Abstract
Sub1 is implicated in transcriptional activation, elongation, and mRNA 3′-end formation in budding yeast. To gain more insight into its function, we performed a synthetic genetic array screen with SUB1 that uncovered genetic interactions with genes involved in the high-osmolarity glycerol (HOG) osmoresponse pathway. We find that Sub1 and the HOG pathway are redundant for survival in moderate osmolarity. Chromatin immunoprecipitation analysis shows that Sub1 is recruited to osmoresponse gene promoters during osmotic shock and is required for full recruitment of TBP, TFIIB, and RNA polymerase II (RNAP II) at a subset of these genes. Furthermore, we detect Sub1 at the promoter of every constitutively transcribed RNAP II and, unexpectedly, at every RNAP III gene tested, but not at the RNAP I-transcribed ribosomal DNA promoter. Significantly, deletion of SUB1 reduced levels of promoter-associated RNAP II or III at these genes, but not TBP levels. Together these data suggest that, in addition to a general role in polymerase recruitment at constitutive RNAP II and RNAP III genes, during osmotic shock, Sub1 facilitates osmoresponse gene transcription by enhancing preinitiation complex formation.
Sub1 (suppressor of TFIIB mutations 1) has been characterized as a regulator of multiple stages of transcription by RNA polymerase II (RNAP II) in budding yeast. It was first identified based on interactions with the general transcription factor (GTF) TFIIB, encoded by the SUA7 gene. Genetically, overexpression of Sub1 suppresses a cold-sensitive SUA7 mutation, and SUB1 deletion is synthetically lethal in strains expressing mutant SUA7 alleles (18, 39). Sub1 was shown to interact with TFIIB and weakly with the TATA-binding protein (TBP). Association with TFIIB implicates Sub1 in transcriptional initiation or activation. Indeed, recombinant Sub1 stimulates RNAP II transcription in vitro (15), and cells overexpressing Sub1 showed increased transcription of a reporter gene driven by certain upstream activation sequences (18). These observations and the characterization of the human homolog of Sub1, PC4, as a transcriptional coactivator (12, 22) have led to speculation that Sub1 functions as a coactivator in budding yeast. Repression of the inducible IMD2 gene was recently shown to require Sub1, implying that Sub1 might also function in transcriptional repression (21).
In addition to its promoter-associated functions, Sub1 has been implicated in other transcription-related processes. Sub1 interacts physically and genetically with Rna15 and Pta1, subunits of the cleavage/polyadenylation factors CFIA and CPF, respectively (7, 14). Overexpression of Sub1 rescues the cleavage defect in extracts made from a strain expressing mutant Pta1, suggesting that Sub1 plays a role in mRNA 3′-end formation (14). However, an analysis of the interaction between Sub1 and Rna15 uncovered a role in antitermination of RNAP II transcription (7). Allele-specific genetic interactions between SUB1 and genes encoding a kinase (KIN28) and phosphatase (FCP1) of the C-terminal domain of the RNAP II largest subunit suggest that Sub1 can regulate transcriptional elongation as well (9). However, whether specific genes or pathways are targeted by Sub1 is not known.
Budding yeast rapidly adapts to changes in extracellular osmotic conditions through the osmotic stress response. Upon exposure to elevated salt concentrations, for example, cells respond by increasing the intracellular concentration of the osmolyte glycerol by decreasing the rate of its export and increasing transcription of genes involved in its biosynthesis (reviewed in reference 17). The transcriptional response to osmotic stress is dominated by the high-osmolarity glycerol (HOG) pathway, which is activated at the cell membrane by detection of high osmolarity and through a signal cascade phosphorylates the mitogen-activated protein kinase (MAPK) Hog1, which is the homolog of mammalian stress-activated MAPK p38 (17, 33). Phosphorylated Hog1 translocates from the cytoplasm to the nucleus, where it associates with a number of osmotic-stress-activated (osmoresponse) genes, including those involved in glycerol biosynthesis, and stimulates their transcription. Hog1 affects transcription by modifying promoter-associated transcription factors but also plays a more direct role in recruitment of RNAP II and the general transcription machinery through an interaction of Hog1 with RNAP II (2). Additionally, Hog1 targets the RPD3 histone deacetylase complex to osmoresponse gene promoters, which leads to histone deacetylation and increased occupancy of RNAP II (11), and in an elongation-related mechanism, Hog1 increases RNAP II levels at coding regions independently of its role at promoters (28).
Despite the importance of Hog1 in the transcriptional response to osmotic stress, activation of a subset of osmoresponse genes is Hog1 independent, and deletion of HOG1 does not entirely abolish expression of most of its target genes (see reference 31 and references therein). The critical osmoresponse gene encoding a key enzyme involved in glycerol synthesis, GPD1, for example, is highly expressed during osmotic shock, and significant levels of osmotic-shock-dependent GPD1 transcription are detected in hog1Δ cells (30). Moderate osmotic stress is supported in hog1Δ cells (25), suggesting that the reduced transcriptional response provided in the absence of Hog1 is sufficient for growth under these conditions. These observations have led to speculation that there are additional signaling pathways that contribute to the full transcriptional response to osmotic shock in parallel with the HOG pathway (30, 32).
To investigate functions of Sub1 further, we carried out a synthetic genetic array (SGA) screen to identify novel genetic interactions with SUB1. Interestingly, the screen uncovered genetic links between SUB1 and genes involved in the HOG pathway, implicating Sub1 in the osmotic stress response. We found that, upon exposure of cells to osmotic stress, Sub1 rapidly associates with osmoresponse gene promoters. We provide evidence that both Hog1 and Sub1 contribute to transcription of osmoresponse genes, with their functions being redundant in conditions of moderate osmolarity. When the HOG pathway is disabled by HOG1 deletion, Sub1 becomes critical for transcription of a subset of osmoresponse genes. Extending these studies, analysis of Sub1 occupancy at several constitutively transcribed genes revealed that Sub1 is present at every RNAP II, as well as RNAP III, gene tested, indicating that Sub1 has a common function in transcription of many genes. Deletion of SUB1 results in reduced levels of both RNAP II and RNAP III at their respective constitutively transcribed genes, without affecting TBP levels or TFIIB levels at RNAP II promoters. In contrast, at a subset of osmoresponse genes, deletion of SUB1 reduced RNAP II levels as well as levels of promoter-associated TBP and TFIIB. Our data suggest that Sub1 functions in the recruitment of polymerases to constitutively transcribed RNAP II and RNAP III genes, while facilitating activation of osmoresponse genes during osmotic shock through the assembly or stabilization of promoter-associated complexes.
MATERIALS AND METHODS
Yeast strains and procedures.
Saccharomyces cerevisiae strains used are listed in Table S1 in the supplemental material. SGA screens were performed twice using duplicate copies of an ordered array of ∼4,700 strains, each with a different nonessential gene deleted, essentially as described previously (35, 36). SUB1, SPT15 (which encodes TBP), and RPC82 were tagged at their genomic loci as previously described (19). Yeast strains expressing tetracycline-repressed genes were derived from the collection of Timothy Hughes (23).
ChIPs.
For each sample, 200 ml of synthetic minimal medium (complete or selective, as appropriate) was inoculated with the appropriate strain and strains were grown to an optical density (595 nm) of 0.6 to 0.8 at 30°C. For osmotic stress, either NaCl to a final concentration of 0.4 M or an equal volume of H2O for controls was added for 5 minutes before cross-linking. The chromatin immunoprecipitation (ChIP) procedure was performed essentially as previously described (20). For detection of hemagglutinin (HA)-tagged proteins, we used 1 μg of anti-HA antibody (Sigma), and 1 μg of anti-Rpb3 monoclonal antibody (Neoclone) was used for detection of RNAP II. TFIIB was detected with 0.8 μl of a previously reported antibody (27). Primer sequences are listed in Table S2 in the supplemental material. Experiments were performed at least three times, and a typical experiment is shown with quantification. Quantification was performed as previously described (20) by determining the ratio of the gene-specific amplification signal to the background amplification signal from the untranscribed region measured in the same reaction for the immunoprecipitated samples. This ratio was then divided by the same ratio determined for the input material, resulting in a normalized value for the change relative to the background value.
