Abstract
Wnt signaling is implicated in a variety of developmental and pathological processes. The molecular mechanisms governing the secretion of Wnt ligands remain to be elucidated. Wntless, an evolutionarily conserved multipass transmembrane protein, is a dedicated secretion factor of Wnt proteins that participates in Drosophila melanogaster embryogenesis. In this study, we show that Xenopus laevis Wntless (XWntless) regulates the secretion of a specific Wnt ligand, XWnt4, and that this regulation is specifically required for eye development in Xenopus. Moreover, the Retromer complex is required for XWntless recycling to regulate the XWnt4-mediated eye development. Inhibition of Retromer function by Vps35 morpholino (MO) resulted in various Wnt deficiency phenotypes, affecting mesoderm induction, gastrulation cell movements, neural induction, neural tube closure, and eye development. Overexpression of XWntless led to the rescue of Vps35 MO-mediated eye defects but not other deficiencies. These results collectively suggest that XWntless and the Retromer complex are required for the efficient secretion of XWnt4, facilitating its role in Xenopus eye development.
The Wnt family of glycoproteins comprises one of the largest families of paracrine factors essential for embryonic development and adult tissue homeostasis (reviewed at the Wnt Homepage, http://www.stanford.edu/∼rnusse/wntwindow.html). It regulates several aspects of biological processes, including cell fate specification, proliferation, migration, and polarity formation (29, 48). The Wnt signaling pathway is initiated by Wnt ligands secreted from Wnt-producing cells. The ligands bind to frizzled receptors and coreceptors expressed on the receiving cells. Wnt ligand perception (5, 24, 46, 49), signaling cascades into receiving cells (8, 27), and the consequences of gene expression (17) or cytoskeletal changes (36) are well documented in various contexts. However, relatively limited information is available about the processes in Wnt-producing cells and extracellular spaces. The establishment of the concentration gradient of the Wnt ligand in the extracellular space is mediated by lipoprotein particle formation (33). The Retromer complex is additionally required in Wnt-producing cells and for long-range secretion of Wnt (12). Porcupine is essential for posttranslational modifications, which may be essential for the proper folding and secretion of Wnt ligands (28).
Recent achievements in Drosophila melanogaster genetics and genomic RNA interference screening have revealed the existence of a new component of the Wnt secretory pathway, specifically, a dedicated secretion factor of Wg designated Wntless (2), Evi (3), or Sprinter (16). Wntless is an evolutionarily conserved multipass transmembrane protein required solely for Wg secretion. Wntless is not essential for the palmitoylation of Wg, indicating that it does not act on functional Wg production, like Porcupine, another evolutionarily conserved multipass transmembrane protein. Wntless is a regulator of intracellular Wg trafficking in Wg-producing cells. Its function is conserved in other species, including Caenorhabditis elegans and humans (2, 3). However, the mechanisms of Wntless action and the specificity for various Wnt isotypes are not well understood at present.
The Retromer complex constitutes the basic machinery for retrograde transport of vesicles from endosomes to the trans-Golgi network (7). In addition to its role in general vesicle transport, the Retromer complex is involved specifically in the initiation of Wnt signaling. Mutation of Vps35, the major component of the Retromer complex, impairs long-range Wnt signaling in C. elegans (12). Recent studies show that the Retromer complex is required for Wntless recycling and thereby contributes to Wnt secretion (4, 15, 32, 35, 50). However, other than its role in Wntless recycling, limited information is available on Retromer complex function in Wnt signaling.
In this study, we show that Xenopus laevis Wntless (XWntless) is functionally equivalent to its Drosophila counterpart in regulating the secretion of Wnt but acts on a specific ligand, XWnt4. The results of phenotypic analysis and secretion assays demonstrate that the function of the Retromer complex in Wnt signaling is not wholly dependent on its participation in Wntless recycling. Our results collectively indicate that Wntless and the Retromer complex are required for Xenopus eye development, possibly via regulation of XWnt4 secretion. However, other Wnt deficiency phenotypes elicited by loss of a Retromer complex component, Vps35, are independent of Wntless recycling.
MATERIALS AND METHODS
DNA, MO oligonucleotides, mRNA, and antisense RNA probe.
MGC132243, the Xenopus homologue of Drosophila Wntless cDNA, was PCR amplified, and the hemagglutinin (HA) epitope was tagged at the 3′ end. These were named XWntless and XWntless-HA. Human Wntless cDNA was PCR amplified, and the myc epitope was tagged at the 3′ end. An antisense morpholino (MO) oligonucleotide of XWntless was generated targeting part of the 5′ untranslated region (UTR) and open reading frame, designed as follows: 5′-AATGATAGCCCCAGCCATACTGTAT-3′. An antisense MO oligonucleotide of Xenopus laevis Vps35 was generated targeting the 5′ UTR and open reading frame sequence which is the region equivalent to the previously described Xenopus tropicalis Vps35 MO target (12), designed as follows: 5′-GGACTGCTGGGTCGTGGGCATCATC-3′. mRNAs were generated by using an mMessage mMachine kit (Ambion), following the manufacturer's protocol. Antisense digoxigenin (DIG)-labeled RNA (Roche) probes were generated as described previously (21).
Xenopus embryo manipulation, in situ hybridization, and histological analysis.
Xenopus laevis eggs were obtained and fertilized as described previously (21). Nieuwkoop and Faber stages were used for the developmental staging (31). mRNAs were expressed by microinjection into the embryos.
In situ hybridization was performed as described previously (18). DIG-labeled antisense RNA probes were hybridized, and the hybridizations were detected by using alkaline phosphatase-conjugated anti-DIG antibody (Roche), stained with BM purple as a substrate (Roche), and photographed.
For histological analysis, embryos were fixed with MEMFA (0.1 M morpholinepropanesulfonic acid [MOPS; pH 7.4], 2 mM EGTA, 1 mM MgSO4, and 4% formaldehyde), dehydrated with methanol, and paraffin molded. Hematoxylin and eosin staining was performed with sectioned samples.
BrdU staining.
Bromodeoxyuridine (BrdU) staining (34) was performed as previously described. For the BrdU in situ section staining, embryos were injected in the belly with BrdU before 3 h of fixation, fixed in MEMFA for 1 h at room temperature, and stored in 100% methanol at −20°C. Fixed embryos were embedded in paraffin. The blocks were sectioned in thicknesses of 10 to 12 μm. Paraffin-embedded sections were dewaxed, and mouse anti-BrdU antibody (BD Biosciences, Pharmingen) was used at a concentration of 1:100. Fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (Sigma) was used for fluorescence detection. In all immunohistochemistry, Hoechst stain (Sigma) was used for nuclear staining at a concentration of 1:5,000.
Secretion assay.
