Abstract
It is well documented that mutations in the MYOCILIN gene may lead to juvenile- and adult-onset primary open-angle glaucoma. However, the functions of wild-type myocilin are still not well understood. To study the functions of human myocilin and its two proteolytic fragments, these proteins were expressed in HEK293 cells. Conditioned medium from myocilin-expressing cells, as well as purified myocilin, induced the formation of stress fibers in primary cultures of human trabecular meshwork or NIH 3T3 cells. Stress fiber-inducing activity of myocilin was blocked by antibodies against myocilin, as well as secreted inhibitors of Wnt signaling, secreted Frizzled-related protein 1 (sFRP1) or sFRP3, and β-catenin small interfering RNA. Interaction of myocilin with sFRP1, sFRP3, and several Frizzled receptors was confirmed by immunoprecipitation experiments and by binding of myocilin to the surface of cells expressing cysteine-rich domains of different Frizzled and sFRPs. Treatment of NIH 3T3 cells with myocilin and its fragments induced intracellular redistribution of β-catenin and its accumulation on the cellular membrane but did not induce nuclear accumulation of β-catenin. Overexpression of myocilin in the eye angle tissues of transgenic mice stimulated accumulation of β-catenin in these tissues. Myocilin and Wnt proteins may perform redundant functions in the mammalian eye, since myocilin modulates Wnt signaling by interacting with components of this signaling pathway.
Glaucoma is a leading cause of blindness in the world. Primary open-angle glaucoma is the most common form of glaucoma. It will affect more than 60 million and blind about 4.5 million people worldwide by the year 2010 (62). It is now well established that a genetic component may contribute to glaucoma, and several glaucoma-associated genes have been identified. The first-identified and the most-studied gene is MYOCILIN (MYOC), which is highly expressed in and secreted by the trabecular meshwork (1, 19, 72, 75, 77, 78), one of the key components of the eye aqueous humor outflow system. Dominant mutations in MYOC may lead to juvenile open-angle glaucoma and are found in 3 to 4% of patients with primary open-angle glaucoma (18, 19). The encoded protein, myocilin, belongs to a family of glycosylated proteins containing a C-terminal olfactomedin domain (57, 90, 91). Olfactomedin domain was originally identified in a glycoprotein isolated from the olfactory epithelium of frogs (71) and subsequently was found in a group of proteins forming a family of olfactomedin domain-containing proteins. The family of olfactomedin domain-containing proteins includes both secreted and membrane-bound proteins that each exhibit a characteristic distribution in different tissues (4, 10, 27, 30, 44, 51, 58, 78, 79). Most of the glaucoma-causing mutations in myocilin are located in the olfactomedin domain (1, 2, 18, 19, 24, 72).
Besides the trabecular meshwork and sclera (1, 77), high levels of MYOC were observed in the ciliary body (1, 13, 39), iris (89), retinal pigment epithelium/choroid (78, 88), and optic nerve (74). Low levels of MYOC expression were detected in several nonocular tissues (75). Myocilin, being a secreted protein, was also found in the aqueous humor, an intraocular fluid responsible for the supply of nutrients and for removal of metabolites from the avascular tissues of the eye anterior segment (63, 65). Some mutations in the MYOC gene lead to the inhibition of mutated myocilin secretion. Secretion of wild-type myocilin also can be reduced or blocked in the presence of mutated myocilin (22, 36, 52). It has been suggested that the intracellular accumulation of myocilin aggregates is deleterious to the trabecular meshwork cells, resulting in the deterioration of their function and subsequent elevation of intraocular pressure (IOP) (38, 49).
Although the MYOC gene has been studied for more than 10 years, the functions of myocilin protein are still not well understood (19, 75). Biochemical data indicate that myocilin may interact with several intracellular and extracellular matrix proteins (16, 37, 48, 61, 73, 78), although the biological significance of such interactions is not clear. The absence of open-angle glaucoma in an elderly woman homozygous for the Arg46Stop mutation (45), as well as the absence of glaucoma in people hemizygous for MYOC (87), suggests that the loss of functional myocilin is not critical for the development of glaucoma or for normal eye functioning. These observations are supported by data in mice with targeted disruption of the Myoc gene. Mice heterozygous and homozygous for a targeted null mutation in Myoc do not have a detectable eye phenotype (42). A 15-fold increase in the levels of normal myocilin in the eyes of transgenic mice also does not lead to the elevation of IOP or glaucoma (26). A glaucoma phenotype appears to be dependent upon expression of mutated myocilin protein in the eye tissues.
In the present study we describe a possible mechanism of myocilin action that may explain many of the previous experimental observations. We demonstrate that myocilin can interact with several Wnt receptors in the Frizzled (Fzd) family, as well as Wnt antagonists in the secreted Frizzled-related protein (sFRP) family and Wnt inhibitory factor 1 (WIF-1). Our data indicate that myocilin modulates the organization of actin cytoskeleton, stimulating the formation of stress fibers through components of Wnt signaling pathways. The organization of actin cytoskeleton is critical for the contractility of the trabecular meshwork and the regulation of IOP. Our data suggest that the absence of a glaucoma phenotype resulting from a myocilin null mutation in the eye may be explained by the compensatory action of Wnt proteins.
MATERIALS AND METHODS
Reagents and DNA constructs.
Recombinant sFRP-1 was purified as previously described (81). Hemagglutinin (HA)-tagged Fzd1, Fzd5, Fzd7, and Fzd10 constructs were generated by subcloning the pertinent open reading frames into pcDNA3.1, using cDNA constructs obtained from the laboratories of Xi He, Jeremy Nathans, and Randall Moon. Fzd5/pcDNA3.1, with an engineered optimal Kozak sequence and an HA tag immediately downstream from the Fzd5 signal peptide sequence, served as the backbone plasmid. Additional Fzd constructs were created by excising the Fzd5 sequence downstream from the HA tag and replacing it with the Fzd1, Fzd7, or Fzd10 open reading frame beginning after the sequence encoding its signal peptide. Fifteen fusion constructs (10 Frizzled and 5 sFRP) containing cysteine-rich domain (CRD), a Myc epitope, and the COOH-terminal glycosylphosphatidylinositol (GPI)-anchoring peptide from decay activating factor were described (70) and kindly provided by J. Nathans (Johns Hopkins University). Human myocilin, myocilin-ΔC and myocilin-ΔN were cloned into the pCS2-FLAG vector. Myocilin-ΔC and myocilin-ΔN fragments were amplified by PCR using pCS2-myocilin-FLAG plasmid DNA (52) as a template. Primers GGCGGATCCTCTGCAATGAGGTTCTTCTGT and CCATCGATGGTCGGGAAGCAGGAACTTC were used for amplification of myocilin-ΔC fragment. Two amplification steps were used to amplify myocilin-ΔN fragment: oligonucleotides GTGTGGGATGTGGGGGCCATTTTGAAGGAGAGC and CCATCGATGGCATCTTGGAGAGCTT were use for the first step, and oligonucleotides GGCGGATCCTCTGCAATGAGGTTCTTCTGT and GCTCTCCTTCAAAATGGCCCCCACATCCCACAC were used for the second step. Purified fragments were digested with BamHI and ClaI and ligated into the pCS2-FLAG vector. The pAPtag-2 vector (GenHunter Cor, Nashville, TN) was used to produce myocilin-alkaline phosphatase (AP) fusion construct. The primers CCAAGCTTTCTGCAATGAGGTTCTTCTGTGCA and CCAGATCTCATCTTGGAGAGCTT were used for amplification. Purified fragment was digested with HindIII/BglII and ligated into the pAPtag-2 vector. The WIF-1-immunoglobulin G (IgG) and WD-IgG constructs (33) were provided by J.-C. Hsieh (State University of New York at Stony Brook). The identity of all constructs was confirmed by sequencing.
Myocilin purification.
HEK293 cells were transiently transfected with FLAG-tagged myocilin constructs and incubated as described above. Serum-containing medium was replaced by serum-free medium after transfection, and cells were incubated for 48 h. Conditioned medium (CM) was collected and myocilin-FLAG proteins were purified using anti-FLAG M2 agarose beads according to the manufacturer's instructions (Sigma, St. Louis, MO). For some experiments, myocilin was further purified by ion-exchange chromatography using HiTrap-SP FF 1-ml columns (GE Healthcare). The purity of the purified proteins was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Transfection and preparation of conditioned media.
HEK293, NIH 3T3, L, and Wnt3a-expressing L cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and G418 (0.4 μg/ml). Trabecular meshwork primary cell lines were cultivated as described previously (80) and were kindly provided by Paul Russell (University of Wisconsin). Transfections were performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol, using 1 μg of DNA/well in a six-well plate (2 × 105 cells/well). At 48 h after transfection, serum-containing DMEM incubation medium was removed, and cells were washed three times with phosphate-buffered saline (PBS) and incubated in serum-free medium for additional 24 h. CM was collected and used directly or frozen at −80°C. Myocilin-depleted CM was prepared by incubation of CM from myocilin-transfected HEK293 cells or Wnt3a-expressing L-cells with monoclonal antibodies to human myocilin (10 μg/ml) for 60 min at room temperature. In some experiments, CM was preincubated with 10 μg of sFRP1 or sFRP3 (R&D Systems, Minneapolis, MN)/ml for 1 h at 37°C before addition to NIH 3T3 cells.