Among repeat experiments, variability in measurements of occupancy is expected because the transcriptional response to osmotic stress occurs very rapidly and lasts only several minutes (1, 31, 32), making it difficult to capture cells at exactly the same state after exposure to NaCl. To address this and other sources of interexperiment variability, we reported the increase in occupancy in treated cells relative to that in mock-treated control cells grown identically and prepared simultaneously with treated cells, within each experiment. The experimental occupancy value was therefore further normalized to the control value (no NaCl) and is indicated as relative occupancy. A similar normalization was used for analyses in which occupancy in wild-type (wt) cells was compared to that in sub1Δ cells to facilitate comparison of the change in occupancy among different genes.
Note that for some osmoresponse genes, particularly those that are highly expressed upon osmotic shock, the intensity of the gene-specific PCR product relative to the intergenic signal appeared reduced in cells exposed to NaCl compared to control cells, as seen in the input set. This difference might reflect differences in the ability to extract these DNA fragments due to changes in chromatin when these genes are transcribed compared to the chromatin of genes in their silent state. As signals were determined as change with respect to background (input) derived from the same chromatin sample, this difference is corrected for.
Reverse transcription-PCR (RT-PCR) analysis.
For preparation of total RNA samples, 50-ml cultures were grown in rich medium to an optical density (595 nm) of 1.0 to 1.5. For osmotic stress, either NaCl to a final concentration of 0.4 M or an equal volume of H2O was added to the cultures for 25 min prior to collection. The extended osmotic shock was used to allow sufficient time for transcripts to accumulate to a detectable level. RNA was isolated as described previously (4). SuperScript II reverse transcriptase (Invitrogen) was used for reverse transcription, with random hexamer oligonucleotides as primers. Primer sequences are listed in Table S2 in the supplemental material. Analyses were performed at least three times, and the results of semiquantitative analyses are shown.
RESULTS
Sub1 interacts genetically with HOG osmoresponse pathway genes.
To gain additional insight into Sub1 function, we carried out an SGA screen (36). Using this method, we generated yeast strains with SUB1 and each of ∼4,700 different nonessential genes deleted. Reduced growth (fitness defects) in any of the double-deletion strains, compared to control strains with each gene deleted individually, indicates a genetic interaction. Among the candidate genes interacting with SUB1 (full results to be reported elsewhere) were a number of genes involved in the HOG osmoresponse pathway, including HOG1, PBS2, SMP1, and PTC1, as well as genes encoding components of the RPD3 histone deacetylase complex, shown to be involved in osmotic stress response, SAP30, SIN3, and PHO23 (data not shown).
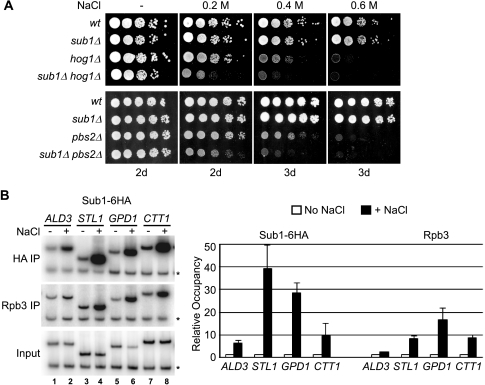
We attempted to confirm the genetic interactions individually but could detect only minor growth defects for some of the HOG pathway genes in the double-deletion strains on standard medium (results not shown). We considered the possibility that, compared to standard growth medium, the medium or conditions used in our SGA screens might have inadvertently triggered a modest stress response and that the genetic interactions were dependent on an activated HOG pathway. We therefore examined the genetic interaction of SUB1 with HOG1 and PBS2 under osmotic stress conditions. PBS2 encodes the sole MAPK kinase in the HOG pathway that phosphorylates Hog1 (5). Progeny of a sporulated diploid cell heterozygous for deletion of both SUB1 and HOG1 or SUB1 and PBS2 were examined by spot assay on rich growth medium or rich medium containing 0.2, 0.4, or 0.6 M NaCl (Fig. 1A). No difference in growth on medium lacking NaCl was apparent, and sub1Δ cells grew as well as wt cells over the range of NaCl concentrations. Both hog1Δ and pbs2Δ cells showed growth defects at 0.4 M NaCl and were unable to grow at 0.6 M NaCl but were unaffected by growth at 0.2 M NaCl. However, cells with both SUB1 and HOG1 or SUB1 and PBS2 deleted showed growth defects at a lower salt concentration than did their hog1Δ and pbs2Δ counterparts. sub1Δ hog1Δ and sub1Δ pbs2Δ cells grew slowly at 0.2 M NaCl and were essentially unable to grow at 0.4 M NaCl. The exacerbation of the osmotic-stress-related growth defect observed in hog1Δ and pbs2Δ cells upon the deletion of SUB1 indicates that SUB1 interacts genetically with the HOG osmoresponse pathway and implicates Sub1 in the osmotic stress response. These data also indicate that, in the absence of the functional HOG pathway, cells require Sub1 for survival in moderate-osmotic-stress conditions (<0.4 M NaCl).
FIG. 1.
Evidence that Sub1 plays a role in osmoregulation. (A) SUB1 interacts genetically with members of the HOG pathway during osmotic stress. Meiotic progeny from sporulation of sub1Δ/SUB1 hog1Δ/HOG1 and sub1Δ/SUB1 pbs2Δ/PBS2 double heterozygous diploids (strains ERYM314 and ERYM441, respectively) were isolated by tetrad dissection. Approximately equal numbers of cells (∼104) of the indicated genotypes were spotted in the first column, with fivefold serial dilutions in each subsequent column, on rich medium (-) or medium containing the indicated concentration of NaCl. Days of growth are indicated at the bottom. (B) Sub1 is targeted to osmoresponse gene promoters after exposure to osmotic stress. Cells expressing Sub1-6HA (strain ERYM318C) were exposed to 0.4 M NaCl (+) or mock treated (−) for 5 min. ChIP analysis was performed using an HA antibody (HA IP) or an antibody recognizing the Rpb3 subunit of RNAP II (Rbp3 IP), and DNA fragments were detected by PCR using primers for the indicated osmoresponse gene promoters. Control primers, recognizing sequences in an untranscribed region of chromosome V, were included in the PCRs (*), and input chromatin was also analyzed by PCR for comparison and quantification. Quantification, indicating occupancy of Sub1-6HA or Rpb3 in NaCl-treated cells relative to occupancy in mock-treated cells, was performed as described in Materials and Methods. Occupancy levels for mock-treated cells (no NaCl) are all normalized to 1 and are shown for comparison. Analysis of Sub1-6HA occupancy at nonstress, constitutively expressed genes is shown in Fig. 6C.
Sub1 is recruited to osmoresponse gene promoters upon osmotic shock.
We next set out to investigate whether Sub1 associates with osmoresponse genes to influence their transcription. To this end, ChIPs were performed on cells with a six-HA-tagged version of Sub1 (Sub1-6HA) replacing wt Sub1 after a moderate osmotic shock and on mock-treated cells. For osmotic shock, cells were treated with 0.4 M NaCl for 5 minutes, which is sufficient for osmoresponse gene induction and Hog1 recruitment (28). Initially, we examined the promoters of four osmoresponse genes that we and others have shown are bound by Hog1 after osmotic shock (3, 26) (data not shown). Sub1-6HA was not detected at the promoters of the ALD3, STL1, GPD1, and CTT1 osmoresponse genes in mock-treated cells. However, after a 5-minute exposure to NaCl, Sub1-6HA cross-linking to each gene dramatically increased (Fig. 1B, HA IP). Signals ranged from approximately 6-fold over background for ALD3 to ∼40-fold for STL1. We also analyzed the association of RNAP II with these genes in the same samples by ChIP using an antibody that recognizes the Rpb3 subunit of RNAP II. As expected, RNAP II was detected at these osmoresponse gene promoters only upon osmotic stress (Fig. 1B, Rpb3 IP).
