Abstract
Notch signaling regulates pancreatic cell differentiation, and mutations of various Notch signaling components result in perturbed pancreas development. Members of the Fringe family of β1,3-N-acetylglucosaminyltransferases, Manic Fringe (MFng), Lunatic Fringe (LFng), and Radical Fringe (RFng), modulate Notch signaling, and MFng has been suggested to regulate pancreatic endocrine cell differentiation. We have characterized the expression of the three mouse Fringe genes in the developing mouse pancreas between embryonic days 9 and 14 and show that the expression of MFng colocalized with the proendocrine transcription factor Ngn3. In contrast, the expression of LFng colocalized with the exocrine marker Ptf1a, whereas RFng was not expressed. Moreover, we show that expression of MFng is lost in Ngn3 mutant mice, providing evidence that MFng is genetically downstream of Ngn3. Gain- and loss-of-function analyses of MFng by the generation of mice that overexpress MFng in early pancreatic progenitor cells and mice with a targeted deletion of MFng provide, however, evidence that MFng is dispensable for pancreas development and function, since no pancreatic defects in these mice were observed.
The Fringe family of β1,3-N-acetylglucosaminyltransferases modulates the activation of the Notch signaling pathway in response to different DSL (Delta, Serrate, Lag2) ligands. The Fringe-mediated addition of N-acetylglucosamine (GlcNAc) to specific epidermal growth factor repeats in the extracellular domains of the Notch receptors is thought to promote interactions with Dll1 ligands, whereas Serrate-Notch interaction is impaired (5, 23, 26). Three vertebrate Fringe genes, Lunatic Fringe (LFng), Radical Fringe (RFng), and Manic Fringe (MFng), that all modify Notch receptors have been described previously (7, 28). The targeted deletion of LFng in mice results in disrupted somitogenesis and perinatal death (36). Less is known about the roles of RFng and MFng. RFng has been reported to inhibit Notch signaling in rat neurons (22) and to affect limb bud development (29). The inactivation of RFng in mutant mice does, however, not perturb somitogenesis, limb bud formation, or mouse development (37). Although the coexpression of MFng with other Notch signaling components in several tissues, including the epidermis, somites, hematopoietic precursors, and the central nervous system, has been described previously (7, 19, 31, 33), a functional role for MFng in mice has not been described.
Signaling through the Notch pathway has been implicated in the control of cell fate in a wide array of developmental processes (7, 18). Upon the binding of the DSL family of membrane-bound ligands, the intracellular domain of Notch is released, and together with RBP-Jκ, the Notch intracellular domain activates the transcription of the Hes and Hes-related families of transcriptional repressors. Several gain- and loss-of-function studies of various Notch signaling components have emphasized a role for Notch signaling during pancreatic development (3, 15, 20). In the early pancreatic epithelium, the Notch ligand gene Dll1 is expressed in a scattered manner (3). Dll1 activates Notch signaling in neighboring cells, resulting in increased Hes1 expression, which in turn represses the expression of the proendocrine gene Ngn3 (3). The signal-receiving cells hence remain as proliferating progenitor cells, whereas Ngn3 expression is allowed in Dll1-expressing cells and these cells subsequently differentiate into endocrine cell types (9). Relatively little is known, however, about a potential role for Fringe proteins in the pancreas. MFng has been reported previously to be expressed in mouse pancreatic proendocrine cells (14, 35), and the forced expression of MFng in chicks is sufficient to promote the endocrine differentiation of pancreatic progenitors and the ectopic differentiation of cells reminiscent of pancreatic endocrine cells from nonpancreatic endoderm tissue (35).
In order to determine in more detail the role of Fringe proteins in mouse pancreatic development, we performed expression and gain- and loss-of-function analyses of MFng in the developing pancreas. We show that MFng is transiently expressed in proendocrine cells of the pancreas and that LFng is expressed predominantly in exocrine acini. The results of the genetic modification of MFng in transgenic mice, by targeted deletion or overexpression specifically in pancreatic progenitor cells, provides evidence that MFng is dispensable for mouse development in general, including the development and function of the pancreas.
MATERIALS AND METHODS
Generation of MFng transgenic mice.
Full-length MFng cDNA (kindly provided by E. Wandzioch) was cloned after the Ipf1/Pdx1 promoter (2). This construct is designated Ipf1-MFng. A second construct, designated Ipf1-MFng-lacZ, was generated by inserting an internal ribosome entry site (IRES) fused to a LacZ reporter gene containing a nuclear localization signal (NLS) (4) after the MFng coding sequence in order to facilitate the detection of transgenic expression. Transgenic mice were generated by pronuclear injection of the purified fragment (1.8 ng/ml) into F2 hybrid oocytes from B6/CBA parents (M&B) (17). Three founders were generated from each of the transgenic constructs. The founder lines that showed the highest levels of transgenic expression from the Ipf1-MFng and Ipf1-MFng-lacZ constructs were chosen for further analysis.
Generation of an MFng-targeting construct.
MFng cDNA was used to screen an RPCI-22 mouse 129s6/SvEvTAC Taconic (female) bacterial artificial chromosome (BAC) library (25) obtained from the BACPAC Resources Center, Children's Hospital Oakland, Oakland, CA. A 10-kb KpnI BAC fragment containing MFng exons 2 to 5 was cloned into pBluescript SK(−), yielding pBS-MFng-KpnI. A 3.6-kbp NcoI fragment containing exons 2 to 4 was blunted into the NheI site at the 3′ end of the loxP-pgk-Neo-loxP cassette of pLNL2 (S. L. Pfaff), yielding pMFng(5′)LNL. Next, a novel KpnI site followed by a third loxP site was introduced into the EcoRI site in the 5′ direction from exon 4. Then a 3.4-kb BsmI-NotI fragment from pBS-MFng-KpnI containing a sequence in the 3′ direction from exon 4 was blunted into the SalI site flanking the 5′ end of the loxP-pgk-Neo-loxP cassette. Finally, an XhoI-HindII fragment containing a herpes simplex virus thymidine kinase cassette was introduced into the XhoI-SalI site flanking the 3′end of the construct.
Generation of an Ngn3-targeting construct.