Immunofluorescence.
NIH 3T3 cells were seeded on the two-well glass chamber slides (Nalge Nunc) in complete DMEM. The medium was removed 4 to 6 h after plating, and cells were washed two to three times with PBS. Cells were incubated with purified myocilin proteins or different CM for 4 h and then fixed with freshly made 3.7% formaldehyde for 10 min at room temperature and permeabilized with 0.1% Triton X-100 in PBS for 5 min. After blocking with 5% of bovine serum albumin (BSA) in PBS at room temperature for 1 h, cells were incubated with rhodamine-phalloidin (Molecular Probes, Eugene, OR) at room temperature for 30 min. An Axioplan-2 fluorescence microscope and an Axio camera (Carl Zeiss MicroImaging, Inc.) were used to detect fluorescence. The images were processed with Adobe Photoshop Elements 2.0 (Adobe, Inc.). For quantification of myocilin-induced stress fiber formation, a baseline of 10 stress fibers per cell was used. Cells containing more than 10 stress fibers were scored as cells with upregulated stress fiber formation. Quantification was performed by two independent observers, yielding similar results. To suppress β-catenin synthesis, cells were transfected with increasing amounts (10 to 80 nM) of β-catenin small interfering RNA (siRNA; 5′-GGCCUGGUUUGAUACUGACCUGUAA-3′) or control siRNA (both from Invitrogen) as described above. β-Catenin labeling was performed with monoclonal β-catenin antibody (1:100; BD Biosciences) and revealed with secondary TRITC (tetramethyl rhodamine isothiocyanate) anti-mouse antibody (1:200; Jackson Immunoresearch Laboratories, Inc., West Grove, PA). Mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg), and the eyes were fixed in 4% paraformaldehyde in 0.1 M PBS (pH 7.4) at 4°C for 24 h before being processed for paraffin embedding. Deparaffinized 6-μm sections were then incubated with primary antibodies for 1 to 2 h at room temperature or overnight at 4°C. Rabbit antiserum against mouse myocilin (52) was affinity purified and used for immunostaining. Signal visualization was performed by incubating sections for 1 h at room temperature with an appropriate secondary antibody conjugated to Cy3 or Alexa 488 fluorophores (1:200; Molecular Probes) diluted in PBS containing 0.5% Triton X-100 and DAPI (4′,6′-diamidino-2-phenylindole; Molecular Probes) for counterstaining.
Western blotting.
Lysates from dissected sclera or drainage structures were separated by 4 to 12% gradient NU-PAGE (Invitrogen). Ten micrograms of each extract was loaded into each well. After separation and transfer to a polyvinylidene difluoride membrane, the blots were incubated with a rabbit polyclonal antibody against Myoc at 1:2,000 dilution or with monoclonal antibodies against β-catenin (BD Bioscience, CA) at a 1: 2,000 dilution. Detection was made by using a SuperSignal WestDura (Pierce, Rockford, IL). The amounts of loaded protein were determined by using immunoblotting for an internal control protein, HSC70 (Santa Cruz). For digital quantification, membranes were scanned by using Typhoon 9410 variable mode image (Amersham Pharmacia Biotech, Piscataway, NJ) and analyzed using Image Scion alpha 4.0.3.2 (Scion Cor, Frederick, MD). All experiments were repeated at least two times.
Binding to cell surface CRD-Myc-GPI.
These experiments were performed according to the described procedure (70). In brief, the CRD-Myc-GPI constructs were transiently transfected into HEK293 cells. Two days after transfection, the coverslips were incubated in fresh growth medium lacking bicarbonate and containing myocilin-AP CM or AP-3Myc-Norrin CM (diluted 1:5). After gentle rocking at 4°C for 2 h, the coverslips were washed four times with PBS supplemented with 1 mM calcium and 1 mM magnesium (PBS/Ca/Mg); fixed in 60% acetone, 3% formaldehyde, and 20 mM HEPES buffer for 30 s; washed twice with PBS/Ca/Mg; and heated in a hybridization chamber at 65°C for 90 min to inactivate endogenous AP. Immobilized AP was visualized by using GeneHunter AP Assay Reagent S (GeneHunter Corp., Nashville, TN).
Coimmunoprecipitation.
HEK293 cells were transiently cotransfected with 4.0 μg of myocilin-FLAG and HA-Fzds, WIF-1-IgG WD-IgG, or sFRP3-Myc-FLAG expression constructs using Lipofectamine 2000. At 48 h after transfection, cells were washed with PBS and lysed in a lysis buffer (0.5% Triton X-100, 0.5% NP-40, 0.25% gelatin, 1× Tris-buffered saline, 1 mM EDTA, 1 mM dithiothreitol, 1 mM Na3VO4, 5 mM NaF, protease inhibitor) for 15 min on ice. Extracts were clarified by centrifugation at 10,000 × g for 10 min. Cleared lysates were subjected to immunoprecipitation with anti-FLAG M2 beads (Sigma). The beads were collected and washed three times in lysis buffer. Immunoprecipitates were analyzed by Western blotting with the indicated antibodies. Mouse hearts were homogenized in lysis buffer and clarified as described above. Cleared lysates were incubated with antiserum against mouse myocilin (52) or control antiserum at 1:500 dilutions for 1 h at 4°C. The antigen-antibody complexes were captured by using protein A-agarose. The beads were collected and processed as described above.
Measurements of Rac1 and JNK activity.
Rac1 activation assays were performed by using a nonradioactive Rac activity assay kit (Upstate Biotechnology, Lake Placid, NY). Briefly, cell lysates were immunoprecipitated with glutathione S-transferase fusion-protein corresponding to the p21-binding domain (residues 67 to 150) of human PAK1 bound to glutathione agarose, subjected to SDS-4 to 12% PAGE, and Western blotted with monoclonal anti-Rac antibody (Upstate Biotechnology). For the Jun N-terminal kinase (JNK) assay, the cell lysates were immunoblotted with an antiphosphorylated JNK antibody (Sigma), which recognizes the active form and the p54 and p46 isoforms of JNKs at a 1:1,000 dilution. Peroxidase-conjugated anti-mouse antibody (GE Healthcare) was used as the secondary antibody. Blots were analyzed using SuperSignal WestDura reagent as described by the manufacturer (Pierce) and quantified on an AlphaImager 3400 (Alphainotech, San Leandre, CA).
Kd (equilibrium dissociation constant) measurements.
For the binding assay, 100 μl of purified sFRP1, sFRP3, and WIF-1 (R&D Systems) at 1 μg/ml were added in triplicate to wells of a MaxiSorp flat-bottom 96-well plate (Nunc, Rochester, NY), followed by incubation overnight. The plate was washed with TBS-T (Tris-HCl [pH 7.0] with 0.05% Tween 20) and incubated with 100 μl of solution containing increasing concentrations (0 to 30 nM) of purified myocilin or Wnt3a for 1 h at room temperature. Plates were washed and incubated with anti-myocilin (R&D Systems) or anti-Wnt3a-biotinylated antibodies (Abcam, Cambridge, MA). Anti-streptavidin-AP antibodies were used as secondary antibodies. AP activity was measured spectrophotometrically. The binding data were analyzed by using GraphPad Prism software.
RESULTS
Myocilin induces reorganization of actin cytoskeleton.
It has been reported that myocilin undergoes an intracellular endoproteolytic cleavage at the C terminus of Arg226 resulting in two fragments of about 22 and 35 kDa (3). The latter fragment contains the C-terminal olfactomedin domain. To study functions of myocilin and its fragments, N- and C-terminal fragments of human myocilin, as well as full-length human myocilin, were cloned into the pCS2 vector in frame with a FLAG epitope. N- and C-terminal myocilin constructs are referred to as myocilin-ΔC and myocilin-ΔN, respectively, here. The myocilin signal peptide was included in the myocilin-ΔN construct to facilitate secretion and purification of the encoded protein (Fig. 1A). Published data suggest that the C-terminal fragment of myocilin is secreted (3, 23, 66), while data concerning secretion of the N-terminal fragment are controversial (23, 66). To test whether N- and C-terminal fragments of myocilin are secreted under the conditions described here, HEK293 cells were transfected with full-length human myocilin. Both N- and C-terminal fragments of myocilin were efficiently secreted and detected in CM using antibody against the N-terminal part of myocilin (52) and anti-FLAG antibodies, respectively (Fig. 1B). Full-length myocilin, myocilin-ΔN, and myocilin-ΔC proteins were purified from the CM of transiently transfected HEK293 cells using FLAG-agarose beads (Fig. 1C).
FIG. 1.
Schematic diagram of myocilin constructs that were used in the present study (A), secretion of different myocilin isoforms (B), and SDS-PAGE analysis of purified myocilin proteins (C). (A) A signal peptide and olfactomedin domain are shown in red and dark blue, respectively. The FLAG epitope is shown in brown. The arrow marks the proteolytic cleavage site. Numbers at the top indicate corresponding positions in the myocilin amino acid sequence. (B) HEK293 cells were transiently transfected with a construct encoding full-length human myocilin, and the presence of different myocilin forms in conditioned medium (lanes 2 and 3) was analyzed 48 h after transfection by Western blotting with antibodies to the N-terminal part of myocilin (lane 2) or FLAG epitope (lane 3). A total of 5 μg of total cell extract was stained with antibodies to the N-terminal part of myocilin (lane 1). (C) A total of 0.5 μg of indicated purified proteins was separated by SDS-4 to 12% PAGE and stained with Coomassie blue.
