Abstract
Angiopoietin 2 (Ang2) was originally shown to be a competitive antagonist for Ang1 of the receptor tyrosine kinase Tie2 in endothelial cells (ECs). Since then, reports have conflicted on whether Ang2 is an agonist or antagonist of Tie2. Here we show that Ang2 functions as an agonist when Ang1 is absent but as a dose-dependent antagonist when Ang1 is present. Exogenous Ang2 activates Tie2 and the promigratory, prosurvival PI3K/Akt pathway in ECs but with less potency and lower affinity than exogenous Ang1. ECs produce Ang2 but not Ang1. This endogenous Ang2 maintains Tie2, phosphatidylinositol 3-kinase, and Akt activities, and it promotes EC survival, migration, and tube formation. However, when ECs are stimulated with Ang1 and Ang2, Ang2 dose-dependently inhibits Ang1-induced Tie2 phosphorylation, Akt activation, and EC survival. We conclude that Ang2 is both an agonist and an antagonist of Tie2. Although Ang2 is a weaker agonist than Ang1, endogenous Ang2 maintains a level of Tie2 activation that is critical to a spectrum of EC functions. These findings may reconcile disparate reports of Ang2's effect on Tie2, impact our understanding of endogenous receptor tyrosine kinase signal transduction mechanisms, and affect how Ang2 and Tie2 are targeted under conditions such as sepsis and cancer.
Angiopoietins, a family of ligands that bind the Tie2 receptor, consist of Ang1, Ang2, and Ang3/Ang4. Ang1 and Ang2 are the best-characterized ligands of Tie2 and were originally shown to be agonistic and antagonistic, respectively (14, 29, 42, 45). The role of Ang1 as a Tie2 agonist is supported by both in vitro and in vivo experiments. In vitro, Ang1 binds to Tie2 and induces its activation via tyrosine phosphorylation. Through the phosphatidylinositol 3-kinase (PI3K)-Akt pathway and others, Ang1 exerts prosurvival, antipermeability, and anti-inflammatory effects on endothelial cells (ECs) (9, 13, 16, 24, 27, 30, 43). In vivo, the fact that Ang1 null embryos die in midgestation with a phenotype nearly identical to that of Tie2 null embryos further confirms that Ang1 is an agonist of Tie2 (9, 40).
Ang2 was originally identified by homology screening as a paralog of Ang1. Through a combination of cell culture and whole-animal studies with Ang2-transgenic mice, it was shown that Ang2 is a naturally occurring competitive antagonist of Tie2. Most notably, Ang2-transgenic embryos died in midgestation with vascular lesions that phenocopied those of Ang1 and Tie2 null embryos (28). Since then, some groups have reported that increased Ang2 may inhibit Ang1/Tie2 signaling in both in vitro and in vivo settings (5, 12, 18, 23, 33, 36, 39, 47), while others have shown that its effects are dependent on dose and context (19, 22, 41). Indeed, in stressed ECs, one recent report suggests that Ang2 may even activate Tie2 signaling in vivo (8).
The current study was undertaken to further elucidate the actions of Ang2 on ECs. We demonstrate herein that exogenous Ang2 activates Tie2 in ECs subjected to normal culture conditions. Moreover, ECs secrete Ang2, which in turn maintains a basal level of Tie2 phosphorylation. We show this by three specific and complementary ways—RNA interference-induced knockdown of Ang2, depletion of secreted Ang2 with a specific neutralizing antibody, and depletion with soluble ectodomain of Tie2 (sTie2). We further use RNA interference against Ang2 to demonstrate that endogenously produced Ang2 drives basal activation of PI3K-Akt in cultured ECs, mediates the promigration action of ECs in a scratch assay, and prevents EC apoptosis following serum deprivation. Ang2 binds Tie2 with less affinity than Ang1 and is less potent than Ang1 in activating Tie2. Either exogenous Ang2 or Ang1 alone acts as a prosurvival factor, with Ang2 again less potent than Ang1. Surprisingly, when added together, Ang2 inhibits Ang1-induced Tie2 phosphorylation and attenuates Ang1's antiapoptotic effect in a dose-dependent manner. Based on these findings, we conclude that Ang2 possesses both partial agonistic as well as antagonistic action on Tie2 in ECs—alone, Ang2 is a weak but necessary activator of Tie2, whereas in the presence of Ang1, Ang2 inhibits Tie2 signaling.