The Ngn3 gene was disrupted by inserting cDNA for humanized Renilla green fluorescent protein (hrGFP; Stratagene) under the translational control of an IRES into exon 2 of Ngn3 (see Fig. S1 in the supplemental material). This strategy simultaneously inactivates Ngn3 and allows the monitoring of Ngn3 promoter activity by using hrGFP fluorescence. A 5.8-kb HindIII-SpeI genomic fragment containing the entire Ngn3 locus and 1.4 kb of upstream and 2.7 kb of downstream sequences was isolated from an RPCI-22 mouse 129s6/SvEvTAC Taconic (female) BAC library (25). The Ngn3 gene was disrupted by the insertion of an IRES-hrGFP-poly(A)-loxP-pgk-Neo-loxP cassette, resulting in an IRES-hrGFP gene knock-in into exon 2 of Ngn3. The IRES-hrGFP gene construct was obtained from pIRES-hrGFP-2A (Stratagene), and the loxP-pgk-Neo-loxP cassette was obtained from pLNL2 (S. L. Pfaff). Recombinant embryonic stem (ES) cell clones were identified through Southern blotting (data not shown).
Generation of MFng- and Ngn3-deficient mice.
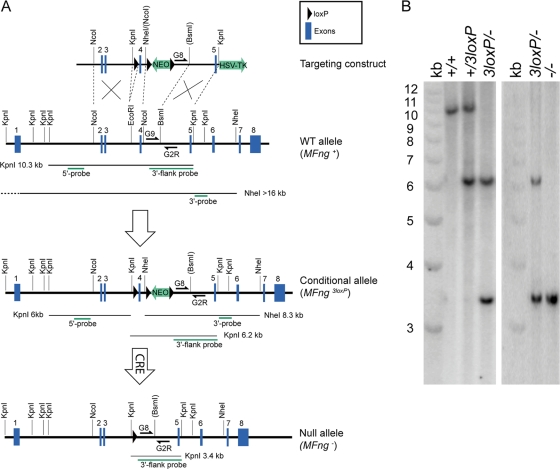
The final targeting constructs (see Fig. 4A; see also Fig. S1 in the supplemental material) were linearized (with NotI for MFng and NotI-XhoI for Ngn3) and introduced into ES cells as described previously (17). Homologous recombinant clones were identified by Southern blotting (see Fig. 4B; also data not shown). The presence of nonhomologous integrations was excluded by analysis with a probe against the Neo gene.
FIG. 4.
Strategy for generating and genotyping MFng conditional and null alleles. (A) The MFng-targeting construct was generated by introducing loxP sites surrounding exon 4, which encodes the DXD glycosyltransferase motif that is required for Fringe function. Homologous recombination and Cre-mediated recombination events were evaluated by Southern blotting using a 5′ probe and a 3′ probe to detect KpnI or NheI restriction fragments as indicated. Routine genotyping of wild-type (WT) and null alleles was performed by PCR using primers MFng-G8, MFng-G9, and MFng-G2R as indicated. HSV-T, herpes simplex virus thymidine kinase cassette. (B) Southern blotting of KpnI-digested genomic DNA probed with the 3′-end-flanking probe (A) detects the wild-type (+), conditional (3loxP), and null (−) alleles of MFng. For construction details, see Materials and Methods.
Genotyping of mice.
The genotypes of mice were determined by PCR analyses of genomic DNA samples extracted from tail biopsy specimens. Primers IPF15′-3 (GGGAAGAGGAGATGTAGACTT), MFng-AR (CCACATAGACATCACGGT), and IPF1-AR (GAGCTGAGCTGGAAGGT) were used simultaneously to amplify 700- and 750-bp PCR fragments corresponding to the wild-type Ipf1 allele and the Ipf1-MFng transgenic construct, respectively. Primers MFng-G9 (ACAGGATCTTAAAACACCACAGT), MFng-G8 (GAAGTTATGTCGATGCCTAACA), and MFng-G2R (CTAAACTTGGGAATACTTGGGA) were used to simultaneously amplify 280- and 230-bp fragments from the MFng wild-type and targeted alleles, respectively. Primers Neo-A (AATGTGTCAGTTTCATAGCC), Ngn3-s (CCACGAAGTGCTCAGTTCCAA), and Ngn3-as (GGGAAGGTGGGCAGGACA) were used to simultaneously amplify 263- and 368-bp fragments from the Ngn3 wild-type and targeted alleles, respectively. The animal studies were approved by the Institutional Animal Care and Use Committee of Umeå University and were conducted in accordance with the guidelines for the care and use of laboratory animals.
In situ hybridization and immunohistochemistry.
In situ hybridization with digoxigenin labeling was performed as described previously (1). The probes used were Ngn3 (2); Notch1, Notch2, Dll1, Hes1, NeuroD, and Serrate1 (3); Hes6 (RIKEN clone no. 2810482C04); Nkx2.2; Hey-L (RIKEN clone no. 1200011B24); Arx (IMAGE clone no. 5715799); MFng (kindly provided by E. Wandzioch); an LFng full-length PCR fragment; and an RFng full-length PCR fragment. Pax4 cDNA was isolated from a βTC1 λ-ZAP express library (kindly provided by Yoav Arava, M. D. Walker lab), and a 950-bp 5′-end SmaI fragment was used as the template for the in situ probe. Immunohistochemistry analysis was performed as described previously (1). The primary antibodies used were rabbit anti-Ipf1/Pdx1 (24), guinea pig antiglucagon (Linco), rabbit antiglucagon (Euro-Diagnostica), rat antisomatostatin (Bender MedSystems), rabbit anti-cleaved caspase 3 (Cell Signaling Technology), guinea pig anti-insulin (Dako), rabbit anti-carboxypeptidase A (anti-CPA; Anawa), rabbit antiamylase (Sigma), rabbit anti-Glut2 (12), rabbit anti-phospho-histone H3 (Upstate Biotechnology), rabbit anti-Nkx6.1 (16), mouse monoclonal pancytokeratin antibody (C2562; Sigma), rabbit anti-Ngn3 (30), rabbit anti-p48 (21), rabbit anti-Isl1 (34), goat antighrelin (Santa Cruz Biotechnology), rabbit anti-Flk1 (RDI), rat antipecam (BD Biosciences), rabbit anti-pancreatic polypeptide (anti-PP; Eurodiagnostics), mouse monoclonal anti-smooth muscle actin antibody (Sigma), and Dolichos biflorus agglutinin (DBA) lectin (Vector Laboratories). The secondary antibodies used were Alexa 488-conjugated anti-guinea pig and anti-rat antibodies (Molecular Probes) and a Cy3-conjugated anti-rabbit antibody (Jackson ImmunoResearch Laboratories, Inc.).
Image processing and quantification.