Since gliomedin, another olfactomedin-domain-containing protein, has been shown to interact with membrane-associated proteins through the olfactomedin domain (14, 15), we tested possible interaction of myocilin and its proteolytic fragments with membrane-associated proteins and effects of myocilin on cell cytoskeleton. Treatment of primary culture of the human trabecular meshwork that was cultivated for 3 h without serum with 1 μg of purified proteins/ml for 3 h dramatically increased the fraction of cells containing actin stress fibers, which were identified by phalloidin staining (Fig. 2A). Myocilin and myocilin-ΔC proteins possessed higher inducing activity than myocilin-ΔN protein (Fig. 2B). To check whether myocilin is able to induce similar changes in other cell lines that are more accessible and suitable for biochemical experiments than trabecular meshwork cells, we used NIH 3T3 cells. Treatment of NIH 3T3 cells with myocilin and its fragments also induced formation of stress fibers similar to those observed in trabecular meshwork cells (not shown).
FIG. 2.
Myocilin-induced formation of actin stress fibers in human trabecular meshwork cells. Cells were treated with 1 μg of the indicated myocilin proteins/ml for 1 h and then stained with rhodamine phalloidin. (B) Quantification of studies corresponding to panel A. The number of cells evaluated is shown at the top of each bar. These experiments were performed three times. Scale bar, 10 μm.
Next, we examined the effects of CM from HEK293 cells that were transiently transfected with cDNAs encoding myocilin and its fragments on the actin cytoskeleton of NIH 3T3 cells. Treatment of NIH 3T3 cells with CM from myocilin- or myocilin-ΔC-expressing cells for 3 h stimulated an induction of actin stress fibers in more than 40% of cells, whereas CM from myocilin-ΔN-expressing cells induced stress fibers in ca. 20% of cells (Fig. 3). CM from HEK293 cells transfected with vector DNA or Ile477Asn myocilin mutant that is not secreted induced stress fiber formation in only ca. 5% of cells (Fig. 3). It is well known that many reagents, including serum (67), transforming growth factor β (56), and Wnt (67), may induce reorganization of actin cytoskeleton of NIH 3T3 cells similar to what is observed after treatment with purified myocilin or myocilin CM. In the conditions that we used, CM from Wnt3a-expressing L cells induced reorganization of actin cytoskeleton in ca. 65% of cells.
FIG. 3.
Myocilin-induced formation of actin stress fibers in NIH 3T3 cells. (A) Cells were treated with CM from HEK293 cells transiently transfected with indicated plasmids for 4 h and then stained with rhodamine phalloidin. Representative fields are shown. (B) Quantification of a typical experiment showing the percentage of cells containing actin stress fibers. The number of cells evaluated is shown at the top of each bar. These experiments were performed four times. Scale bar, 10 μm.
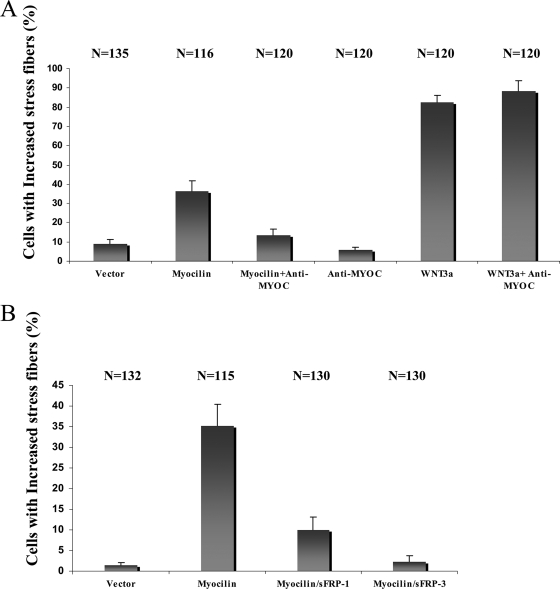
To prove that changes in the actin cytoskeleton were induced by myocilin and not other proteins present in CM of transiently transfected cells, CM was treated with antibodies raised against human myocilin before addition to cells as described in Materials and Methods. Treatment with anti-myocilin antibodies significantly reduced the fraction of NIH 3T3 cells containing stress fibers, whereas the addition of these antibodies to the CM of Wnt3a-expressing cells did not have any effect (Fig. 4A). We concluded that myocilin is the agent responsible for the actin cytoskeleton reorganization in NIH 3T3 cells and trabecular meshwork cells and that myocilin-ΔC plays a more prominent role in this reorganization than does myocilin-ΔN.
FIG. 4.
Effects of myocilin antiserum (A) and sFRPs (B) on actin stress fibers formation in NIH 3T3 cells. Myocilin antiserum at 10 μg/ml (A) or the indicated sFRPs at 10 μg/ml (B) was preincubated with myocilin CM for 30 min before addition to NIH 3T3 cells. The number of cells evaluated is shown at the top of each bar. These experiments were performed twice.
Myocilin may modulate Wnt signaling.
Our previous results indicated that other members of the family of olfactomedin-domain-containing proteins, olfactomedin 1 and olfactomedin 3, also known as optimedin, showed some similarities to the action of Wnt proteins (46, 58). Therefore, we tested whether myocilin may act through components of Wnt signaling pathways. First, we determined whether secreted inhibitors of Wnt signaling—sFRP1 (17, 64) or sFRP3, also known as FrzB (31, 47, 64, 84)—are able to block stress fiber formation induced by myocilin. Preincubation of myocilin CM with purified sFRP1 or sFRP3 (10 μg/ml) inhibited the formation of stress fibers in NIH 3T3 cells, with sFRP3 showing a stronger inhibitory effect (Fig. 4B). These data suggested that the reorganization of actin cytoskeleton by myocilin may occur through components of Wnt signaling pathways.
Stabilization and translocation of β-catenin protein to the nucleus is a well-established mechanism of Wnt signaling (9, 50). However, nuclear translocation of β-catenin does not appear to be required for rearrangement of the actin cytoskeleton (12, 67). Similar to the action of Wnt5a (67), CM containing full-length myocilin, myocilin-ΔC or myocilin-ΔN proteins did not induce nuclear accumulation of β-catenin in cell culture, although they did stimulate an accumulation of β-catenin at the cellular membrane in more than 50% of treated cells (Fig. 5A to D). Since NIH 3T3 cells contain a significant pool of β-catenin, we did not observe a significant increase in the levels of total β-catenin, as judged by Western blotting experiments (data not shown). However, treatment of CHO cells, which normally express a low level of β-catenin, with myocilin CM led to a significant increase in β-catenin levels similar to that observed after Wnt3a CM treatment (Fig. 5E). β-Catenin is essential for myocilin-induced stress fiber formation, since the reduction of β-catenin levels by siRNA treatment (Fig. 5F) decreased the fraction of cells containing stress fibers to below the numbers observed in control cells (Fig. 5G).
FIG. 5.
Changes in subcellular localization of β-catenin in response to myocilin treatment and effects of β-catenin inhibition on stress fiber formation. NIH 3T3 cells were treated with CM from cells expressing no myocilin (A), myocilin (B), myocilin-ΔC (C), or myocilin-ΔN for 3 h and stained with antibodies to β-catenin. Note the membrane localization of β-catenin in panels B to D. These experiments were performed four times. (E) Changes in the levels of total β-catenin in CHO cells treated with CM from Wnt3a- or myocilin-expressing cells. (F) Changes in the levels of total β-catenin in NIH 3T3 cells treated with β-catenin siRNA (10, 20, 40, and 80 nM) as described in Materials and Methods. (G) Reduction of stress fiber formation in NIH 3T3 cells after reduction of β-catenin level by 80 nM siRNA. Scale bar, 10 μm.
The in vivo effect of myocilin on β-catenin distribution was tested in transgenic mice that were produced using bacterial artificial chromosome DNA containing full-length human MYOC gene, as well as 89 and 51 kb of the 5′- and 3′-flanking sequences, respectively. This bacterial artificial chromosome DNA reproduced the expression pattern of endogenous Myoc gene (93). In the tissues of the eye angle and aqueous humor of adult animals, the combined levels of human and mouse myocilin proteins were ∼3-fold higher in transgenic animals than the levels of mouse myocilin in nontransgenic littermates. Elevated levels of myocilin did not induce morphological changes or lead to elevation of intraocular pressure in the eyes of transgenic mice. Staining of the eye sections with antibodies against β-catenin demonstrated that overexpression of myocilin stimulated the upregulation of β-catenin in the eye angle tissues and sclera in the eyes of transgenic mice (Fig. 6A and B). The immunostaining results were confirmed in Western blotting experiments (Fig. 6C and D). Transgenic mice demonstrated a >4-fold increase in β-catenin level in the angle tissues and a >2-fold increase in the sclera compared to their wild-type littermates.
FIG. 6.
Upregulation of β-catenin in the eyes of 20-month-old wild-type and transgenic mice. Paraffin sections of control (A) and transgenic (B) eyes were stained with antibodies to β-catenin (1:100 dilution) and DAPI. Three pairs of animals were analyzed. A typical staining pattern is shown. cb, ciliary body; i, iris; tm, trabecular meshwork. (C) Western blot analysis of β-catenin expression in the eye angle tissues and sclera of wild-type and transgenic mice. (D) Quantification of the results shown in panel B. WT, wild type; AG, eye angle tissues; SL, sclera.
