Abstract
Proper mitotic progression is crucial for maintenance of genomic integrity in proliferating cells and is regulated through an intricate series of events, including protein phosphorylation governed by a complex network of protein kinases. One kinase family implicated in the regulation of mitotic progression is protein kinase CK2, a small family of enzymes that is overexpressed in cancer and induces transformation in mice and cultured fibroblasts. CK2α, one isoform of the catalytic subunits of CK2, is maximally phosphorylated at four sites in nocodazole-treated cells. To investigate the effects of CK2α phosphorylation on mitotic progression, we generated phosphospecific antibodies against its mitotic phosphorylation sites. In U2OS cells released from S-phase arrest, these antibodies reveal that CK2α is most highly phosphorylated in prophase and metaphase. Phosphorylation gradually decreases during anaphase and becomes undetectable during telophase and cytokinesis. Stable expression of phosphomimetic CK2α (CK2α-4D, CK2α-4E) results in aberrant centrosome amplification and chromosomal segregation defects and loss of mitotic cells through mitotic catastrophe. Conversely, cells expressing nonphosphorylatable CK2α (CK2α-4A) show a decreased ability to arrest in mitosis following nocodazole treatment, suggesting involvement in the spindle assembly checkpoint. Collectively, these studies indicate that reversible phosphorylation of CK2α requires precise regulation to allow proper mitotic progression.
Proper progression through mitosis is mediated by a complex web of signaling pathways that ensure faithful division of genetic material. Deregulation of these pathways can lead to aneuploidy and genetic instability, resulting in tumorigenesis (16). Protein kinase CK2 is a pleiotropic serine/threonine kinase that is upregulated in a variety of human cancers (reviewed in reference 13) and possesses oncogenic properties in mice and fibroblast cultures (20, 33). The kinase is generally found as a tetramer with two catalytic subunits (CK2α and/or CK2α′) and two regulatory subunits (CK2β) (12). CK2 is involved in signaling pathways controlling multiple cellular processes, including cell cycle control and cell survival (reviewed in reference 21). In these pathways, CK2 has a multitude of different interacting proteins and substrates, and subsequently, information on the precise regulation of CK2 has been elusive.
Expression of CK2 is essential for viability in both yeast and slime mold (17, 34) and is required for progression through the G1/S and G2/M transitions of the yeast cell cycle (14, 34). In mammalian cells, there are requirements for CK2 at the G0/G1, G1/S, and G2/M phases of the cell cycle (25, 26, 35). CK2α, one of the catalytic subunits of CK2, contains four proline-directed phosphorylation sites (T344, T360, S362, and S370) that are phosphorylated in nocodazole-arrested cells (4, 24). The reactions are catalyzed in vitro by the mitotic cyclin-dependent kinase Cdk1, which is believed to be the kinase responsible in cells (4). These phosphorylation sites are located on the extended C-terminal tail of CK2α, which is not present in CK2α′ (29). This difference between isoforms suggests some functional specialization for the catalytic subunits of CK2. Interestingly, while mice lacking CK2α′ are viable (44), CK2α knockout results in embryonic lethality (27). The CK2α C-terminal phosphorylation sites are conserved in birds and mammals, further supporting the idea that they play an important role in regulating the function of CK2 (29).
To examine the phosphorylation of CK2α in mitosis, we generated phosphospecific antibodies against its phosphorylation sites. We show that CK2α is phosphorylated in mitotic cells. This phosphorylation occurs mainly in prophase and metaphase, decreases through anaphase, and is absent in telophase and cytokinesis. To gain insight into the function of CK2α phosphorylation in mitosis, cell lines with tetracycline-regulated expression of phosphorylation site mutant forms of CK2α with either phosphomimetic glutamic acid or aspartic acid substitutions (CK2α-4D, CK2α-4E) or with nonphosphorylatable alanine substitutions (CK2α-4A) were examined. Expression of phosphomimetic mutant CK2α proteins resulted in aberrant centrosome amplification, chromosomal segregation defects, and loss of mitotic cells through mitotic catastrophe. Nonphosphorylatable CK2α expression did not show these effects, but cells showed a decreased ability to arrest following spindle insult by nocodazole treatment. Taken together, these results show that proper temporal regulation of CK2α phosphorylation is required for proper mitotic progression and highlight a role for CK2α phosphorylation in the maintenance of spindle integrity and control of cell division.
MATERIALS AND METHODS
Antibodies.
Polyclonal antibodies against phosphorylated CK2α were raised in New Zealand White rabbits against phosphorylated peptides (pT344, CANSSVPpTSGG; pT360/pS362, CISSVPpTPpSPL; pS370, CRRRLAGpSPVI) coupled to keyhole limpet hemocyanin by Covance Research Products, Inc. (Denver, PA). Nonphosphospecific antibodies were immunodepleted from the antisera on SulfoLink resin (Pierce) conjugated to nonphosphorylated versions of the above peptides. Phosphospecific antibodies were isolated from the resultant flowthrough by affinity purification with phosphorylated peptides. Polyclonal anti-CK2α, anti-CK2β, and anti-Cdk1 antisera have been previously described (22). Monoclonal antibody 12CA5, which reacts against the hemagglutinin (HA) epitope, was purchased from Roche. The hybridoma producing the 9E10 monoclonal antibody directed against the Myc epitope, developed by J. M. Bishop, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences, University of Iowa, Iowa City. CK2α, phospho-histone H3 (serine 10), cyclin B1, and cytochrome c antibodies were purchased from Santa Cruz Biotechnology. Pericentrin antibodies were purchased from AbCam. Monoclonal antibodies against β-tubulin were a generous gift from Lina Dagnino (Department of Pharmacology, University of Western Ontario). Goat anti-rabbit antibody (GAR)- or goat anti-mouse antibody-horseradish peroxidase secondary antibodies were purchased from Bio-Rad. Fluorescein isothiocyanate (FITC)-GAR was from Sigma, and Texas Red-GAR and Alexa Fluor 488-goat anti-mouse antibody were from Molecular Probes. Fluorescent secondary antibodies for immunoblot detection were purchased from LI-COR Biosciences.
Plasmid constructs.
The CK2α-HA/Myc-CK2β bidirectional plasmid in pBI (Clontech) and phosphorylation site mutant CK2α proteins in pRc/CMV (Invitrogen) have been previously described (31, 42). To introduce the CK2α phosphorylation site mutations into the tetracycline-responsive, bidirectional pBI vector, the pRc/CMV vectors were cut with restriction endonucleases BstBI and BlpI (New England BioLabs) to release a 1,040-bp insert containing the mutations at the C-terminal phosphorylation sites. The CK2α-HA/Myc-CK2β bidirectional plasmid was digested in the same manner, and the mutated inserts were ligated into the wild-type plasmid, thus replacing a wild-type CK2α C terminus with a mutant C terminus. All plasmids were verified by DNA sequencing.
Generation and maintenance of cell lines.
UTA6 cells were derived from the human osteosarcoma U2OS cell line and express the tetracycline transactivator (tTA) fusion protein (a generous gift from Christoph Englert, Forschungszentrum Karlsruhe, Karlsruhe, Germany) (9). CK2α-HA- and CK2α-KD-HA (kinase dead)-expressing cell lines have been previously described (42). Cell lines with tetracycline-regulated expression of HA-tagged phosphorylation site mutant CK2α proteins were generated by cotransfection of CK2α-4D-HA, CK2α-4E-HA, or CK2α-4A-HA with plasmid pTK-hyg (Clontech) in the presence of tetracycline. Drug selection with 500 μg/ml hygromycin and 460 μg/ml G418 (Life Technologies Inc.) was started at 48 h after transfection. Once stably transfected colonies had formed, they were picked and transferred to 96-well dishes. Colonies were expanded and tested for tight inducible expression of phosphorylation site mutant CK2α proteins by Western blot analysis. Cells were maintained in Dulbecco's modified Eagle medium (Sigma) with 10% fetal calf serum (Invitrogen), antibiotic supplements (0.1 mg/ml streptomycin and 100 U/ml penicillin; Life Technologies Inc.), and 1.5 μg/ml tetracycline (Sigma). To achieve cell synchronization in S phase, cells were treated by double-thymidine block with two 16-h treatments with 2 mM thymidine (Sigma) separated by a 10-h incubation without thymidine. To achieve cell synchronization in G2/M, cells were released from S-phase arrest for 8 h and then treated with 40 ng/ml nocodazole (Sigma) for 18 h.