Secretion assays of myc-tagged XWnt1, XWnt3a, XWnt4, XWnt5a, XWnt8, and XWnt11 were performed in an animal cap assay system modified from a previous protocol (23). Four-cell-stage embryos were injected in the animal pole region of all blastomeres with mRNAs or MO as indicated. At the blastula to early gastrula stages, animal caps were dissected in calcium- and magnesium-free MBS (88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 10 mM HEPES, pH 7.4) and incubated in the same solution for 3 h (secretion assays with XWntless MO) or for 6 to 9 h (secretion assays with Vps35 MO) with slow rotation. Supernatants and cells were collected and subjected to Western blotting with mouse monoclonal anti-myc antibody (1:1,000; Santa Cruz) or mouse monoclonal antiactin antibody (Santa Cruz).
RESULTS
Cloning and expression pattern of XWntless in Xenopus laevis.
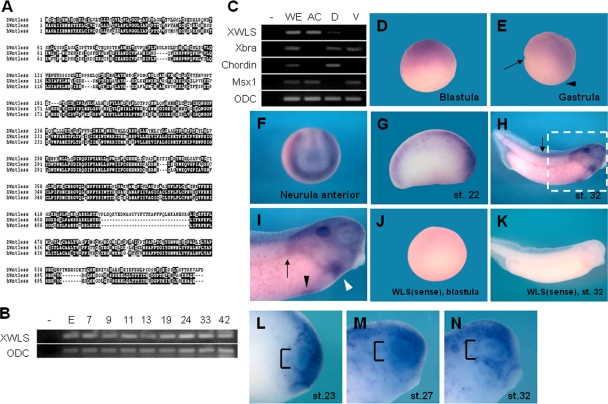
Wntless/Evi/Sprinter was initially identified as a dedicated Wnt secretion factor in the invertebrate Drosophila melanogaster. During Drosophila embryogenesis, mutations in wntless/evi/sprinter specifically impair Wg signaling, inducing phenotypes such as a loss-of-wing margin and segment polarity defects (2, 3, 16). To determine the roles of Wntless in Wnt signaling during vertebrate embryogenesis, we isolated a homologue in Xenopus. Xenopus laevis MGC132243, or XWntless, is a putative Wntless homolog displaying 41.7% and 74.5% amino acid sequence identity with the Drosophila and human proteins, respectively (Fig. 1A). To establish the roles of XWntless during Xenopus embryogenesis, we initially examined its expression patterns. Temporal expression analyzed by using reverse transcription-PCR (RT-PCR) disclosed that XWntless was expressed maternally (egg), which continued after the onset of zygotic gene expression (stage 9) to the tadpole stage (stage 42) (Fig. 1B). Spatially, XWntless was enriched in the animal hemisphere of the early cleavage embryo, which persisted until the late gastrula stage (Fig. 1C, D, and E). The dorsal and the ventral marginal zones were almost devoid of XWntless expression during the gastrula stage (Fig. 1C and E). At the neurula stage, strong expression was evident at the border of the neural plate and dorsal midline (Fig. 1F). After the neurula stage, XWntless was detected in various organs, including the eye, liver, heart, pronephros, otic vesicle, and dorsal neural tube (Fig. 1G to I). Dynamic expression occurred in the developing eye. In particular, XWntless was expressed in the eye field, from stages 23 to 27. From stage 30, XWntless expression was confined to distinct regions, i.e., the central part and border of the eye (Fig. 1L to N).
FIG. 1.
Dynamic expression pattern of XWntless during Xenopus embryogenesis. (A) Sequence similarity among Drosophila Wntless (DWntless), Xenopus Wntless (XWntless), and human Wntless (hWntless). (B) Temporal expression pattern of XWntless was analyzed by RT-PCR. Numbers indicate developmental stages. E, egg stage; −, without reverse transcription. (C) Spatial expression pattern of XWntless was analyzed by RT-PCR of dissected tissues of gastrula embryo. −, without reverse transcription; WE, whole embryo; AC, animal cap explant; D, dorsal marginal explant; V, ventral marginal explant. Xbra was used for marginal expression control, Chordin for dorsal marginal expression control, Msx1 for animal cap and ventral marginal expression control, and ODC for loading control. (D to I) Expression pattern of XWntless was analyzed by in situ hybridization. (D and E) XWntless is expressed at the animal hemisphere at blastula and gastrula stage. XWntless is not detected at the dorsal and ventral marginal zone of gastrula embryo (arrow and arrowhead, respectively). (F) At neurula stage, XWntless is expressed broadly throughout the dorsal side of the embryo and strongly at the borders of lateral and anterior neural plate. (G) At stage 22, XWntless is expressed at the eye primordium and neural tube. (H and I) At stage 32, XWntless is expressed at the liver (black arrowhead), heart (white arrowhead), pronephros (arrow in panel I), and dorsal neural tube, including brain and spinal cord (arrow in panel H). Panel I is an enlarged image of the portion of panel H enclosed by the white dashed line. (J and K) XWntless sense probe was hybridized as a control. (L to N) Eye expression of XWntless is dynamically changing. At stages 23 and 27, XWntless is expressed broadly at the eye field, and at stage 32, XWntless is restricted to distinct regions, the center and the border of the eye. WLS, XWntless; st., stage.
Functions of XWntless during Xenopus eye development.
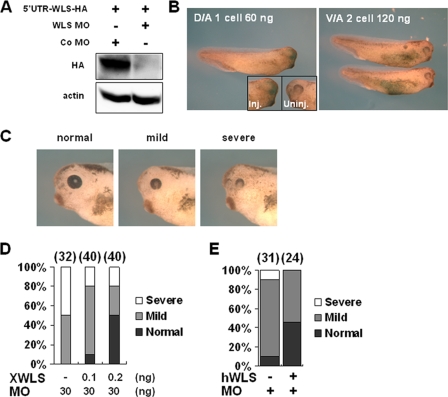
To establish the possible roles of XWntless during Xenopus embryogenesis, loss-of-function experiments were performed using an antisense MO against XWntless. XWntless MO specifically blocked the translation of ectopic XWntless, which contains an entire MO target site (5′ UTR-WLS-HA) (Fig. 2A). Following injection of XWntless MO into the dorso-animal region of eight-cell-stage embryos, eye defects developed. However, the injection of up to 120 ng XWntless MO into the ventro-animal region of eight-cell-stage embryos did not affect normal development (Fig. 2B). The eye phenotype was effectively rescued in a dose-dependent manner by coexpression of XWntless mRNA (XWntless-HA) at stage 42 (Fig. 2C and D). These phenotypic results suggest that XWntless regulates normal eye development. Human Wntless mRNA can also rescue the eye defects induced by XWntless MO in Xenopus (Fig. 2C and E), suggesting that vertebrate Wntless functions are conserved among species.
FIG. 2.