These data indicate that myocilin may increase β-catenin levels and that β-catenin is essential for stress fiber formation. However, similar to Wnt5a, stress fiber formation after myocilin treatment does not require nuclear accumulation of β-catenin, suggesting that myocilin may act through noncanonical Wnt signaling pathways.
Myocilin binding to Fzd receptors and sFRPs.
sFRPs block Wnt signaling either by interacting with Wnt proteins to prevent their binding to Fzd receptors or by forming complexes with Fzd receptors, thus disrupting their interaction with Wnt ligands (41). The available data suggest that the CRDs of sFRPs are sufficient for these interactions (41). To test the possible binding of myocilin to the CRDs of different Fzd receptors and sFRPs, we used a myocilin-alkaline phosphatase fusion protein (myocilin-AP) as a probe. Norrin-AP (70) was used as a positive control. HEK293 cells were transfected with plasmids encoding the CRDs of ten known Fzd receptors and five sFRPs. These CRDs were cloned in frame with the Myc epitope and GPI-anchoring peptide (CRD-Myc-GPI) (70). Staining of transfected cells with anti-Myc antibodies demonstrated that different CRD-Myc-GPI proteins accumulated at the surface of transfected cells at similar levels (not shown). CM from cells expressing myocilin-AP or Norrin-AP was collected 48 h after transfection, and the activity of the AP was measured as described in Materials and Methods. The AP activity was ∼10-fold higher for Norrin than for myocilin (results not shown). CM was added for 2 h to cells transfected with CRD-Myc-GPI, and the activities of AP bound to cell membranes were visualized by staining using nitroblue tetrazolium/BCIP (5-bromo-4-chloro-3-indolylphosphate) as a substrate. Norrin-AP, as expected from the published results (70), efficiently bound to Fzd4 CRD, whereas myocilin-AP bound to Fzd1, Fzd3, Fzd4, Fzd7, Fzd10, sFRP2, sFRP3, and sFRP5 (Fig. 7). These results were consistent with our conclusion that sFRP3 produces a stronger inhibitory effect on stress fiber formation than does sFRP1 (see Fig. 4).
FIG. 7.
Binding of myocilin-AP to the CRDs of different Fzds and sFRPs. Myocilin-AP CM was incubated with live HEK293 cells transfected with the indicated CRD-Myc-GPI constructs. The AP activity was visualized by staining with nitro blue tetrazolium/BCIP. AP-Norrin CM was used as a control. These experiments were repeated three times. The results of a typical experiment are shown.
The interaction of myocilin with sFRP3 and several Fzds was confirmed in coimmunoprecipitation experiments. HEK293 cells were cotransfected with sFRP3-FLAG-Myc and myocilin-FLAG or myocilin-ΔC-FLAG constructs. CM was collected 48 h after transfection, and protein complexes were immunoprecipitated with Myc antibodies. Both full-length myocilin and myocilin-ΔC were detected in the complexes with sFRP3 (Fig. 8A). It is interesting that the cleavage products were also precipitated with sFRP3 when full-length myocilin was used. The higher intensity of sFRP3 bands in the presence of myocilin (Fig. 8A, right panel) suggests that myocilin may stabilize sFRP3 or increase its secretion. To verify that myocilin and sFRPs are able to interact in a natural environment, we immunoprecipitated extracts from mouse heart tissue with antibodies to mouse myocilin. Both sFRP1 and sFRP3 were coimmunoprecipitated with myocilin from heart extracts (Fig. 8B, C). The qualitative difference in the interaction of myocilin with sFRP1 and sFRP3 in the myocilin-AP versus cellular extract binding assays may be due to the use of only the CRD in the former assay, whereas full-length sFRPs were used in the latter assay.
FIG. 8.
Physical interaction of myocilin with Fzd receptors and antagonists of Wnt signaling. HEK293 cells were cotransfected with the indicated constructs (A and D). Mouse heart extracts were used in panels B and C. CM was collected 48 h after transfection in the case of secreted antagonists and treated with Myc antibodies (A). (B and C) Heart extracts were immunoprecipitated with antibodies to mouse myocilin. After immunoprecipitation, proteins were eluted from the beads, separated by SDS-PAGE, and then probed with antibodies to sFRP3 (B) or sFRP1 (C). (D) HEK293 cells were lysed, immunoprecipitated with anti-FLAG beads, and analyzed as described above with antibodies to HA for detection. Shown are Western blots of CM (A, right) or cell lysates (B to D, lower panels) before immunoprecipitation probed with the indicated antibodies. These experiments were repeated more than two times.
To test possible interaction of myocilin with Fzds, HEK293 cells were cotransfected with myocilin-FLAG and various HA-tagged Fzd constructs and lysed 48 h after transfection. Protein complexes were immunoprecipitated with anti-FLAG-agarose. Fzd1, Fzd7, and Fzd10 receptors were detected in the complexes with myocilin, whereas Fzd5 did not form complexes with myocilin (Fig. 8D).
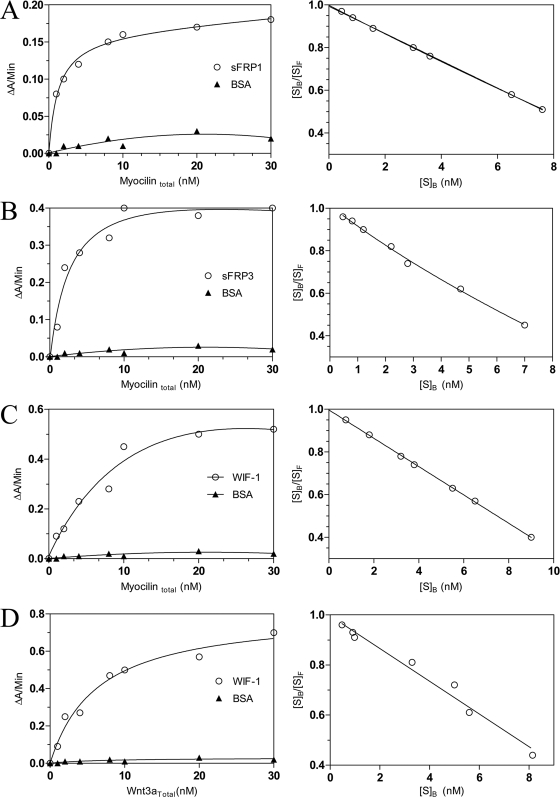
To quantify the binding affinities of myocilin for sFRP1 and sFRP3 and compare them with binding affinities of Wnt3a, we used 96-well plates that were coated with purified sFRP1, sFRP3, or BSA as a control. The reported binding affinities of Wnt3a with sFRP1 and sFRP3 are 11 and 8 nM, respectively (86). The plates were incubated with increasing concentrations of purified FLAG-tagged myocilin (see Materials and Methods). Binding of myocilin was detected using biotinylated anti-myocilin antibodies. Little binding was detected on BSA-coated wells, while sFRP1- and sFRP3-coated plates demonstrated hyperbolic binding curves (Fig. 9). Linear Scatchard plots of binding data indicated noncooperative interaction with Kd values of 15 ± 0.5 nM and 12 ± 0.7 nM for sFRP1 and sFRP3, respectively.
FIG. 9.
Binding of myocilin to sFRP1, sFRP3, WIF-1, and BSA (left panels). The binding of Wnt3a to WIF-1 and BSA is shown for comparison. The vertical axis shows the increase in absorbance at 405 nm. The right panels represent Scatchard plots of the data for myocilin binding to corresponding proteins. The vertical axis shows the ratio of bound to free myocilin; the horizontal axis shows the concentration of bound myocilin. These experiments were repeated three times.
On the basis of all of these results, we concluded that myocilin interacts with several Fzd and sFRP proteins, that the CRDs mediate most of these interactions, and that the binding affinities of myocilin to sFRP1 and sFRP3 are similar to those of Wnt3a.
Myocilin binding to the WD domain of WIF-1 protein.
There are several structurally unrelated antagonists of Wnt signaling that are able to interact with Wnt proteins (41). We tested whether myocilin, similar to Wnts and olfactomedin 1 (58), binds to WIF-1. HEK293 cells were cotransfected with constructs encoding myocilin-FLAG and WIF-1 or the Wnt-binding domain (WD) of WIF-1 fused to the human IgG heavy chain (Fig. 10). Since both myocilin and WIF-1 are secreted proteins, coimmunoprecipitation was performed from the CM of transfected cells. Our results demonstrated that myocilin interacts with WIF-1 and that WD domain is sufficient for such interactions. The binding affinity of myocilin to WIF-1 was quantified by using the same method that was used to quantify its binding to sFRPs. Wnt3a protein was used in these experiments for comparison. The calculated Kd values of WIF-1 binding to myocilin and Wnt3a were 16 ± 0.9 nM and 15 ± 0.2 nM, respectively (Fig. 9). We concluded that myocilin interacts with WIF-1 with a binding affinity similar to that for Wnt3a and that the WD domain is sufficient for this interaction.
FIG. 10.