Cell lysis and immunoprecipitation.
Cells were lysed on ice in NP-40 lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% NP-40, 1 μg/ml pepstatin A, 1 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaF, 4 mM Na2VO4). The lysates were sonicated on ice in three 10-s bursts and then centrifuged in a Beckman TL100.2 rotor at 55,000 rpm for 15 min. For immunoprecipitation, 1 mg of total cell lysate was incubated with 2 μl of 12CA5 anti-HA antibody or 5 μl of anti-Cdk1 antiserum bound to protein A-Sepharose for 1 h at 4°C. Beads were washed four times in lysis buffer, and proteins were eluted in boiling sample buffer.
Immunoblot analysis.
The protein concentration of each sample was determined with the BCA protein assay (Pierce). Equal amounts of protein lysate were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis by the method of Laemmli (19). Proteins were transferred to polyvinylidene difluoride membranes (Boehringer Mannheim) for 1 h at 15 V and 0.3 A with the Trans-Blot semidry electrophoretic transfer apparatus (Bio-Rad). Immunoblotting was performed by blocking for 1 h in 5% bovine serum albumin in Tris-buffered saline-Triton X-100, followed by overnight incubation with the primary antibody. Immune complexes were detected either by incubation with horseradish peroxidase-linked secondary antibodies and detection by chemiluminescence or by incubation with fluorophore-linked secondary antibodies and detection on a LI-COR near-infrared fluorescent scanner and Odyssey V3.0 software.
Kinase assays.
CK2 activity was measured in whole-cell extracts with a synthetic peptide substrate (RRRDDDSDDD) (23). Assays were performed for 5 or 10 min at 30°C in a final reaction mixture volume of 30 μl containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol, 0.1 mM ATP (specific activity, 500 to 1,000 cpm/pmol; ICN), and 0.1 mM substrate peptide. Reactions were initiated by the addition of 2 or 4 μg of cell extract. The reactions were terminated by spotting 10 μl of the reaction mixture onto P81 phosphocellulose paper. Samples were washed four times in 1% phosphoric acid and once in 95% ethanol. Activity was detected with a Beckman LS 5801 scintillation counter. Cdk1 activity was measured with histone H1 (Calbiochem) as described in reference 22.
Growth curve construction.
CK2α-HA-, CK2α-4D-HA-, CK2α-4E-HA-, and CK2α-4A-HA-expressing cells were seeded into six-well dishes at a starting density of 20,000 cells per well. Protein expression was induced, and complete medium was added in the presence or absence of 1.5 μg/ml tetracycline. The medium was changed every 3 days. On days 0, 1, 3, 5, and 7, the cells were harvested with 5 mM EDTA in phosphate-buffered saline (PBS) and counted with a hemocytometer. Trypan blue (Gibco) was used to distinguish viable from nonviable cells.
Cell cycle analysis.
CK2α-HA-, CK2α-4D-HA-, CK2α-4E-HA-, and CK2α-4A-HA-expressing cells were arrested in S phase by double-thymidine block. Upon release from the thymidine block, protein expression was induced and complete medium was added to the cells in the presence or absence of 1.5 μg/ml tetracycline. For 24 h, floating and adherent cells were collected every 2 h with 5 mM EDTA in PBS and fixed in 70% ethanol overnight at −20°C. Cells were then washed with PBS and stained with propidium iodide (PI) staining solution (0.1% sodium citrate, 0.1% Triton X-100, 50 μg/ml PI [Sigma], 0.1 mg/ml DNase-free RNase A [Sigma]) for 20 min at 37°C. Nocodazole-arrested cells were fixed and stained as described above. Cells stained with phospho-histone H3 were harvested 12 h after thymidine release and induction and then fixed as described above. After washes with PBS, PBS-0.5% Triton X-100, and PBS-5% bovine serum albumin (PBSBA), cells were incubated for 2 h with 1 μg/ml phospho-histone H3 in PBSBA. Positive cells were detected with GAR-FITC (1:200; Sigma) in PBSBA. Cells were then counterstained with PI as described above. Cells were analyzed on a Becton Dickinson fluorescence-activated cell sorter (FACScan) with Cell Quest Pro software (Becton Dickinson). In each sample, 20,000 events in a specific gated region were counted. Data analysis was carried out with FlowJo software (Tree Star).
Immunostaining and microscopy.
Cells used for immunostaining were grown on poly-l-lysine-coated coverslips (Bio-Rad). For mitochondrial staining, cells were incubated for 30 min with 200 nM MitoTracker Deep Red 633 FM prior to fixation. Following fixation with 50:50 methanol-acetone, coverslips were incubated in 0.1% glycine in PBS and blocked in 5% FBS in PBS. Primary and secondary antibody incubations were performed at 37°C for 1 h and 30 min, respectively. After washing with PBS, DNA was stained with 4′,6′-diamidino-2-phenylindole (DAPI) and coverslips were mounted onto microscope slides with AirVol. Cells were visualized on a Zeiss META 510 LSM confocal microscope. Z-series images with a thickness of 0.6 μm were captured and processed with Zeiss software and Adobe Photoshop.
Trypan blue viability assays.
Cells were arrested by thymidine block, and protein expression was induced as described above. Twenty-four hours after induction, floating and adherent cells were collected with 5 mM EDTA in PBS and stained with 0.4% trypan blue stain (Gibco). Total and nonviable cells were counted with a hemocytometer.
RESULTS
Generation and characterization of phosphospecific CK2α antibodies.
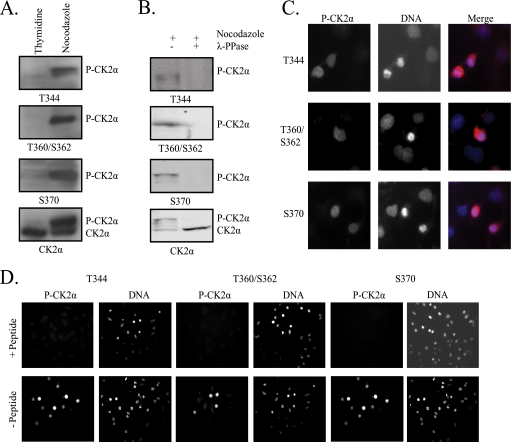
To investigate mitotic phosphorylation of CK2α, we generated polyclonal antibodies targeting the four C-terminal phosphorylation sites. Three antibodies were made, targeting the T344, T360/S362, and S370 phosphorylation sites, respectively. Following affinity purification, the antibodies were tested for phosphospecificity. Phosphorylation of CK2α can be detected after gel electrophoresis by its slight mobility decrease (Fig. 1A, CK2α immunoblot). Lysates from cells arrested in S phase by a thymidine block show no mobility shift due to phosphorylation and, accordingly, show no reactivity when immunoblotted with phosphospecific CK2α antibodies (Fig. 1A, thymidine lanes). Lysates from cells arrested in mitosis by nocodazole treatment show robust reactivity toward phosphospecific CK2α antibodies at a position corresponding to the phosphorylated portion of total CK2α (Fig. 1A, nocodazole lanes). Additionally, all three phosphospecific antibodies show no cross-reactivity to unphosphorylated CK2α. To confirm that these antibodies are specifically detecting phosphorylation, we next immunoblotted lysates from nocodazole-arrested cells after dephosphorylation with λ-phosphatase. As shown in Fig. 1B, treatment with λ-phosphatase resulted in loss of the mobility shift associated with CK2α phosphorylation and loss of reactivity to phosphospecific CK2α antibodies. To test the ability of the phosphospecific antibodies to detect phosphorylated CK2α in cells, nocodazole-arrested cells were fixed and immunostained with phosphospecific CK2α antibodies. DNA was stained with DAPI. All three antibodies exclusively stain mitotic cells, recognized by the condensed nature of the chromosomes (Fig. 1C). The signal is specific to phosphorylated CK2α, as preincubation of the antibodies with the phosphorylated peptides used in antibody generation completely blocks detection by immunostaining (Fig. 1D). To test for cross-reactivity among the three antibodies, we also preincubated each antibody with peptides targeting the other phosphorylation sites. When peptides targeted the same phosphorylation site as the antibody, antibody reactivity was blocked, but peptides targeting other phosphorylation sites not targeted by a particular antibody did not inhibit antibody reactivity (see Fig. S1 in the supplemental material).
FIG. 1.