MATERIALS AND METHODS
Materials.
Chemicals were purchased from Sigma-Aldrich unless otherwise specified. Recombinant human Ang2, Ang1, and sTie2 (soluble ectodomain of Tie2), anti-human Tie1, Tie2, and phospho(p)Tie2 (Y922) antibodies, and human Ang1 and Ang2 enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN). Specific small interfering RNAs (siRNAs) (Ang2-82 and Ang2-84) for human Ang2 and universal negative control siRNAs were purchased from Applied Biosystems (Foster City, CA). Ang2-specific neutralizing antibody, whose avidity and specificity for Ang2 have been previously validated (34), is a gift from Amgen. Anti-Akt and -pAkt antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti-glyceraldehyde-3-phosphate dehydrogenase and p-tyrosine (pTyro) (4G10) antibodies were obtained from Chemicon (Jaffrey, NH). Caspase-3/CPP32 (K-105) and caspase-9/Mch6 (K-118) fluorometric assay kits were purchased from Biovision (Mountain View, CA). A PI3K activity assay kit (K-1000) was purchased from Echelon Biosciences Inc. (Salt Lake City, UT).
EC culture studies.
Human umbilical vein ECs (HUVECs) were purchased from Cascade Biologics, Portland, OR, and cultured in full medium of the EBM-2-MV kit (Lonza, Basel, Switzerland). Cells with fewer than 10 passages were used in the current study. HUVECs were cultured in full medium (FM) until confluent and then stimulated with Ang2 or Ang1 for 30 min (with appropriate concentrations) before cell lysates were prepared as described previously (48). When sTie2 was used to neutralize secreted Ang2 in the culture supernatant, HUVECs were exposed to 1,000 ng/ml sTie2 for 2, 4, or 6 h before preparation of the cells lysates. Ang2-specific (Ang2-82 and Ang2-84) and universal negative control (NC) siRNAs were transfected into 80%-confluent HUVECs at a dose of 50 nmol (incubation time, 2.5 h) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). HUVECs transfected with siRNAs were left in FM for 48 h before cell lysates were prepared or additional experiments were conducted. For Ang2 neutralizing antibody experiments, HUVECs were exposed to antibody at 10 or 20 μg/ml for 1 h before cell lysates were prepared. For apoptosis experiments, HUVECs were exposed to either serum-free EBM2 medium (SFM) or FM for 8 h before cell lysates and conditioned medium were collected for Ang2 ELISA, caspase-3, and/or caspase-9 activity assays.
Tie2 and Tie1 phosphorylation assay.
Tie2 and Tie1 phosphorylation levels were analyzed with immunoprecipitation (IP)-Western blotting as described previously (48). Briefly, cell lysate of equal amounts of protein in 1 ml of radioimmunoprecipitation assay buffer were used for IP. After overnight incubation at 4°C with either anti-human Tie2 or Tie1 (1 to 2 μg/sample) antibodies, the protein-immunoglobulin G (IgG) complexes were pulled down using protein G beads (Dynal Invitrogen, Carlsbad, CA) and subjected to electrophoresis, followed by blotting with anti-pTyro antibody and then anti-Tie2 or -Tie1 thereafter. Reverse IP for measuring of Tie1 or -2 phosphorylation was performed using either normal mouse IgG2b or mouse monoclonal anti-pTyro (4G10, IgG2b) for IP and anti-Tie2 or -Tie1 for Western blotting thereafter (48). The intensities of bands on X-ray films were analyzed using Image-J image analysis package software (NIH, Bethesda, MD).
Binding assays of Ang2 or Ang1 with Tie2 receptor.