XWntless (WLS) MO specifically inhibits eye development. (A) WLS MO specifically inhibited translation of ectopic WLS, which contained an entire MO target site (5′ UTR-WLS-HA). Actin was used as a loading control. Standard control MO (Co MO) was used as a control. (B) A 60-ng amount of WLS MO injected at dorso-animal (D/A) region resulted in the complete-loss-of-eye phenotype on the MO-injected side. Ventro-animal (V/A) injection of MO did not produce marked changes in morphology. Inj., WLS MO-injected side; Uninj., uninjected side. (C) WLS morphants showed various eye defects at stages 39 and 42. Phenotypic index of eye defects: mild, small-sized eye; severe, reduced eye and loss of normal structure of the eye. (D) XWntless-HA mRNA (XWLS) that lacks the first seven nucleotides of MO target sites (XWLS) rescued WLS morphant phenotypes dose dependently when coinjected with WLS MO (MO). Phenotypes were counted at stage 42. Numbers above bars are numbers of embryos used for analysis. (E) Human WLS mRNA (hWLS; 100 pg) can rescue Xenopus WLS MO (MO)-mediated eye defects. Phenotypes were counted at stage 39. +, present; −, absent.
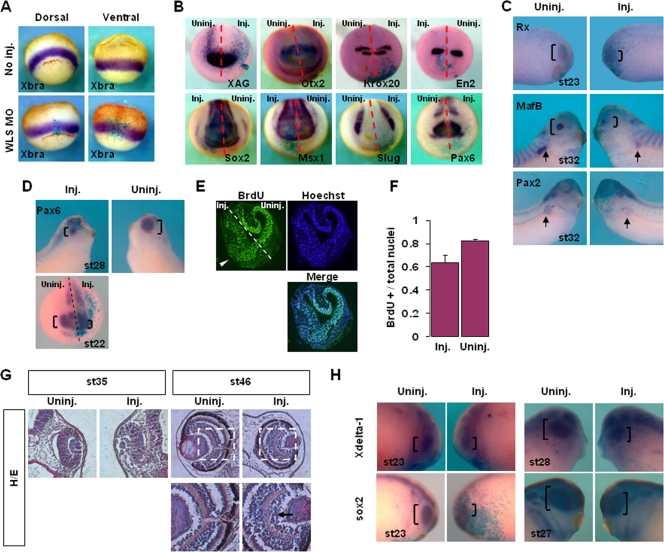
Interestingly, knockdown of XWntless did not affect the expression of the mesodermal marker Xbra (Fig. 3A); the neural plate marker Sox2; anteroposterior neural patterning markers XAG, Otx2, Krox20, En2, and Pax6; the neural plate border marker Msx1; or the neural crest marker Slug (Fig. 3B). Gastrulation defects, including the blockade of convergent extension movements, truncation of the dorsal axis, and inhibition of blastopore closure, were not observed, even at the highest doses examined (120 ng of XWntless MO) (Fig. 3A and B; data not shown). Several Wnt ligands are implicated in cell fate specification and motility during gastrulation and neurulation, including XWnt8 in mesoderm induction (10), neural induction (1), and neural crest induction (44) and XWnt5a and XWnt11 in organizer formation (42, 47), mesoderm induction (42, 45), gastrulation cell movements, and blastopore closure (41, 45). The failure of XWntless depletion to cause defects in these processes implies that the protein is not required for the activities of several Wnt proteins, including XWnt5a, XWnt8, and XWnt11.
FIG. 3.
XWntless is required for a subset of Wnt-related developmental processes. XWntless morphants were analyzed at various stages in views of Wnts-related developmental processes. Uninj., uninjected side; Inj., XWntless MO-injected side; st, stage. Brackets indicate eye region. (A) XWntless morphants of gastrula stage were analyzed by Xbra expression to see Wnt-mediated mesoderm induction. WLS, XWntless. (B) XWntless MO was injected unilaterally and analyzed with various neural markers at neurula stage. Dashed lines indicate midline of the embryos. (C) Unilaterally injected XWntless morphants were analyzed for eye and pronephros markers. Arrows indicate pronephros expression of MafB and Pax2. (D) Unilaterally injected XWntless morphants were analyzed for Pax6 expression. Dashed line indicates midline of the embryo. (E) BrdU incorporation was stained in stage 23 embryo. The number of proliferating cells at the eye primordium was reduced on the XWntless MO-injected side (white arrowhead). Dashed line indicates midline of the embryo. (F) Ratio of proliferating cells to total cells at the eye primordium as shown in panel E. Three independent samples were counted in XWntless MO-injected side and in uninjected side. Standard deviations are indicated by error bars. BrdU+, BrdU positive. (G) Unilaterally injected XWntless morphant eyes were sectioned at stage 35 and at stage 46. Hematoxylin and eosin staining (H/E) was performed. Regions enclosed with white dashed lines are enlarged below. Arrow indicates ganglion cell layer of XWntless morphant eye. Lower panels are enlarged images of portions of panels above enclosed by white dashed lines. (H) Retinal proneural markers sox2 and Xdelta-1 were analyzed in unilaterally injected XWntless morphants at stage 23, stage 27, or stage 28.
Eye defects elicited by the loss of XWntless were further analyzed by using molecular markers of the eye. XWntless MO was injected unilaterally at the two-cell stage, and in situ hybridization was performed. The expression of the retinal markers Rx (26) and Pax6 and the lens marker MafB (20) was suppressed on the XWntless MO-injected side (Fig. 3C and D). Interestingly, pronephric tubule expressions of MafB (11) and Pax2 (38) were also inhibited upon XWntless depletion (Fig. 3C). The results collectively indicate that XWntless is required for a subset of Wnt-mediated developmental processes, in particular, eye and pronephros development.
We further explored eye defects, focusing on aspects of retinal neurogenesis. A specific Wnt signaling receptor, Fz5, is required for retinal neurogenesis (40, 43), raising the possibility that the eye defects of XWntless morphants are caused by alterations in Wnt signaling during retinal neurogenesis. The proliferation of retinal progenitors on the XWntless MO-injected side was decreased in comparison to their proliferation on the uninjected side, as is evident from BrdU incorporation at stage 23 in the eye primordium (Fig. 3E). We assessed the proportion of BrdU-positive cells relative to the total number of cells in the developing eye primordium. XWntless depletion led to a significant reduction in the proportion of BrdU-positive cells, with 82% (standard deviation, 0.8%; n = 4) of cells proliferating on the uninjected side and only 64% (standard deviation, 7%; n = 4) on the XWntless MO-injected side (Fig. 3F). We additionally observed structural anomalies in the eye on the XWntless-depleted side. The optic cup was disorganized and the lens size reduced in eye sections at stage 35 (Fig. 3G). At a later stage, when almost all the neuron types had emerged (stage 46) (25), outer ganglion cells were abnormally expanded and disorganized in the eye on the XWntless MO-injected side (Fig. 3G). Finally, the retinal proneural markers Xdelta-1 and Sox2 (43) were reduced only on the XWntless MO-injected side (Fig. 3H). Based on these results, we conclude that the eye defects induced by loss of XWntless can be attributed to interference with retinal neurogenesis, at least in part.
Requirement of XWntless for XWnt4 secretion and function.