Generation of phosphospecific CK2α antibodies. (A) Lysates from cells arrested in S phase by a double-thymidine block or in mitosis by nocodazole treatment were immunoblotted with phosphospecific CK2α antibodies targeting phosphorylated T344, T360/S362, or S370. Total CK2α was detected with a CK2α antibody. (B) Lysates from nocodazole-arrested cells were left untreated or dephosphorylated with λ-phosphatase (λ-PPase) before immunoblotting as in panel A. (C) Nocodazole-arrested cells were fixed and immunostained with phosphospecific CK2α antibodies. DNA was stained with DAPI. Mitotic cells are recognizable by the condensation of chromosomes. Magnification, ×40. (D) Phosphospecific CK2α antibodies were preincubated with phosphorylated peptides corresponding to the appropriate phosphorylation site before immunostaining of nocodazole-arrested cells as in panel C. DNA is stained with DAPI. Magnification, ×20. P-CK2α, phosphorylated CK2α.
CK2α is phosphorylated in prophase and metaphase and dephosphorylated during anaphase.
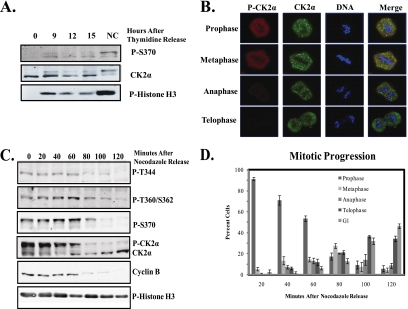
While CK2α has long been known to be maximally phosphorylated in nocodazole-treated cells on the basis of its shift in electrophoretic mobility (4, 24), phosphospecific antibodies provide new opportunities to directly evaluate the extent of phosphorylation as mitosis progresses in a nonarrested cell. To test whether CK2α phosphorylation occurs in all mitotic cells, we compared the proportion of CK2α phosphorylated in cells undergoing normal mitotic progression versus those arrested in mitosis by nocodazole treatment. U2OS cells were arrested in S phase by a double-thymidine block and released into the cell cycle for 9, 12, or 15 h. At these time points, mitotic cells were collected by mitotic shake off and analyzed for CK2α phosphorylation with the phospho-S370 antibody (Fig. 2A). When equal amounts of cell lysates were subjected to Western blot analysis, CK2α was found to be phosphorylated in mitotic cells, albeit at lower levels than in cells treated with nocodazole. This result was also obtained with a total CK2α antibody, with mitotic cells exhibiting the characteristic shift seen upon phosphorylation. Phospho-histone H3 (serine 10) was used as a marker of mitosis.
FIG. 2.
CK2α phosphorylation occurs in mitotic cells during prophase and metaphase. (A) U2OS cells were synchronized in S phase by a double-thymidine block and released into mitosis for 9, 12, or 15 h before harvest. Lysates were immunoblotted with phosphospecific CK2α antibody P-S370. Total CK2α is also shown. Mitotic cells were detected with a phospho-histone H3 (Ser 10) antibody. (B) U2OS cells were synchronized as in panel A, fixed 12 h after thymidine release, and immunostained with antibodies against phosphorylated CK2α (P-CK2α) (red) and total CK2α (green). DNA was stained with DAPI. Magnification, ×63. (C) U2OS cells were arrested in mitosis by nocodazole treatment, washed, and harvested at 20-min intervals after the removal of nocodazole. Lysates were immunoblotted with antibodies against phosphorylated CK2α, total CK2α, cyclin B1 to detect the onset of anaphase, and phospho-histone H3 (serine 10) to detect mitotic cells. (D) Cells were arrested in mitosis as in panel C and plated on slides following release. After fixation and DAPI staining, cells were scored for mitotic stage based on DNA morphology. At least 100 cells were scored for each time point in each of three replicate experiments.
We next examined whether CK2α phosphorylation was occurring throughout mitosis. To achieve this, U2OS cells were arrested in S phase by double thymidine release, released from the double-thymidine block for 12 h, and then fixed for immunostaining with phosphospecific and total CK2α antibodies. Staining with CK2α revealed granular staining in all stages of mitosis, the significance of which remains to be investigated. Notably, as shown in Fig. 2B, cells in prophase and metaphase show robust phospho-CK2α staining, which decreases rapidly in anaphase and is undetectable in telophase and cytokinesis. To confirm this result, cells were arrested in prophase by nocodazole treatment to enable tracking of the phosphorylation in a synchronized population. After removal of nocodazole, cells were harvested at 20-min intervals to track CK2α phosphorylation through mitosis by Western blot analysis with phosphospecific CK2α antibodies (Fig. 2C). Phosphorylation was initially strong and decreased after 80 min of progression. This result is also shown with a CK2α antibody, with mitotic cells exhibiting the characteristic shift seen upon CK2α phosphorylation. This correlated well with the onset of anaphase, as cyclin B expression decreases just prior to the onset of CK2α dephosphorylation. Histone H3, which remains phosphorylated at serine 10 until the completion of cytokinesis (7), served as a marker for late mitosis. To determine the average mitotic stage of cells at each time point, cells were incubated on coverslips after release from nocodazole arrest and sorted into mitotic stages based on DNA morphology (Fig. 2D). The majority of the cells remained in prophase and metaphase until the 60-min time point and progressed into anaphase at 80 to 100 min after release. From these results, we concluded that CK2α is phosphorylated in mitotic cells, but at lower levels than in nocodazole-arrested cells. This phosphorylation is temporally regulated, with maximal phosphorylation in prophase and metaphase and dephosphorylation occurring during anaphase.
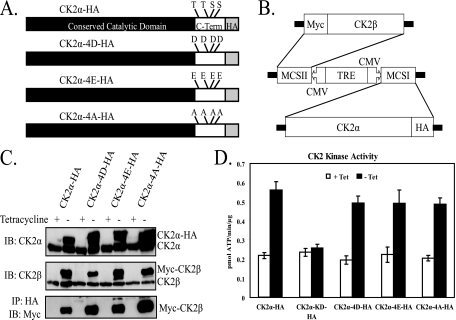
Characterization of cell lines with inducible expression of phosphorylation site mutant CK2α proteins.
The above-described results indicate that temporal regulation of CK2α phosphorylation may be important in mitotic progression. To study the role of the phosphorylation of CK2α in cell cycle progression, all four proline-directed phosphorylation sites unique to the CK2α C terminus (T344, T360, S362, and S370) were mutated to either aspartic acid (CK2α-4D) or glutamic acid (CK2α-4E) in order to mimic the phosphorylated state. Additionally, the phosphorylation sites were mutated to alanine (CK2α-4A) in order to generate a nonphosphorylatable form of CK2α (Fig. 3A). UTA6 cells with stable, tetracycline-regulated expression of phosphorylation site mutant CK2α proteins were generated with a bidirectional plasmid expressing both HA-tagged phosphorylation site mutant CK2α protein and Myc-tagged CK2β (Fig. 3B). This bidirectional system has the advantage of maintaining the stoichiometry of the CK2 tetramer, and tetracycline regulation ensures tight control over expression of the mutant proteins. Myc-CK2β coimmunoprecipitates with CK2α-HA, indicating that these exogenously expressed proteins can interact in the cell (Fig. 3C). However, it is possible that these exogenous CK2 subunits also form complexes with endogenous subunits to form mixed CK2 tetramers. In cell lysates, the presence of phosphorylation site mutant CK2α proteins caused an increase in kinase activity comparable to the increase seen with wild-type CK2α-HA. Thus, these phosphorylation site mutations do not appear to affect the enzymatic activity of CK2α (Fig. 3D).
FIG. 3.
Inducible expression of phosphorylation site mutant CK2α proteins. (A) CK2α C-terminal phosphorylation sites (Thr344, Thr360, Ser362, and Ser370) were mutated to aspartic acid (CK2α-4D-HA), glutamic acid (CK2α-4E-HA), or alanine (CK2α-4A-HA). C-Term, C terminus. (B) HA-tagged CK2α mutant proteins were stably expressed from a tetracycline-regulated bidirectional vector along with Myc-tagged CK2β. MCSI/II, multiple cloning sites I and II; CMV, cytomegalovirus promoter; TRE, tetracycline-responsive element. (C) Inducible expression of tetrameric complexes containing CK2α-HA, CK2α-4D-HA, CK2α-4E-HA, CK2α-4A-HA, and Myc-CK2β. Cells were incubated for 24 h in the presence (+) or absence (−) of tetracycline. Equal amounts of lysate were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to immunoblot analysis with antibodies targeted against CK2α and CK2β or immunoprecipitated (IP) with a 12CA5 (anti-HA) antibody and immunoblotted (IB) with an anti-Myc antibody. (D) Lysates were prepared from cells with tetracycline-regulated expression of CK2α-HA, CK2α-KD-HA (kinase dead), CK2α-4D-HA, CK2α-4E-HA, and CK2α-4A-HA cultured in the presence (+) or absence (−) of tetracycline (Tet) for 24 h. Lysates were incubated with a synthetic peptide substrate of CK2 (RRRDDDSDDD) and [γ-32P]ATP. Kinase activities are the average of four determinations. The error bars indicate 1 standard deviation from the mean.