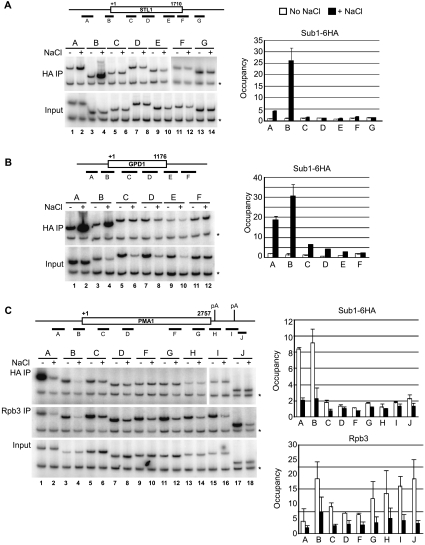
In addition to its promoter-associated function, Sub1 has been implicated in other transcription-related processes (see the introduction). We wished therefore to determine more precisely the locations along osmoresponse genes to which Sub1 was recruited after osmotic shock. To investigate this, ChIP analysis was performed using several primer pairs along the STL1 and GPD1 genes. As shown in Fig. 2A and B, Sub1-6HA was detected only on these genes after exposure to osmotic stress, and predominantly at the promoter regions of both genes. For GPD1, significantly lower levels of Sub1-6HA were also detected at coding regions, although less cross-linking was detected with increased distance from the promoter (Fig. 2B). Together these data demonstrate that Sub1 is rapidly recruited to the promoters of osmoresponse genes in response to osmotic shock and suggest that Sub1 is involved in their transcription.
FIG. 2.
Sub1 associates predominantly with the promoters of transcribed genes. Detailed ChIP analysis of Sub1-6HA occupancy along the osmoresponse genes STL1 (A) and GPD1 (B) and the constitutively transcribed gene PMA1 (C) was performed. Analysis and quantification were performed as for Fig. 1B using the primer pairs indicated in the gene diagrams. (A and B) Sub1-6HA occupies STL1 and GPD1 genes only during osmotic stress, and Sub1-6HA occupancy is restricted to promoter-proximal regions (primer pair B for both genes), although significantly lower levels of Sub1-6HA are detected at coding regions of GPD1 (primer pairs C through E). (C) Consistent with previous studies that showed that PMA1 transcription is downregulated during the osmotic stress response (31), Rpb3 cross-linking was significantly reduced after exposure to NaCl (Rpb3 IP). Under nonstress conditions, Sub1-6HA was detected only at promoter-proximal regions of PMA1 (HA IP, primer pairs A and B). In NaCl-treated cells, promoter-associated Sub1-6HA cross-linking essentially disappeared (HA IP). *, control primers.
Sub1 functions in transcription of genes activated by osmotic shock.
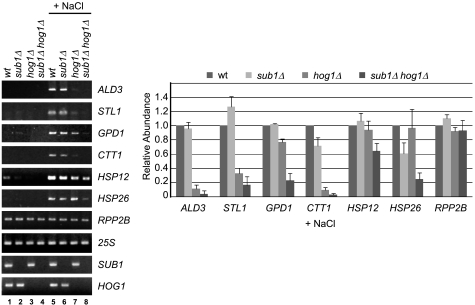
We next examined the effect of deletion of SUB1 and/or HOG1 on expression of osmoresponse genes after osmotic shock. Steady-state RNA levels of osmoresponse genes were measured by RT-PCR in wt, sub1Δ, hog1Δ, and sub1Δ hog1Δ cells (from the same strains as those shown in Fig. 1A) after addition of NaCl and in mock-treated cells (Fig. 3). In addition to measuring transcript levels of the osmoresponse genes examined in Fig. 1B, we measured transcript levels of two general stress response genes, the heat shock genes HSP12 and HSP26, and, as controls, levels of transcripts of the constitutively transcribed ribosomal protein gene RPP2B and of the 25S rRNA and SUB1 and HOG1 transcripts themselves. Both HSP12 and HSP26 are induced after a shift to high osmolarity (31). No significant levels of transcripts from most of the genes were detected in cells growing under normal growth conditions (lanes 1 to 4). A low level of transcription was detected for HSP12 in untreated wt cells (lane 1); interestingly, this depended on both SUB1 and HOG1 (compare lane 1 with lanes 2 to 4 for HSP12). As expected, addition of NaCl triggered transcription of the osmoresponse genes, including HSP26, and greatly stimulated transcription of HSP12 in wt cells (compare lane 1 with lane 5). Deletion of SUB1 (lane 6) had an overall modest effect, with most genes tested showing little reduction in transcript level. Transcription of HSP26, however, showed a significant dependence on Sub1 (compare lanes 5 and 6). The effect of SUB1 deletion on transcription did not result in a growth defect, as sub1Δ and wt cells grew equally well in medium containing NaCl (Fig. 1A). Deletion of HOG1 had a greater effect, with most genes showing a partial decrease or a nearly complete loss of transcripts (compare lanes 5 and 7). Nonetheless, we did detect stress-dependent transcription of a subset of these genes in the absence of Hog1, and transcription from such genes must be sufficient for hog1Δ cells to survive in moderate concentrations of NaCl, as seen in Fig. 1A. In sub1Δ hog1Δ cells, which cannot support growth on NaCl-containing medium, we observed the most dramatic effect on transcription compared to wt. Transcripts of stress response genes GPD1 and HSP26 that were detected in hog1Δ cells were further reduced by the deletion of SUB1 (compare lanes 7 and 8), with approximately two- to fourfold fewer transcripts in sub1Δ hog1Δ cells than in hog1Δ cells. Our results indicate that both Sub1 and Hog1 contribute to the full transcriptional response to osmotic stress and that Sub1 is critical for stress-dependent transcription of a subset of osmoresponse genes in hog1Δ cells.
FIG. 3.
Sub1 contributes to the transcription of osmoresponse genes. Total RNA was isolated from the four yeast strains used in Fig. 1A (top), of the indicated genotypes, after exposure to NaCl (+ NaCl) or mock treatment. Levels of osmoresponse gene transcripts (as indicated) were determined by RT-PCR. In addition, transcript levels of the constitutively transcribed RPP2B gene and RNAP I-transcribed 25S rRNA in each sample were determined as a control. The deletion of SUB1 and HOG1 was confirmed by detection of their transcripts only in the appropriate samples. Semiquantitative analysis of relative abundance after NaCl exposure is at the right, with values for each gene set normalized to wt levels after correction for total RNA levels in each sample, as estimated by the 25S rRNA signal.
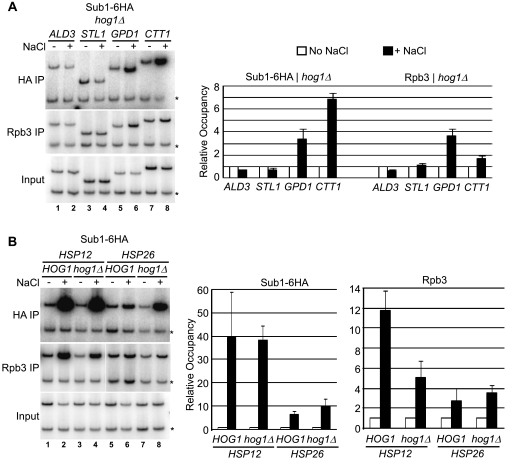
We next determined whether Hog1 is involved in recruiting Sub1 to osmoresponse gene promoters. A strain expressing Sub1-6HA was generated in the hog1Δ background, these cells were either exposed to NaCl or mock treated, and ChIP experiments were performed. First we examined the occupancy of Sub1-6HA at the promoters of the four osmoresponse genes examined in Fig. 1B. In contrast to what we observed in the presence of Hog1, we did not detect Sub1-6HA at the ALD3 or STL1 gene promoters in hog1Δ cells after osmotic shock (Fig. 4A, HA IP lanes 1 to 4), indicating that Hog1 is necessary for Sub1 recruitment. Sub1-6HA was detected, however, at the GPD1 and CTT1 promoters after osmotic shock, but at reduced levels for GPD1 compared to those when Hog1 was present (compare Fig. 1B, lanes 5 to 8, with 4A). Next, we examined the promoters of the general stress response genes HSP12 and HSP26, both of which showed Hog1-independent transcription under osmotic stress (Fig. 3). In wt cells (HOG1+), Sub1-6HA associated with both HSP12 and HSP26 genes after osmotic shock, with relatively high levels for HSP12 (Fig. 4B, HA IP). Deletion of HOG1 did not greatly affect Sub1-6HA occupancy at HSP12, whereas for HSP26, which showed a significant dependence on Sub1 for its transcription (Fig. 3), we in fact detected increased Sub1-6HA cross-linking in hog1Δ cells (compare lanes 5 to 8). These results indicate that, in response to osmotic shock, Sub1 is recruited to a subset of osmoresponse genes independently of Hog1.