Photoshop CS software was used to generate pseudocolored in situ hybridization-immunohistochemistry composite pictures and to process image data. The colocalization of immunohistochemical and in situ hybridization signals was quantified manually using in situ hybridization-immunohistochemistry composites from every fifth section of embryonic day 9 (E9) embryos or from single E14 sections containing >50 Ngn3+ cells. Area quantification of immunohistochemical stainings was performed using Easy Image Analysis software (Tekno Optik AB, Sweden). All quantification data are presented as averages ± standard errors of the means (SEM).
Glucose measurements.
Glucose tolerance was measured following the intraperitoneal injection of anesthetized mice with 2 g of glucose/kg of body weight after overnight fasting. Blood glucose levels were measured using a Glucometer Elite (Bayer Inc.).
RESULTS
MFng and LFng are expressed in the developing mouse pancreas.
In order to further define mechanisms regulating Notch signaling in the developing pancreas, we analyzed the expression of the Notch-modulating Fringe genes LFng, MFng, and RFng. Between E9.5 and E14.5, MFng expression was detected in scattered cells of both the pancreatic mesenchyme and epithelium (Fig. 1A). The scattered epithelial expression pattern was reminiscent of the expression patterns of Dll1 and Ngn3 (Fig. 1B and C), and colabeling with Ngn3-specific antibodies revealed that epithelial MFng expression colocalized with Ngn3 (Fig. 1D and H). LFng expression was first observed at E14.5 in developing acinar structures of the pancreatic epithelium that coexpressed Ptf1a (also known as p48) (Fig. 1E and I). In contrast, RFng expression was not detectable in the developing pancreatic epithelium at any developmental stage (Fig. 1F and L and data not shown).
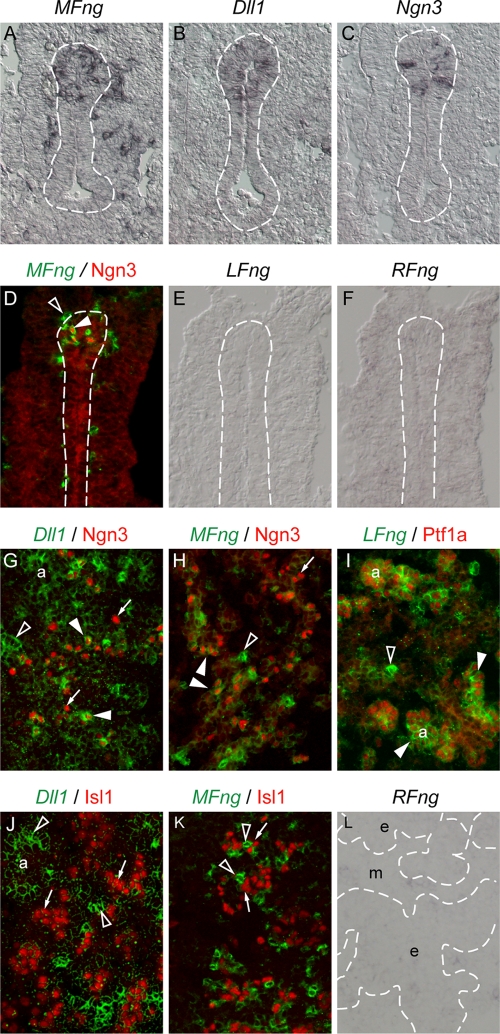
FIG. 1.
Expression of MFng in Ngn3+ proendocrine cells of the developing pancreas. E10.5 (A to C), E9.5 (D to F), and E14.5 (G to L) wild-type pancreases were analyzed by in situ hybridization. (A to C) MFng (black in panel A), Dll1 (black in panel B), and Ngn3 (black in panel C) are expressed in scattered cells within the dorsal pancreas at E10.5. (D to F) MFng (green pseudocolor in panel D) is coexpressed with Ngn3 (red immunohistochemical staining in panel D) at E9.5, whereas the expression of LFng (E) and RFng (F) is not detected. (G, H, J, and K) Dll1 (green pseudocolor in panels G and J) and MFng (green pseudocolor in panels H and K) are transiently expressed in proendocrine cells expressing Ngn3 (red immunohistochemical staining in panels G and H) but downregulated in differentiated endocrine cells expressing Isl1 (red immunohistochemical staining in panels J and K). (I and L) LFng (green pseudocolor in panel I) is coexpressed with Ptf1a (red immunohistochemical staining in panel I) in exocrine acinar cells, whereas RFng (L) is not expressed in pancreatic epithelia at E14.5. Broken lines (A to F and L) delimit pancreatic epithelia. Open arrowheads mark cells positive for in situ hybridization but negative for Ngn3, Isl1, or Ptf1a. Closed arrowheads mark cells positive for in situ hybridization and Ngn3 or Ptf1a. Arrows mark cells negative for in situ hybridization but positive for Ngn3 or Isl1. a, acinar exocrine cells; e, pancreatic epithelia; m, mesenchyme.
Immunohistochemical double labeling with cell-specific markers revealed that virtually all epithelial MFng-expressing cells coexpressed Ngn3 between E9.5 and E14.5 (Fig. 1D and H). Conversely, most Ngn3+ cells (87% ± 2%; n = 3) at E9.5 also expressed MFng (Fig. 1D). At E14.5, however, only subsets of Ngn3+ cells (51% ± 3% [n = 3] and 25% ± 3% [n = 3], respectively) coexpressed MFng and Dll1 (Fig. 1G and H). Moreover, the expression of MFng or Dll1 could not be detected in more mature endocrine cells expressing Isl1 (Fig. 1J and K), suggesting that MFng and Dll1 become downregulated as proendocrine cells mature or differentiate. Taken together, these data raise the possibility that the Notch signaling pathway is modulated by LFng in acinar progenitors and by MFng in endocrine progenitor cells.
MFng expression is dependent on Ngn3.
Previous studies with chicks have suggested that MFng functions upstream of Ngn3 to induce endocrine differentiation (35). Whereas our expression analyses verify that the expression patterns of MFng and Ngn3 colocalize, the temporal order of MFng expression and Ngn3 expression could not be determined from the results of these studies. To investigate the epigenetic relationship between MFng and Ngn3 expression, we next analyzed MFng expression in mice deficient in Ngn3 function. The Ngn3-deficient mice were generated as described in Materials and Methods and depicted in Fig. S1 in the supplemental material. In agreement with the findings in previous publications (13), mice homozygous for the Ngn3 allele disrupted by the IRES-hrGFP gene cassette, hereafter designated Ngn3−/−, showed a complete absence of differentiated pancreatic endocrine cell types (data not shown). Analyses of MFng expression revealed that it was absent in Ngn3−/− E10.5 and E14.5 pancreatic epithelia (Fig. 2B′ and C′). In contrast, MFng+ cells were still present in the pancreatic mesenchymes of Ngn3−/− mice (Fig. 2B′ and C′). These results provide evidence that MFng is genetically downstream of Ngn3 in the developing pancreatic epithelium.