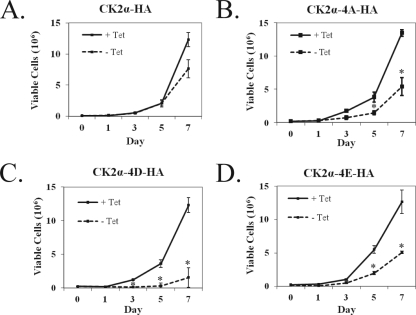
Expression of phosphorylation site mutant CK2α proteins results in decreased proliferation.
To determine if mutation of the four conserved CK2α phosphorylation sites had any effect on cell proliferation, we analyzed the growth of cells expressing CK2α-HA, CK2α-4D-HA, CK2α-4E-HA, or CK2α-4A-HA in combination with Myc-CK2β. Protein expression was induced by removal of tetracycline from the culture medium, and cells were counted over a 7-day period to examine proliferative capacity compared to that of cells grown in the absence of CK2α overexpression. Cells expressing wild-type CK2α-HA showed a slight decrease in proliferation over the 7-day period (Fig. 4A), consistent with previous results (42). In the three phosphorylation site mutant cell lines, the proliferation defects were more marked than in their nonexpressing counterparts (Fig. 4B, C, and D). To ensure that the observed growth defects were not artifacts from random integration events in the construction of the stable cell lines, growth curves were also prepared with additional cell lines expressing each mutant protein. These cell lines also exhibited decreased growth upon the expression of mutant CK2α proteins (see Fig. S2 in the supplemental material). The decreased proliferation observed upon the expression of phosphorylation site mutant CK2α proteins suggests that CK2α phosphorylation does have a functional effect on cellular proliferation.
FIG. 4.
Cell proliferation profiles of inducible wild-type and mutant CK2α cell lines show decreased growth with phosphorylation site mutant protein expression. Cells expressing CK2α-HA (A), CK2α-4A-HA (B), CK2α-4D-HA (C), and CK2α-4E-HA (D) were seeded into six-well dishes at 2 × 104/well and cultured in the presence (+) or absence (−) of 1.5 μg/μl tetracycline (Tet) at day 0. The growth medium was changed every 3 days. Cell counts were obtained in triplicate for a period of 7 days. The results represent the average of three independent experiments. The error bars indicate 1 standard deviation from the mean. An asterisk indicates a significant difference between samples with and without tetracycline (P < 0.05).
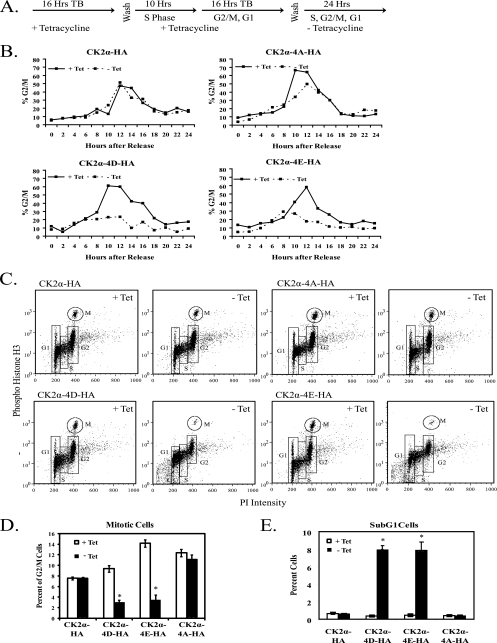
As expression of phosphorylation site mutant CK2α proteins caused varied decreases in proliferation, we next sought to determine if any specific point in the cell cycle was perturbed upon expression. Cells expressing CK2α-HA, CK2α-4D-HA, CK2α-4E-HA, and CK2α-4A-HA were grown in the presence or absence of tetracycline and arrested in S phase by a double-thymidine block. Upon release from the double-thymidine block, mutant CK2α protein expression was induced (Fig. 5A). Cells were fixed at 2-h intervals for 24 h and analyzed by flow cytometry. Samples from each stable cell line without protein induction showed similar cell cycle progression profiles, with a maximum amount of cells with 4N DNA content 8 to 12 h after release from the thymidine block (Fig. 5B). Profiles of cells expressing phosphomimetic mutant CK22α proteins showed a maximum amount of cells with 4N DNA content at slightly earlier time points, and all three phosphorylation site mutants showed a marked decrease in cells with 4N DNA content. The CK2α-4A-HA-expressing cells show a modest decrease in mitotic cells at the 10- and 12-h time points, while the CK2α-4D-HA- and CK2α-4E-HA-expressing cells show more marked decreases in mitotic cells from the 10-h time point onward. This indicates that expression of phosphorylation site mutant CK2α proteins may affect the timing of transit to G2/M and lead to loss of cells in mitosis.
FIG. 5.
Expression of phosphomimetic mutant CK2α proteins leads to loss of mitotic cells. (A) CK2α-HA, CK2α-4D-HA, CK2α-4E-HA, and CK2α-4A-HA cells were synchronized with a double-thymidine block (TB) and then cultured in the presence (+ Tet) or absence (− Tet) of tetracycline for 24 h. (B) Cell cycle profiles of synchronized CK2α cell lines. The 0-h time point was designated the time immediately after the double-thymidine block. Cells were harvested and fixed at 2-h intervals, stained with PI, and analyzed by flow cytometry. The percentage of cells in G2/M was taken from the percentage of cells with 4N DNA content. (C) Phospho-histone H3 (serine 10) staining of cells 12 h after release from a double-thymidine block and induction of phosphorylation site mutant CK2α proteins. Cells were fixed and stained with phospho-histone H3 (serine 10) antibody, an FITC-GAR secondary antibody, and PI. Samples were analyzed by flow cytometry. (D) Graphical representation of the percentage of G2/M cells in mitosis 12 h after release from a double-thymidine block. (E) Graphical representation of the amount of cells with sub-G1 levels of DNA 12 h after release from a double-thymidine block. In panels D and E, significant differences between cells of each line in the presence or absence of tetracycline are denoted by asterisks (P > 0.05 by pairwise analysis of variance).
The loss of mitotic cells observed could have several explanations, including cell cycle arrest in either S phase or G2/M or loss of cells due to cell death. When cells were induced for protein expression 6 h before release from a thymidine block, there were no differences in cell cycle profiles compared to those of cells induced at the time of release (see Fig. S3 in the supplemental material). This indicates that expression of phosphorylation site mutant CK2α proteins does not affect S-phase entry or progression. To investigate whether the loss of mitotic cells is due to G2 arrest, we employed phospho-histone H3 (serine 10) staining to distinguish between G2 cells and mitotic cells (43). Cells were released from a double-thymidine block, and protein expression was induced. At the 12-h time point, cells were fixed and analyzed for phospho-histone H3 positivity and DNA content. Representative histograms are shown in Fig. 5C. The cell cycle distribution of each cell line, averaged from three independent experiments, is shown in Table 1. Recapitulating the results shown in Fig. 5B, expression of CK2α-HA had no effect on the percentage of cells in mitosis. Compared to the total percentage of cells in G2/M, expression of CK2α-4D-HA and CK2α-4E-HA resulted in an almost complete loss of mitotic cells (Fig. 5D). The decrease in mitotic cells did not accompany a corresponding increase in G2 cells, as would be expected if the loss of mitotic cells were due to a G2/M arrest. In fact, the amount of G2 cells also significantly decreases upon the expression of CK2α-4D-HA or CK2α-4E-HA (Table 1). Interestingly, the CK2α-4D-HA and CK2α-4E-HA cell lines showed an accumulation of cells with sub-G1 levels of DNA upon the expression of phosphomimetic CK2α (Fig. 5E). No accumulation of sub-G1 cells was observed with either CK2α-HA or CK2α-4A-HA expression. Therefore, we conclude that the loss of mitotic cells observed upon expression of the phosphomimetic CK2α forms is not due to cell cycle arrest in S phase or G2 phase but instead may be attributed to cell death.