FIG. 4.
Hog1-independent recruitment of Sub1 to osmoresponse genes. (A) A strain with HOG1 deleted (hog1Δ) and expressing tagged Sub1 (Sub1-6HA, strain ERYM369) was exposed to osmotic shock (+ NaCl) or mock treated (no NaCl), and levels of Sub1-6HA and RNAP II occupancy (HA IP and Rpb3 IP, respectively) at the promoter regions of the indicated osmoresponse genes were determined and quantified as in Fig. 1B. (B) Occupancy of Sub1-6HA and RNAP II at the HSP12 and HSP26 genes in HOG1 and hog1Δ cells (strains ERYM318C and ERYM369, respectively) in mock-treated cells and in response to osmotic shock was determined and quantified as in Fig. 1B. *, control primers.
We also examined Rpb3 occupancy at the stress response genes in the absence of Hog1. In hog1Δ cells, significant levels of Rpb3 were detected after osmotic shock only for GPD1, HSP26, and HSP12 (Fig. 4A and B, Rpb3 IP). Deletion of HOG1 resulted in reduced Rpb3 association at GPD1 (compare Fig. 4A with 1B) and HSP12 (Fig. 4B, Rbp3 IP, compare lanes 2 and 4) compared to that for wt cells,. However, as for Sub1-6HA, the low level of Rpb3 occupying HSP26 modestly increased in hog1Δ cells (compare lanes 6 and 8). Our ChIP analysis of RNAP II occupancy generally correlates well with the RT-PCR transcript analysis of Fig. 3, with transcripts detected only for GPD1, HSP26, and HSP12 in hog1Δ cells and HOG1 deletion not greatly affecting HSP26 transcription. Our results demonstrate that Sub1 shows a stress-dependent association with promoters of osmoresponse genes that are transcribed in the absence of the HOG pathway.
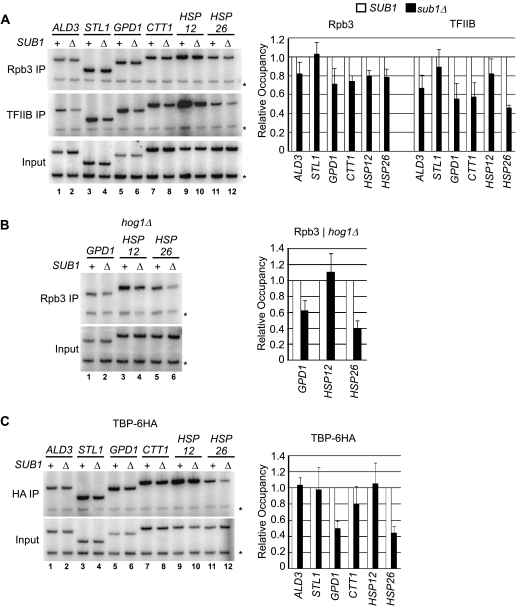
Sub1 affects TBP, TFIIB, and RNAP II occupancy at some osmoresponse genes.
To explore possible mechanisms by which promoter-associated Sub1 enhances transcription of osmoresponse genes, we tested whether Sub1 is involved in recruitment of RNAP II or GTFs. By ChIP analysis, we first measured levels of RNAP II cross-linked at osmoresponse genes in wt and sub1Δ cells after exposure to NaCl. Deletion of SUB1 caused an overall modest reduction in RNAP II recruitment to most active osmoresponse gene promoters (Fig. 5A, Rpb3 IP). Since the effect of Sub1 on transcription was most obvious in hog1Δ cells (Fig. 3), we determined the effect of SUB1 deletion in hog1Δ cells on RNAP II recruitment to the genes that were expressed independently of Hog1 (GPD1, HSP12, and HSP26). A significant reduction in RNAP II occupancy at the GPD1 and HSP26 promoters, but not at the HSP12 promoter, was detected (Fig. 5B). This is consistent with our transcript analysis in Fig. 3, in which transcript levels of GPD1 and HSP26 in hog1Δ cells were significantly reduced in the absence of Sub1. This indicates that Sub1 functions in RNAP II recruitment to a subset of osmoresponse genes that are activated independently of Hog1.
FIG. 5.
SUB1 deletion suppresses recruitment of RNAP II, TBP, and TFIIB at active osmoresponse genes. (A) SUB1+ and sub1Δ cells were treated with NaCl, and RNAP II and TFIIB occupancy at the promoters of the indicated osmoresponse genes was determined (Rpb3 IP and TFIIB IP, respectively). ChIP analysis and quantification (indicating occupancy of Rpb3 or TFIIB in sub1Δ cells relative to occupancy in wt cells) were performed as for Fig. 1B. (B) Three genes showing osmotic-shock-dependent transcription in the absence of Hog1 (Fig. 3) were analyzed for RNAP II occupancy (Rpb3 IP) in hog1Δ cells in the presence or absence of Sub1 (using strains derived from the experiment in Fig. 1A). Cells were exposed to NaCl and analyzed by ChIP as in Fig. 1B. (C) A strain expressing tagged TBP (TBP-6HA) was generated in SUB1+ and sub1Δ strain backgrounds (strains ERYM481D and ERYM542), and the effect of SUB1 deletion on TBP recruitment to the indicated osmoresponse genes was determined 5 minutes after the addition of NaCl by ChIP, as in Fig. 1B. *, control primers.
We next determined the effect of SUB1 deletion on recruitment of the GTFs TFIIB and TBP to the osmoresponse gene promoters. Occupancy of TFIIB was measured in the same samples used to measure Rpb3 levels using an anti-TFIIB antibody (27) (Fig. 5A, TFIIB IP). Compared to what we observed with Rpb3, deletion of SUB1 had an overall greater effect on TFIIB occupancy (Fig. 5A, TFIIB IP). Deletion of SUB1 reduced TFIIB cross-linking by 30 to 50% for most genes, with a more modest reduction for STL1 and HSP12. To measure TBP occupancy, we generated a strain expressing a C-terminally six-HA-tagged version of TBP (TBP-6HA). This strain has a doubling time approximately twice as long as that in wt cells, indicating that the C-terminal HA tag affects TBP function (data not shown). However, recruitment of TBP-6HA to promoters, as measured by ChIP, was as expected (see Fig. S1 in the supplemental material) and as reported by others (see, e.g., references 20 and 34). Therefore, this strain was used for comparing TBP recruitment to promoters in wt versus sub1Δ backgrounds. As expected, TBP-6HA was detected at each osmoresponse gene after exposure to NaCl (Fig. 5C; see Fig. S1 in the supplemental material). Deletion of SUB1 did not have a general effect on TBP-6HA recruitment, with no effect observed at the ALD3, STL1, and HSP12 genes and only a modest reduction in TBP-6HA occupancy observed at the CTT1 promoter (Fig. 5C). However, significantly less TBP-6HA cross-linked to GPD1 and HSP26 promoters in sub1Δ cells than in wt cells (compare lanes 5 and 11 with 6 and 12). Importantly, both GPD1 and HSP26 showed Hog1-independent transcription that greatly depended on Sub1 (Fig. 3). These data indicate that Sub1 has a gene-specific function in the recruitment of TBP to promoters. The effect of SUB1 deletion on TBP occupancy at the GPD1 and HSP26 promoters was apparent in hog1Δ cells, where RNAP II recruitment and transcription of these genes were dependent on Sub1 (Fig. 3 and 5B).
Sub1 associates with constitutively expressed RNAP II and RNAP III genes.