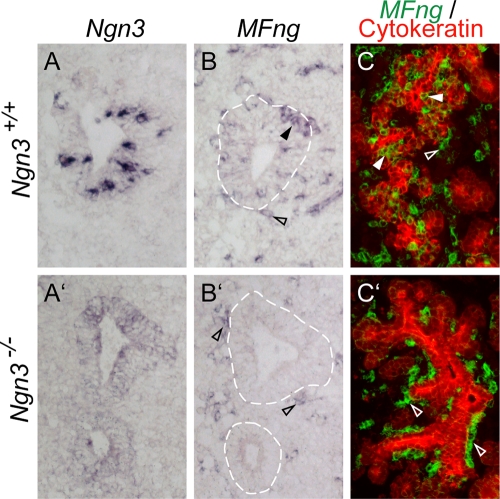
FIG. 2.
Loss of MFng expression in Ngn3−/− pancreas. E10.5 (A to B′) and E14.5 (C and C′) pancreases of wild-type (A to C) and Ngn3-deficient (A′ to C′) mice were analyzed by in situ hybridization. (A and B) In wild-type pancreas at E10.5, Ngn3 and MFng mRNAs (blue) are detected in scattered epithelial cells. The expression of MFng is also detected in mesenchymal cells (open arrowhead in panel B). (A′ and B′) In Ngn3−/− pancreatic epithelia at E10.5, mutant Ngn3 mRNA is weakly detected (blue in panel A′) whereas MFng mRNA (blue in panel B′) is not detected. The expression of MFng (open arrowheads in panel B′) is retained in mesenchymal cells. (C and C′) In wild-type E14.5 embryos, MFng (green pseudocolor) is expressed both in the pancytokeratin-positive pancreatic epithelium (red) and within the surrounding mesenchyme (C). In Ngn3−/− pancreas, MFng expression is retained only in the mesenchymal cells (C′). Broken lines in panels B and B′ delineate pancreatic epithelia. Closed arrowheads mark MFng-positive pancreatic epithelial tissue. Open arrowheads mark MFng-positive pancreatic mesenchyme tissue.
Forced expression of MFng in pancreatic progenitor cells does not perturb pancreatic development.
In order to investigate the role for MFng in mouse pancreatic development, we generated transgenic mice, designated Ipf1-Mfng mice, expressing MFng under the control of the Ipf1/Pdx1 promoter (2), which is active in both pancreatic progenitor cells and differentiated β-cells as they appear during development. A construct containing an IRES-lacZ cassette following MFng was also generated in order to facilitate the detection of the transgenic expression (for further details, see Materials and Methods). The MFng open reading frame in the final constructs was verified by sequencing, and the transgenic expression of MFng was verified by β-galactosidase staining and/or in situ hybridization in five of six transgenic lines. At E10.5, strong uniform expression of MFng throughout the pancreatic epithelia of Ipf1-MFng embryos was detected (Fig. 3A and A ′). In both wild-type and Ipf1-MFng E10.5 embryos, cells strongly double positive for Dll1 and Ngn3 were observed scattered among pancreatic Ngn3-negative epithelial cells expressing Dll1 at low levels (Fig. 3B and B′). The epithelial areas of Ngn3+ cells and glucagon-positive cells in Ipf1-MFng transgenic mice and in wild-type littermates at E10.5 were similar (Fig. 3F). At E14.5, ectopic MFng expression was observed primarily in the acinar tissue and β-cells and only low levels could be detected in the undifferentiated epithelium (Fig. 3C and C′). Immunohistochemical analyses of Ngn3+ proendocrine cells and differentiated endocrine cells expressing glucagon, insulin, or ghrelin revealed no apparent differences between wild-type and Ipf1-MFng pancreases (Fig. 3D to E′).
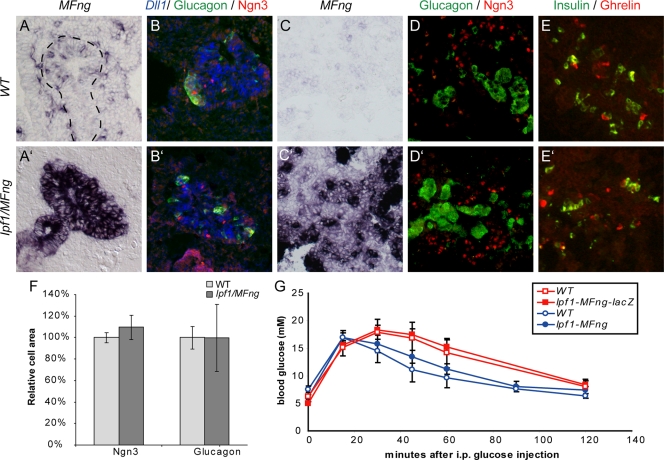
FIG. 3.
Normal endocrine cell differentiation and β-cell function in Ipf1-MFng transgenic mice. (A to E′) Expression of progenitor and differentiation markers in the pancreases of wild-type (WT) (A to E) and Ipf1-MFng (A′ to E′) mice at E10.5 (A to B′) and E14.5 (C to E′). (A to B′) At E10.5, the forced expression of MFng (compare panels A and A′) does not affect the expression of Dll1 (blue pseudocolor in panels B and B′), Ngn3 (red immunohistochemical staining in panels B and B′), or glucagon (green immunohistochemical staining in panels B and B′). (C to E′) At E14.5, Ipf1-MFng pancreases show normal expression of Ngn3 and glucagon (red and green immunohistochemical staining, respectively, in panels D and D′) and insulin and ghrelin (green and red immunohistochemical staining, respectively, in panels E and E′). (F) Quantification of the epithelial areas occupied by Ngn3+ cells and glucagon-positive α-cells at E10.5. Values are presented relative to wild-type values and are averages ± SEM (n = 3). (G) The clearance of glucose from the blood after the intraperitoneal (i.p.) injection of Ipf1-MFng-lacZ transgenic (n = 7) and wild-type (n = 5) mice and Ipf1-MFng transgenic (n = 4) and wild-type (n = 4) mice with glucose was normal. Values are shown with SEM. Broken lines in panel A delimit pancreatic epithelia.
