid and transient elevations in levels of IL-15 and IFN-α that occurred in the majority of subjects as viremia increased, the more prolonged elevations in TNF-α and IL-18 levels that paralleled viremia, and the later-peaking elevation in IL-10 levels.
FIG. 4.
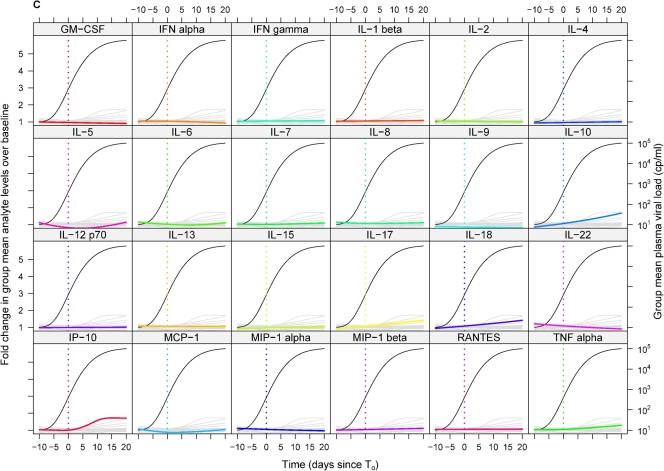
Mean change relative to baseline in plasma analyte levels over time in groups of subjects infected with HIV, HBV, and HCV. The mean changes relative to baseline in plasma levels of 24 different analytes over time (relative to T0) in the entire group of subjects infected with HIV (A), HBV (B), or HCV (C) are shown. Each panel includes a composite of the results for all 24 analytes, with data for a single analyte (as indicated by the label above) highlighted in color and data for other analytes shown in gray. The mean plasma viral load in the entire group of subjects (viral nucleic acid copies [cp]/ml, plotted on a log scale) is also shown in each panel (black line). Panels are ordered alphabetically by analyte.
FIG. 5.
Scaled proportional group mean change relative to baseline over time in plasma levels of selected analytes in subjects acutely infected with HIV. The proportional change relative to baseline in plasma levels of selected analytes (see key) and the viral load over time in the entire group of HIV-infected subjects are shown. The peak heights for different analytes are scaled according to the percentage of HIV-infected subjects studied who exhibited an elevation in plasma levels of the analyte concerned. Time is plotted in days relative to T0. cp, copies.
Reduced and delayed elevations in cytokine and chemokine levels in acute HBV and HCV infections.
Elevations in cytokine/chemokine levels were observed in a lower proportion of individuals acutely infected with HBV and HCV than with HIV, with the difference being particularly marked for HBV (Table 1). For example, whereas rapid elevations in IFN-α and IL-15 levels were observed in the majority of HIV-infected plasma donors, elevations in the levels of these cytokines occurred in ≤50% of the HBV- or HCV-infected subjects, and when they did occur, they were typically of much lower magnitude. There was a corresponding lack of detection of type I IFN activity (data not shown), which is indicative of a block(s) to acute-phase upregulation of innate cytokine production in HBV and HCV infections. Furthermore, where elevations in cytokine levels were observed in HBV- or HCV-infected individuals, they typically occurred with delayed kinetics compared to those in HIV infection (Table 1 and Fig. 3C and D and E and F, respectively). For example, although TNF-α levels became elevated in the majority of HBV- and HCV-infected subjects, the median day of first elevation in both these infections (12.5 days for HBV and 11 days for HCV) was significantly later than that found for HIV infection (day 6) (P = 0.0399 and 0.0042 for the comparisons with HBV and HCV, respectively).
HBV evoked the “quietest” acute-phase cytokine and chemokine response of the three infections studied: of the 30 analytes measured, only 3 had elevated levels in >50% of subjects during the time frame studied (IL-18, TNF-α, and IL-10, each elevated in 6 of 10 donors). Not only were relatively few elevations in cytokine/chemokine levels observed in acute HBV infection, but those that did occur were typically of lower magnitude than the corresponding elevations during acute HIV infection (Table 3). The low frequency, low magnitude, and lack of synchronicity of elevations in plasma analyte levels in the HBV-infected subjects resulted in very little perturbation of the group mean levels of any analyte over the time frame studied (Fig. 4B).