TABLE 1.
Cell cycle distribution of cells expressing phosphorylation site mutant CK2α proteins 12 h after release from a thymidine block
| Cell line and treatment | % of cellsc
|
||||
|---|---|---|---|---|---|
| Sub-G1 | G1 | S | G2a | Mb | |
| CK2α-HA | |||||
| Plus tetracycline | 0.64 | 32.04 | 13.77 | 48.79 | 4.01 |
| Minus tetracycline | 0.59 | 29.89 | 15.16 | 49.60 | 4.13 |
| CK2α-4D-HA | |||||
| Plus tetracycline | 0.36 | 28.91 | 17.97 | 46.79 | 4.85 |
| Minus tetracycline | 7.92 | 33.21 | 24.98 | 32.27 | 0.97 |
| CK2α-4E-HA | |||||
| Plus tetracycline | 0.44 | 31.71 | 15.47 | 44.08 | 7.26 |
| Minus tetracycline | 7.85 | 33.05 | 22.77 | 34.40 | 1.23 |
| CK2α-4A-HA | |||||
| Plus tetracycline | 0.41 | 31.22 | 16.36 | 44.67 | 6.80 |
| Minus tetracycline | 0.35 | 31.29 | 15.45 | 46.59 | 5.91 |
Cells with 4N DNA content negative for phospho-histone H3 staining.
Cells with 4N DNA content positive for phospho-histone H3 staining.
Boldface indicates significant differences between samples with and without tetracycline (P < 0.01).
Phosphomimetic CK2α expression causes cell death by mitotic catastrophe.
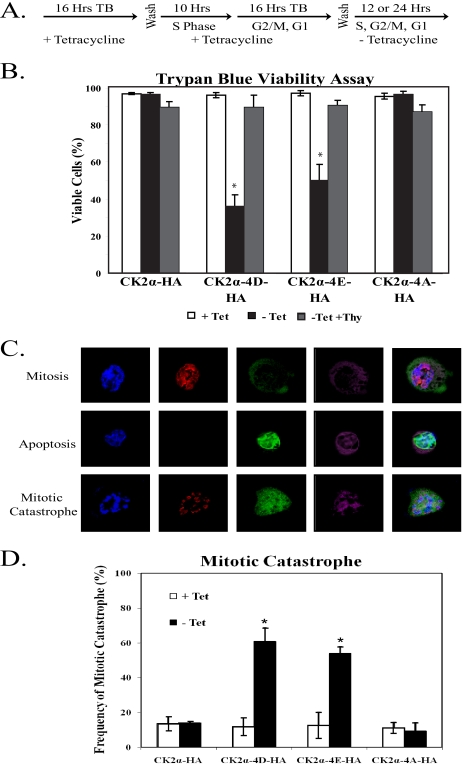
Cell cycle analysis of cells expressing phosphomimetic mutant CK2α proteins showed a dramatic increase in cells with sub-G1 amounts of DNA, indicating cell death. To quantitate the extent of cell death upon phosphomimetic CK2α expression, we arrested cells in S phase by double-thymidine block and induced protein expression upon release into the cell cycle (Fig. 6A). Cells were allowed to progress through mitosis and were stained 24 h after thymidine release with trypan blue. In this assay, viable cells remain unstained while nonviable cells are stained dark blue. While the wild-type CK2α-HA cell line and the nonphosphorylatable CK2α-4A-HA cell line showed no decrease in the amount of viable cells upon CK2α expression, the CK2α-4D-HA and CK2α-4E-HA cell lines showed significant decreases in viability upon protein expression (Fig. 6B). To ensure that the observed cell death was related to cell cycle progression and was not a direct result of phosphomimetic CK2α expression, cells were induced for protein expression for 24 h in a continuous thymidine-induced S-phase arrest. As shown in Fig. 6B, there was little evidence of cell death in phosphomimetic CK2α-expressing cells arrested in S phase. Therefore, the cell death observed in the phosphomimetic CK2α-expressing cell lines requires both protein expression and progression through the cell cycle. Since the cell death observed was cell cycle dependent and seemed to involve the specific loss of mitotic cells, we hypothesized that the loss of mitotic cells is due to induction of mitotic catastrophe. To confirm that cells expressing CK2α-4D-HA or CK2α-4E-HA are dying during the process of cell division, we employed immunostaining to detect cells that are simultaneously mitotic and apoptotic. To identify mitotic cells, we immunostained cells with a phospho-histone H3 (serine 10) antibody. We used cytochrome c release from the mitochondria as a marker of early apoptosis, combining immunostaining with a cytochrome c antibody with MitoTracker Deep Red 633 FM staining to visualize the mitochondria. A representative cell in the process of both mitosis and apoptosis is shown in Fig. 6C. As shown in Fig. 6D, expression of CK2α-4D-HA or CK2α-4E-HA caused a large increase in mitotic catastrophe over baseline levels. Expression of neither CK2α-HA nor CK2α-4A-HA caused any increase in the amount of mitotic catastrophe observed. From this we conclude that expression of phosphomimetic mutant CK2α proteins results in cell death by mitotic catastrophe.
FIG. 6.
Phosphomimetic CK2α expression causes cell death by mitotic catastrophe. (A) CK2α-HA, CK2α-4D-HA, CK2α-4E-HA, and CK2α-4A-HA cells were synchronized in S phase by a double-thymidine block (TB). Upon release, the cells were induced for CK2α expression and analyzed 12 h (panels C and D) or 24 h (panel B) after release into the cell cycle. (B) Cells were incubated for 24 h after release from thymidine (Thy) treatment and stained with trypan blue to assess viability. The percentage of cells excluding blue staining (indicating cell viability) was tabulated for at least 200 cells in each of three independent experiments. Cells were also maintained in S phase by continued thymidine treatment and assayed for viability. The error bars indicate 1 standard deviation from the mean. Significant differences between cells of each line in the presence or absence of tetracycline (Tet) are denoted by asterisks (P > 0.05 by pairwise analysis of variance). (C) Cells expressing wild-type or mutant CK2α were fixed after 12 h of cell cycle progression and immunostained with antibodies against phospho-histone H3 (P-Histone H3) serine 10 (to show mitosis), and cytochrome c (Cyt C; to show apoptosis). Mitochondria were stained with MitoTracker Deep Red 633 FM to visualize cytochrome c release. (D) The percentage of cells exhibiting features of mitotic catastrophe was tabulated for at least 100 mitotic cells in each of three independent experiments. The error bars indicate 1 standard deviation from the mean. Significant differences between cells of each line in the presence or absence of tetracycline are denoted by asterisks (P > 0.05 by pairwise analysis of variance).
Mitotic defects induced by phosphomimetic CK2α expression include aberrant spindle formation and missegregation of chromosomes.
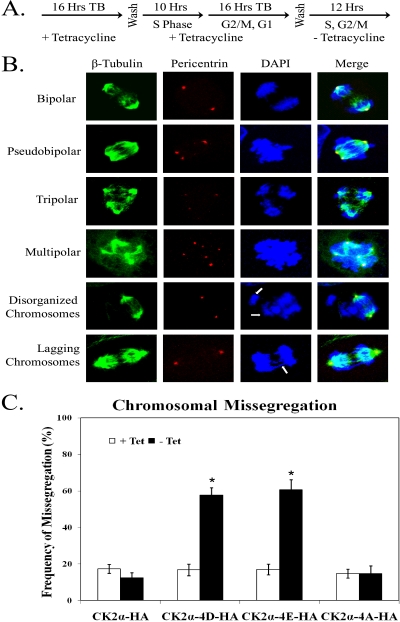
Expression of phosphomimetic mutant CK2α proteins caused a dramatic increase in cell death by mitotic catastrophe. To further examine the mitotic defects caused by expression of CK2α-4D-HA or CK2α-4E-HA, cells were arrested at S phase, released into mitosis, and induced for expression of phosphorylation site mutant CK2α proteins (Fig. 7A). Cells were then fixed and immunostained with antibodies against β-tubulin and pericentrin to visualize the mitotic spindle and the centrosomes, respectively (5). Interestingly, cells expressing phosphomimetic forms of CK2α show multiple centrosomes, resulting in the formation of pseudobipolar, tripolar, and multipolar cells (Fig. 7B). The aberrant numbers of centrosomes in these cells appear to be due to centrosome fragmentation and not overduplication, as cells expressing CK2α-4D-HA or CK2α-4E-HA display proper numbers of centrosomes in S phase (data not shown). Even among the proportion of phosphomimetic CK2α-4D-HA- and CK2α-4E-HA-expressing cells that displayed two centrosomes, we noted abnormalities in chromosomal segregation, including chromosomes that did not seem to line up properly at the metaphase plate and lagging chromosomes during separation in anaphase. When these varied phenotypes were quantified, approximately half of the cells expressing CK2α-4D-HA or CK2α-4E-HA showed defects in chromosomal segregation (Fig. 7C). The dramatic phenotypes observed upon expression of phosphomimetic CK2α indicate that CK2α phosphorylation may be involved in the maintenance of centrosome integrity and proper chromosomal segregation.