Consistent with the lack of developmental defects in Ipf1-MFng transgenic mice, mice from all transgenic lines were healthy and reproductive and showed no signs of perturbed glucose homeostasis when exposed to exogenous glucose by intraperitoneal injection (Fig. 3G). Immunohistochemical analysis of pancreases from adult Ipf1-MFng transgenic mice also revealed that the gross pancreatic histology and the expression of exocrine enzymes (CPA and amylase) and endocrine hormones were normal (data not shown). Thus, the overexpression of MFng in early mouse pancreatic progenitor cells does not perturb pancreatic development, endocrine cell differentiation, or adult β-cell function.
Targeted deletion of MFng does not perturb embryonic development.
In order to assess a potential requirement of MFng for pancreas development and/or endocrine cell differentiation, we next generated mice in which MFng could be conditionally inactivated. The ability of the Fringe proteins to glycosylate the Notch receptor critically depends on the glycosyltransferase motif DXD (5, 23), which in MFng is encoded by exon 4. A three-loxP-site targeting construct was prepared with loxP sites flanking exon 4, followed by a neomycin resistance cassette and a third loxP site (Fig. 4A). Following Cre-mediated excision of exon 4, the splicing of exon 3 directly onto exon 5 is predicted to result in a frameshift and a premature stop codon in exon 5. The construct was introduced into ES cells, and recombinant clones were used to generate mice carrying the conditional MFng allele with three loxP sites (MFng3loxP). To inactivate MFng specifically in the pancreas, the MFng+/3loxP mice were bred with transgenic mice expressing the Cre recombinase fused to a nuclear localization signal (NLS-Cre) under the control of the Ipf1/Pdx1 promoter (Ipf1/nlsCRE mice). However, the breeding of male MFng+/3loxP mice with female Ipf1/nlsCRE mice generated offspring carrying a recombinant MFng allele, MFng− (Fig. 4B), presumably due to leaky expression of NLS-Cre in female germ cells. Mice heterozygous for the MFng− allele were next crossed to generate MFng−/− mice, which turned out to be viable, healthy, and reproductive, providing evidence that MFng function is not critical for mouse development and thus making the conditional inactivation strategy redundant. Hence, subsequent analyses were performed using MFng−/− mice.
Normal organization and function of pancreases in adult MFng−/− mice.
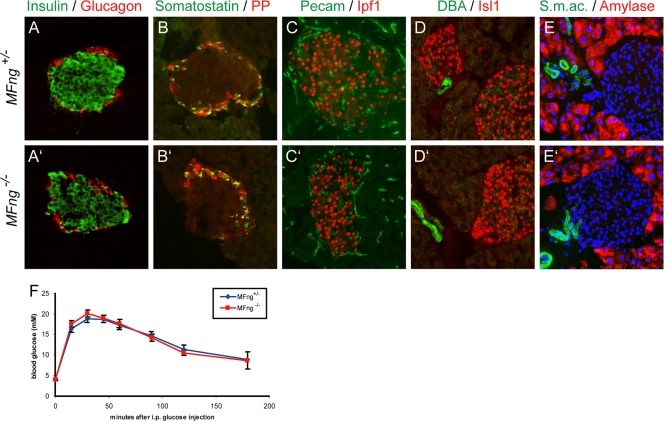
Analyses of pancreases from adult MFng heterozygous and homozygous mutant mice showed that gross pancreatic histology, including that of endocrine islets and acinar tissue and ducts, was normal in MFng−/− mice (Fig. 5). The distribution and numbers of the different endocrine cell types, as defined by the expression of insulin, glucagon, somatostatin, and PP, were also normal in MFng homozygous mice (Fig. 5A to B′ and data not shown). The expression of the β-cell markers Nkx6.1, Ipf1/Pdx1, and Glut2 and the panendocrine marker Isl1, as well as the expression of the exocrine markers amylase, CPA, and Ptf1a and the ductal marker DBA, in pancreases from adult MFng-deficient mice was indistinguishable from that observed in pancreases from heterozygous littermates (Fig. 5C to E′ and data not shown). We next analyzed the vascularization of the pancreas in MFng mutant mice since MFng is expressed in endothelial cells of developing blood vessels (data not shown). The blood vessel markers pecam, smooth muscle actin, and Flk1 all showed normal expression in the pancreases of MFng−/− mutant mice compared to that in the pancreases of MFng+/− mice (Fig. 5C, C′, E, and E′ and data not shown). No variations in cell proliferation or apoptosis, as assessed by using phospho-histone H3 or cleaved caspase 3, respectively, in pancreases of adult MFng−/− mutant mice compared to that in pancreases of heterozygous littermates were observed (data not shown). Finally, β-cell function was assessed by the administration of intraperitoneal glucose injections. MFng-deficient mice cleared exogenous glucose as efficiently as heterozygous littermates, providing evidence for normal β-cell function and blood glucose level homeostasis in MFng−/− mutant mice (Fig. 5F). Thus, MFng appears to be dispensable for mouse pancreas development and function.
FIG. 5.
Pancreatic histology and β-cell function in adult MFng−/− pancreas are normal. (A to E′) Immunohistochemical analysis of the endocrine hormones insulin, glucagon, somatostatin, and PP (as indicated in panels A to B′), the endocrine markers Ipf1 and Isl1 (red in panels C to D′), the vasculature markers pecam and smooth muscle actin (s.m.ac.; green in panels C, C′, E, and E′), the duct marker DBA (green in panels D and D′), and the exocrine enzyme amylase (red in panels E and E′) in pancreases of MFng+/− (A to E) and MFng −/− (A′ to E′) mice. Nuclear staining with DAPI (4′,6-diamidino-2-phenylindole; blue) is shown in panels E and E′. (F) The patterns of the clearance of glucose from the blood after the intraperitoneal (i.p.) injection of MFng+/− (n = 13) and MFng−/− (n = 8) mice with glucose were similar. Values are shown with SEM.
Normal pancreatic development of MFng−/− pancreas.