Physical interaction of myocilin and WIF-1. HEK293 cells were cotransfected with the indicated constructs. CM was collected 48 h after transfection, and protein complexes were precipitated with FLAG antibody beads. Proteins eluted from the beads were separated by SDS-PAGE and probed with peroxidase-conjugated antibodies to human IgG (upper panel). The lower panel shows a Western blot of the CM before immunoprecipitation probed with anti-FLAG antibodies. These experiments were repeated two times.
Rac1 and JNK are activated by Myoc CM.
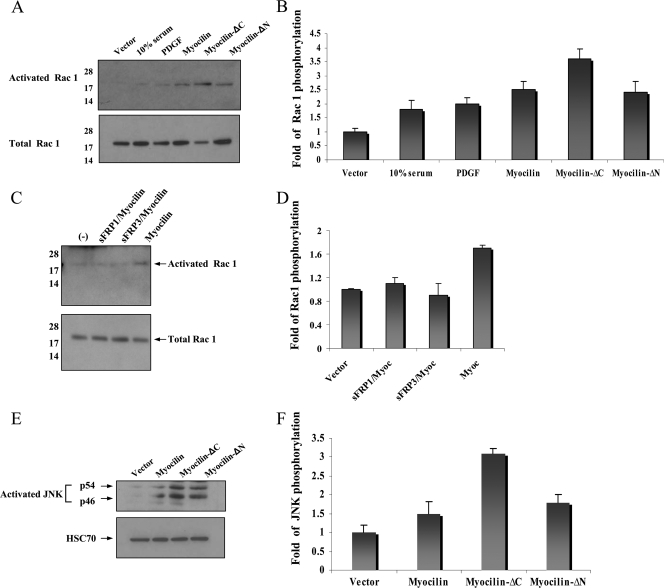
Modification of actin cytoskeleton by the noncanonical Wnt planar cell polarity pathway occurs through activation of the small GTPases RhoA or Rac. Activation of Rho involves Daam1 protein that binds to the PDZ domain of Dishevelled (Dvl). Activation of Rac1 requires the carboxy-terminal DEP domain of Dvl and stimulates JNK activity (28). Active GTP-bounds forms of RhoA and Rac1 were measured as described in Materials and Methods. The levels of active RhoA were low in NIH 3T3 cells treated with control or myocilin CMs for 1 h (data not shown). The levels of active Rac1 were increased after treatment with myocilin CM compared to control CM, with a maximal 3.7-fold increase after about 1 h (Fig. 11A). Full-length myocilin, myocilin-ΔC, and myocilin-ΔN were all able to induce Rac1 activity, with myocilin-ΔC demonstrating the highest stimulatory activity (Fig. 11B). Control Western blotting experiments demonstrated that similar levels of myocilin and its fragments were present in CM (not shown). To test whether myocilin-induced Rac1 activation involves components of Wnt signaling pathways, CM from myocilin-expressing cells was preincubated with sFRP1 or sFRP3 for 20 min before addition to NIH 3T3 cells. Such treatments reduced the levels of active Rac1 to the level observed in control cells (Fig. 11C and D), indicating that activation of Rac1 by myocilin treatment may occur through components of the Wnt signaling pathway. Supporting this conclusion, all tested myocilin constructs induced activation of JNK in NIH 3T3 cells, with myocilin-ΔC producing the highest stimulatory effect (Fig. 11E and F).
FIG. 11.
Myocilin activates Rac1 and JNK. The indicated myocilin CMs were added to NIH 3T3 cells for 30 min, and the levels of activated Rac1 (A) and JNK (E) were measured. The ratio between activated and total Rac1 was about 1:100 for myocilin-ΔC. Preincubation of CM with sFRP1 or sFRP3 eliminated activation of Rac1 by myocilin (C). Panels B, D, and F represent quantification of the results shown in panels A, C, and E, respectively. These experiments were repeated at least twice.
DISCUSSION
In the present study we describe a possible mechanism of myocilin action. Our data demonstrate that myocilin may act as a modulator of Wnt signaling through interactions with several Wnt receptors in the Fzd family and antagonists of Wnt signaling, sFRPs and WIF-1 (Fig. 12). Wnt signaling is critical in developing and adult multicellular organisms and may cross talk with other essential signaling pathways (9, 25, 32). It is involved in cell fate determination, differentiation, cell division, axonal growth, and cell survival. Deregulation of Wnt signaling can lead to cancerous transformation (5). Nineteen Wnt genes encoding secreted proteins were identified in mammals (9, 60). Wnt proteins act as ligands and interact with Fzd receptors and several coreceptors belonging to different families of proteins (6). Ten Fzd genes were identified in mammals (34). Wnts and their receptors show specificity of binding and dynamic expression patterns in the course of development and in adults. Several Wnt, Fzd, and sFRP proteins are expressed in developing and adult mammalian eyes, and it is well established that Wnt signaling plays an important role in normal eye development and in several eye pathologies (11, 43). In particular, Wnt signaling pathways contribute to retinal degeneration, cataract, exudative vitreoretinopathy, ocular tumor, and several congenital eye disorders. Recently, upregulation of sFRP1 has been demonstrated in the trabecular meshwork of people with open-angle glaucoma (85). Overexpression of sFRP1 in the mouse eye after intravitreous injection led to rapid elevation of IOP (85).
FIG. 12.
Schematic diagram of myocilin action. Myocilin may bind Wnt antagonists WIF-1 and sFRPs and compete with Wnt for binding to several Fzd receptors. Proteins that are affected by myocilin treatment are indicated in red. Thin uninterrupted lines with two arrows indicate proteins that interact with each other.
Unlike Wnt genes, the MYOC gene is expressed late in development in both mice and humans. Therefore, the absence of MYOC expression should not affect Wnt signaling during embryonic eye development. Myocilin may start to compete with Wnt proteins postnatally. Although it is difficult to evaluate the relative amounts of myocilin and Wnt proteins in the adult trabecular meshwork, it appears that levels of MYOC RNA may be higher than the levels of Wnt RNAs (77). Our data suggest that the binding affinities of myocilin to sFRP1 and sFRP3 proteins are only slightly lower than those of the Wnt3a protein. It is possible that in the adult trabecular meshwork myocilin and Wnt proteins are equal players in the regulation of Wnt signaling. In many other tissues where myocilin is also expressed (skeletal muscle and brain, for example), its relative expression might be weaker than the expression of Wnt proteins, and myocilin may play a minor role in the regulation of Wnt signaling in these tissues.
Our data demonstrate that myocilin specifically interacts only with some Fzd receptors and sFRPs but not with others. Among Fzd receptors tested by two techniques, coimmunoprecipitation and AP staining, myocilin interacted with Fzd1, Fzd7, and Fzd10 but not with Fzd5. Our data indicate that myocilin may have a broader specificity of binding to different CRDs compared to norrin and, in this respect, is more similar to Xenopus Wnt8, which bound to Fzd4, Fzd5, Fzd7, and Fzd8 in AP staining experiments (70). At present, we do not know whether extracellular loops between transmembrane segments of Fzd receptors contribute to the specificity and affinity of binding of myocilin or whether CRDs alone determine the specificity and affinity of binding. Published data suggest that deletion of the CRD in Drosophila did not eliminate signaling through Fzd receptors (7). Fzd1 and Fzd7 receptors have been reported to be expressed in the cultured trabecular meshwork cells (85, 92). It is interesting that Fzd7 receptor, similar to myocilin, is upregulated by glucocorticoids (35). Fzd7 is highly conserved through evolution (82) and is able to activate both canonical and noncanonical Wnt signaling pathways (53). Two sites of myocilin binding to trabecular meshwork were reported: a saturable site with high affinity (Kd = 4.3 nM) and a nonsaturable site with low affinity (Kd = 23 nM). Only one site with low affinity was observed in fibroblasts (59). It is possible that one or more Fzd receptors contributes to these interactions.
The canonical Wnt signaling pathway relies on the stabilization of β-catenin and its translocation to the nucleus, where it drives transcription of target genes. Although myocilin does not stimulate the activity of the TOPFLASH reporter in transfected HEK293 cells (unpublished observations) and its addition to NIH 3T3 cells does not lead to nuclear accumulation of β-catenin (see Fig. 5), we cannot exclude the possibility that myocilin may modulate canonical Wnt signaling when appropriate receptors and coreceptors are present. Some Wnt proteins, e.g., Wnt5a, may act as an activator or inhibitor of transcription depending upon the repertoire of receptors and coreceptors present (54). We are currently testing the ability of Fzd1, Fzd7, and known Wnt coreceptors to affect β-catenin-dependent transcription in the presence of myocilin.
It has been suggested that sFRP1, sFRP2, and sFRP5 comprise a subfamily that has distinct characteristics in comparison to sFRP3 and sFRP4, probably due to the specificity of binding to Wnt ligands (21, 68). According to the myocilin-AP binding assays, myocilin interacts with sFRPs from both subfamilies. In this assay, myocilin did not interact with the CRD of sFRP1 but interacted well with the CRD of sFRP3. However, when full-length sFRP was used, myocilin was able to interact with both sFRP1 and sFRP3.
It appears that sFRP1 and sFRP3 are not highly expressed in the human trabecular meshwork, retinal pigmented epithelial cells and iris, the sites of myocilin expression (77, 88). As noted above, elevated expression of sFRP1 in the trabecular meshwork has been associated with increased IOP and glaucoma (85). The mechanism of sFRP1 action might involve the inhibition of both Wnt and myocilin signaling.