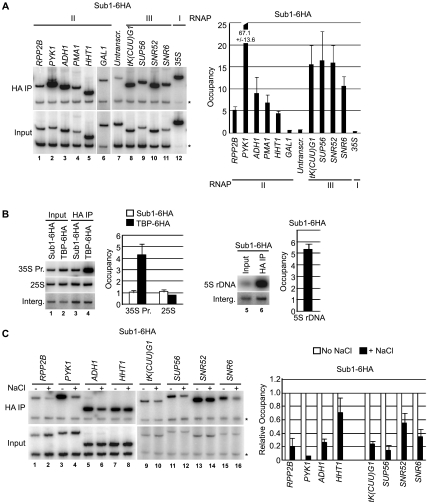
The observation that Sub1 is recruited to the promoters of osmoresponse genes when they are transcriptionally active led us to investigate whether Sub1 is present at other transcriptionally active genes. Previous studies reported Sub1 association with the PYK1, ADH1 and PMA1 genes (9, 24). Using the SUB1-6HA strain, we repeated the analysis on these genes. As expected, Sub1-6HA was enriched at the promoter region of each of these genes, from approximately 7-fold over background for PMA1 to >60-fold for PYK1 (Fig. 6A, lanes 2 to 4). Additionally, we determined whether Sub1 occupied other RNAP II-transcribed genes. Indeed, Sub1-6HA was detected at the ribosomal protein gene, RPP2B, and the histone H3 gene, HHT1, both at approximately fivefold greater than background (Fig. 6A, lanes 1 and 5). We did not detect Sub1-6HA at the promoter of an inactive gene, the transcriptionally repressed GAL1 gene (lane 6). We next performed a detailed analysis of Sub1 occupancy on the relatively long (2,757-nucleotide coding sequence) RNAP II-transcribed gene PMA1 using nine PCR primer pairs situated throughout the gene's length. Sub1-6HA was detectable only at promoter-proximal positions on PMA1 (Fig. 2C), in a pattern similar to what we observed on the transcriptionally active osmoresponse genes STL1 and GPD1. Our findings demonstrate that Sub1 associates with the promoters of transcriptionally active RNAP II genes.
FIG. 6.
Sub1 is detected at both constitutively transcribed RNAP II and at RNAP III genes. (A) Occupancy of Sub1-6HA at the indicated constitutively transcribed genes was determined by ChIP as in Fig. 1B. RNAP II transcribes the highly transcribed PYK1, ADH1, and PMA1 genes; the ribosomal protein gene RPP2B; the histone H3 gene, HHT1; and the inducible GAL1 gene, which is repressed in the conditions used. RNAP III-transcribed genes that were tested include genes for tRNAs [tk(CUU)G1 and SUP56], a snoRNA (SNR52), and U6 snRNA (SNR6). The RNAP I-transcribed 35S rDNA promoter region was also examined (35S). Because the 35S sequence is highly repeated, we examined 10-fold less of the immunoprecipitated material by PCR for this gene. An untranscribed region of chromosome VII was used as a control (Untranscr.). *, control primers. (B) Occupancy of Sub1-6HA and TBP-6HA at the rDNA gene promoter region (35S Pr.) and internally at the 25S rRNA-encoding region (25S) was determined. The intergenic control region (Interg.) was analyzed in separate PCRs with 10-fold-more material than for the 35S promoter and 25S regions. As for panel A, quantification indicates change with respect to background (input), and therefore a value of 1 indicates no signal over background. Occupancy of Sub1-6HA at the 5S rDNA gene (RDN5-1), as shown at the right, was likewise determined. (C) Sub1 evacuates constitutively transcribed RNAP II and III genes in response to osmotic stress. Levels of Sub1-6HA occupation at the indicated RNAP II and III genes in mock-treated cells and in cells exposed to 0.4 M NaCl for 5 min were determined by ChIP, as in Fig. 1B. A similar osmotic-stress-dependent reduction in Sub1-6HA occupancy was detected at the PMA1 promoter (see Fig. 2C).
As controls for the analysis of Sub1 occupancy on RNAP II genes, we examined chromosomal regions in which we did not expect to find Sub1. This included an untranscribed region of chromosome VII, several RNAP III genes, and the RNAP I-transcribed 35S ribosomal DNA (rDNA) promoter region. Sub1-6HA was not detected at the untranscribed DNA or at the 35S promoter (Fig. 6A, lanes 7 and 12). Strikingly, however, we did detect strong signals (>10-fold) for Sub1-6HA at all RNAP III genes tested, including genes for tRNAs [tk(CUU)G1 and SUP56], a snoRNA (SNR52), and U6 snRNA (SNR6) (Fig. 6A, lanes 8 to 11). PC4, the human homolog of Sub1, had previously been implicated in RNAP III transcription by in vitro experiments (38); however, a role for PC4 (or Sub1) in RNAP III transcription in vivo has not been reported. All the RNAP III genes tested are distant enough on the chromosome from the nearest RNAP II genes that we are confident the Sub1-6HA signal was specific to that gene and not an artifact of ChIP resolution limitations.
The RNAP I-transcribed rDNA gene is situated within the rDNA repeat unit that is present at approximately 150 copies per cell, making it difficult to compare occupancy at this region with that at a single-copy control intergenic region, as performed in our ChIP experiments. To examine more rigorously whether Sub1 occupies the 35S promoter at the rDNA gene, we repeated our analysis by comparing Sub1 cross-linking with TBP cross-linking at both the 35S promoter region and a downstream portion of the gene, comprising the 25S rRNA-encoding region of the locus (Fig. 6B, left). ChIP analysis indicated that, compared to input chromatin, there was no enrichment for either the 35S or 25S regions in the Sub1-6HA immunoprecipitation. In contrast, our analysis showed that TBP-6HA occupies the 35S promoter region but not the internal 25S portion of the gene, as expected. We also determined whether Sub1-6HA occupies the 5S rDNA gene, which is transcribed by RNAP III (RDN5-1) and is situated ∼2 kb from the RNAP I promoter within the rDNA repeat. Significant levels of Sub1-6HA were detected at this locus (Fig. 6B, right), which indicates that the absence of Sub1 from the RNAP I promoter is not due to effects specific to this region of chromatin. Taken together, our data indicate that Sub1 is recruited to active RNAP II and III genes but not the RNAP I-transcribed rDNA gene.
An analysis of the transcriptional response to osmotic shock identified hundreds of genes (including constitutively transcribed genes) whose expression is either up- or downregulated under osmotic stress conditions (31). Considering the rapid association of Sub1 with osmoresponse gene promoters during osmotic shock, we asked whether exposure of cells to NaCl affected its association with constitutively transcribed RNAP II and III genes. We measured levels of Sub1-6HA at the constitutive RNAP II and III genes tested in Fig. 6A by ChIP in mock-treated cells and in cells 5 minutes after exposure to NaCl. In contrast to what we observed with osmoresponse genes, osmotic shock caused a marked decrease in Sub1-6HA cross-linking to each RNAP II and III gene tested (Fig. 6C and 2C). This is consistent with the finding that most chromatin-associated proteins immediately dissociate from DNA upon osmotic shock and reassemble within 10 to 30 min (29). While this causes a transient but dramatic reduction in transcription of nonstress genes (29), it likely does not cause a significant change in steady-state transcript levels for most genes, as we show for RPP2B transcripts after exposure to NaCl for 25 min (Fig. 3). Taken together, our data indicate that within 5 minutes of exposure to osmotic shock Sub1 rapidly evacuates constitutively transcribed genes and accumulates at the promoters of osmoresponse genes.
Sub1 affects RNAP II, but not TFIIB and TBP, levels at constitutively transcribed genes.
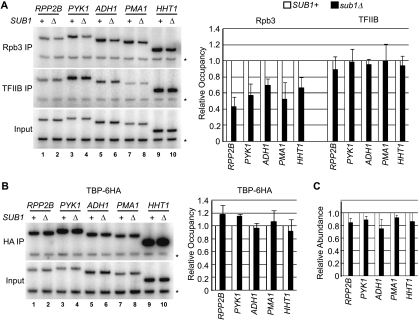
To determine how Sub1 affects transcription by RNAP II at constitutively transcribed genes, we measured Rpb3 levels on the promoters of active genes in wt and sub1Δ cells. Each of the five genes examined in Fig. 6A was assayed, and for each we detected a reduction in the level of Rpb3 at its promoter in the absence of Sub1 (Fig. 7A). The magnitude of the reduction was approximately twofold on average. These data are in agreement with our previous study in which levels of RNAP II, as measured with the 8WG16 antibody (which recognizes the unphosphorylated C-terminal domain), at the ADH1 and ACT1 genes decreased in the absence of Sub1 (9).