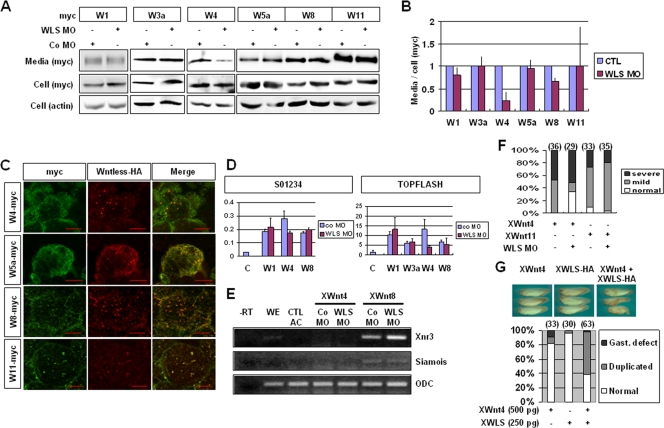
In view of the above finding that XWntless knockdown phenotypes are not associated with all Xenopus Wnt activities, we attempted to identify the specific requirements of XWntless for specific Xenopus Wnts. To establish the specificity of XWntless, we measured Wnt protein levels by Western blot analysis of the culture medium of animal caps expressing various Wnt isoforms ectopically. The injection of XWntless MO caused inhibition of ectopic secretion of XWnt4 but not the other Wnts examined (XWnt1, XWnt3a, XWnt5a, XWnt8, and XWnt11) (Fig. 4A and B). The specific requirement of XWntless for XWnt4 cannot be attributed to specific binding, since similar colocalization patterns of XWntless-HA with myc-tagged XWnt4 and other XWnts (XWnt5a, XWnt8, and XWnt11) were observed in animal cap tissues (Fig. 4C). These results suggest that XWntless is not required for the secretion of a group of Wnts consisting of XWnt1, XWnt3a, XWnt5a, XWnt8, and XWnt11 but is specifically required for XWnt4.
FIG. 4.
Specific requirement of XWntless in the secretion and function of XWnt4. (A) Secretions of ectopically overexpressed, epitope-tagged XWnt1, XWnt3a, XWnt4, XWnt5a, XWnt8, and XWnt11 were measured by Western blotting. XWntless MO and control MO (Co MO) were injected as indicated. (B) Secreted Wnts were quantified by using ImageJ software (available at http://rsb.info.nih.gov/ij; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD). Ratios of proteins in media and cells were used for comparison. All control lanes were normalized to 1. At least three independent assays were performed and used for analysis. Error bars indicate standard deviations. (C) Animal cap explants were subjected to immunostaining with anti-myc and anti-HA antibodies. All the Wnts tested were colocalized with XWntless in animal cap explants. Scale bars indicate 20 μm. (D) Siamois promoter (S01234) and TOPFLASH reporter activities were assayed by using a dual luciferase assay system. pRLTK Renilla vector was used as an internal control. Error bars indicate standard deviations. (E) RT-PCR analysis of animal cap explants injected with mRNAs and MO as indicated was performed. ODC was used as a loading control. −RT, without reverse transcription; WE, whole embryo; AC, animal cap explant. (F) Gastrulation defects were counted at stage 20. mild, blastopore closure defect and normal axis elongation; severe, blastopore closure defect and dorsal axis truncation. Numbers above bars are numbers of embryos used for analysis. (G) Ectopic partial-axis-inducing activity of XWnt4 was enforced by coinjection with XWntless mRNA (XWLS). WLS, XWntless; +, present; −, absent; W1, XWnt1; W3a, XWnt3a; W4, XWnt4; W5a, XWnt5a; W8, XWnt8; W11, XWnt11; CTL, control; Gast, gastrulation; Duplicated, duplicated axis.
The specificity of XWntless to XWnt4 was further analyzed with reporter, phenotypic rescue, and ectopic trunk-inducing assays. First, endogenous siamois and Xnr3 genes and exogenous siamois promoter (S01234) and TOPFLASH reporter activation were measured by using RT-PCR and a luciferase assay. Although ectopic XWnt4 could not enhance the endogenous siamois and Xnr3 genes' activation, ectopic XWnt4 displayed canonical Wnt-like activity, as is evident from exogenous S01234 promoter and TOPFLASH activation. XWntless MO specifically inhibited XWnt4-mediated S01234 and TOPFLASH activation but not activation by XWnt1 or XWnt8 and XWnt3a-mediated TOPFLASH activation (Fig. 4D). XWntless MO did not affect the activation of Xnr3 and siamois by XWnt8 overexpression, either (Fig. 4E). We additionally performed phenotypic analysis of the in vivo function of XWntless on specific Wnts. Gastrulation defects were induced by XWnt4 and XWnt11 following injection into the dorso-animal region. XWntless MO specifically rescued several of the XWnt4-mediated (34%; n = 29) but not XWnt11-mediated gastrulation defects (Fig. 4F). The ectopic induction of secondary axis assay is a typical method to monitor canonical Wnt activity. XWnt4 could not induce complete secondary axis upon ventral expression. However, secondary trunk or partial axis was induced by a high-dosage XWnt4 injection (500 pg) with relatively low frequency (Fig. 4G) (9%; n = 33). The ability of XWnt4 to induce partial secondary axis was enforced by coexpression of XWntless (Fig. 4G) (60%; n = 63). Overall, these results support the hypothesis that XWntless is specific to XWnt4 but not to XWnt1, XWnt3a, XWnt5a, XWnt8, or XWnt11.
Relationship between XWntless and the Retromer complex in the regulation of Wnt signaling.
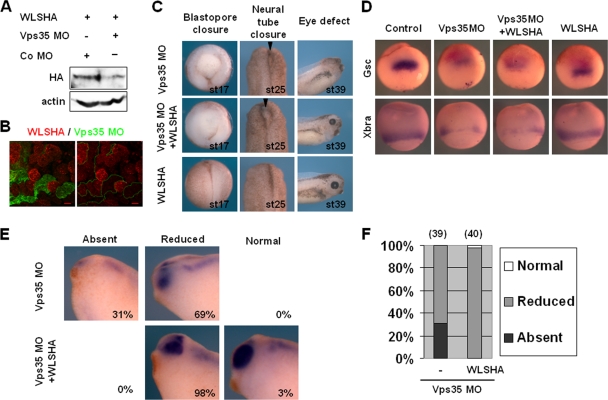
The Retromer complex is required for Wntless recycling and contributes to Wnt secretion (4, 14, 15, 32, 35, 50). In Xenopus tropicalis, the loss of a Retromer complex component, Vps35, induces several Wnt deficiency phenotypes (12). Interestingly, these Vps35 knockdown phenotypes were not consistent with those of the Wntless morphants used in this study (Fig. 2 and 3). In view of these discrepancies, we investigated the roles of the Retromer complex in Wnt signaling during Xenopus laevis embryogenesis. Loss of Vps35 in Xenopus laevis led to results similar to previous findings in terms of XWntless stability (4, 15, 32, 35, 50) and developmental defects (12). The ectopic XWntless-HA level was decreased upon coinjection with Vps35 MO, supporting the model that the Retromer complex recycles XWntless in Xenopus embryos as well (Fig. 5A and B). Injection of Vps35 MO into the dorsal marginal region of a four-cell-stage embryo produced multiple defects in gastrula and neurula embryos, including failure of blastopore and neural tube closure (Fig. 5C). These defects are reminiscent of those caused by altered noncanonical XWnt5a or XWnt11 signaling. Eye defects were additionally observed in Vps35 morphants (Fig. 5C, E, and F), similar to those in XWntless morphants (Fig. 2 and 3). Interestingly, overexpression of XWntless did not rescue Vps35 MO-induced defects in blastopore or neural tube closure but reversed eye defects (Fig. 5C, E, and F). These results suggest that the Retromer complex and XWntless cooperate to regulate eye development, whereas early patterning processes during gastrulation and neurulation are dependent on the Retromer complex but not XWntless.