TABLE 3.
Median baseline and highest recorded level of each analyte and median day at which the highest analyte level was recorded in the HBV-infected subjects studied with detectable elevations in the analyte
| Analyte | Median baseline level (pg/ml) | Median highest recorded level (pg/ml) | Median day of highest recorded level |
|---|---|---|---|
| IP-10 | 324.8 | 734.3 | 7 |
| IL-15 | 2 | 3.7 | 22 |
| TNF-α | 4.5 | 8.3 | 18 |
| IFNα | 3.1 | 5.5 | 11.5 |
| MCP-1 | 51.3 | 80.8 | 21 |
| IL-1 Rα | 50.2 | 179.3 | 12 |
| MIP-1α | 2.4 | 7.3 | 30 |
| IL-10 | 9.6 | 29 | 19 |
| IL-8 | 4.3 | 9.7 | 21.5 |
| IL-17 | 3.9 | 13.1 | 1 |
| IL-6 | 8.1 | 10.1 | 14 |
| IFN-γ | 20.2 | 22.2 | 15 |
| Eotaxin | 64.1 | 81 | 10 |
| IL-18 | 176.6 | 254.5 | 15 |
| MIP-1β | 58.5 | 95.1 | 14.5 |
| VEGF | 8.6 | 36.9 | 13 |
| IL-4 | 43.5 | 75.1 | 22 |
| FGF basic | 42.9 | 83.9 | 8.5 |
| G-CSF | 13.8 | 21.7 | 22 |
| IL-22 | 12.8 | 22.7 | 21 |
| GM-CSF | 5.3 | 7.1 | 20 |
| IL-7 | 10.8 | 22 | 23.5 |
| IL-9 | 155.8 | 665.5 | 17.5 |
| IL-12(p70) | 8.8 | 13.2 | 24.5 |
| IL-2 | 6.6 | 9.1 | 28.5 |
| IL-5 | 6.1 | 8 | 19 |
| IL-13 | 10.8 | 14 | 20.5 |
| IL-1β | 2.4 | 2.8 | 20 |
| PDGF-BB | 382.5 | 1,231 | 21 |
| RANTES | 5,341 | 10,879 | 29 |
There were more changes in plasma cytokine/chemokine levels in acute HCV infection than in acute HBV infection although still less dramatic perturbations than in acute HIV infection. Analytes that had elevated levels in >50% of the HCV-infected subjects included TNF-α and IL-18 (8 of 10 subjects); IP-10, MIP-1β, IL-10, and IL-22 (7 of 10 subjects); and RANTES, IFN-γ, and IL-6 (6 of 10 subjects). Interestingly, the chemokines MIP-1β and RANTES were elevated in a higher proportion of individuals acutely infected with HCV than with HIV. The kinetics of elevation of cytokine levels in acute HCV infection were also markedly delayed compared to those in acute HIV infection (Fig. 3E and F). The median time of initial elevation of 16 of the 30 analytes studied was between days 6 and 8 post-T0 in acute HIV infection, whereas only 3 of 30 analytes had a median time of first elevation of day 8 or earlier in acute HCV infection. Additionally, the hierarchy of cytokine induction differed between acute HCV and acute HIV infection (Fig. 3 and Table 1); e.g., TNF-α was among the first group of cytokines/chemokines to be elevated in acute HIV infection, but the first elevation in TNF-α was preceded by elevations in levels of multiple other analytes in acute HCV infection. RANTES was among the first analytes to be elevated in acute HCV infection (median day of first elevation, 8), but levels increased relatively late in acute HIV infection (median day of first elevation, 16). As in acute HBV infection, elevations of plasma cytokine levels in acute HCV infection were typically of lower magnitude than in acute HIV infection (Table 4). The low proportion of responding subjects and lack of synchronicity of elevations in analyte levels again resulted in little change being apparent in the group mean plasma levels of any analyte over the time frame studied (Fig. 4C).
TABLE 4.