One hundred microliters of sTie2 (2.0 μg/ml) in phosphate-buffered saline (PBS) was coated on a 96-well plate overnight at 4°C, and the strips were blocked with 1% bovine serum albumin in PBS for 3 h at room temperature. Ang2 and Ang1 in 100 μl of PBS with appropriate concentrations were added to coated strips and incubated for 2 h at room temperature. The Ang2 or Ang1 captured by sTie2 was detected by sequential incubation of goat anti-Ang2 or goat anti-Ang1 primary antibodies, respectively, and horseradish peroxidase-conjugated anti-goat secondary antibody. The absorbance was measured at 450 nm and plotted against the concentrations of the ligands used to generate binding curves. In competition binding assays, the binding of 500 ng/ml of Ang2 or 100 ng/ml of Ang1 to sTie2 (2.0 μg/ml) immobilized on the solid phase of ELISA strips was performed in the presence or absence of 100 ng/ml Ang1 or 500 ng/ml of Ang2, respectively.
Endothelial migration assay.
HUVECs seeded 48 h earlier on six-well plates were subjected to a 5-mm-wide scratch in each well; the uniform width of each scratch was immediately confirmed by light microscopy. Cells were left in FM for another 3 days, after which photomicrographs were taken to measure the widths of the remaining cell-free gaps. The ratios of cell-free width at day 3/day 0 were calculated and are expressed in arbitrary units, with that of NC-siRNA-exposed cells accepted as being equal to 1.0. Presented results are averaged across six assays per condition.
Matrigel capillary tube formation assay.
Capillary tube-like structures were formed overnight after HUVECs (2.5 × 104 cells in 300 μl full medium) were inoculated in 48-well plate precoated with 100 μl Matrigel (BD Biosciences, Franklin Lakes, NJ) as described previously (48). The images of tubes formed were taken 24 h later. The percentage of area covered by tubes formed is analyzed using the Image-J image analysis package software (NIH, Bethesda, MD).
Caspase-3 and caspase-9 activity assays.
Samples were prepared using the lysis buffer provided with the kits, and samples with equal volumes (50 μl) were used to measure caspase-3 and caspase-9 activities, following the manufacturer's protocol. Protein concentrations were measured using a bicinchoninic acid (BCA) assay (Pierce, Rockford, IL), and fluorometric readings of caspase-3 and caspase-9 activities were adjusted by protein content. Final values of caspase-3 and caspase-9 activities are expressed in arbitrary units, with that of HUVECs in FM accepted as being equal to 100%. The activities reported in SFM are reported as percentages of activity noted in FM.
PI3K activity assay.
PI3K activity is measured by means of measuring the formation of (PI3,4,5)P3 from PI(4,5)P2. Cell lysates were prepared using radioimmunoprecipitation assay buffer containing both protease and phosphatase inhibitors. PI3K in cell lysates was first pulled down using anti-PI3K (p85) by IP. Then, the activity of the purified PI3K was measured using a competitive ELISA in which the signal is inversely proportional to the amount of PI(3,4,5)P3 produced. The PI3K activity is presented as pmol of PI(3,4,5)P3 per mg protein in the original cell lysate.
Phospho- and total-Akt protein assay.
Total and phospho-Akt levels were analyzed by means of Western blotting, utilizing antibodies directed against total and phospho-specific Akt protein as described previously (48).
Statistical analysis.
Values are presented as means ± standard deviations. Means were compared either by using an unpaired Student t test or by one-way analysis of variance followed by a t test. A P value of <0.05 was considered statistically significant.
RESULTS
Exogenous Ang2 induces Tie2 phosphorylation.