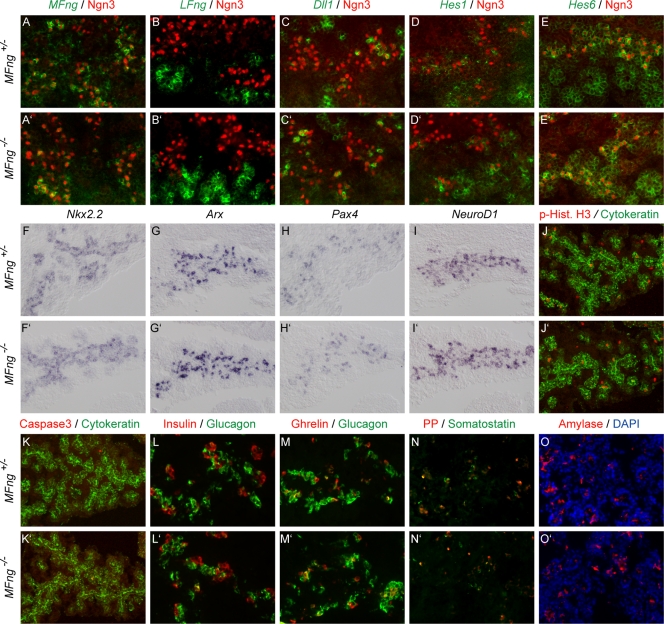
In order to investigate a possible transient embryonic phenotype, as has been described previously for the conditional inactivation of RBP-Jκ (11), we next analyzed E10.5 to E16.5 pancreases derived from MFng−/− and MFng+/− embryos. No difference in the number of Ngn3+ proendocrine cells between MFng−/− and MFng+/− pancreases was observed (Fig. 6A to E ′). Ngn3+ proendocrine cells exhibited normal expression of the Notch ligand gene Dll1 and the Notch signaling-responsive gene Hes1 and its inhibitor Hes6 (Fig. 6C to E′ and data not shown). These results demonstrate that MFng is not required for the generation of Ngn3+ proendocrine cells or for their transcriptional regulation of Dll1, Hes1, and Hes6. In MFng−/− embryos, no ectopic expression of LFng or RFng within the pancreatic epithelium at E10.5 and E14.5 was observed, arguing against compensatory expression of LFng and RFng in MFng−/− pancreas (Fig. 6A to B′ and data not shown). The expression of the Notch ligand Serrate1 and the Notch receptors Notch1 and Notch2 in the pancreatic epithelia of E14.5 MFng−/− embryos was unaltered compared to that in wild-type embryos (data not shown). These results show that the loss of MFng function is not compensated for by other Fringe genes and that it does not lead to altered expression of key Notch signaling components.
FIG. 6.
Normal pancreatic development in MFng−/− mice. (A to E′) In situ hybridization of Notch signaling genes (green pseudocolor in A to E′) in E14.5 pancreases and proendocrine cells expressing Ngn3 (red immunohistochemistry staining) in MFng+/− (A to E) and MFng−/− (A′ to E′) mice. Mutant MFng mRNA was detected at reduced levels (compare panels A and A′), no compensatory upregulation of LFng (green pseudocolor in panels B and B′) was observed, and the expression of Dll1 and Hes1 and Hes6 (as indicated in panels C to E′) was unaltered. (F to I′) In situ hybridization of progenitor cell markers Nkx2.2, Arx, Pax4, and NeuroD1 (blue, as indicated). (J to K′) Immunohistochemical analysis of proliferation and apoptosis markers phospho-histone H3 (red in panels J and J′) and cleaved caspase 3 (red in panels K and K′) in E14.5 pancreatic epithelia (indicated by green pancytokeratin immunohistochemistry staining). (L to O′) Immunohistochemical analyses of endocrine hormones (as indicated in panels L to N′) and the exocrine enzyme amylase (O and O′). Nuclear staining with DAPI (blue) is shown in panels O and O′.
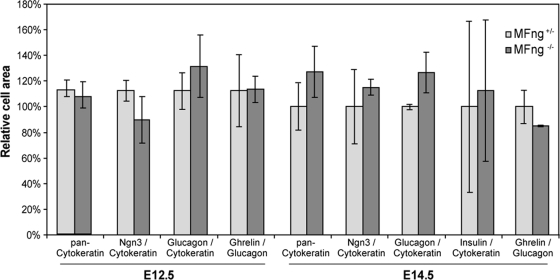
A potential role for MFng in the specification of proendocrine cell identity was assessed by analyzing the expression of the transcription factor genes, such as Nkx2.2, Arx, Pax4, and NeuroD1, known to control pancreatic endocrine cell specification (6, 8, 27). The expression of Nkx2.2, Arx, Pax4, and NeuroD1 was unaltered in pancreatic progenitor cells and in Ngn3+ proendocrine cells of MFng−/− mice at E10.5 and E14.5 (Fig. 6F to I′ and data not shown). Next, a possible role for MFng in controlling the expansion and differentiation of specified proendocrine cells was investigated. No variations in progenitor cell apoptosis or proliferation, as assayed by using cleaved caspase 3 or phospho-histone H3, respectively, was observed, however (Fig. 6J to K′). The differentiation of glucagon-positive α-cells between E10.5 and E16.5 (Fig. 6L to M′ and data not shown) and the onset of the expression of the later-appearing pancreatic endocrine hormones (insulin, ghrelin, somatostatin, and PP) and exocrine markers (Ptf1a, CPA, and amylase) at E14.5 and E16.5 (Fig. 6L to O′ and data not shown) in MFng−/− mice were similar to those in heterozygous littermates. The quantification of pancreatic progenitors, proendocrine cells, and differentiated α- and β-cells from E12.5 and E14.5 pancreases revealed no significant differences between MFng−/− and MFng+/− mice (Fig. 7). Taken together, these results show that MFng is dispensable for the normal specification, proliferation, differentiation, and survival of pancreatic progenitors during embryonic development.
FIG. 7.
Quantification of endocrine cell types in E12.5 and E14.5 MFng −/− pancreases. No significant difference in the total epithelial area, the area occupied by Ngn3+ proendocrine cells, or the area occupied by endocrine cell types expressing glucagon, insulin, or ghrelin between MFng+/− and MFng−/− embryos at E12.5 or E14.5 were found (n = 3). Data are averages ± SEM.
DISCUSSION
During pancreatic development, Notch signaling plays a central role in the control of cell proliferation and differentiation. Transient expression of the Notch-modulating enzyme MFng precedes the appearance of differentiated endocrine cell types in the anterior pituitary and neurons in the olfactory epithelium (S. Norlin, unpublished data) and has also been reported to occur in immature neurons as they exit the ventricular zone (19). Thus, these expression patterns are suggestive of a conserved role for MFng in various neural and endocrine progenitor cell populations. In vitro studies have also suggested that MFng acts by inhibiting Notch signaling in chick endoderm, thereby triggering the differentiation of both pancreatic and nonpancreatic progenitor cells into endocrine cells (35). Here, we have investigated the role of MFng in the developing mouse pancreas in vivo.