FIG. 7.
Phosphomimetic CK2α expression results in aberrant mitotic spindle formation and missegregation of chromosomes. (A) CK2α-HA, CK2α-4D-HA, CK2α-4E-HA, and CK2α-4A-HA cells were synchronized in S phase by a double-thymidine block. Upon release, the cells were induced for CK2α expression and fixed 12 h after release into the cell cycle. (B) Cells were immunostained with antibodies against pericentrin (to visualize centrosomes) (red) and β-tubulin (to visualize microtubules) (green). DNA was stained with DAPI. (C) The percentage of mitoses with missegregation of chromosomes was tabulated for at least 100 anaphase cells in each of three independent experiments. The error bars indicate 1 standard deviation from the mean. Significant differences between cells of each line in the presence or absence of tetracycline (Tet) are denoted by asterisks (P > 0.05 by pairwise analysis of variance).
Nonphosphorylatable CK2α expression abrogates the SAC.
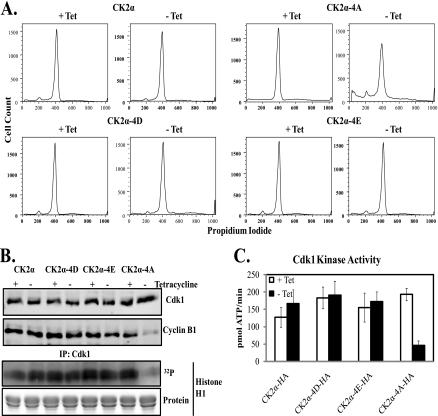
Phosphorylation of CK2α was originally identified in cells treated with nocodazole and therefore with an activated spindle assembly checkpoint (SAC) (36). Because of this, we examined whether disruption of normal CK2α phosphorylation would affect the ability of cells to arrest in mitosis after microtubule disruption by nocodazole treatment. Cells were treated with nocodazole in the presence or absence of phosphorylation site mutant CK2α protein expression. After fixation and PI staining, cell cycle analysis was employed. While expression of wild-type or phosphomimetic CK2α had no effect on the ability of the cells to arrest in mitosis, we observed a significant number of cells expressing CK2α-4A-HA that lost the ability to arrest after spindle damage, resulting in a decrease in G2/M cells and increases in both sub-G1 and G1 cells (Fig. 8A). The average percentages of cells of each line in each stage of the cell cycle are shown in Table 2. To confirm the loss of G2/M arrest in cells expressing nonphosphorylatable CK2α, Cdk1 activity was investigated (41). Upon immunoprecipitation of Cdk1, cells expressing CK2α-4A-HA showed both decreased expression of cyclin B1 and decreased phosphorylation of histone H1, indicating that these cells have an abrogated SAC (Fig. 8B). The average Cdk1 activity from three independent experiments is shown in Fig. 8C. We conclude that proper phosphorylation of CK2α is required to maintain the SAC in response to spindle damage.
FIG. 8.
Expression of nonphosphorylatable CK2α abrogates the SAC. (A) Cell cycle profiles of cells expressing CK2α-HA, CK2α-4D-HA, CK2α-4E-HA, or CK2α-4A-HA that were arrested in mitosis by nocodazole treatment in the presence or absence of tetracycline (Tet), followed by fixation and PI staining. Samples were analyzed by flow cytometry. (B) Cdk1 was immunoprecipitated from lysates prepared from nocodazole-arrested cells expressing CK2α-HA, CK2α-4D-HA, CK2α-4E-HA, or CK2α-4A-HA. Immunoprecipitates (IP) were immunoblotted for Cdk1 and cyclin B1 or used in kinase assays with histone H1 and [γ-32P]ATP. Phosphorylation is shown by a representative autoradiograph. Total histone H1 is shown by Coomassie blue staining. (C) Graphical representation of Cdk1 kinase activity. Results are the average of three independent experiments. Error bars indicate 1 standard deviation from the mean.
TABLE 2.
Cell cycle distribution of nocodazole-arrested cells expressing phosphorylation site mutant CK2α proteins
| Cell line and treatment | % of cellsa
|
|||
|---|---|---|---|---|
| Sub-G1 | G1 | S | G2/M | |
| CK2α-HA | ||||
| Plus tetracycline | 7.00 | 6.33 | 10.57 | 88.26 |
| Minus tetracycline | 7.01 | 6.04 | 11.50 | 73.50 |
| CK2α-4D-HA | ||||
| Plus tetracycline | 3.89 | 3.13 | 10.88 | 71.80 |
| Minus tetracycline | 4.28 | 7.33 | 17.00 | 63.29 |
| CK2α-4E-HA | ||||
| Plus tetracycline | 3.40 | 5.81 | 11.41 | 76.86 |
| Minus tetracycline | 4.92 | 8.91 | 18.84 | 73.59 |
| CK2α-4A-HA | ||||
| Plus tetracycline | 5.24 | 5.57 | 12.37 | 82.64 |
| Minus tetracycline | 14.87 | 10.93 | 24.74 | 51.41 |
Boldface indicates significant differences between samples with and without tetracycline (P < 0.01).
DISCUSSION
In this study, we have investigated the role of CK2α phosphorylation in mitosis. Our data indicate that precise regulation of these phosphorylation events is required for proper mitotic progression. Through generation of phosphospecific antibodies against four phosphorylation sites known to be phosphorylated in nocodazole-arrested cells (4, 24), we show that these sites are also phosphorylated in cells progressing through normal mitosis. Mitotic phosphorylation is strongest during prophase and metaphase and decreases during anaphase, becoming undetectable by telophase and cytokinesis. The temporal pattern of phosphorylation observed matches the temporal activation of Cdk1, the kinase believed to be responsible for these phosphorylation events (24).
We next sought to determine the function of CK2α phosphorylation by observation of phenotypes associated with the expression of either phosphomimetic mutant CK2α proteins (CK2α-4D-HA and CK2α-4E-HA) or nonphosphorylatable mutant CK2α (CK2α-4A-HA). Proliferation curves showed decreased growth in all three lines compared with the expression of CK2α-HA, with particular defects shown in the phosphomimetic cell lines. Subsequent cell cycle analysis showed sub-G1 cells upon phosphomimetic CK2α expression, indicating that mitotic cells were dying in a cell cycle-dependent manner. Upon CK2α-4D-HA or CK2α-4E-HA expression, mitotic cells undergo mitotic catastrophe, a type of cell death that occurs in mitosis due to a deficiency in cell cycle checkpoint control or cellular damage (6). This may serve as a mechanism to eliminate cells that, if permitted to progress through mitosis, would result in aneuploid daughter cells. The stimulus for induction of mitotic catastrophe may be the centrosomal amplification observed in phosphomimetic protein-expressing cells, leading to aberrant spindle formation and chromosomal missegregation. Similar results have recently been observed in Drosophila melanogaster, as silencing of the CkIIα gene by RNA interference causes mitotic abnormalities, including centrosome abnormalities and lagging chromatids (3). In mammalian cells, CK2 has been shown to colocalize with the centrosomes (10, 30) and mitotic spindle (18, 46), and many components of the mitotic machinery interact with and/or are substrates of CK2, including β-tubulin, microtubule-associated proteins 1A and 1B, Tau, condensin, and protein phosphatase 2A (2, 11, 15, 39). Recent proteomic investigations have identified CK2 as a component of both the centrosome and the spindle midbody (1, 38). Phosphoproteomic analysis of mitotic spindles recently identified a number of possible CK2 substrates, including both known CK2 substrates such as topoisomerase IIα and HSP-90 and novel substrates such as septin 2, INCENP, and MAP7 (32). While the mechanism by which aberrant CK2α phosphorylation leads to centrosomal defects is unknown, it is clear that proper regulation ensures spindle organization and maintains genomic integrity.