Median baseline and highest recorded level of each analyte and median day at which the highest analyte level was recorded in the HCV-infected subjects studied with detectable elevations of levels of analyte
| Analyte | Median baseline level (pg/ml) | Median highest recorded level (pg/ml) | Median day of highest recorded level |
|---|---|---|---|
| IP-10 | 600.9 | 3532.0 | 30.0 |
| IL-15 | 1.7 | 2.5 | 22.5 |
| TNF-α | 5.0 | 8.9 | 28.5 |
| IFN-α | 4.7 | 7.2 | 4.0 |
| MCP-1 | 45.4 | 73.3 | 23.0 |
| IL-1Rα | 105.8 | 151.7 | 27.0 |
| MIP-1α | 2.3 | 5.8 | 25.0 |
| IL-10 | 12.9 | 51.0 | 32.0 |
| IL-8 | 4.4 | 8.7 | 31.0 |
| IL-17 | 2.2 | 61.6 | 14.0 |
| IL-6 | 7.9 | 14.3 | 23.0 |
| IFN-γ | 18.0 | 30.3 | 23.0 |
| Eotaxin | 46.0 | 59.1 | 30.0 |
| IL-18 | 209.5 | 395.3 | 25.0 |
| MIP-1β | 42.7 | 84.6 | 30.0 |
| VEGF | 8.9 | 20.2 | 18.5 |
| IL-4 | 24.7 | 36.4 | 17.5 |
| FGF basic | 14.4 | 57.6 | 34.0 |
| G-CSF | 31.6 | 44.7 | 27.0 |
| IL-22 | 14.1 | 31.3 | 31.5 |
| GM-CSF | 4.1 | 8.1 | 12.0 |
| IL-7 | 3.0 | 7.1 | 17.5 |
| IL-9 | 39.3 | 96.1 | 13.0 |
| IL-12(p70) | 6.5 | 10.8 | 23.0 |
| IL-2 | 8.3 | 14.1 | 23.0 |
| IL-5 | 0.7 | 1.0 | 16.0 |
| IL-13 | 17.9 | 39.7 | 17.5 |
| IL-1β | 2.3 | 4.4 | 17.5 |
| PDGF-BB | 1,246.2 | 2,621.9 | 19.0 |
| RANTES | 8,509.0 | 17,467.8 | 11.0 |
In summary, there were marked differences in the pattern of elevations in cytokine/chemokine levels observed in plasma during the phase of exponential viral amplification in acute HIV, HBV, and HCV infections, with a rapid induction of classic innate cytokines followed by multiple other cytokines and chemokines in acute HIV infection, remarkably little perturbation in plasma cytokine/chemokine levels in acute HBV infection, and a delayed response of more-intermediate magnitude in acute HCV infection.
DISCUSSION
The availability of plasma panels from donors who acquired HIV, HBV, or HCV infection has enabled an unprecedented comparative analysis of the induction of plasma cytokines and chemokines during the acute phase of exponential viral replication in each of these infections. The increase in viremia in primary HIV infection was found to be associated with an ordered sequence of elevations in plasma levels of multiple cytokines and chemokines, including rapid and transient elevations in IFN-α and IL-15 levels; a large increase in IP-10 levels; rapid and more-sustained increases in TNF-α and MCP-1 levels; more slowly initiated elevations in levels of additional proinflammatory factors including IL-6, IL-8, IL-18, and IFN-γ; and a late-peaking increase in levels of the immunoregulatory cytokine IL-10. In contrast, the exponential increase in viremia in acute HBV and HCV infections was not associated with a similarly pronounced rapid increase in plasma levels of classic innate cytokines such as IFN-α and IL-15. In acute HBV infection, there was relatively little perturbation in plasma cytokine and chemokine levels over the entire time frame studied, with TNF-α, IL-8, and IL-10 being the only factors with elevated levels in >50% of subjects. In acute HCV infection, there were delayed increases in levels of cytokines and chemokines including TNF-α, IFN-γ, IL-6, IL-10, IL-18, IP-10, MIP-1β, and RANTES.