FIG. 7.
Sub1 affects RNAP II recruitment at constitutively transcribed genes without affecting TBP and TFIIB occupancy. Levels of RNAP II and TFIIB (A) and TBP (B) at the indicated constitutively transcribed RNAP II genes in SUB1+ and sub1Δ cells were measured. RNAP II (Rpb3 IP) and TFIIB ChIPs were performed using the same chromatin samples, whereas TBP was measured using chromatin from a strain expressing TBP-6HA, as in Fig. 5C. *, control primers. (C) Total RNA was isolated from SUB1+ and sub1Δ cells, and steady-state transcript levels were determined by RT-PCR. Semiquantitative analysis is shown, with values for the sub1Δ set normalized to corresponding wt levels after correction for total RNA levels in each sample, as estimated from the 25S rRNA signal.
We next measured the occupancy of TBP and TFIIB at the promoters of the constitutive genes to determine whether the reduction in RNAP II occupancy in sub1Δ cells corresponded to a drop in promoter-associated factors. As we did for the osmoresponse genes in Fig. 5, we used ChIP to measure TBP-6HA and TFIIB levels at the constitutively transcribed gene promoters in wt and sub1Δ cells (Fig. 7A and B). Both TBP-6HA and TFIIB were detected at each promoter. Unexpectedly, however, in contrast to what we observed for Rpb3 and at the osmoresponse genes, deletion of SUB1 had no significant effect on the levels of promoter-associated TBP-6HA or TFIIB at any of the five genes tested. This indicates that Sub1 affects RNAP II association with constitutively transcribed genes at a step after recruitment of GTFs.
We measured steady-state transcript levels of the five constitutively transcribed genes to determine whether a twofold reduction in RNAP II occupancy affected transcript levels. Transcript levels in wt and sub1Δ cells were determined by RT-PCR, with regions of the RNAP I-transcribed 25S rRNA and SUB1 mRNA measured as controls (Fig. 7C and data not shown). Although we observed an overall reduction in transcript levels, the effect was minor, ranging from ∼10 to 25% fewer transcripts. We interpret this to indicate that a twofold reduction in polymerase density at promoters affects steady-state transcript levels only modestly, with sufficient transcripts accumulating over time such that a major difference was not observed. We did not expect to see a greater overall effect on constitutive transcription because sub1Δ cells grow as well as wt cells in standard growth conditions (Fig. 1A).
Sub1 affects RNAP III, but not TBP, levels at RNAP III genes.
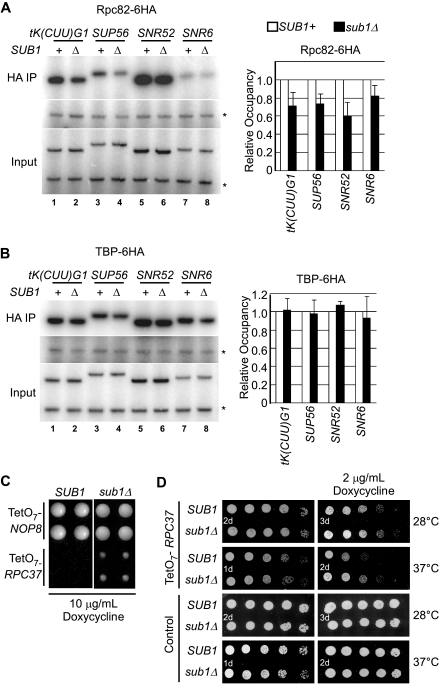
The observation that Sub1 occupies RNAP III genes led us to investigate whether Sub1 affects RNAP III recruitment to its target genes, as we observed for RNAP II. To this end, we generated a strain that expresses the Rpc82 subunit of RNAP III tagged with a C-terminal six-HA tag, Rpc82-6HA. Levels of Rpc82-6HA cross-linking to the four RNAP III genes examined in Fig. 6A in wt and sub1Δ cells were determined. As with RNAP II, deletion of SUB1 caused an overall reduction in RNAP III association with target genes, ranging from ∼20 to 40% less Rpc82-6HA cross-linking to promoters in sub1Δ cells (Fig. 8A). However, an analysis of TBP-6HA occupancy at these genes in wt and sub1Δ cells indicated no effect of Sub1 on TBP association (Fig. 8B). These data suggest that Sub1 stimulates RNAP III recruitment after the association of TBP to RNAP III promoters, just as we observed for constitutively transcribed RNAP II genes. Despite this, measurements of steady-state levels of transcripts of the four target genes assayed by ChIP indicated that deletion of SUB1 had no significant effect on transcript accumulation (see Fig. S2 in the supplemental material), perhaps reflecting the stability of these transcripts and/or an effect of growth conditions.
FIG. 8.
Involvement of Sub1 in RNAP III transcription. (A) Levels of RNAP III association with the indicated genes in SUB1+ and sub1Δ cells expressing a tagged version of Rpc82 (Rpc82-6HA, strains ERYM573A and ERYM574A, respectively) were determined by ChIP. (B) Analysis of TBP levels associated with the indicated genes was performed as for Fig. 7B. To facilitate analysis, 10-fold-less immunoprecipitated material was analyzed by PCR in the HA immunoprecipitation set than for the corresponding intergenic set in panels A and B. *, control primers. (C) Colony size comparison for a strain in which the gene expressing the Rpc37 subunit of RNAP III (TetO7-RPC37) is under the control of a promoter repressed by tetracycline and an isogenic strain lacking SUB1, both grown on medium containing 10 μg/ml doxycycline for 48 h. Quadruplicate analysis is shown, and, for comparison, a similar analysis of the TetO7-NOP8 strain, in which the NOP8 gene (unrelated to RNAP III transcription) is under the control of the tetracycline-repressed promoter, was performed. (D) Spot analysis (as in Fig. 1A) of the TetO7-RPC37 SUB1 and sub1Δ strains on control medium and medium containing 2 μg/ml doxycycline at 28°C and 37°C. The same analysis was performed on wt cells, unaffected by growth in doxycycline, as a control. Days of growth are indicated on each photograph.
Supporting the physiological significance of the above results, we also obtained genetic evidence for a functional role for Sub1 in RNAP III transcription. We screened a set of nearly 800 yeast strains, each expressing a different essential gene under the control of a tetracycline-repressed promoter (TetO7 [21]) for genetic interactions with SUB1 in an SGA-type analysis. Reduced expression of the tetracycline-regulated gene in each strain by growth on medium containing the tetracycline analog doxycycline causes a range of growth defects, from no observable effect to severe impairment (21), and enhancement or suppression of these defects by deletion of SUB1 indicates a genetic interaction. The screen revealed that, among the interactions detected, deletion of SUB1 partially suppressed the severe growth defect associated with reduced expression of RPC37, which encodes a subunit of RNAP III (Fig. 8C and data not shown). We repeated the analysis of RPC37 using a concentration of doxycycline at which growth of the TetO7-RPC37 strain could be detected. At 2 μg/ml doxycycline, the modest growth defect of the TetO7-RPC37 strain was slightly suppressed by deletion of SUB1 (Fig. 8D). However, the suppressive effect of SUB1 deletion was more obvious when cells were stressed by growth at 37°C (Fig. 8D). These analyses confirm that severe growth defects associated with reduced expression of RPC37 are partially suppressed by deletion of SUB1 and that SUB1 and RPC37 interact genetically. The growth defect associated with reduced availability of Rpc37, and presumably RNAP III, may be partially alleviated by reduced demand for RNAP III in sub1Δ cells, because there is less RNAP III associated with target genes (Fig. 8A).