As CK2α phosphorylation occurs mainly during prophase and metaphase, we theorize that the defects seen upon the expression of phosphorylation site mutant CK2α proteins may be due to loss of temporal control of phosphorylation. During normal cell division, CK2α remains unphosphorylated until the onset of prophase, when phosphorylation occurs. In the presence of spindle abnormalities, CK2α phosphorylation is at a maximum. Once all chromosomes are lined up at the metaphase plate, CK2α is dephosphorylated as the cell enters anaphase and remains dephosphorylated until the next cell division. Defects seen upon the expression of phosphorylation site mutant CK2α proteins may represent the consequences of loss of temporal regulation of phosphorylation. When CK2α seems phosphorylated before the onset of prophase, decreased centrosome integrity leads to abnormal spindle formation and chromosomal missegregation. Cells expressing phosphomimetic CK2α also undergo mitotic catastrophe, either as a result of these spindle abnormalities or, alternatively, when CK2α seems to remain phosphorylated after the metaphase-anaphase transition. Expression of nonphosphorylatable CK2α results in loss of cell cycle arrest after spindle damage, indicating that CK2α phosphorylation plays a role in maintaining the SAC. This result was particularly interesting. Cells expressing CK2α-4A-HA did not display dramatic mitotic defects, showing only a slight decrease in proliferation compared to cells expressing wild-type CK2α. This corresponds well to the finding that not all CK2α seems to be phosphorylated in a normally dividing cell and indicates that only a subset of CK2α needs to be modified to fulfill its mitotic purposes. However, upon treatment of cells with nocodazole, when CK2α phosphorylation is at maximum levels, expression of nonphosphorylatable CK2α neutralizes the activated SAC, allowing cells to progress through mitosis even in the presence of endogenous phosphorylated CK2α. The involvement of CK2 in SAC signaling has been previously demonstrated, as depletion of CK2 activity compromises SAC arrest after nocodazole treatment (37). However, the role of CK2α phosphorylation has not been examined.
Interestingly, expression of phosphorylation site mutant CK2α proteins elicits mitotic phenotypes even in the presence of comparable amounts of endogenous CK2α. Since the mutant proteins are capable of forming complexes with Myc-CK2β and presumably incorporate into mixed tetramers with endogenous CK2 subunits, this suggests that phosphorylation site mutant CK2α proteins may have a dominant effect over endogenous, and presumably phosphorylated, CK2α. This dominant effect may be mediated through blocking or encouraging phosphorylation-dependent interactions between CK2 and mitotic proteins. It seems likely that the CK2α phosphorylation sites serve as a regulatory mechanism, likely through forming an interaction site for the binding of other proteins. This has been shown to be the case for Pin1, a cis/trans peptidyl-prolyl isomerase with a number of mitotic substrates. Pin1 selectively isomerizes proline residues adjacent to phosphorylated serine or threonine residues (45). While C-terminal phosphorylation of CK2α does not affect the general kinase activity of CK2, as measured by kinase assays with a substrate peptide, it is plausible that phosphorylation at these sites may be important in the regulation of CK2 activity against particular substrates, as is the case for topoisomerase IIα. The modulation of CK2 kinase activity toward topoisomerase IIα is dependent on Pin1 binding, as a complex consisting of CK2, Pin1, and topoisomerase IIα is formed (31). It remains unknown whether this regulatory mechanism is involved in selective binding and modulation of additional CK2 substrates. Recently, a proteomic screen of Plk1 polo box domain binding proteins identified CK2α as a phosphorylation-dependent mitotic binding partner for Plk1 (28). Plk1 is a mitotic kinase with multiple roles in mitotic progression (40). Interestingly, the protein sequence surrounding the CK2α T344 phosphorylation site corresponds to the consensus sequence for Plk1 polo box domain binding (8). Further investigation of the interplay between these two mitotic kinases may reveal precise roles for CK2α phosphorylation in mitosis.
Overall, we have shown that CK2α is phosphorylated during mitotic progression and that these phosphorylation events do, indeed, have a regulatory role in the process of cell division. Substitution of these residues leads to mitotic catastrophe, defects in centrosome amplification, and abrogation of the SAC. Future work will focus on the mechanisms by which CK2α phosphorylation regulates these events. Collectively, these results offer evidence of a role for reversible phosphorylation of CK2α in the control of cell division.
Supplementary Material
Acknowledgments
We thank Kristin Chadwick and Jamie Simek for technical help and Eric Ball, Fred Dick, and Ryan Mohan for helpful discussion. Flow cytometry was performed at the London Regional Flow Cytometry Facility at Robarts Research Institute. Confocal microscopy was performed in the laboratory of Dale Laird.
This work was supported by an operating grant from the Canadian Cancer Society to D.W.L. N.A.S. has been supported by a Canada Graduate Scholarship from the Canadian Institutes of Health Research and by funding from the Canadian Institutes of Health Research Strategic Training Program in Cancer Research. D.R.D. was supported by an Ontario Graduate Scholarship in Science and Technology.
We do not have any financial interests related to this work.
Footnotes
Published ahead of print on 2 February 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Andersen, J. S., C. J. Wilkinson, T. Mayor, P. Mortensen, E. A. Nigg, and M. Mann. 2003. Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426570-574. [DOI] [PubMed] [Google Scholar]
- 2.Avila, J., L. Ulloa, J. Gonzalez, F. Moreno, and J. Diaz-Nido. 1994. Phosphorylation of microtubule-associated proteins by protein kinase CK2 in neuritogenesis. Cell. Mol. Biol. Res. 40573-579. [PubMed] [Google Scholar]
- 3.Bettencourt-Dias, M., R. Giet, R. Sinka, A. Mazumdar, W. G. Lock, F. Balloux, P. J. Zafiropoulos, S. Yamaguchi, S. Winter, R. W. Carthew, M. Cooper, D. Jones, L. Frenz, and D. M. Glover. 2004. Genome-wide survey of protein kinases required for cell cycle progression. Nature 432980-987. [DOI] [PubMed] [Google Scholar]
- 4.Bosc, D. G., E. Slominski, C. Sichler, and D. W. Litchfield. 1995. Phosphorylation of casein kinase II by p34cdc2. Identification of phosphorylation sites using phosphorylation site mutants in vitro. J. Biol. Chem. 27025872-25878. [DOI] [PubMed] [Google Scholar]
- 5.Bourke, E., H. Dodson, A. Merdes, L. Cuffe, G. Zachos, M. Walker, D. Gillespie, and C. G. Morrison. 2007. DNA damage induces Chk1-dependent centrosome amplification. EMBO Rep. 8603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castedo, M., J. L. Perfettini, T. Roumier, K. Andreau, R. Medema, and G. Kroemer. 2004. Cell death by mitotic catastrophe: a molecular definition. Oncogene 232825-2837. [DOI] [PubMed] [Google Scholar]
- 7.Crosio, C., G. M. Fimia, R. Loury, M. Kimura, Y. Okano, H. Zhou, S. Sen, C. D. Allis, and P. Sassone-Corsi. 2002. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol. Cell. Biol. 22874-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elia, A. E., L. C. Cantley, and M. B. Yaffe. 2003. Proteomic screen finds pSer/pThr-binding domain localizing Plk1 to mitotic substrates. Science 2991228-1231. [DOI] [PubMed] [Google Scholar]
- 9.Englert, C., X. Hou, S. Maheswaren, P. Bennett, C. Ngwu, G. G. Re, A. J. Garvin, M. R. Rosner, and D. A. Haber. 1995. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 144662-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faust, M., J. Gunther, E. Morgenstern, M. Montenarh, and C. Gotz. 2002. Specific localization of the catalytic subunits of protein kinase CK2 at the centrosomes. Cell. Mol. Life Sci. 592155-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faust, M., N. Schuster, and M. Montenarh. 1999. Specific binding of protein kinase CK2 catalytic subunits to tubulin. FEBS Lett. 46251-56. [DOI] [PubMed] [Google Scholar]