As only plasma samples were available from the donors studied here, it was not possible to address the cellular sources of the elevated levels of cytokines and chemokines. However, the pattern of elevations in cytokine levels observed during acute HIV infection would be consistent with a “textbook” response involving the sequential activation of dendritic cells (DCs), other innate cell subsets, and adaptive responses. Plasmacytoid DCs (pDCs), which act as the principal source of systemic IFN-α production in many viral infections, are activated to produce cytokines following the endocytosis of HIV virions in vitro (4) and may have been triggered by systemic viral spread to produce the early elevations in plasma IFN-α, IL-15, and TNF-α levels. Myeloid DCs (mDCs) are classically responsible for the slower and more-prolonged secretion of an array of cytokines including IL-12, IL-18, and TNF-α. Notably, little elevation in IL-12(p70) was detected during the viral ramp-up phase in acute HIV infection. This could be a consequence of the pronounced type I IFN response, which has been shown to limit IL-12 production by conventional DCs in other virus infections (13), or may be a consequence of HIV infection of mDCs, which blocks their maturation and IL-12 production in vitro (19). The lack of a strong, early IL-12 response may promote HIV-1 persistence: the administration of recombinant IL-12 to macaques during acute SIV infection enhanced both NK and CD8+ T-cell responses and resulted in lower viral loads and enhanced survival (2). The deficit in early IL-12 production aside, the initial DC activation and robust cytokine production in HIV infection were likely involved in activating other cell subsets, including monocytes and natural killer (NK), NKT, and T/B cells. Interestingly, the chemokines MIP-1α, MIP-1β, and RANTES, which possess anti-HIV activity (11), were induced in only a low proportion of HIV-infected subjects during the time frame studied. One potential target of immunotherapy would be to induce a rapid increase in the levels of these chemokines in acute HIV infection.
In contrast to HIV infection, strong elevations in plasma levels of innate cytokines were not detected as viremia increased in acute HBV and HCV infections. As the plasma donor panels gave a good coverage of the time frame from day −20 to day +20 relative to the first detection of plasma viremia in each infection, systemic elevations in plasma cytokine/chemokine levels occurring during the eclipse phase and initial period of acute viral expansion should have been captured. It thus appears that a high-level systemic elevation of innate cytokines such as IFN-α does not occur in acute HBV and HCV infections. Both HBV and HCV replicate primarily in the liver, as opposed to the widespread distribution of virus in acute HIV infection, which may contribute to the difference in systemic innate activation in these infections. Additionally, HBV and HCV possess strategies for blocking type I IFN production in the cells which they infect (7, 16, 29, 48). However, pDCs are triggered to produce IFN-α upon the recognition of viral components, without the need for infection. This suggests that HBV and HCV may also impair pDC function in vivo, a hypothesis supported by a recent study demonstrating that HCV can inhibit Toll-like receptor 9-stimulated IFN-α production by pDCs (but not mDCs) by a mechanism that does not require pDC infection (41). The delayed elevations in plasma levels of certain cytokines and chemokines that we observed in acute HCV infection may result from a slow activation of mDCs and other innate cell subsets in the absence of pDC-derived cytokines. In contrast, HBV may possess mechanisms for suppressing even this.
The cascade of cytokine production that occurs in acute HIV infection may contribute to the control of viral replication directly and/or indirectly via the activation of other effector mechanisms. For example, type I IFNs mediate antiviral activity against HIV in vitro (37) and have been shown to inhibit HIV replication in severe combined immunodeficiency (SCID) mice transplanted with human cells (28). Proinflammatory cytokines including TNF-α, IL-6, and IL-22 upregulate the production of acute-phase reactants such as serum amyloid A that can also inhibit HIV replication (34). Type I IFNs and other innate cytokines including IL-15 and IL-18 also mediate important immunostimulatory effects in early infection, acting on both innate and adaptive cell subsets. IL-15 treatment during acute SIV infection resulted in an increase in NK cell and SIV-specific CD8+ T-cell numbers at peak viremia and a reduction in the number of SIV-infected cells in lymph nodes (35).