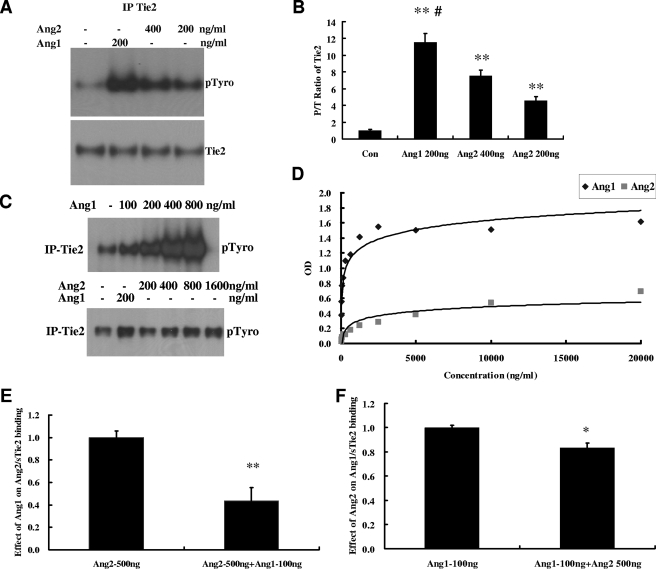
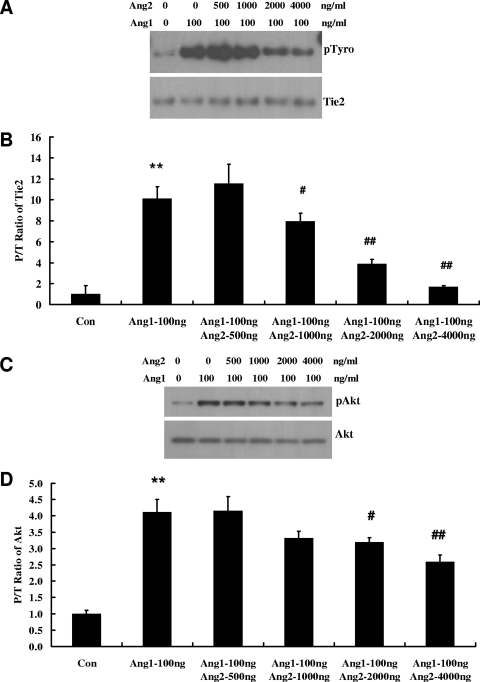
HUVECs under basal conditions had a low level of Tie2 phosphorylation (Fig. 1A). When stimulated for 30 min with 200 or 400 ng/ml of Ang2, a 4.6- or 7.5-fold increase of phospho-Tie2 (pTie2) was induced (Fig. 1A and B). As a positive control, 200 ng/ml of Ang1 induced an 11.5-fold increase of pTie2, significantly greater than that induced by 400 ng/ml of Ang2. Similar data were also obtained when using human dermal microvascular ECs (see Fig. S1 in the supplemental material). Ang1-mediated Tie2 phosphorylation increased in a dose-dependent fashion between 0 and 800 ng/ml; in contrast, Ang2-mediated Tie2 phosphorylation was less prominent and saturated at about 800 ng/ml (Fig. 1C). We then performed binding studies for Ang2 and Ang1 with sTie2 using solid-phase binding assays as described in Materials and Methods. Under these conditions, the concentrations of ligands bound to achieve 50% saturation were 106 ng/ml for Ang1 and 2,018 ng/ml for Ang2 (Fig. 1D). To further demonstrate that Ang1 has a higher binding affinity for sTie2, competition binding assays of Ang1 and Ang2 with sTie2 were performed. In the presence of 100 ng/ml (1/5 of the Ang2 concentration) of Ang1, the binding of 500 ng/ml of Ang2 to sTie2 was reduced by 57% (P < 0.01) compared to that in the absence of Ang1 (Fig. 1E). On the other hand, the binding of 100 ng/ml of Ang1 to Tie2 was reduced by only 17% (P < 0.05) in the presence of 500 ng/ml of Ang2 compared to that in the absence of Ang2 (Fig. 1F). These data suggest that Ang2, compared with Ang1, binds to Tie2 with a lower affinity and that this may account for the weaker activation of the Tie2 receptor.
FIG. 1.