DISCUSSION
Our data indicate that Sub1 functions in the recruitment of polymerases to active RNAP II and III genes and also uncovers specific conditions in which the normally nonessential Sub1 becomes critical. We found that, in the absence of the HOG pathway, cells require Sub1 for survival under moderate osmolarity. Our analysis suggests that, without the HOG pathway, expression of necessary osmoresponse genes is possible only because the cell turns to an alternative pathway that makes use of Sub1 to target RNAP II to those genes. Supporting this, previous studies have postulated that Hog1-independent pathways participate in the stress-dependent expression of osmoresponse genes, such as GPD1 (1, 30). GPD1, which encodes a key enzyme involved in glycerol biosynthesis, is essential during osmotic stress (1), and we found that, in hog1Δ cells, both RNAP II recruitment to GPD1 and HSP26 expression depend on Sub1. The function of Sub1 in the osmotic stress response might therefore represent redundancy necessary to ensure survival if the HOG pathway fails.
Sub1 employs distinct, gene-specific mechanisms to facilitate transcription initiation. Whereas deletion of SUB1 did not affect TBP and TFIIB association with constitutively transcribed RNAP II gene promoters, TBP and TFIIB levels at the GPD1 and HSP26 promoters were both significantly reduced after induction by NaCl. This suggests that Sub1 uses a different strategy, involving preinitiation complex assembly or stability, for targeting RNAP II to these genes, which would then facilitate their rapid induction during osmotic stress. Physical interactions between Sub1 and GTFs (18, 39) might be required for targeting Sub1 to active promoters, whereas in the osmotic stress response, rapid targeting of Sub1 to promoters, perhaps by stress-responsive transcription factors, might activate transcription by recruiting GTFs through the same Sub1-GTF interactions. Recruitment of Sub1 to the inducible ARG1 promoter (our unpublished data) suggests that pathways besides the osmoregulation pathway might employ Sub1 for rapid induction. However, our large-scale genetic analysis did not reveal additional Sub1-dependent gene activation pathways (our unpublished data), suggesting specificity in its function in the osmoresponse pathway.
Our detailed analysis of Sub1 occupancy on the transcriptionally active STL1, GPD1, and PMA1 genes revealed that Sub1 is strongly promoter associated. In our previous studies, cells expressing a plasmid-encoded version of an N-terminally three-HA-tagged Sub1 were used in ChIP experiments to show that 3HA-Sub1 associates with the constitutively transcribed ACT1, ADH1, and PMA1 genes (9). In that study, approximately equal levels of 3HA-Sub1 at the promoter and at downstream positions of the coding regions of those genes were detected. We believe those results were due to elevated Sub1 levels in the cell, due to additional expression from a plasmid. When we compared Sub1 occupancy in a strain expressing C-terminally tagged Sub1 from the SUB1 locus with that in a strain expressing C-terminally tagged Sub1 from a plasmid, we found the plasmid-derived Sub1 cross-linked at coding regions, whereas the chromosomally expressed Sub1 did not (data not shown). Although this indicates the potential for Sub1 to associate with the elongating transcriptional machinery, consistent with a role in elongation (9), our current results suggest that Sub1 is predominantly promoter localized when expressed from its natural promoter. In another study, cells expressing a tandem affinity purification (TAP)-tagged Sub1 (from the chromosomal SUB1 locus) were used to determine the occupancy of Sub1 along the PYK1 gene (24). High levels of Sub1-TAP were detected at the PYK1 promoter, low levels at a coding region, and an intermediate level near the polyadenylation site. The physical interaction of Sub1 with the cleavage/polyadenylation machinery (7, 14) supports a role for Sub1 at the 3′ ends of genes, although this may also reflect the presence of certain cleavage/polyadenylation factors at promoters (8).
Intriguingly, we detected Sub1 at every type of RNAP III gene that we tested. Very few other factors are found at both RNAP II and RNAP III promoters; these are limited to TBP (reviewed in reference 16) and five common subunits of the polymerases themselves (6, 10, 37). Additionally, in a recent genome-wide analysis, the RNAP II initiation and elongation factor TFIIS was detected at RNAP III genes, where it may act as a general RNAP III transcription factor (13). To our knowledge, however, besides TFIIS, a yeast transcription factor that regulates transcription of all types of RNAP II and RNAP III genes, but not RNAP I genes, has not yet been reported. A previous study detected PC4 in a complex containing the RNAP III general transcription factor TFIIIC and found it to be a potent stimulator of RNAP III transcription in vitro using a reconstituted transcription system (38). Our observations therefore place Sub1 in an evolutionarily conserved and unusual position, as a putative transcriptional regulator for RNAP II and III.
Under normal growth conditions, we detected Sub1 at all constitutively transcribed RNAP II and RNAP III genes tested, correlating association of Sub1 with sites of active transcription. In general, promoters with high levels of Sub1 corresponded to genes that are highly transcribed and are associated with high levels of RNAP II (this study and our unpublished data), although in some cases there is a disproportionately high level of Sub1 (e.g., at the PYK1 promoter). This general correlation supports a role for Sub1 in facilitating the recruitment of polymerases to transcribed genes after the assembly of promoter complexes, including TBP (and TFIIB for RNAP II promoters). It is curious that Sub1, so abundantly and ubiquitously present at so many genes, would have only modest effects on transcription, as measured by steady-state transcript analysis. However, our data support the idea that an important function for Sub1 might involve facilitating the mobilization of transcription apparatuses during conditions of stress, such as exposure to NaCl. During osmotic shock, the expression of hundreds of genes is either up- or downregulated (31). Our analysis indicates that the association of Sub1 with constitutively transcribed genes is reduced, while Sub1 accumulates at osmoresponse genes. As the transcriptional response to osmotic stress is transient (1, 30, 31), this could allow for a temporary and modest reduction in the availability of RNAP II at constitutive genes, while facilitating recruitment of RNAP II to stress-induced genes and preinitiation complex assembly at critical genes. According to this model, Sub1 is involved in fine-tuning the level of polymerases at RNAP II and III genes, while poising the cell for changes in transcription patterns necessary when certain stresses are encountered. It will be key to determine what targets Sub1 to actively transcribed promoters in a general manner and during activation of specific genes in order to understand how Sub1 functions in both situations. In any event, our current study has uncovered novel promoter-associated functions for a protein with multiple roles at many stages of gene expression.
Supplementary Material
Acknowledgments
We thank Elizabeth Miller (Columbia University) and Jennifer Haynes and Brenda Andrews (University of Toronto) for strains and plasmids and Michael Hampsey (Robert Wood Johnson Medical School) for generously supplying the TFIIB antibody. We thank Patricia Richard for insightful discussions and for comments on the manuscript.
E. Rosonina is a Research Fellow of the Terry Fox Foundation through an award from the National Cancer Institute of Canada. This work was supported by grants from the National Institutes of Health to J.L.M. and I.M.W.