No information is available about the long-term control of viral replication in the subjects whose acute responses were studied here, but whereas HIV persists in the vast majority of infected individuals, HCV typically persists in only ∼60 to 80% of infected adults, and HBV persists in ∼10% of infected adults (38), indicating that the rapid induction of a robust systemic cytokine response is not a prerequisite for preventing the establishment of virus persistence. Moreover, high-level proinflammatory cytokine/chemokine production during acute viral infections can be associated with enhanced pathogenesis, as observed in human infections with avian influenza A (H5N1) virus (14) and severe acute respiratory syndrome coronavirus (9). The pronounced cytokine/chemokine response induced during acute HIV infection may likewise have detrimental effects. HIV replication is promoted by immune activation; hence, the burst of production of immunostimulatory cytokines in acute infection may enhance early virus replication. Although IL-15 treatment during acute SIV infection enhanced early immune responses, it also increased the activation and proliferation of CD4+ T cells, which was associated with the establishment of a higher persisting viral load and accelerated disease progression (35). IL-15 treatment also abrogated the ability of vaccination to decrease set-point viremia in macaques (26). Many of the other proinflammatory cytokines induced in acute HIV infection have also been shown to promote HIV replication in vitro and may do likewise in vivo.
Innate cytokines induced in acute HIV infection may also contribute more directly to the CD4+ T-cell apoptosis that occurs at this time. Although the apoptosis of HIV-infected CD4+ T cells may restrict viral spread, cytokines are also thought to drive the “bystander” destruction of uninfected CD4+ T cells, and widespread acute-phase CD4+ T-cell destruction is a hallmark of pathogenic immunodeficiency virus infections in humans and primates. Type I IFNs can induce cellular apoptosis both directly and indirectly, e.g., the HIV-stimulated production of IFN-α induces CD4+ T-cell expression of TRAIL, the binding of which to DR5 is thought to contribute to CD4+ T-cell loss in HIV infection (25). Notably, the elevation in IFN-α levels in acute HIV infection is accompanied by a peak in plasma TRAIL levels (18). Likewise, TNF-α can drive apoptosis through binding to cellular TNF receptors, and peak plasma levels of apoptotic microparticles, TNFR2, and Fas ligand are observed at the time of maximal TNF-α elevation, when viremia is at its peak (18).
Consistent with high-level systemic cytokine production in acute HIV infection causing immunopathology, there is a much more immunosuppressive immune profile in the acute phase of nonpathogenic immunodeficiency virus infections in primates. For example, nonpathogenic SIVagm infection of African green monkeys is associated with a rapid and strong induction of transforming growth factor β1 (TGF-β1) and forkhead box (Fox) P3 expression, followed by an increase in IL-10 levels, with no increase in TNF-α and only a transient increase in IFN-γ transcription. In contrast, in pathogenic SIVmac infection in rhesus macaques, there is a delayed increase in IL-10 levels accompanied by only a moderate increase in levels of TGF-β (27). The latter pattern resembles acute HIV infection in humans, where we observed a late peak in plasma IL-10 levels and were unable to detect any elevation in levels of TGF-β in assays performed on a subset of subjects (data not shown). The late-peaking IL-10 response may not only be too late to subdue immunopathogenic levels of proinflammatory cytokine production but may also contribute to HIV persistence by driving the exhaustion of virus-specific CD8+ T cells (8, 15).
In summary, our current study reveals an impressive and broad “cytokine storm” in acute HIV infection, which, in the vast majority of infected subjects, leads to failure to clear the virus. Although some of the cytokines/chemokines produced in acute HIV infection may contribute to the control of viral replication, the exaggerated cytokine response likely also contributes to the early immunopathology of the infection and associated long-term consequences.
Acknowledgments
We thank Mike Busch and Greg Chiklis for supplying viral load data for the plasma panels from HCV- and HBV-infected donors, respectively; Steve Plonk for arranging for viral load testing of the plasma panels from HIV-infected donors; and Linda Harris for help with the database. We also thank Barton Haynes, Andrew McMichael, and George Shaw for many helpful discussions and for comments on the manuscript.
This work was supported by funding from the Division of AIDS, NIAID, NIH (grant AI67854). P.B. is a Jenner Institute Investigator and receives salary support from a Jenner Institute senior fellowship.
Footnotes
Published ahead of print on 28 January 2009.