Our data indicate that myocilin affects noncanonical Wnt signaling and reorganization of the actin cytoskeleton. The addition of extracellular myocilin to human trabecular meshwork cells or mouse NIH 3T3 cells induced the formation of stress fibers. Surprisingly, myocilin-ΔC protein that occurs naturally and does not have the olfactomedin domain is as efficient as full-length myocilin in the induction of stress fibers. Currently, we do not know whether full-length myocilin and myocilin-ΔC perform different biological functions. It has been reported that different forms of olfactomedin 1, with or without the olfactomedin domain, may have distinct roles in neuronal differentiation (55, 58).
Our findings vary from the results described by Shen et al. (69), who reported that overexpression of myocilin in transfected human trabecular meshwork cells induced a loss of stress fibers and focal adhesion. These differences may be due to differences in the level of myocilin expression, the time of cell exposure to myocilin (days versus hours), the sites of myocilin application (intracellular versus extracellular), and the conditions in which the cells were cultivated (serum-containing medium versus serum-free medium).
Reorganization of actin cytoskeleton involves activation of Rac1 and JNK. It has been reported that Rac1 may stabilize the cadherin-β-catenin complex (20). Our data also suggest that myocilin-induced activation of Rac1 is accompanied by the stabilization of β-catenin and its accumulation at the cellular membrane (see Fig. 5). Similarly, others have observed an increase in dephosphorylated and total β-catenin at the plasma membrane after activation of Wnt signaling (29). Much evidence indicates that the state of the actin cytoskeleton of trabecular meshwork cells is important in determining aqueous humor resistance. Increased aqueous humor resistance may lead to elevation of IOP, which is the main risk factor in glaucoma. Cytochalasin B or D and misakinolide A, drugs that disrupt the actin cytoskeleton, decrease outflow resistance in living monkey and organ-cultured human eyes (40, 76, 83). Treatment of human ocular anterior segment with dexamethasone promoted formation of cross-linked actin network and was accompanied by elevation of IOP (8). The actin cytoskeleton is essential for establishing proper cell contacts with extracellular matrix and with each other through interactions with the components of focal adhesions and adherens junctions. Modulation of Wnt signaling by myocilin may provide a useful tool in the reorganization of cytoskeleton of trabecular meshwork cells and regulation of intraocular pressure. However, our results do not exclude the possibility that myocilin may regulate other pathways in addition to Wnt signaling.
In conclusion, myocilin may be a new modulator of Wnt signaling. It appeared relatively late in evolution, since definitive myocilin orthologs have not been identified in lower vertebrates. Myocilin and Wnt proteins may have redundant functions in the postnatal mammalian eye. Redundancy of function is common for biologically important processes, and myocilin may provide another example of this phenomenon. When myocilin is deleted, Wnt proteins or other members of the family of olfactomedin domain-containing proteins may play a compensatory role and mask the potentially deleterious effects of myocilin deletion.
Acknowledgments
We thank J. Nathans and J.-C. Hsieh for the expression constructs and P. Russell for trabecular meshwork cell lines.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Eye Institute and National Cancer Institute.
Footnotes
Published ahead of print on 2 February 2009.
REFERENCES
- 1.Adam, M. F., A. Belmouden, P. Binisti, A. P. Brezin, F. Valtot, A. Bevhetoille, J.-C. Dascotte, B. Copin, L. Gomez, A. Chaventre, J.-F. Bach, and H.-J. Garchon. 1997. Recurrent mutations in a single exon encoding the evolutionary conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum. Mol. Genet. 62091-2097. [DOI] [PubMed] [Google Scholar]
- 2.Alward, W. L., Y. H. Kwon, C. L. Khanna, A. T. Johnson, S. S. Hayreh, M. B. Zimmerman, J. Narkiewicz, J. L. Andorf, P. A. Moore, J. H. Fingert, V. C. Sheffield, and E. M. Stone. 2002. Variations in the myocilin gene in patients with open-angle glaucoma. Arch. Ophthalmol. 1201189-1197. [DOI] [PubMed] [Google Scholar]
- 3.Aroca-Aguilar, J. D., F. Sanchez-Sanchez, S. Ghosh, M. Coca-Prados, and J. Escribano. 2005. Myocilin mutations causing glaucoma inhibit the intracellular endoproteolytic cleavage of myocilin between amino acids Arg226 and Ile227. J. Biol. Chem. 28021043-21051. [DOI] [PubMed] [Google Scholar]
- 4.Barembaum, M., T. A. Moreno, C. LaBonne, J. Sechrist, and M. Bronner-Fraser. 2000. Noelin-1 is a secreted glycoprotein involved in generation of the neural crest. Nature Cell Biol. 2219-225. [DOI] [PubMed] [Google Scholar]
- 5.Barker, N., and H. Clevers. 2006. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 5997-1014. [DOI] [PubMed] [Google Scholar]
- 6.Cadigan, K. M., and Y. I. Liu. 2006. Wnt signaling: complexity at the surface. J. Cell Sci. 119395-402. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C. M., W. Strapps, A. Tomlinson, and G. Struhl. 2004. Evidence that the cysteine-rich domain of Drosophila Frizzled family receptors is dispensable for transducing Wingless. Proc. Natl. Acad. Sci. USA 10115961-15966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, A. F., D. Brotchie, A. T. Read, P. Hellberg, S. English-Wright, I. H. Pang, C. R. Ethier, and I. Grierson. 2005. Dexamethasone alters F-actin architecture and promotes cross-linked actin network formation in human trabecular meshwork tissue. Cell Motil. Cytoskeleton 6083-95. [DOI] [PubMed] [Google Scholar]
- 9.Clevers, H. 2006. Wnt/beta-catenin signaling in development and disease. Cell 127469-480. [DOI] [PubMed] [Google Scholar]
- 10.Danielson, P. E., S. Forss-Petter, E. L. Battenberg, L. deLecea, F. E. Bloom, and J. G. Sutcliffe. 1994. Four structurally distinct neuron-specific olfactomedin-related glycoproteins produced by differential promoter utilization and alternative mRNA splicing from a single gene. J. Neurosci. Res. 38468-478. [DOI] [PubMed] [Google Scholar]
- 11.De Iongh, R. U., H. E. Abud, and G. R. Hime. 2006. WNT/Frizzled signaling in eye development and disease. Front. Biosci. 112442-2464. [DOI] [PubMed] [Google Scholar]
- 12.Endo, Y., V. Wolf, K. Muraiso, K. Kamijo, L. Soon, A. Uren, M. Barshishat-Kupper, and J. S. Rubin. 2005. Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J. Biol. Chem. 280777-786. [DOI] [PubMed] [Google Scholar]
- 13.Escribano, J., J. Ortego, and M. Coca-Prados. 1995. Isolation and characterization of cell-specific cDNA clones from a subtractive library of the ocular ciliary body of a single normal human donor: transcription and synthesis of plasma proteins. J. Biochem. 118921-931. [DOI] [PubMed] [Google Scholar]
- 14.Eshed, Y., K. Feinberg, D. J. Carey, and E. Peles. 2007. Secreted gliomedin is a perinodal matrix component of peripheral nerves. J. Cell Biol. 177551-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eshed, Y., K. Feinberg, S. Poliak, H. Sabanay, O. Sarig-Nadir, I. Spiegel, J. R. Bermingham, Jr., and E. Peles. 2005. Gliomedin mediates Schwann cell-axon interaction and the molecular assembly of the nodes of Ranvier. Neuron 47215-229. [DOI] [PubMed] [Google Scholar]
- 16.Fautsch, M. P., A. M. Vrabel, and D. H. Johnson. 2006. The identification of myocilin-associated proteins in the human trabecular meshwork. Exp. Eye Res. 821046-1052. [DOI] [PubMed] [Google Scholar]
- 17.Finch, P. W., X. He, M. J. Kelley, A. Uren, R. P. Schaudies, N. C. Popescu, S. Rudikoff, S. A. Aaronson, H. E. Varmus, and J. S. Rubin. 1997. Purification and molecular cloning of a secreted, Frizzled-related antagonist of Wnt action. Proc. Natl. Acad. Sci. USA 946770-6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fingert, J. H., E. Heon, J. M. Liebmann, T. Yamamoto, J. E. Craig, J. Rait, K. Kawase, S. T. Hoh, Y. M. Buys, J. Dickinson, R. R. Hockey, D. Williams-Lyn, G. Trope, Y. Kitazawa, R. Ritch, D. A. Mackey, W. L. Alward, V. C. Sheffield, and E. M. Stone. 1999. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum. Mol. Genet. 8899-905. [DOI] [PubMed] [Google Scholar]
- 19.Fingert, J. H., E. M. Stone, V. C. Sheffield, and W. L. Alward. 2002. Myocilin glaucoma. Surv. Ophthalmol. 47547-561. [DOI] [PubMed] [Google Scholar]