Ang2 binds and induces Tie2 activation in HUVECs. (A) Confluent HUVECs were stimulated with Ang2 and Ang1 at the indicated concentrations for 30 min, and cell lysates were prepared as described above and immunoprecipitated with Tie2. After IP, Western blotting for phosphotyrosine and total Tie2 was performed. Representative blots of three independent experiments are depicted. (B) Phospho/total Tie2 (P/T) ratio for bands in panel A (means ± standard deviations; n = 3). (C) Dose response for Ang1- and Ang2-mediated Tie2 phosphorylation. Shown is a representative blot. (D) Binding curves of Ang1 and Ang2 for sTie2 were performed as described in Materials and Methods. (E) Inhibition of Ang2/Tie2 binding by Ang1. (F) Inhibition of Ang1/Tie2 binding by Ang2.
Endogenous Ang2 is an autocrine agonist of Tie2.
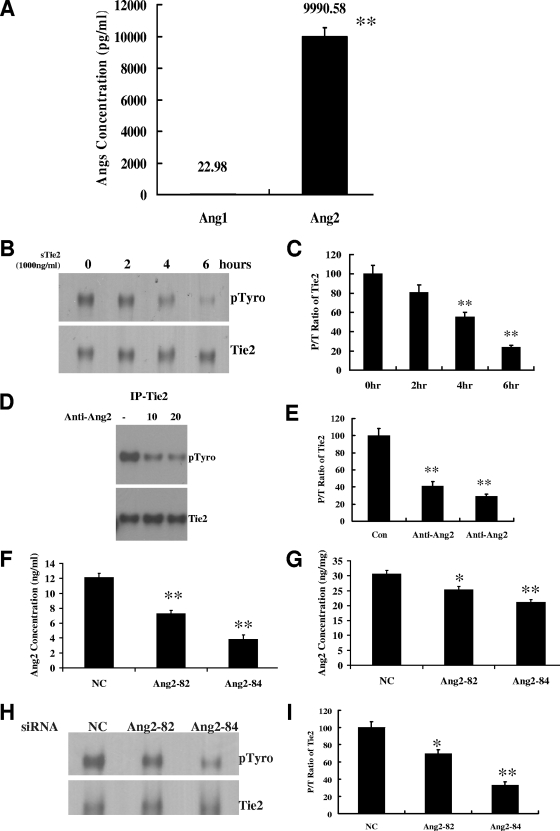
We first determined the endogenous production of Ang2 and Ang1 by HUVECs grown in fresh FM for 24 h. The Ang2 concentration in the 24-h-conditioned medium was 450-fold higher than that of Ang1 (9,990.59 pg/ml versus 22.98 pg/ml; P < 0.01) (Fig. 2A). Since Ang-2 was the dominantly expressed Tie2 ligand in this system, we next used sTie2 to counteract the effect of secreted Ang2. When normal HUVECs were exposed to 1,000 ng/ml of sTie2 for 2, 4, or 6 h, we observed a time-dependent reduction in pTie2 (Fig. 2B and C). To eliminate the possibility of an artifact arising from sTie2 binding of undetected Ang1, we repeated the depletion experiment with a neutralizing antibody that binds Ang2 with 1,000-fold greater avidity than Ang1 (34). After a 1-h incubation, we observed a dose-dependent reduction of pTie2 in those cells (Fig. 2D and E). The ability of this antibody to neutralize Ang2 but not Ang1 was confirmed using exogenous Ang2 (see Fig. S2 in the supplemental material). Finally, we used two specific siRNAs (Ang2-82 and Ang2-84) to knock down Ang2 expression. Both constructs reduced Ang2 concentrations in conditioned medium and cell lysates compared to those for NC-siRNA-treated cells (Fig. 2F and G). The pTie2 levels were significantly lower in both Ang2-82 siRNA-treated (69% of NC-siRNA-treated cells; P < 0.05) and Ang2-84 siRNA-treated (33% of NC-siRNA-treated cells; P < 0.01) HUVECs (Fig. 2H and I). These data suggest that Ang2 secreted by ECs acts in an autocrine fashion to maintain basal Tie2 phosphorylation.
FIG. 2.