REFERENCES
- 1.Abel, K., D. M. Rocke, B. Chohan, L. Fritts, and C. J. Miller. 2005. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J. Virol. 7912164-12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari, A. A., A. E. Mayne, J. B. Sundstrom, P. Bostik, B. Grimm, J. D. Altman, and F. Villinger. 2002. Administration of recombinant rhesus interleukin-12 during acute simian immunodeficiency virus (SIV) infection leads to decreased viral loads associated with prolonged survival in SIVmac251-infected rhesus macaques. J. Virol. 761731-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barcellini, W., G. P. Rizzardi, G. Poli, G. Tambussi, C. Velati, P. L. Meroni, A. G. Dalgleish, and A. Lazzarin. 1996. Cytokines and soluble receptor changes in the transition from primary to early chronic HIV type 1 infection. AIDS Res. Hum. Retrovir. 12325-331. [DOI] [PubMed] [Google Scholar]
- 4.Beignon, A. S., K. McKenna, M. Skoberne, O. Manches, I. DaSilva, D. G. Kavanagh, M. Larsson, R. F. Gorelick, J. D. Lifson, and N. Bhardwaj. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J. Clin. Investig. 1153265-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biglino, A., A. Sinicco, B. Forno, A. M. Pollono, M. Sciandra, C. Martini, P. Pich, and P. Gioannini. 1996. Serum cytokine profiles in acute primary HIV-1 infection and in infectious mononucleosis. Clin. Immunol. Immunopathol. 7861-69. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, P., and N. Bhardwaj. 2008. Innate immune responses in primary HIV-1 infection. Curr. Opin. HIV AIDS 336-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breiman, A., N. Grandvaux, R. Lin, C. Ottone, S. Akira, M. Yoneyama, T. Fujita, J. Hiscott, and E. F. Meurs. 2005. Inhibition of RIG-I-dependent signaling to the interferon pathway during hepatitis C virus expression and restoration of signaling by IKKɛ. J. Virol. 793969-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks, D. G., M. J. Trifilo, K. H. Edelmann, L. Teyton, D. B. McGavern, and M. B. Oldstone. 2006. Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 121301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron, M. J., L. Ran, L. Xu, A. Danesh, J. F. Bermejo-Martin, C. M. Cameron, M. P. Muller, W. L. Gold, S. E. Richardson, S. M. Poutanen, B. M. Willey, M. E. DeVries, Y. Fang, C. Seneviratne, S. E. Bosinger, D. Persad, P. Wilkinson, L. D. Greller, R. Somogyi, A. Humar, S. Keshavjee, M. Louie, M. B. Loeb, J. Brunton, A. J. McGeer, the Canadian SARS Research Network, and D. J. Kelvin. 2007. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 818692-8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centlivre, M., M. Sala, S. WainHobson, and B. Berkhout. 2007. In HIV-1 pathogenesis the die is cast during primary infection. AIDS 211-11. [DOI] [PubMed] [Google Scholar]
- 11.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MPI-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 2701811-1815. [DOI] [PubMed] [Google Scholar]
- 12.Cox, D., and D. Oakes. 1984. Analysis of survival data. Chapman & Hall, Boca Raton, FL.
- 13.Dalod, M., T. P. Salazar-Mather, L. Malmgaard, C. Lewis, C. Asselin-paturel, F. Briere, G. Trinchieri, and C. A. Biron. 2002. Interferon alpha/beta and interleukin 12 responses to viral infection: pathways regulating dendritic cell cytokine expression. J. Exp. Med. 195517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong, M. D., C. P. Simmons, T. T. Thanh, V. M. Hien, G. J. Smith, T. N. Chau, D. M. Hoang, N. V. Chau, T. H. Khanh, V. C. Dong, P. T. Qui, B. V. Cam, Q. H. Do, Y. Guan, J. S. Pereis, N. T. Chinh, T. T. Hien, and J. Farrar. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 121203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ejrnaes, M., C. M. Filippi, M. M. Martinic, E. M. Ling, L. M. Togher, S. Crotty, and M. G. von Herrath. 2006. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 2032461-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foy, E., K. Li, R. Sumpter, Jr., Y. M. Loo, C. L. Johnson, C. Wang, P. M. Fish, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Control of antiviral defences through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. USA 1022986-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaines, H., M. A. von Sydow, L. V. von Stedingk, G. Biberfeld, B. Bottiger, L. O. Hansson, P. Lundbergh, A. B. Sonnerborg, J. Wasserman, and O. O. Strannegaard. 1990. Immunological changes in primary HIV-1 infection. AIDS 4995-999. [DOI] [PubMed] [Google Scholar]
- 18.Gasper-Smith, N., D. M. Crossman, J. F. Whitesides, N. Mensali, J. S. Ottinger, S. G. Plonk, M. A. Moody, G. Ferrari, K. J. Weinhold, S. E. Miller, C. F. Reich III, L. Qin, S. G. Self, G. M. Shaw, T. N. Denny, L. E. Jones, D. S. Pisetsky, and B. F. Haynes. 2008. Induction of plasma (TRAIL), TNFR2, Fas ligand, and plasma microparticles after human immunodeficiency virus type 1 (HIV-1) transmission: implications for HIV-1 vaccine design. J. Virol. 827700-7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granelli-Piperno, A., A. Golebiowska, C. Trumpfheller, F. P. Siegal, and R. M. Steinman. 2004. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc. Natl. Acad. Sci. USA 1017669-7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graziosi, C., K. R. Gantt, M. Vaccarezza, J. F. Demarest, M. Daucher, M. S. Saag, G. M. Shaw, T. C. Quinn, O. J. Cohen, C. C. Welbon, G. Pantaleo, and A. S. Fauci. 1996. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc. Natl. Acad. Sci. USA 934386-4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green, P., and B. W. Silverman. 1994. Nonparametric regression and generalized linear models: a roughness penalty approach. Chapman & Hall, Boca Raton, FL.
- 22.Guo, W. 2002. Functional mixed effects models. Biometrics 58121-128. [DOI] [PubMed] [Google Scholar]
- 23.Haase, A. T. 2005. Perils a mucosal front lines for HIV and SIV and their hosts. Nat. Rev. Immunol. 5783-792. [DOI] [PubMed] [Google Scholar]
- 24.Hazenberg, M. D., S. A. Otto, B. H. van Benthem, M. T. Roos, R. A. Coutinho, J. M. Lange, D. Hamann, M. Prins, and F. Miedema. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 171881-1888. [DOI] [PubMed] [Google Scholar]
- 25.Herbeuval, J. P., A. W. Hardy, A. Boasso, S. A. Anderson, M. J. Dolan, M. Dy, and G. M. Shearer. 2005. Regulation of TNF-related apoptosis-inducing ligand on primary CD4+ T cells by HIV-1: role of type 1 IFN-producing plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 10213974-13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hryniewicz, A., D. A. Price, M. Moniuszko, A. Boasso, Y. Edghill-Spano, S. M. West, D. Venzon, M. Vaccari, W. P. Tsai, E. Tryniszewska, J. Nacsa, F. Villinger, A. A. Ansari, C. J. Trindade, M. Morre, D. Brooks, P. Arlen, H. J. Brown, C. M. Kitchen, J. A. Zack, D. C. Douek, G. M. Shearer, M. G. Lewis, R. A. Koup, and G. Franchini. 2007. Interleukin-15 but not interleukin-7 abrogates vaccine-induced decrease in virus level in simian immunodeficiency virus mac251-infected macaques. J. Immunol. 1783492-3504. [DOI] [PubMed] [Google Scholar]
- 27.Kornfeld, C., M. J.-Y. Ploquin, I. Pandrea, A. Faye, R. Onanga, C. Apetrei, V. Poaty-Mavoungou, P. Rouquet, J. Estaquier, L. Mortara, J.-F. Desoutter, C. Butor, R. Le Grand, P. Roques, F. Simon, F. Barre-Sinoussi, O. M. Diop, and M. C. Muller-Trutwin. 2005. Antiinflammatory profiles during primary HIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Investig. 1151082-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapenta, C., S. M. Santini, E. Proietti, P. Rizza, M. Logozzi, M. Spada, S. Parlato, S. Fais, P. M. Pitha, and F. Belardelli. 1999. Type 1 interferon is a powerful inhibitor of in vivo HIV-1 infection and preserves human CD4+ T cells from virus-induced depletion in SCID mice transplanted with human cells. Virology 26378-88. [DOI] [PubMed] [Google Scholar]