FIG. 5.
XWntless-dependent and -independent roles of the Retromer complex during Xenopus development. (A) Ectopic XWntless-HA mRNA (1 ng) was injected with or without Vps35 MO (20 ng) into the two blastomeres of two-cell-stage embryo. At stage 11, embryos were harvested and lysates were subjected to Western blotting with anti-HA antibody. Actin was used as a loading control. +, present; −, absent; Co, control. (B) Embryos were injected first with XWntless-HA mRNA (1 ng) in two blastomeres of the two-cell stage. Later, at the eight-cell stage, the same embryos were injected with Vps35 MO (20 ng) and lineage tracer green fluorescent protein mRNA in one blastomere of the eight-cell stage. At stage 11, animal caps were dissected and subjected to immunostaining with anti-HA antibody. (C) Phenotypic analysis of Vps35 morphants with or without XWntless mRNA coinjection. Blastopore closure defects were analyzed at stage 17 and neural tube closure defects at stage 25 from dorsal marginal zone of injected embryos, and eye defects were analyzed at stage 39 from dorso-animal injected embryos. Injection dose: sVps35 MO, 20 ng; XWntless mRNA, 333 pg. Arrowheads, unclosed neural tubes. (D) Injections were performed into dorsal marginal region. Gsc and Xbra expression levels were analyzed by in situ hybridization at stage 10.5 or 11. Injection dose: Vps35 MO, 20 ng; XWntless mRNA, 333 pg. (E) Vps35 MO (20 ng) and XWntless mRNA (333 pg) were injected bilaterally into two blastomeres of dorsal animal and subjected to in situ hybridization with Pax6 at stage 35. Absent, no Pax6 expression at the eye; Reduced, reduced Pax6 expression at the eye; Normal, normal Pax6 expression at the eye. (F) Graphic representation of the results shown in panel D. Numbers above bars are numbers of embryos used for analysis. −, without XWntless; WLS, XWntless.
We further analyzed Vps35 morphants in detail to determine Wnt signaling activities in vivo by monitoring changes in the expression of various marker genes. Vps35 MO inhibited organizer formation and mesoderm induction at early gastrula, as evident from the reduced expression of Gsc and Xbra. XWntless overexpression did not stimulate the recovery of diminished levels of Gsc and Xbra caused by Vps35 MO (Fig. 5D). Eye defects were analyzed by examining Pax6 expression. Loss of Vps35 resulted in reduced or absent Pax6 expression. These defects were significantly rescued by coexpression of XWntless (Fig. 5E and F). We further examined changes in various neural markers, since Wnt signaling plays multiple roles during neurulation, such as neural induction, neural crest induction, and anteroposterior neural patterning. Inhibition of neural crest induction and the concomitant expansion of neural plate are diagnostic of impaired canonical Wnt8 activity (19). Unilateral injection of Vps35 MO into two-cell-stage embryos resulted in reduction of neural crest and expansion of neural plate, as observed from altered expression of the neural crest marker Twist, neural plate marker Sox2, and neural plate border marker Msx1 (Fig. 6A). Additionally, anteroposterior neural patterning markers XAG, En2, Krox20, HoxB9, and Wnt4 were reduced on the Vps35 MO-injected side. The expression domain of the forebrain marker Otx2 was expanded, but the level of expression diminished. Pax6 expression at the hindbrain region was reduced, but its expression at the anterior-most neural plate was expanded because of neural plate expansion by Vps35 MO injection. Intriguingly, overexpression of XWntless did not rescue all the changes in marker expression of Vps35 morphants at the neurula stage (Fig. 6A), suggesting that the requirement of the Retromer complex for Wnt signaling during Xenopus neurulation is not dependent on its function in XWntless recycling.
FIG. 6.
Requirements of the Retromer complex for Wnt signaling during early Xenopus development. (A) Embryos were unilaterally injected with Vps35 MO (20 ng) alone or together with XWntless-HA mRNA (333 pg), with LacZ mRNA. At neurula stage, LacZ-stained embryos were subjected to in situ hybridization with Twist, Sox2, XAG, Otx2, En2, Krox20, HoxB9, Msx1, Pax6, or Wnt4 probe. Arrows indicate the injected sides. Dashed lines indicate the midlines of the injected embryos. (B) Embryos were unilaterally injected with Vps35 MO (20 ng) alone or together with β-catenin mRNA (500 pg) or XDsh mRNA (500 pg), with LacZ mRNA. At neurula stage, LacZ-stained embryos were subjected to in situ hybridization with Twist, Sox2, or En2 probe. Arrows indicate the injected sides. Dashed lines indicate the midlines of the treated embryos. (C) Two-cell-stage embryos were bilaterally injected at animal pole and raised until stage 12. Whole embryos were harvested and subjected to Western blotting for anti-phospho-Smad1 and anti-phospho-ERK1/2. Anti-Smad1, anti-ERK, and antiactin were used as loading controls. p, phospho. (D) Secretions of ectopic XWnt1, XWnt3a, XWnt5a, XWnt8, and XWnt11 were measured by Western blotting. Vps35 MO and XWntless-HA mRNA were coinjected with myc-tagged Wnts as indicated. W1, XWnt1; W3a, XWnt3a; W5a, XWnt5a; W8, XWnt8; W11, XWnt11; +, present; −, absent; WLS, XWntless.
To establish whether the early patterning defects of Vps35 morphants are a direct consequence of impaired canonical Wnt signaling, we performed rescue experiments by coinjecting β-catenin or Dishevelled (XDsh). Coexpression of β-catenin or XDsh restored the reduced Twist and En2 levels. Sox2 expansion was partially rescued upon β-catenin or XDsh coexpression (Fig. 6B). Other signaling pathways, including BMP and FGF, were not affected by the Retromer complex, since Vps35 MO induced no changes in the phosphorylation states of BMP signal transducer Smad1 and FGF signal transducer ERK1 (Fig. 6C). We hypothesize that the Retromer complex is required for Wnt signaling during gastrulation (Fig. 5C and D) and neurulation (Fig. 5C and 6A) by influencing secretion of XWnt5a, XWnt8, and/or XWnt11 in an XWntless-independent manner. However, we detected no differences in the secretion levels of ectopic XWnt1, XWnt3a, XWnt5a, XWnt8, and XWnt11 between wild-type and Vps35 morphant animal cap tissues (Fig. 6D). This finding suggests that the mechanism of Retromer action on early patterning processes is distinct from its effects on Wnt secretion.