Our data provide evidence that Ngn3 function is required for MFng expression, suggesting that MFng function is downstream rather than upstream of Ngn3 in mouse pancreatic proendocrine cells. Consistent with such a scenario, and assuming that the transgenic MFng mRNA is properly translated, our data show that the forced expression of MFng in early mouse pancreatic progenitors is not sufficient to induce Ngn3 expression and endocrine cell differentiation. Thus, it appears unlikely that the ability of MFng to induce endocrine differentiation when ectopically expressed in chick endoderm is related to the function of MFng in mouse Ngn3+ pancreatic cells. Instead, the forced expression of MFng in chick endodermal cells may result in the ectopic inhibition of Notch signaling, which has been shown to result in precocious and ectopic endocrine cell differentiation in mouse (3, 10, 20, 32). The contradicting results from mouse and chick experiments may be explained by differences in available ligands, as MFng inhibits Notch activation by Serrate but not by Dll1. In early mouse pancreatic progenitor cells, Dll1, but not Serrate1, is expressed (3), whereas in chick gut epithelial cells, the expression of both Dll1 and Serrate2 has been reported to occur (35). Thus, in chick, ectopic MFng may inhibit Serrate-Notch signaling, leading to endocrine differentiation, whereas in mouse, MFng may rather act to potentiate Notch signaling by Dll1. Alternatively, differences in the tissue distribution patterns of ectopically and transgenically expressed MFng enzymes may explain the contrasting results. A key feature of Notch-mediated lateral inhibition is the amplification of differences in Notch activation between neighboring cells. In E8.5 to E10.5 Ipf1-MFng transgenic mice, all pancreatic progenitor cells express MFng, which can modulate Notch signaling equally in all progenitor cells. In electroporated chick endoderm, the mosaic ectopic expression of MFng in pancreatic progenitor cells may introduce an imbalance of Notch activity between transfected and nontransfected cells, which then may be sufficient to trigger endocrine differentiation.
Proendocrine expression of MFng depends on Ngn3 function and appears to be downregulated as endocrine cells differentiate. Thus, we expected MFng function to be associated with Ngn3+ proendocrine cell maturation. The targeted deletion of MFng did not, however, result in any detectable perturbations in pancreatic development, cell differentiation, or function. Although we cannot exclude the possibility that the translation product of the remaining exons 1 to 3 may retain unknown activities separate from the glycosyltransferase activity of MFng, these results argue against a role for MFng in the specification, generation, and differentiation of the endocrine cell lineages. In the pancreas, there is no apparent overlap in the expression patterns of the different Fringe family members. Moreover, we failed to detect any compensatory changes in the expression of other Fringe genes and of pancreatic Notch pathway genes. Importantly, we also failed to observe any changes in the expression of the Notch target gene Hes1 or its inhibitor Hes6. Similarly, Hes1 expression in chick gut endoderm is apparently unaltered after the ectopic expression of MFng (35), raising the possibility that the effect of MFng in chick endoderm is not mediated by Notch signaling via Hes1.
The targeted deletion of LFng in mouse results in increased perinatal death and severe defects related to impaired somitogenesis (36). In contrast, no obvious phenotype due to the deletion of RFng has been reported, and mice deficient in both LFng and RFng display phenotypes similar to those of LFng single knockouts (37). Thus, although the MFng expression pattern is suggestive of a role for MFng in the differentiation of various progenitor cell populations, we here show that the inactivation of MFng does not result in any gross perturbations of mouse development. Further detailed analysis of MFng-deficient mice with respect to the development and function of other MFng-expressing tissues, such as the anterior pituitary and olfactory epithelia, may nevertheless provide further insight regarding the function of MFng in mouse. Presently, however, it appears that MFng, like RFng, is dispensable for mouse development.
Supplementary Material
Acknowledgments
We thank the Umeå Transgene Core Facility, U. Valtersson, and E. Pålsson for technical assistance and members of our laboratory for technical instructions, suggestions, and helpful discussions.
This work was supported by grants from the Swedish Research Council, the Juvenile Diabetes Research Foundation, and the Kempe Foundation (to H.E).
Footnotes
Published ahead of print on 17 February 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ahlgren, U., J. Jonsson, and H. Edlund. 1996. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development 1221409-1416. [DOI] [PubMed] [Google Scholar]
- 2.Apelqvist, A., U. Ahlgren, and H. Edlund. 1997. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr. Biol. 7801-804. [DOI] [PubMed] [Google Scholar]
- 3.Apelqvist, A., H. Li, L. Sommer, P. Beatus, D. J. Anderson, T. Honjo, M. Hrabe de Angelis, U. Lendahl, and H. Edlund. 1999. Notch signaling controls pancreatic cell differentiation. Nature 400877-881. [DOI] [PubMed] [Google Scholar]
- 4.Arber, S., B. Han, M. Mendelsohn, M. Smith, T. M. Jessell, and S. Sockanathan. 1999. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron 23659-674. [DOI] [PubMed] [Google Scholar]
- 5.Bruckner, K., L. Perez, H. Clausen, and S. Cohen. 2000. Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature 406411-415. [DOI] [PubMed] [Google Scholar]
- 6.Chao, C. S., Z. L. Loomis, J. E. Lee, and L. Sussel. 2007. Genetic identification of a novel NeuroD1 function in the early differentiation of islet alpha, PP and epsilon cells. Dev. Biol. 312523-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, B., A. Bashirullah, L. Dagnino, C. Campbell, W. W. Fisher, C. C. Leow, E. Whiting, D. Ryan, D. Zinyk, G. Boulianne, C. C. Hui, B. Gallie, R. A. Phillips, H. D. Lipshitz, and S. E. Egan. 1997. Fringe boundaries coincide with Notch-dependent patterning centres in mammals and alter Notch-dependent development in Drosophila. Nat. Genet. 16283-288. [DOI] [PubMed] [Google Scholar]