Endogenous Ang2 is an autocrine agonistic ligand of Tie2 in endothelial cells. (A) Ang2 and Ang1 concentrations in HUVECs' 24-h-conditioned medium were measured by ELISA (n = 10). (B and C) Soluble (s)Tie2 (1,000 ng/ml) was added to culture medium of confluent HUVECs and incubated for the indicated times. The P/T ratio of Tie2 was analyzed by IP (with anti-Tie2)-Western blotting (anti-pTyro and total Tie2). Representative blots of three independent experiments (B) are depicted, along with the P/T ratio (C) of Tie2 (means ± standard deviations; n = 3). (D and E) The level of pTie2 in HUVECs treated with Ang2 neutralizing (0, 10, or 20 μg/ml) antibody was analyzed by IP (anti-Tie2)-Western blotting (anti-pTyro and total Tie2). Representative blots of three independent experiments are depicted (D) along with the P/T ratio (E) of Tie2 (means ± standard deviations; n = 3). (F and G) Two specific siRNAs for human Ang2 (Ang2-82 and Ang2-84), as well as a universal negative control siRNA (NC siRNA), were transfected into HUVECs, and 48 h later, Ang2 levels in culture supernatant (F) and cell lysates (G) of HUVECs were measured by ELISA (n = 6). (H and I) The level of pTie2 in HUVECs treated with Ang2 siRNAs was analyzed by IP (anti-Tie2)-Western blotting (anti-pTyro and total Tie2). Representative blots of three independent experiments are depicted (H), along with the P/T ratio (I) of Tie2 (means ± standard deviations; n = 3). *, P value of <0.05; **, P value of <0.01.
Endogenous Ang2 activates the PI3K-Akt pathway.
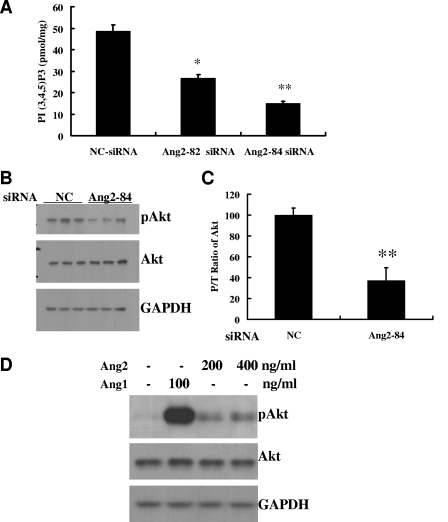
Having observed by three methods that endogenous Ang2 is necessary for basal activation of Tie2 in ECs, we next studied the downstream consequences of this autocrine loop by focusing on the PI3K-Akt signal cascade, a major pathway for transducing the prosurvival and promigratory signals of Tie2 in ECs. A significant reduction of PI3K activity was observed in both Ang2-82-siRNA-treated (45% reduction; P < 0.01) and Ang2-84-siRNA-treated (69% reduction; P < 0.01) HUVECs compared to that in NC-siRNA treated cells (Fig. 3A). Furthermore, the activation of Akt—as measured by comparing phospho- and total Akt (P/T ratio)—was significantly decreased in Ang2-84-siRNA-treated cells (37% of NC-siRNA-treated cells; P < 0.01; n = 3) (Fig. 3B and C). Figure 3D demonstrates that exogenous Ang2 also induces Akt phosphorylation, albeit at a weaker level than that with exogenous Ang1.
FIG. 3.
Ang2/Tie2 signaling activates the PI3K/Akt pathway. (A) PI3K activities of HUVECs treated with Ang2 siRNAs. Forty-eight hours after HUVECs in 10-cm petri dishes were transfected with NC, Ang2-82, or Ang2-84 siRNA, cell lysates were prepared for analysis of PI3K activity as described in Materials and Methods (means ± standard deviations; n = 3). (B and C) Total and phospho-Akt levels of HUVECs treated with Ang2 siRNAs. Phospho-Akt and total Akt were compared in NC and Ang2-84 siRNA-treated HUVECs. Western blots of three independent samples are depicted, along with the P/T ratio of Akt (n = 3). (D) Exogenous Ang2 induces Akt phosphorylation. HUVECs were stimulated with Ang2 at the indicated concentrations for 30 min, and phospho- and total Akt levels were compared. Ang1 (100 ng/ml) was used as a positive control. Glyceraldehyde-3-phosphate dehydrogenase was used as a loading control. *, P value of <0.05; **, P value of <0.01.