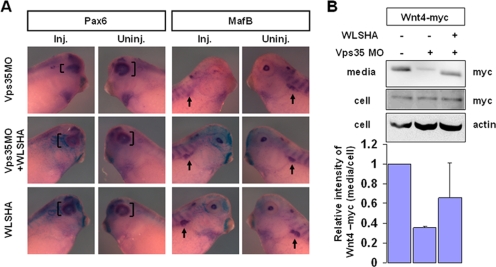
Next, we investigated roles of the Retromer complex in Wnt signaling at later stages of development. At the tailbud stage, Pax6 expression in the eye was reduced on the Vps35 MO-injected side. In this case, overexpression of XWntless rescued Pax6 levels (Fig. 7A), consistent with the results shown in Fig. 5E and F. MafB expression in the lens and pronephros was suppressed on the Vps35 MO-injected side and recovered upon XWntless overexpression (Fig. 7A). Concomitantly, ectopic XWnt4 secretion was reduced in the presence of Vps35 MO and restored by coexpression of XWntless (Fig. 7B), suggesting that the Retromer complex regulates XWnt4 secretion by recycling XWntless in Xenopus. These results collectively suggest that the early patterning defects of Vps35 morphants during gastrulation and neurulation are not caused by impaired XWntless recycling, while eye and pronephros defects induced by Vps35 MO are attributed to impaired XWntless recycling, which hinders XWnt4 secretion.
FIG. 7.
Cooperative roles of the Retromer complex and XWntless for Wnt signaling during Xenopus eye development. (A) At tailbud stage, LacZ-stained embryos were subjected to in situ hybridization with Pax6 and MafB probes. Inj., injected side; Uninj., uninjected side of the same embryo. Bracket indicates the expression domain of Pax6. Arrow indicates pronephros expression of MafB. Injection dose: Vps35 MO, 10 ng; XWntless mRNA, 333 pg. (B) Secretion of ectopic XWnt4 was measured by Western blotting. Vps35 MO and XWntless-HA mRNA were coinjected with myc-tagged XWnt4 as indicated. Relative intensities of the blots were measured by using ImageJ software. Error bars show standard deviations. +, present; −, absent; WLS, XWntless.
DISCUSSION
Despite extensive studies of Wnt signaling, limited information is available on the mechanisms of Wnt ligand secretion. Recently, two new components, Wntless and the Retromer complex, have been identified in the Wnt secretory pathway. Wntless is a dedicated secretion factor of Wnts (2, 3, 16), and the Retromer complex is required for Wntless recycling from endosomes to the trans-Golgi network, facilitating the reuse of Wntless for prolonged Wnt secretion (4, 15, 32, 35, 50). In this study, we present evidence that Xenopus Wntless is not a dedicated secretion factor for all Wnts but acts specifically on XWnt4. In addition, the Retromer complex is required for Wnt signaling activity during Xenopus gastrulation and neurulation independently of XWntless recycling. Based on the results of secretion assay and rescue experiments, we suggest that the Retromer complex recycles XWntless, thus regulating XWnt4 secretion during Xenopus eye development.
XWntless, a dedicated secretion factor of a subset of Xenopus Wnt isoforms.
Here, we employed a Xenopus model system to measure XWnt1, XWnt3a, XWnt4, XWnt5a, XWnt8, and XWnt11 secreted directly from animal cap tissues and analyze knockdown phenotypes. Our data show that XWnt1, XWnt3a, XWnt5a, XWnt8, and XWnt11 are not dependent on XWntless for secretion (Fig. 4A and B) or in vivo function (Fig. 3A and B). This limited requirement of XWntless for Xenopus Wnts argues against the “pan-Wnt requirement of Wntless” concept (2-4). The inconsistency between our findings and those of others on Wntless specificity may be attributed to differences in the model species used. Indeed, orthologs of XWnt8 and XWnt11 are not found in Drosophila and C. elegans, in which Wntless function has been suggested. Further studies of the molecular mechanisms of the interactions and functional correlation between Wntless and Wnts are required in various other model species to elucidate the molecular mechanism of Wntless specificity. Additionally, some recent reports also point out the possibility that some Wnts are secreted independently of Wntless. First, Ching et al. reported that WntD is secreted independently of Wntless in Drosophila. They found that WntD is not lipid modified and thus is not subjected to the known secretory pathway through Porcupine and Wntless but to an alternative pathway through Rab1 (9). Second, Bolognesi et al. reported that Tribolium castaneum WntD/8 (Tc-WntD/8) may be secreted independently of Tc-Wntless in T. castaneum. They showed by epistatic analysis that Tc-WntD/8 function is dependent on Porcupine but not on Wntless (6). Collectively, our results and the recently published results of others suggest that Wntless is required not for pan-Wnt secretion but for secretion of a limited number of Wnts.
We have employed Xenopus embryogenesis as a model system to study developmental-stage-dependent and tissue context-dependent requirements of Wntless. Unexpectedly, XWntless is specifically required for eye development (Fig. 2 and 3) but not for several early patterning processes, including organizer formation, mesoderm induction, neural induction, neural crest induction, gastrulation cell movements, and early neural anteroposterior patterning (Fig. 3A and B). The Xenopus Wnt ligands XWnt5a, XWnt8, and XWnt11 participate in organizer formation, mesoderm induction, gastrulation cell movements, neural induction, anteroposterior neural patterning, and neural crest induction (1, 10, 22, 30, 39, 41, 42, 44, 45). The lack of effects on these processes in XWntless morphants implies that XWntless is dispensable for in vivo functions of XWnt5a, XWnt8, and XWnt11. We cannot entirely exclude the possibility that maternal XWntless protein may compensate XWntless MO-mediated knockdown of XWntless mRNA translation until early gastrula stages. However, a maternal contribution might not have an effect at relatively later stages, i.e., at neurula stages. Thus, we conclude that at least for zygotic Wnt5a, Wnt8, and Wnt11, XWntless does not affect their secretion. While the Wnt ligands involved in eye development remain to be identified, XWnt4 and at least one another canonical Wnt function in Xenopus eye development (13, 26, 37, 43). In the literature, loss of XWnt4 resulted in complete loss of the eye at tadpole stage and reduced eye marker expression at early neurula stage (26). Although the eye phenotype of the XWnt4 morphant is more severe than that of the XWntless morphant, the two are comparable in the aspect of abnormal eye development. Additionally, XWnt4 is the only Xenopus Wnt ligand that functions in pronephros development (38), and XWntless depletion leads to a reduction in the expression of the pronephric tubule markers MafB and Pax2 (Fig. 3C). These phenotypes of XWntless morphants support the hypothesis that XWntless is required for the in vivo functioning of XWnt4.
Wntless-dependent and -independent roles of the Retromer complex in Wnt signaling.