- 20.Fukata, M., S. Kuroda, M. Nakagawa, A. Kawajiri, N. Itoh, I. Shoji, Y. Matsuura, S. Yonehara, H. Fujisawa, A. Kikuchi, and K. Kaibuchi. 1999. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J. Biol. Chem. 27426044-26050. [DOI] [PubMed] [Google Scholar]
- 21.Galli, L. M., T. Barnes, T. Cheng, L. Acosta, A. Anglade, K. Willert, R. Nusse, and L. W. Burrus. 2006. Differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2, and Sfrp-3. Dev. Dyn. 235681-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gobeil, S., M. A. Rodrigue, S. Moisan, T. D. Nguyen, J. R. Polansky, J. Morissette, and V. Raymond. 2004. Intracellular sequestration of hetero-oligomers formed by wild-type and glaucoma-causing myocilin mutants. Investig. Ophthalmol. Vis. Sci. 453560-3567. [DOI] [PubMed] [Google Scholar]
- 23.Goldwich, A., C. R. Ethier, D. W. Chan, and E. R. Tamm. 2003. Perfusion with the olfactomedin domain of myocilin does not affect outflow facility. Investig. Ophthalmol. Vis. Sci. 441953-1961. [DOI] [PubMed] [Google Scholar]
- 24.Gong, G., O. Kosoko-Lasaki, G. R. Haynatzki, and M. R. Wilson. 2004. Genetic dissection of myocilin glaucoma. Hum. Mol. Genet. 13R91-R102. [DOI] [PubMed] [Google Scholar]
- 25.Gordon, M. D., and R. Nusse. 2006. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 28122429-22433. [DOI] [PubMed] [Google Scholar]
- 26.Gould, D. B., L. Miceli-Libby, O. V. Savinova, M. Torrado, S. I. Tomarev, R. S. Smith, and S. W. John. 2004. Genetically increasing myoc expression supports a necessary pathologic role of abnormal proteins in glaucoma. Mol. Cell. Biol. 249019-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graveel, C. R., S. R. Harkins-Perry, L. G. Acevedo, and P. J. Farnham. 2003. Identification and characterization of CRG-L2, a new marker for liver tumor development. Oncogene 221730-1736. [DOI] [PubMed] [Google Scholar]
- 28.Habas, R., I. B. Dawid, and X. He. 2003. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 17295-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hendriksen, J., M. Jansen, C. M. Brown, H. van der Velde, M. van Ham, N. Galjart, G. J. Offerhaus, F. Fagotto, and M. Fornerod. 2008. Plasma membrane recruitment of dephosphorylated β-catenin upon activation of the Wnt pathway. J. Cell Sci. 1211793-1802. [DOI] [PubMed] [Google Scholar]
- 30.Hillier, B. J., and V. D. Vacquier. 2003. Amassin, an olfactomedin protein, mediates the massive intercellular adhesion of sea urchin coelomocytes. J. Cell Biol. 160597-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoang, B., M. Moos, Jr., S. Vukicevic, and F. P. Luyten. 1996. Primary structure and tissue distribution of FRZB, a novel protein related to Drosophila frizzled, suggest a role in skeletal morphogenesis. J. Biol. Chem. 27126131-26137. [DOI] [PubMed] [Google Scholar]
- 32.Hoppler, S., and C. L. Kavanagh. 2007. Wnt signalling: variety at the core. J. Cell Sci. 120385-393. [DOI] [PubMed] [Google Scholar]
- 33.Hsieh, J. C., L. Kodjabachian, M. L. Rebbert, A. Rattner, P. M. Smallwood, C. H. Samos, R. Nusse, I. B. Dawid, and J. Nathans. 1999. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature 398431-436. [DOI] [PubMed] [Google Scholar]
- 34.Huang, H. C., and P. S. Klein. 2004. The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol. 5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurson, C. J., J. S. Butler, D. T. Keating, D. W. Murray, D. M. Sadlier, J. M. O'Byrne, and P. P. Doran. 2007. Gene expression analysis in human osteoblasts exposed to dexamethasone identifies altered developmental pathways as putative drivers of osteoporosis. BMC Musculoskelet. Disord. 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobson, N., M. Andrews, A. R. Shepard, D. Nishimura, C. Searby, J. H. Fingert, G. Hageman, R. Mullins, B. L. Davidson, Y. H. Kwon, W. L. Alward, E. M. Stone, A. F. Clark, and V. C. Sheffield. 2001. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum. Mol. Genet. 10117-125. [DOI] [PubMed] [Google Scholar]
- 37.Joe, M. K., S. Sohn, Y. R. Choi, H. Park, and C. Kee. 2005. Identification of flotillin-1 as a protein interacting with myocilin: implications for the pathogenesis of primary open-angle glaucoma. Biochem. Biophys. Res. Commun. 3361201-1206. [DOI] [PubMed] [Google Scholar]
- 38.Joe, M. K., S. Sohn, W. Hur, Y. Moon, Y. R. Choi, and C. Kee. 2003. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem. Biophys. Res. Commun. 312592-600. [DOI] [PubMed] [Google Scholar]
- 39.Karali, A., P. Russell, F. H. Stefani, and E. R. Tamm. 2000. Localization of myocilin/trabecular meshwork-inducible glucocorticoid response protein in the human eye. Investig. Ophthalmol. Vis. Sci. 41729-740. [PubMed] [Google Scholar]
- 40.Kaufman, P. L., and K. A. Erickson. 1982. Cytochalasin B and D dose-outflow facility response relationships in the cynomolgus monkey. Investig. Ophthalmol. Vis. Sci. 23646-650. [PubMed] [Google Scholar]
- 41.Kawano, Y., and R. Kypta. 2003. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 1162627-2634. [DOI] [PubMed] [Google Scholar]
- 42.Kim, B. S., O. V. Savinova, M. V. Reedy, J. Martin, Y. Lun, L. Gan, R. S. Smith, S. I. Tomarev, S. W. John, and R. L. Johnson. 2001. Targeted disruption of the myocilin gene (Myoc) suggests that human glaucoma-causing mutations are gain of function. Mol. Cell. Biol. 217707-7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubo, F., and S. Nakagawa. 2008. Wnt signaling in retinal stem cells and regeneration. Dev. Growth Differ. 50245-251. [DOI] [PubMed] [Google Scholar]
- 44.Kulkarni, N. H., C. A. Karavanich, W. R. Atchley, and R. R. Anholt. 2000. Characterization and differential expression of a human gene family of olfactomedin-related proteins. Genet. Res. 7641-50. [DOI] [PubMed] [Google Scholar]
- 45.Lam, D. S., Y. F. Leung, J. K. Chua, L. Baum, D. S. Fan, K. W. Choy, and C. P. Pang. 2000. Truncations in the TIGR gene in individuals with and without primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 411386-1391. [PubMed] [Google Scholar]
- 46.Lee, H. S., and S. I. Tomarev. 2007. Optimedin induces expression of N-cadherin and stimulates aggregation of NGF-stimulated PC12 cells. Exp. Cell Res. 31398-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leyns, L., T. Bouwmeester, S. H. Kim, S. Piccolo, and E. M. De Robertis. 1997. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88747-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li, Y., J. D. Aroca-Aguilar, S. Ghosh, F. Sanchez-Sanchez, J. Escribano, and M. Coca-Prados. 2006. Interaction of myocilin with the C-terminal region of hevin. Biochem. Biophys. Res. Commun. 339797-804. [DOI] [PubMed] [Google Scholar]
- 49.Liu, Y., and D. Vollrath. 2004. Reversal of mutant myocilin non-secretion and cell killing: implications for glaucoma. Hum. Mol. Genet. 131193-1204. [DOI] [PubMed] [Google Scholar]
- 50.Logan, C. Y., and R. Nusse. 2004. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20781-810. [DOI] [PubMed] [Google Scholar]
- 51.Loria, P. M., J. Hodgkin, and O. Hobert. 2004. A conserved postsynaptic transmembrane protein affecting neuromuscular signaling in Caenorhabditis elegans. J. Neurosci. 242191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malyukova, I., H. S. Lee, R. N. Fariss, and S. I. Tomarev. 2006. Mutated mouse and human myocilins have similar properties and do not block general secretory pathway. Investig. Ophthalmol. Vis. Sci. 47206-212. [DOI] [PubMed] [Google Scholar]
- 53.Medina, A., W. Reintsch, and H. Steinbeisser. 2000. Xenopus frizzled 7 can act in canonical and non-canonical Wnt signaling pathways: implications on early patterning and morphogenesis. Mech. Dev. 92227-237. [DOI] [PubMed] [Google Scholar]
- 54.Mikels, A. J., and R. Nusse. 2006. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 4e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreno, T. A., and M. Bronner-Fraser. 2005. Noelins modulate the timing of neuronal differentiation during development. Dev. Biol. 288434-447. [DOI] [PubMed] [Google Scholar]
- 56.Moustakas, A., and C. Stournaras. 1999. Regulation of actin organisation by TGF-β in H-ras-transformed fibroblasts. J. Cell Sci. 1121169-1179. [DOI] [PubMed] [Google Scholar]
- 57.Mukhopadhyay, A., S. Talukdar, A. Bhattacharjee, and K. Ray. 2004. Bioinformatic approaches for identification and characterization of olfactomedin related genes with a potential role in pathogenesis of ocular disorders. Mol. Vis. 10304-314. [PubMed] [Google Scholar]