Footnotes
Published ahead of print on 9 February 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Albertyn, J., S. Hohmann, J. M. Thevelein, and B. A. Prior. 1994. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol. Cell. Biol. 144135-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alepuz, P. M., E. de Nadal, M. Zapater, G. Ammerer, and F. Posas. 2003. Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II. EMBO J. 222433-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alepuz, P. M., A. Jovanovic, V. Reiser, and G. Ammerer. 2001. Stress-induced map kinase Hog1 is part of transcription activation complexes. Mol. Cell 7767-777. [DOI] [PubMed] [Google Scholar]
- 4.Amberg, D. C., D. J. Burke, and J. N. Strathern. 2006. Yeast RNA isolation: small-scale. Cold Spring Harbor protocol. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. doi: 10.1101/pdb.prot4155. [DOI]
- 5.Brewster, J. L., T. de Valoir, N. D. Dwyer, E. Winter, and M. C. Gustin. 1993. An osmosensing signal transduction pathway in yeast. Science 2591760-1763. [DOI] [PubMed] [Google Scholar]
- 6.Buhler, J. M., F. Iborra, A. Sentenac, and P. Fromageot. 1976. Structural studies on yeast RNA polymerases. Existence of common subunits in RNA polymerases A(I) and B(II). J. Biol. Chem. 2511712-1717. [PubMed] [Google Scholar]
- 7.Calvo, O., and J. L. Manley. 2001. Evolutionarily conserved interaction between CstF-64 and PC4 links transcription, polyadenylation, and termination. Mol. Cell 71013-1023. [DOI] [PubMed] [Google Scholar]
- 8.Calvo, O., and J. L. Manley. 2003. Strange bedfellows: polyadenylation factors at the promoter. Genes Dev. 171321-1327. [DOI] [PubMed] [Google Scholar]
- 9.Calvo, O., and J. L. Manley. 2005. The transcriptional coactivator PC4/Sub1 has multiple functions in RNA polymerase II transcription. EMBO J. 241009-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carles, C., I. Treich, F. Bouet, M. Riva, and A. Sentenac. 1991. Two additional common subunits, ABC10 alpha and ABC10 beta, are shared by yeast RNA polymerases. J. Biol. Chem. 26624092-24096. [PubMed] [Google Scholar]
- 11.De Nadal, E., M. Zapater, P. M. Alepuz, L. Sumoy, G. Mas, and F. Posas. 2004. The MAPK Hog1 recruits Rpd3 histone deacetylase to activate osmoresponsive genes. Nature 427370-374. [DOI] [PubMed] [Google Scholar]
- 12.Ge, H., and R. G. Roeder. 1994. Purification, cloning, and characterization of a human coactivator, PC4, that mediates transcriptional activation of class II genes. Cell 78513-523. [DOI] [PubMed] [Google Scholar]
- 13.Ghavi-Helm, Y., M. Michaut, J. Acker, J.-C. Aude, P. Thuriaux, M. Werner, and J. Soutourina. 2008. Genome-wide location analysis reveals a role of TFIIS in RNA polymerase III transcription. Genes Dev. 221934-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He, X., A. U. Khan, H. Cheng, D. L. Pappas, Jr., M. Hampsey, and C. L. Moore. 2003. Functional interactions between the transcription and mRNA 3′ end processing machineries mediated by Ssu72 and Sub1. Genes Dev. 171030-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry, N. L., D. A. Bushnell, and R. D. Kornberg. 1996. A yeast transcriptional stimulatory protein similar to human PC4. J. Biol. Chem. 27121842-21847. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez, N. 1993. TBP, a universal eukaryotic transcription factor? Genes Dev. 71291-1308. [DOI] [PubMed] [Google Scholar]
- 17.Hohmann, S., M. Krantz, and B. Nordlander. 2007. Yeast osmoregulation. Methods Enzymol. 42829-45. [DOI] [PubMed] [Google Scholar]
- 18.Knaus, R., R. Pollock, and L. Guarente. 1996. Yeast SUB1 is a suppressor of TFIIB mutations and has homology to the human co-activator PC4. EMBO J. 151933-1940. [PMC free article] [PubMed] [Google Scholar]
- 19.Knop, M., K. Siegers, G. Pereira, W. Zachariae, B. Winsor, K. Nasmyth, and E. Schiebel. 1999. Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast 15963-972. [DOI] [PubMed] [Google Scholar]
- 20.Komarnitsky, P., E. J. Cho, and S. Buratowski. 2000. Different phosphorylated forms of RNA polymerase II and associated mRNA processing factors during transcription. Genes Dev. 142452-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koyama, H., E. Sumiya, M. Nagata, T. Ito, and K. Sekimizu. 2008. Transcriptional repression of the IMD2 gene mediated by the transcriptional co-activator Sub1. Genes Cells 131113-1126. [DOI] [PubMed] [Google Scholar]
- 22.Kretzschmar, M., K. Kaiser, F. Lottspeich, and M. Meisterernst. 1994. A novel mediator of class II gene transcription with homology to viral immediate-early transcriptional regulators. Cell 78525-534. [DOI] [PubMed] [Google Scholar]
- 23.Mnaimneh, S., A. P. Davierwala, J. Haynes, J. Moffat, W. T. Peng, W. Zhang, X. Yang, J. Pootoolal, G. Chua, A. Lopez, M. Trochesset, D. Morse, N. J. Krogan, S. L. Hiley, Z. Li, Q. Morris, J. Grigull, N. Mitsakakis, C. J. Roberts, J. F. Greenblatt, C. Boone, C. A. Kaiser, B. J. Andrews, and T. R. Hughes. 2004. Exploration of essential gene functions via titratable promoter alleles. Cell 11831-44. [DOI] [PubMed] [Google Scholar]
- 24.Nedea, E., X. He, M. Kim, J. Pootoolal, G. Zhong, V. Canadien, T. Hughes, S. Buratowski, C. L. Moore, and J. Greenblatt. 2003. Organization and function of APT, a subcomplex of the yeast cleavage and polyadenylation factor involved in the formation of mRNA and small nucleolar RNA 3′-ends. J. Biol. Chem. 27833000-33010. [DOI] [PubMed] [Google Scholar]
- 25.O'Rourke, S. M., and I. Herskowitz. 2004. Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol. Biol. Cell 15532-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pascual-Ahuir, A., K. Struhl, and M. Proft. 2006. Genome-wide location analysis of the stress-activated MAP kinase Hog1 in yeast. Methods 40272-278. [DOI] [PubMed] [Google Scholar]
- 27.Pinto, I., W. H. Wu, J. G. Na, and M. Hampsey. 1994. Characterization of sua7 mutations defines a domain of TFIIB involved in transcription start site selection in yeast. J. Biol. Chem. 26930569-30573. [PubMed] [Google Scholar]
- 28.Proft, M., G. Mas, E. de Nadal, A. Vendrell, N. Noriega, K. Struhl, and F. Posas. 2006. The stress-activated Hog1 kinase is a selective transcriptional elongation factor for genes responding to osmotic stress. Mol. Cell 23241-250. [DOI] [PubMed] [Google Scholar]
- 29.Proft, M., and K. Struhl. 2004. MAP kinase-mediated stress relief that precedes and regulates the timing of transcriptional induction. Cell 118351-361. [DOI] [PubMed] [Google Scholar]
- 30.Rep, M., J. Albertyn, J. M. Thevelein, B. A. Prior, and S. Hohmann. 1999. Different signalling pathways contribute to the control of GPD1 gene expression by osmotic stress in Saccharomyces cerevisiae. Microbiology 145(Pt. 3)715-727. [DOI] [PubMed] [Google Scholar]
- 31.Rep, M., M. Krantz, J. M. Thevelein, and S. Hohmann. 2000. The transcriptional response of Saccharomyces cerevisiae to osmotic shock. Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J. Biol. Chem. 2758290-8300. [DOI] [PubMed] [Google Scholar]
- 32.Rep, M., V. Reiser, U. Gartner, J. M. Thevelein, S. Hohmann, G. Ammerer, and H. Ruis. 1999. Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol. Cell. Biol. 195474-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saito, H., and K. Tatebayashi. 2004. Regulation of the osmoregulatory HOG MAPK cascade in yeast. J. Biochem. 136267-272. [DOI] [PubMed] [Google Scholar]
- 34.Schroder, P. A., and M. J. Moore. 2005. Association of ribosomal proteins with nascent transcripts in S. cerevisiae. RNA 111521-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong, A. H., and C. Boone. 2006. Synthetic genetic array analysis in Saccharomyces cerevisiae. Methods Mol. Biol. 313171-192. [DOI] [PubMed] [Google Scholar]
- 36.Tong, A. H., M. Evangelista, A. B. Parsons, H. Xu, G. D. Bader, N. Page, M. Robinson, S. Raghibizadeh, C. W. Hogue, H. Bussey, B. Andrews, M. Tyers, and C. Boone. 2001. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 2942364-2368. [DOI] [PubMed] [Google Scholar]
- 37.Valenzuela, P., G. I. Bell, F. Weinberg, and W. J. Rutter. 1976. Yeast DNA dependent RNA polymerases I, II and III. The existence of subunits common to the three enzymes. Biochem. Biophys. Res. Commun. 711319-1325. [DOI] [PubMed] [Google Scholar]
- 38.Wang, Z., and R. G. Roeder. 1998. DNA topoisomerase I and PC4 can interact with human TFIIIC to promote both accurate termination and transcription reinitiation by RNA polymerase III. Mol. Cell 1749-757. [DOI] [PubMed] [Google Scholar]
- 39.Wu, W. H., I. Pinto, B. S. Chen, and M. Hampsey. 1999. Mutational analysis of yeast TFIIB. A functional relationship between Ssu72 and Sub1/Tsp1 defined by allele-specific interactions with TFIIB. Genetics 153643-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.