Recently, several groups independently reported a specific function of the Retromer complex in Wntless recycling and thus in Wnt secretion (4, 14, 15, 32, 35, 50). Here, we show that most Wnt deficiency phenotypes of Vps35 morphants are not caused by impaired XWntless recycling. The gastrulation defects affecting convergent extension movements, mesoderm induction, and neural patterning defects, including neural plate expansion and reduction of the neural crest, can be attributed to impaired XWnt5a, XWnt8, and XWnt11 signaling. However, high levels of XWntless expression could not rescue these defects (Fig. 5C and D and 6A), and ectopic secretion of these Wnts was not affected by loss of Vps35 (Fig. 6D). Notably, the Retromer complex was originally reported to be required for long-range secretion but not short-range secretion of Wnts (12). In our experimental setting, we cannot distinguish between long-range and short-range secretion of Wnts. Thus, it is possible that the Retromer complex is required for XWnt5a, XWnt8, and XWnt11 signaling by influencing long-range Wnt delivery. Alternatively, it is possible that Wnt-receiving cells require the Retromer complex. Neither of these two hypotheses can be excluded at this stage, and further studies are required to clarify the detailed mechanisms of Retromer complex function in XWnt5a, XWnt8, and XWnt11 signaling.
Interestingly, eye defects of Vps35 morphants can be rescued by XWntless overexpression (Fig. 5C, E, and F and 7A). Our data, in conjunction with the results of others (4, 14, 15, 32, 35, 50), show that the Retromer complex is required for Wntless recycling to maintain the cellular Wntless level (Fig. 5A and B). The specific ability of XWntless to rescue eye defects of Vps35 morphants can be explained by its requirement for XWnt4 secretion and the importance of the Retromer complex in XWntless recycling. In line with this hypothesis, XWnt4 secretion was impaired by Vps35 depletion, which was restored by coexpression of XWntless, albeit not to a significant extent (Fig. 7B). We suggest that the eye defects of Vps35 and XWntless morphants are caused in the same way, i.e., by effects on XWnt4 secretion.
In conclusion, we suggest a possible mechanism by which XWntless and the Retromer complex affect Wnt signaling. XWnt4 is guided to extracellular space by XWntless. The Retromer complex functions to recycle XWntless from the plasma membrane to the trans-Golgi network (4, 15, 32, 35, 50), where XWntless acts specifically on at least XWnt4. XWnt1, XWnt3a, XWnt5a, XWnt8, and XWnt11 are secreted in an XWntless-independent manner, but it is possible that the Retromer complex functions in the long-range secretion of these Wnt proteins and/or is required by the respective receiving cells for the signaling cascade.
Acknowledgments
We thank Dan Kessler, J. B. Gurdon, Masanori Taira, Randall Moon, and Tomas Pieler for generous gifts of DNA constructs. We also thank Jae-Seong Yang, Young-Yun Kong, Bon-Kyoung Koo, and members of our laboratories for helpful comments, Gun-Hwa Kim, Seung Joon Lee, Edmond Changkyun Park, Gun-Sik Cho, and Inchul Yeo for careful reading of the manuscript.
This work was supported by the Pure Basic Research Group (070-2005-C00115) of KRF and the Brain Korea 21 project. Eek-hoon Jho was supported by a grant from Korea Research Foundation (KRF-2006-312-C00339).
Footnotes
Published ahead of print on 17 February 2009.
REFERENCES
- 1.Baker, J. C., R. S. Beddington, and R. M. Harland. 1999. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 133149-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banziger, C., D. Soldini, C. Schutt, P. Zipperlen, G. Hausmann, and K. Basler. 2006. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125509-522. [DOI] [PubMed] [Google Scholar]
- 3.Bartscherer, K., N. Pelte, D. Ingelfinger, and M. Boutros. 2006. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125523-533. [DOI] [PubMed] [Google Scholar]
- 4.Belenkaya, T. Y., Y. Wu, X. Tang, B. Zhou, L. Cheng, Y. V. Sharma, D. Yan, E. M. Selva, and X. Lin. 2008. The retromer complex influences wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev. Cell 14120-131. [DOI] [PubMed] [Google Scholar]
- 5.Bhanot, P., M. Brink, C. H. Samos, J. C. Hsieh, Y. Wang, J. P. Macke, D. Andrew, J. Nathans, and R. Nusse. 1996. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382225-230. [DOI] [PubMed] [Google Scholar]
- 6.Bolognesi, R., L. Farzana, T. D. Fischer, and S. J. Brown. 2008. Multiple Wnt genes are required for segmentation in the short-germ embryo of Tribolium castaneum. Curr. Biol. 181624-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonifacino, J. S., and R. Rojas. 2006. Retrograde transport from endosomes to the trans-Golgi network. Nat. Rev. Mol. Cell Biol. 7568-579. [DOI] [PubMed] [Google Scholar]
- 8.Chen, W., D. ten Berge, J. Brown, S. Ahn, L. A. Hu, W. E. Miller, M. G. Caron, L. S. Barak, R. Nusse, and R. J. Lefkowitz. 2003. Dishevelled 2 recruits beta-Arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science 3011391-1394. [DOI] [PubMed] [Google Scholar]
- 9.Ching, W., H. C. Hang, and R. Nusse. 2008. Lipid-independent secretion of a Drosophila Wnt protein. J. Biol. Chem. 28317092-17098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christian, J. L., J. A. McMahon, A. P. McMahon, and R. T. Moon. 1991. Xwnt-8, a Xenopus Wnt-1/Int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development 1111045-1055. [DOI] [PubMed] [Google Scholar]
- 11.Coolen, M., K. Sii-Felice, O. Bronchain, A. Mazabraud, F. Bourrat, S. Retaux, M. P. Felder-Schmittbuhl, S. Mazan, and J. L. Plouhinec. 2005. Phylogenomic analysis and expression patterns of large Maf genes in Xenopus tropicalis provide new insights into the functional evolution of the gene family in osteichthyans. Dev. Genes Evol. 215327-339. [DOI] [PubMed] [Google Scholar]
- 12.Coudreuse, D. Y., G. Roel, M. C. Betist, O. Destree, and H. C. Korswagen. 2006. Wnt gradient formation requires retromer function in Wnt-producing cells. Science 312921-924. [DOI] [PubMed] [Google Scholar]
- 13.de Iongh, R. U., H. E. Abud, and G. R. Hime. 2006. WNT/Frizzled signaling in eye development and disease. Front. Biosci. 112442-2464. [DOI] [PubMed] [Google Scholar]
- 14.Eaton, S. 2008. Retromer retrieves wntless. Dev. Cell 144-6. [DOI] [PubMed] [Google Scholar]
- 15.Franch-Marro, X., F. Wendler, S. Guidato, J. Griffith, A. Baena-Lopez, N. Itasaki, M. M. Maurice, and J. P. Vincent. 2008. Wingless secretion requires endosome-to-Golgi retrieval of Wntless/Evi/Sprinter by the retromer complex. Nat. Cell Biol. 10170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman, R. M., S. Thombre, Z. Firtina, D. Gray, D. Betts, J. Roebuck, E. P. Spana, and E. M. Selva. 2006. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development 1334901-4911. [DOI] [PubMed] [Google Scholar]