- 58.Nakaya, N., H. S. Lee, Y. Takada, I. Tzchori, and S. I. Tomarev. 2008. Zebrafish olfactomedin 1 regulates retinal axon elongation in vivo and is a modulator of Wnt signaling pathway. J. Neurosci. 287900-7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen, T. D., P. Chen, W. D. Huang, H. Chen, D. Johnson, and J. R. Polansky. 1998. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J. Biol. Chem. 2736341-6350. [DOI] [PubMed] [Google Scholar]
- 60.Nusse, R. 2005. Wnt signaling in disease and in development. Cell Res. 1528-32. [DOI] [PubMed] [Google Scholar]
- 61.Peters, D. M., K. Herbert, B. Biddick, and J. A. Peterson. 2005. Myocilin binding to Hep II domain of fibronectin inhibits cell spreading and incorporation of paxillin into focal adhesions. Exp. Cell Res. 303218-228. [DOI] [PubMed] [Google Scholar]
- 62.Quigley, H. A., and A. T. Broman. 2006. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 90262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao, P. V., R. R. Allingham, and D. L. Epstein. 2000. TIGR/myocilin in human aqueous humor. Exp. Eye Res. 71637-641. [DOI] [PubMed] [Google Scholar]
- 64.Rattner, A., J. C. Hsieh, P. M. Smallwood, D. J. Gilbert, N. G. Copeland, N. A. Jenkins, and J. Nathans. 1997. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc. Natl. Acad. Sci. USA 942859-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Russell, P., E. R. Tamm, F. J. Grehn, G. Picht, and M. Johnson. 2001. The presence and properties of myocilin in the aqueous humor. Investig. Ophthalmol. Vis. Sci. 42983-986. [PubMed] [Google Scholar]
- 66.Sanchez-Sanchez, F., F. Martinez-Redondo, J. D. Aroca-Aguilar, M. Coca-Prados, and J. Escribano. 2007. Characterization of the intracellular proteolytic cleavage. Biol. Chem. 28227810-27824. [DOI] [PubMed] [Google Scholar]
- 67.Sato, A., D. K. Khadka, W. Liu, R. Bharti, L. W. Runnels, I. B. Dawid, and R. Habas. 2006. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development 1334219-4231. [DOI] [PubMed] [Google Scholar]
- 68.Satoh, W., M. Matsuyama, H. Takemura, S. Aizawa, and A. Shimono. 2008. Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/β-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis 4692-103. [DOI] [PubMed] [Google Scholar]
- 69.Shen, X., T. Koga, B. C. Park, N. SundarRaj, and B. Y. Yue. 2008. Rho GTPase and cAMP/protein kinase A signaling mediates myocilin-induced alterations in cultured human trabecular meshwork cells. J. Biol. Chem. 283603-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smallwood, P. M., J. Williams, Q. Xu, D. J. Leahy, and J. Nathans. 2007. Mutational analysis of Norrin-Frizzled4 recognition. J. Biol. Chem. 2824057-4068. [DOI] [PubMed] [Google Scholar]
- 71.Snyder, D. A., A. M. Rivers, H. Yokoe, B. P. Menco, and R. R. Anholt. 1991. Olfactomedin: purification, characterization, and localization of a novel olfactory glycoprotein. Biochemistry 309143-9153. [DOI] [PubMed] [Google Scholar]
- 72.Stone, E. M., J. H. Fingert, W. M. Alward, T. D. Nguyen, J. R. Polansky, S. F. Sunden, D. Nishimura, A. F. Clark, A. Nystuen, B. E. Nichols, D. A. Mackey, R. Ritch, J. W. Kalenak, E. R. Craven, and V. C. Sheffield. 1997. Identification of a gene that causes primary open angle glaucoma. Science 275668-670. [DOI] [PubMed] [Google Scholar]
- 73.Surgucheva, I., B. C. Park, B. Y. Yue, S. Tomarev, and A. Surguchov. 2005. Interaction of myocilin with gamma-synuclein affects its secretion and aggregation. Cell. Mol. Neurobiol. 251009-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swiderski, R. E., J. L. Ross, J. H. Fingert, A. F. Clark, W. L. Alward, E. M. Stone, and V. C. Sheffield. 2000. Localization of MYOC transcripts in human eye and optic nerve by in situ hybridization. Investig. Ophthalmol. Vis. Sci. 413420-3428. [PubMed] [Google Scholar]
- 75.Tamm, E. R. 2002. Myocilin and glaucoma: facts and ideas. Prog. Retin. Eye Res. 21395-428. [DOI] [PubMed] [Google Scholar]
- 76.Tian, B., B. Geiger, D. L. Epstein, and P. L. Kaufman. 2000. Cytoskeletal involvement in the regulation of aqueous humor outflow. Investig. Ophthalmol. Vis. Sci. 41619-623. [PubMed] [Google Scholar]
- 77.Tomarev, S. I., G. Wistow, V. Raymond, S. Dubois, and I. Malyukova. 2003. Gene expression profile of the human trabecular meshwork: NEIBank sequence tag analysis. Investig. Ophthalmol. Vis. Sci. 442588-2596. [DOI] [PubMed] [Google Scholar]
- 78.Torrado, M., R. Trivedi, R. Zinovieva, I. Karavanova, and S. I. Tomarev. 2002. Optimedin: a novel olfactomedin-related protein that interacts with myocilin. Hum. Mol. Genet. 111291-1301. [DOI] [PubMed] [Google Scholar]
- 79.Tsuda, H., N. Sasai, M. Matsuo-Takasaki, M. Sakuragi, Y. Murakami, and Y. Sasai. 2002. Dorsalization of the neural tube by Xenopus tiarin, a novel patterning factor secreted by the flanking nonneural head ectoderm. Neuron 33515-528. [DOI] [PubMed] [Google Scholar]
- 80.Tumminia, S. J., K. P. Mitton, J. Arora, P. Zelenka, D. L. Epstein, and P. Russell. 1998. Mechanical stretch alters the actin cytoskeletal network and signal transduction in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 391361-1371. [PubMed] [Google Scholar]
- 81.Uren, A., F. Reichsman, V. Anest, W. G. Taylor, K. Muraiso, D. P. Bottaro, S. Cumberledge, and J. S. Rubin. 2000. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. J. Biol. Chem. 2754374-4382. [DOI] [PubMed] [Google Scholar]
- 82.Vincan, E., R. K. Swain, T. Brabletz, and H. Steinbeisser. 2007. Frizzled7 dictates embryonic morphogenesis: implications for colorectal cancer progression. Front. Biosci. 124558-4567. [DOI] [PubMed] [Google Scholar]
- 83.Wang, H., X. Liu, L. Guo, B. T. Gabelt, P. Y. Lee, S. M. Podos, N. Wang, and P. L. Kaufman. 2007. Effects of MISA A on actin cytoskeleton of cultured HTM cells and intraocular pressure of rats and glaucomatous monkeys. Curr. Eye Res. 32843-850. [DOI] [PubMed] [Google Scholar]
- 84.Wang, S., M. Krinks, K. Lin, F. P. Luyten, and M. Moos, Jr. 1997. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 88757-766. [DOI] [PubMed] [Google Scholar]
- 85.Wang, W. H., L. G. McNatt, I. H. Pang, J. C. Millar, P. E. Hellberg, M. H. Hellberg, H. T. Steely, J. S. Rubin, J. H. Fingert, V. C. Sheffield, E. M. Stone, and A. F. Clark. 2008. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J. Clin. Investig. 1181056-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wawrzak, D., M. Metioui, E. Willems, M. Hendrickx, E. de Genst, and L. Leyns. 2007. Wnt3a binds to several sFRPs in the nanomolar range. Biochem. Biophys. Res. Commun. 3571119-1123. [DOI] [PubMed] [Google Scholar]
- 87.Wiggs, J. L., and D. Vollrath. 2001. Molecular and clinical evaluation of a patient hemizygous for TIGR/MYOC. Arch. Ophthalmol. 1191674-1678. [DOI] [PubMed] [Google Scholar]
- 88.Wistow, G., S. L. Bernstein, M. K. Wyatt, R. N. Fariss, A. Behal, J. W. Touchman, G. Bouffard, D. Smith, and K. Peterson. 2002. Expressed sequence tag analysis of human RPE/choroid for the NEIBank Project: over 6000 non-redundant transcripts, novel genes and splice variants. Mol. Vis. 8205-220. [PubMed] [Google Scholar]
- 89.Wistow, G., S. L. Berstein, S. Ray, M. K. Wyatt, A. Behal, J. W. Touchman, G. Bouffard, D. Smith, and K. Peterson. 2002. Expressed sequence tag analysis of adult human iris for the NEIBank Project: steroid-response factors and similarities with retinal pigment epithelium. Mol. Vis. 8185-195. [PubMed] [Google Scholar]
- 90.Yokoe, H., and R. R. Anholt. 1993. Molecular cloning of olfactomedin, an extracellular matrix protein specific to olfactory neuroepithelium. Proc. Natl. Acad. Sci. USA 904655-4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeng, L. C., Z. G. Han, and W. J. Ma. 2005. Elucidation of subfamily segregation and intramolecular coevolution of the olfactomedin-like proteins by comprehensive phylogenetic analysis and gene expression pattern assessment. FEBS Lett. 5795443-5453. [DOI] [PubMed] [Google Scholar]
- 92.Zhao, X., K. E. Ramsey, D. A. Stephan, and P. Russell. 2004. Gene and protein expression changes in human trabecular meshwork cells treated with transforming growth factor-beta. Investig. Ophthalmol. Vis. Sci. 454023-4034. [DOI] [PubMed] [Google Scholar]
- 93.Zhou, Y., O. Grinchuk, and S. I. Tomarev. 2008. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Investig. Ophthalmol. Vis. Sci. 491932-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]