- 12.Gietz, R. D., K. C. Graham, and D. W. Litchfield. 1995. Interactions between the subunits of casein kinase II. J. Biol. Chem. 27013017-13021. [DOI] [PubMed] [Google Scholar]
- 13.Guerra, B., and O. G. Issinger. 1999. Protein kinase CK2 and its role in cellular proliferation, development and pathology. Electrophoresis 20391-408. [DOI] [PubMed] [Google Scholar]
- 14.Hanna, D. E., A. Rethinaswamy, and C. V. Glover. 1995. Casein kinase II is required for cell cycle progression during G1 and G2/M in Saccharomyces cerevisiae. J. Biol. Chem. 27025905-25914. [DOI] [PubMed] [Google Scholar]
- 15.Hériché, J. K., F. Lebrin, T. Rabilloud, D. Leroy, E. M. Chambaz, and Y. Goldberg. 1997. Regulation of protein phosphatase 2A by direct interaction with casein kinase 2α. Science 276952-955. [DOI] [PubMed] [Google Scholar]
- 16.Kastan, M. B., and J. Bartek. 2004. Cell-cycle checkpoints and cancer. Nature 432316-323. [DOI] [PubMed] [Google Scholar]
- 17.Kikkawa, U., S. K. O. Mann, R. A. Firtel, and T. Hunter. 1992. Molecular cloning of casein kinase II α subunit from Dictyostelium discoideum and its expression in the life cycle. Mol. Cell. Biol. 125711-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krek, W., G. Maridor, and E. A. Nigg. 1992. Casein kinase II is a predominantly nuclear enzyme. J. Cell Biol. 11643-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 20.Landesman-Bollag, E., P. L. Channavajhala, R. D. Cardiff, and D. C. Seldin. 1998. p53 deficiency and misexpression of protein kinase CK2α collaborate in the development of thymic lymphomas in mice. Oncogene 162965-2974. [DOI] [PubMed] [Google Scholar]
- 21.Litchfield, D. W. 2003. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem. J. 3691-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litchfield, D. W., F. J. Lozeman, M. F. Cicirelli, M. Harrylock, L. H. Ericsson, C. J. Piening, and E. G. Krebs. 1991. Phosphorylation of the beta subunit of casein kinase II in human A431 cells. Identification of the autophosphorylation site and a site phosphorylated by p34cdc2. J. Biol. Chem. 26620380-20389. [PubMed] [Google Scholar]
- 23.Litchfield, D. W., F. J. Lozeman, C. Piening, J. Sommercorn, K. Takio, K. A. Walsh, and E. G. Krebs. 1990. Subunit structure of casein kinase II from bovine testis. Demonstration that the alpha and alpha′ subunits are distinct polypeptides. J. Biol. Chem. 2657638-7644. [PubMed] [Google Scholar]
- 24.Litchfield, D. W., B. Luscher, F. J. Lozeman, R. N. Eisenman, and E. G. Krebs. 1992. Phosphorylation of casein kinase II by p34cdc2 in vitro and at mitosis. J. Biol. Chem. 26713943-13951. [PubMed] [Google Scholar]
- 25.Lorenz, P., R. Pepperkok, W. Ansorge, and W. Pyerin. 1993. Cell biological studies with monoclonal and polyclonal antibodies against human casein kinase II subunit beta demonstrate participation of the kinase in mitogenic signaling. J. Biol. Chem. 2682733-2739. [PubMed] [Google Scholar]
- 26.Lorenz, P., R. Pepperkok, and W. Pyerin. 1994. Requirement of casein kinase 2 for entry into and progression through early phases of the cell cycle. Cell. Mol. Biol. Res. 40519-527. [PubMed] [Google Scholar]
- 27.Lou, D. Y., I. Dominguez, P. Toselli, E. Landesman-Bollag, C. O'Brien, and D. C. Seldin. 2008. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol. Cell. Biol. 28131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowery, D. M., K. R. Clauser, M. Hjerrild, D. Lim, J. Alexander, K. Kishi, S. E. Ong, S. Gammeltoft, S. A. Carr, and M. B. Yaffe. 2007. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J. 262262-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozeman, F. J., D. W. Litchfield, C. Piening, K. Takio, K. A. Walsh, and E. G. Krebs. 1990. Isolation and characterization of human cDNA clones encoding the alpha and the alpha′ subunits of casein kinase II. Biochemistry 298436-8447. [DOI] [PubMed] [Google Scholar]
- 30.McKendrick, L., D. Milne, and D. Meek. 1999. Protein kinase CK2-dependent regulation of p53 function: evidence that the phosphorylation status of the serine 386 (CK2) site of p53 is constitutive and stable. Mol. Cell. Biochem. 191187-199. [PubMed] [Google Scholar]
- 31.Messenger, M. M., R. B. Saulnier, A. D. Gilchrist, P. Diamond, G. J. Gorbsky, and D. W. Litchfield. 2002. Interactions between protein kinase CK2 and Pin1. Evidence for phosphorylation-dependent interactions. J. Biol. Chem. 27723054-23064. [DOI] [PubMed] [Google Scholar]
- 32.Nousiainen, M., H. H. Sillje, G. Sauer, E. A. Nigg, and R. Korner. 2006. Phosphoproteome analysis of the human mitotic spindle. Proc. Natl. Acad. Sci. USA 1035391-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orlandini, M., F. Semplici, R. Ferruzzi, F. Meggio, L. A. Pinna, and S. Oliviero. 1998. Protein kinase CK2α′ is induced by serum as a delayed early gene and cooperates with Ha-ras in fibroblast transformation. J. Biol. Chem. 27321291-21297. [DOI] [PubMed] [Google Scholar]
- 34.Padmanabha, R., J. L.-P. Chen-Wu, D. E. Hanna, and C. V. C. Glover. 1990. Isolation, sequencing, and disruption of the yeast CKA2 gene: casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol. Cell. Biol. 104089-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pepperkok, R., P. Lorenz, W. Ansorge, and W. Pyerin. 1994. Casein kinase II is required for transition of G0/G1, early G1, and G1/S phases of the cell cycle. J. Biol. Chem. 2696986-6991. [PubMed] [Google Scholar]
- 36.Rieder, C. L., and H. Maiato. 2004. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 7637-651. [DOI] [PubMed] [Google Scholar]
- 37.Sayed, M., S. Pelech, C. Wong, A. Marotta, and B. Salh. 2001. Protein kinase CK2 is involved in G2 arrest and apoptosis following spindle damage in epithelial cells. Oncogene 206994-7005. [DOI] [PubMed] [Google Scholar]
- 38.Skop, A. R., H. Liu, J. Yates III, B. J. Meyer, and R. Heald. 2004. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 30561-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takemoto, A., K. Kimura, J. Yanagisawa, S. Yokoyama, and F. Hanaoka. 2006. Negative regulation of condensin I by CK2-mediated phosphorylation. EMBO J. 255339-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Vugt, M. A., and R. H. Medema. 2005. Getting in and out of mitosis with Polo-like kinase-1. Oncogene 242844-2859. [DOI] [PubMed] [Google Scholar]
- 41.Varetti, G., and A. Musacchio. 2008. The spindle assembly checkpoint. Curr. Biol. 18R591-R595. [DOI] [PubMed] [Google Scholar]
- 42.Vilk, G., R. B. Saulnier, R. St Pierre, and D. W. Litchfield. 1999. Inducible expression of protein kinase CK2 in mammalian cells. Evidence for functional specialization of CK2 isoforms. J. Biol. Chem. 27414406-14414. [DOI] [PubMed] [Google Scholar]
- 43.Xu, B., S. T. Kim, D. S. Lim, and M. B. Kastan. 2002. Two molecularly distinct G2/M checkpoints are induced by ionizing irradiation. Mol. Cell. Biol. 221049-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu, X., P. A. Toselli, L. D. Russell, and D. C. Seldin. 1999. Globozoospermia in mice lacking the casein kinase II α′ catalytic subunit. Nat. Genet. 23118-121. [DOI] [PubMed] [Google Scholar]
- 45.Yaffe, M. B., M. Schutkowski, M. Shen, X. Z. Zhou, P. T. Stukenberg, J. U. Rahfeld, J. Xu, J. Kuang, M. W. Kirschner, G. Fischer, L. C. Cantley, and K. P. Lu. 1997. Sequence-specific and phosphorylation-dependent proline isomerization: a potential mitotic regulatory mechanism. Science 2781957-1960. [DOI] [PubMed] [Google Scholar]
- 46.Yu, I. J., D. L. Spector, Y. S. Bae, and D. R. Marshak. 1991. Immunocytochemical localization of casein kinase II during interphase and mitosis. J. Cell Biol. 1141217-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.