- 29.Li, K., E. Foy, J. C. Ferreon, M. Nakamura, A. C. Ferreon, M. Ikeda, S. C. Ray, M. Gale Jr., and S. M. Lemon. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA 1022992-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 4341148-1152. [DOI] [PubMed] [Google Scholar]
- 31.Malnati, M. S., G. Tambussi, E. Clerici, S. Polo, M. Algeri, V. Nardese, L. Furci, A. Lazzarin, and P. Lusso. 1997. Increased plasma levels of the C-C chemokine RANTES in patients with primary HIV-1 infection. J. Biol. Regul. Homeost. Agents 1140-42. [PubMed] [Google Scholar]
- 32.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 4341093-1097. [DOI] [PubMed] [Google Scholar]
- 33.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 2721167-1170. [DOI] [PubMed] [Google Scholar]
- 34.Misse, D., H. Yssel, D. Trabattoni, C. Oblet, S. Lo Caputo, F. Mazzotta, J. Pene, J. P. Gonzalez, M. Clerici, and F. Veas. 2007. IL-22 participates in an innate anti-HIV-1 host-resistance network through acute-phase protein induction. J. Immunol. 178407-415. [DOI] [PubMed] [Google Scholar]
- 35.Mueller, Y. M., D. H. Do, S. R. Altork, C. M. Artlett, E. J. Gracely, C. D. Katsetos, A. Legido, F. Villinger, J. D. Altman, C. R. Brown, M. G. Lewis, and P. D. Katsikis. 2008. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J. Immunol. 180350-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norris, P. J., B. L. Pappalardo, B. Custer, G. Spotts, F. M. Hecht, and M. P. Busch. 2006. Elevations in IL-10, TNF-alpha, and IFN-gamma from the earliest point of HIV type 1 infection. AIDS Res. Hum. Retrovir. 22757-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitha, P. M. 1994. Multiple effects of interferon on the replication of human immunodeficiency virus type 1. Antivir. Res. 24205-219. [DOI] [PubMed] [Google Scholar]
- 38.Rehermann, B., and M. Nascimbeni. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5215-229. [DOI] [PubMed] [Google Scholar]
- 39.Rizzardi, G. P., W. Barcellini, G. Tambussi, F. Lillo, M. Malnati, L. Perrin, and A. Lazzarin. 1996. Plasma levels of soluble CD30, tumour necrosis factor (TNF)-alpha and TNF receptors during primary HIV-1 infection: correlation with HIV-1 RNA and the clinical outcome. AIDS 10F45-F50. [DOI] [PubMed] [Google Scholar]
- 40.Roe, B., S. Coughlan, J. Hassan, A. Grogan, G. Farrell, S. Norris, C. Bergin, and W. W. Hall. 2007. Elevated serum levels of interferon-gamma-inducible protein-10 in patients coinfected with hepatitis C virus and HIV. J. Infect. Dis. 1961053-1057. [DOI] [PubMed] [Google Scholar]
- 41.Shiina, M., and B. Rehermann. 2008. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology 47385-395. [DOI] [PubMed] [Google Scholar]
- 42.Sinicco, A., A. Biglino, M. Sciandra, B. Forno, A. M. Pollono, R. Raiteri, and P. Gioannini. 1993. Cytokine network and acute primary HIV-1 infection. AIDS 71167-1172. [DOI] [PubMed] [Google Scholar]
- 43.Storey, J. 2002. A direct approach to false discovery rates. J. R. Stat. Soc. 64479-498. [Google Scholar]
- 44.Thiebaut, R., and H. Jacqmin-Gadda. 2004. Mixed models for longitudinal left-censored repeated measures. Comput. Methods Programs Biomed. 74255-260. [DOI] [PubMed] [Google Scholar]
- 45.Verbeke, G., and G. Molenberghs. 2000. Linear mixed models for longitudinal data. Springer-Verlag, New York, NY.
- 46.Vidoni, P. 2006. Response prediction in mixed effects models. J. Stat. Planning Inference 1363948-3966. [Google Scholar]
- 47.von Sydow, M., A. Sonnerborg, H. Gaines, and O. Strannegard. 1991. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res. Hum. Retrovir. 7375-380. [DOI] [PubMed] [Google Scholar]
- 48.Whitten, T. M., A. T. Quets, and R. H. Schloemer. 1991. Identification of the hepatitis B factor that inhibits expression of the beta interferon gene. J. Virol. 654699-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]