- 17.Gordon, M. D., and R. Nusse. 2006. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 28122429-22433. [DOI] [PubMed] [Google Scholar]
- 18.Harland, R. M. 1991. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 36685-695. [DOI] [PubMed] [Google Scholar]
- 19.Heeg-Truesdell, E., and C. LaBonne. 2006. Neural induction in Xenopus requires inhibition of Wnt-beta-catenin signaling. Dev. Biol. 29871-86. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi, S., and K. Yasuda. 2001. Distinct roles of maf genes during Xenopus lens development. Mech. Dev. 101155-166. [DOI] [PubMed] [Google Scholar]
- 21.Kim, G. H., and J. K. Han. 2007. Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J. 262513-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ku, M., and D. A. Melton. 1993. Xwnt-11: a maternally expressed Xenopus wnt gene. Development 1191161-1173. [DOI] [PubMed] [Google Scholar]
- 23.Lee, H. X., A. L. Ambrosio, B. Reversade, and E. M. De Robertis. 2006. Embryonic dorsal-ventral signaling: secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell 124147-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Y., and G. Bu. 2005. LRP5/6 in Wnt signaling and tumorigenesis. Future Oncol. 1673-681. [DOI] [PubMed] [Google Scholar]
- 25.Locker, M., M. Agathocleous, M. A. Amato, K. Parain, W. A. Harris, and M. Perron. 2006. Hedgehog signaling and the retina: insights into the mechanisms controlling the proliferative properties of neural precursors. Genes Dev. 203036-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurus, D., C. Heligon, A. Burger-Schwarzler, A. W. Brandli, and M. Kuhl. 2005. Noncanonical Wnt-4 signaling and EAF2 are required for eye development in Xenopus laevis. EMBO J. 241181-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikels, A. J., and R. Nusse. 2006. Wnts as ligands: processing, secretion and reception. Oncogene 257461-7468. [DOI] [PubMed] [Google Scholar]
- 28.Miura, G. I., J. Buglino, D. Alvarado, M. A. Lemmon, M. D. Resh, and J. E. Treisman. 2006. Palmitoylation of the EGFR ligand Spitz by Rasp increases Spitz activity by restricting its diffusion. Dev. Cell 10167-176. [DOI] [PubMed] [Google Scholar]
- 29.Moon, R. T., B. Bowerman, M. Boutros, and N. Perrimon. 2002. The promise and perils of Wnt signaling through beta-catenin. Science 2961644-1646. [DOI] [PubMed] [Google Scholar]
- 30.Moon, R. T., R. M. Campbell, J. L. Christian, L. L. McGrew, J. Shih, and S. Fraser. 1993. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development 11997-111. [DOI] [PubMed] [Google Scholar]
- 31.Nieuwkoop, P. D., and J. Faber. 1967. Normal table of Xenopus laevis (Daudin). North Holland, Amsterdam, The Netherlands.
- 32.Pan, C. L., P. D. Baum, M. Gu, E. M. Jorgensen, S. G. Clark, and G. Garriga. 2008. C. elegans AP-2 and retromer control Wnt signaling by regulating MIG-14/Wntless. Dev. Cell 14132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panakova, D., H. Sprong, E. Marois, C. Thiele, and S. Eaton. 2005. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 43558-65. [DOI] [PubMed] [Google Scholar]
- 34.Perron, M., S. Kanekar, M. L. Vetter, and W. A. Harris. 1998. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev. Biol. 199185-200. [DOI] [PubMed] [Google Scholar]
- 35.Port, F., M. Kuster, P. Herr, E. Furger, C. Banziger, G. Hausmann, and K. Basler. 2008. Wingless secretion promotes and requires retromer-dependent cycling of Wntless. Nat. Cell Biol. 10178-185. [DOI] [PubMed] [Google Scholar]
- 36.Povelones, M., and R. Nusse. 2002. Wnt signalling sees spots. Nat. Cell Biol. 4E249-E250. [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen, J. T., M. A. Deardorff, C. Tan, M. S. Rao, P. S. Klein, and M. L. Vetter. 2001. Regulation of eye development by frizzled signaling in Xenopus. Proc. Natl. Acad. Sci. USA 983861-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saulnier, D. M., H. Ghanbari, and A. W. Brandli. 2002. Essential function of Wnt-4 for tubulogenesis in the Xenopus pronephric kidney. Dev. Biol. 24813-28. [DOI] [PubMed] [Google Scholar]
- 39.Shibata, M., M. Itoh, H. Hikasa, S. Taira, and M. Taira. 2005. Role of crescent in convergent extension movements by modulating Wnt signaling in early Xenopus embryogenesis. Mech. Dev. 1221322-1339. [DOI] [PubMed] [Google Scholar]
- 40.Sumanas, S., and S. C. Ekker. 2001. Xenopus frizzled-5: a frizzled family member expressed exclusively in the neural retina of the developing eye. Mech. Dev. 103133-136. [DOI] [PubMed] [Google Scholar]
- 41.Tada, M., and J. C. Smith. 2000. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 1272227-2238. [DOI] [PubMed] [Google Scholar]
- 42.Tao, Q., C. Yokota, H. Puck, M. Kofron, B. Birsoy, D. Yan, M. Asashima, C. C. Wylie, X. Lin, and J. Heasman. 2005. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120857-871. [DOI] [PubMed] [Google Scholar]
- 43.Van Raay, T. J., K. B. Moore, I. Iordanova, M. Steele, M. Jamrich, W. A. Harris, and M. L. Vetter. 2005. Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron 4623-36. [DOI] [PubMed] [Google Scholar]
- 44.Villanueva, S., A. Glavic, P. Ruiz, and R. Mayor. 2002. Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev. Biol. 241289-301. [DOI] [PubMed] [Google Scholar]
- 45.Wallingford, J. B., K. M. Vogeli, and R. M. Harland. 2001. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int. J. Dev. Biol. 45225-227. [PubMed] [Google Scholar]
- 46.Wehrli, M., S. T. Dougan, K. Caldwell, L. O'Keefe, S. Schwartz, D. Vaizel-Ohayon, E. Schejter, A. Tomlinson, and S. DiNardo. 2000. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407527-530. [DOI] [PubMed] [Google Scholar]
- 47.Westfall, T. A., R. Brimeyer, J. Twedt, J. Gladon, A. Olberding, M. Furutani-Seiki, and D. C. Slusarski. 2003. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/beta-catenin activity. J. Cell Biol. 162889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wodarz, A., and R. Nusse. 1998. Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 1459-88. [DOI] [PubMed] [Google Scholar]
- 49.Wu, C. H., and R. Nusse. 2002. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J. Biol. Chem. 27741762-41769. [DOI] [PubMed] [Google Scholar]
- 50.Yang, P. T., M. J. Lorenowicz, M. Silhankova, D. Y. Coudreuse, M. C. Betist, and H. C. Korswagen. 2008. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev. Cell 14140-147. [DOI] [PubMed] [Google Scholar]