- 8.Collombat, P., J. Hecksher-Sorensen, J. Krull, J. Berger, D. Riedel, P. L. Herrera, P. Serup, and A. Mansouri. 2007. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J. Clin. Investig. 117961-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edlund, H. 2001. Factors controlling pancreatic cell differentiation and function. Diabetologia 441071-1079. [DOI] [PubMed] [Google Scholar]
- 10.Fujikura, J., K. Hosoda, H. Iwakura, T. Tomita, M. Noguchi, H. Masuzaki, K. Tanigaki, D. Yabe, T. Honjo, and K. Nakao. 2006. Notch/Rbp-j signaling prevents premature endocrine and ductal cell differentiation in the pancreas. Cell Metab. 359-65. [DOI] [PubMed] [Google Scholar]
- 11.Fujikura, J., K. Hosoda, Y. Kawaguchi, M. Noguchi, H. Iwakura, S. Odori, E. Mori, T. Tomita, M. Hirata, K. Ebihara, H. Masuzaki, A. Fukuda, K. Furuyama, K. Tanigaki, D. Yabe, and K. Nakao. 2007. Rbp-j regulates expansion of pancreatic epithelial cells and their differentiation into exocrine cells during mouse development. Dev. Dyn. 2362779-2791. [DOI] [PubMed] [Google Scholar]
- 12.Goulley, J., U. Dahl, N. Baeza, Y. Mishina, and H. Edlund. 2007. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 5207-219. [DOI] [PubMed] [Google Scholar]
- 13.Gradwohl, G., A. Dierich, M. LeMeur, and F. Guillemot. 2000. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc. Natl. Acad. Sci. USA 971607-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu, G., J. M. Wells, D. Dombkowski, F. Preffer, B. Aronow, and D. A. Melton. 2004. Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development 131165-179. [DOI] [PubMed] [Google Scholar]
- 15.Hald, J., J. P. Hjorth, M. S. German, O. D. Madsen, P. Serup, and J. Jensen. 2003. Activated Notch1 prevents differentiation of pancreatic acinar cells and attenuate endocrine development. Dev. Biol. 260426-437. [DOI] [PubMed] [Google Scholar]
- 16.Hart, A., S. Papadopoulou, and H. Edlund. 2003. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev. Dyn. 228185-193. [DOI] [PubMed] [Google Scholar]
- 17.Hogan, B., R. Beddington, F. Costantini, and E. Lacy. 1994. Manipulating the mouse embryo. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 18.Irvine, K. D. 1999. Fringe, Notch, and making developmental boundaries. Curr. Opin. Genet. Dev. 9434-441. [DOI] [PubMed] [Google Scholar]
- 19.Ishii, Y., S. Nakamura, and N. Osumi. 2000. Demarcation of early mammalian cortical development by differential expression of fringe genes. Brain Res. Dev. Brain Res. 119307-320. [DOI] [PubMed] [Google Scholar]
- 20.Jensen, J., E. E. Pedersen, P. Galante, J. Hald, R. S. Heller, M. Ishibashi, R. Kageyama, F. Guillemot, P. Serup, and O. D. Madsen. 2000. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2436-44. [DOI] [PubMed] [Google Scholar]
- 21.Li, H., and H. Edlund. 2001. Persistent expression of Hlxb9 in the pancreatic epithelium impairs pancreatic development. Dev. Biol. 240247-253. [DOI] [PubMed] [Google Scholar]
- 22.Mikami, T., Y. Ohnaka, A. Nakamura, A. Kurosaka, and N. Itoh. 2001. Radical fringe negatively modulates Notch signaling in postmitotic neurons of the rat brain. Brain Res. Mol. Brain Res. 86138-144. [DOI] [PubMed] [Google Scholar]
- 23.Moloney, D. J., V. M. Panin, S. H. Johnston, J. Chen, L. Shao, R. Wilson, Y. Wang, P. Stanley, K. D. Irvine, R. S. Haltiwanger, and T. F. Vogt. 2000. Fringe is a glycosyltransferase that modifies Notch. Nature 406369-375. [DOI] [PubMed] [Google Scholar]
- 24.Ohlsson, H., K. Karlsson, and T. Edlund. 1993. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 124251-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osoegawa, K., M. Tateno, P. Y. Woon, E. Frengen, A. G. Mammoser, J. J. Catanese, Y. Hayashizaki, and P. J. de Jong. 2000. Bacterial artificial chromosome libraries for mouse sequencing and functional analysis. Genome Res. 10116-128. [PMC free article] [PubMed] [Google Scholar]
- 26.Panin, V. M., V. Papayannopoulos, R. Wilson, and K. D. Irvine. 1997. Fringe modulates Notch-ligand interactions. Nature 387908-912. [DOI] [PubMed] [Google Scholar]
- 27.Prado, C. L., A. E. Pugh-Bernard, L. Elghazi, B. Sosa-Pineda, and L. Sussel. 2004. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc. Natl. Acad. Sci. USA 1012924-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rampal, R., A. S. Li, D. J. Moloney, S. A. Georgiou, K. B. Luther, A. Nita-Lazar, and R. S. Haltiwanger. 2005. Lunatic fringe, manic fringe, and radical fringe recognize similar specificity determinants in O-fucosylated epidermal growth factor-like repeats. J. Biol. Chem. 28042454-42463. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Esteban, C., J. W. Schwabe, J. De La Pena, B. Foys, B. Eshelman, and J. C. Belmonte. 1997. Radical fringe positions the apical ectodermal ridge at the dorsoventral boundary of the vertebrate limb. Nature 386360-366. [DOI] [PubMed] [Google Scholar]
- 30.Selander, L., and H. Edlund. 2002. Nestin is expressed in mesenchymal and not epithelial cells of the developing mouse pancreas. Mech. Dev. 113189-192. [DOI] [PubMed] [Google Scholar]
- 31.Singh, N., R. A. Phillips, N. N. Iscove, and S. E. Egan. 2000. Expression of notch receptors, notch ligands, and fringe genes in hematopoiesis. Exp. Hematol. 28527-534. [DOI] [PubMed] [Google Scholar]
- 32.Sumazaki, R., N. Shiojiri, S. Isoyama, M. Masu, K. Keino-Masu, M. Osawa, H. Nakauchi, R. Kageyama, and A. Matsui. 2004. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat. Genet. 3683-87. [DOI] [PubMed] [Google Scholar]
- 33.Thelu, J., P. Rossio, and B. Favier. 2002. Notch signaling is linked to epidermal cell differentiation level in basal cell carcinoma, psoriasis and wound healing. BMC Dermatol. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thor, S., J. Ericson, T. Brannstrom, and T. Edlund. 1991. The homeodomain LIM protein Isl-1 is expressed in subsets of neurons and endocrine cells in the adult rat. Neuron 7881-889. [DOI] [PubMed] [Google Scholar]
- 35.Xu, Y., S. Wang, J. Zhang, A. Zhao, B. Z. Stanger, and G. Gu. 2006. The fringe molecules induce endocrine differentiation in embryonic endoderm by activating cMyt1/cMyt3. Dev. Biol. 297340-349. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, N., and T. Gridley. 1998. Defects in somite formation in lunatic fringe-deficient mice. Nature 394374-377. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, N., C. R. Norton, and T. Gridley. 2002. Segmentation defects of Notch pathway mutants and absence of a synergistic phenotype in lunatic fringe/radical fringe double mutant mice. Genesis 3321-28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.