Endogenous Ang2 is a prosurvival factor.
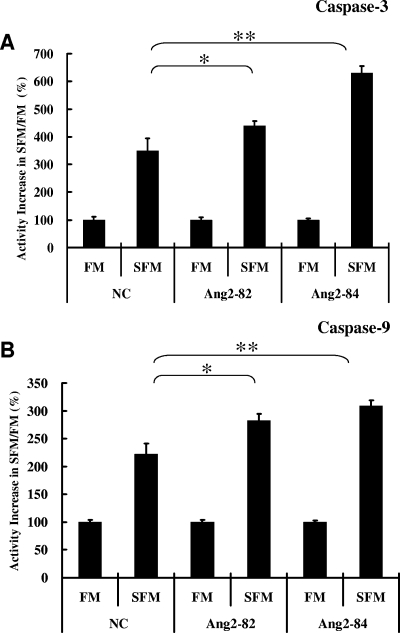
To further assess the biological relevance of Ang2/Tie2 basal signaling in ECs, we studied the effect of this pathway on resistance to serum deprivation-induced apoptosis. In NC-siRNA-treated HUVECs, 8 h of exposure to SFM increased caspase-3 activity 3.5-fold (P < 0.01) over that for the same cells kept in FM, verifying the apoptosis-inducing effect of SFM exposure (Fig. 4A). Caspase-3 activities were even more strongly elevated when comparing Ang2-siRNA-treated cells exposed to SFM with those exposed to FM—4.4-fold for Ang2-82 (P < 0.05) and 6.3-fold for Ang2-84 (P < 0.01) (Fig. 4A). A similar pattern of changes was confirmed by measuring caspase-9 activity under these conditions (Fig. 4B).
FIG. 4.
Caspase-3 and caspase-9 activities in Ang2 siRNA-treated HUVECs under serum deprivation. Caspase-3 (A) or caspase-9 (B) activity was measured using by using a fluorospectrophotometer as described in Materials and Methods. Forty-eight hours after HUVECs in six-well plates were transfected with NC, Ang2-82, or Ang2-84 siRNA, cells were exposed to FM or SFM for eight further hours before cell lysates were prepared and caspase-3 and caspase-9 activities were measured (n = 8). *, P value of <0.05; **, P value of <0.01.
Endogenous Ang2 is a promigration factor.
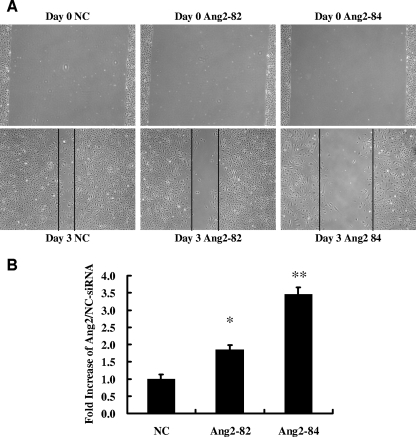
Since Tie2 is known to mediate not only EC survival but also migration responses (1, 3), we tested the role of the autocrine Ang2 pathway in an in vitro migration assay. HUVECs treated with either Ang2-82 siRNA or Ang2-84 siRNA demonstrated significantly less migration than NC-siRNA-treated cells as measured by the width of the cell-free band persisting 3 days after a 5-mm-wide scratch was placed (Fig. 5A and B).
FIG. 5.
Endogenous Ang2 is promigratory for HUVECs. (A) Forty-eight hours after HUVECs in six-well plates were transfected with NC, Ang2-82, or Ang2-84 siRNA, a 5-mm-wide scratch was made in each well and cells were left in FM for another 3 days before the images of cells were taken to measure the width of the remaining cell-free band. (B) The ratios of width at day 3/day 0 were calculated, and that of NC-siRNA-treated cells was expressed as an arbitrary value of 1 (n = 6). *, P value of <0.05; **, P value of <0.01.
Ang2 antagonizes the activity of Ang1 on ECs.
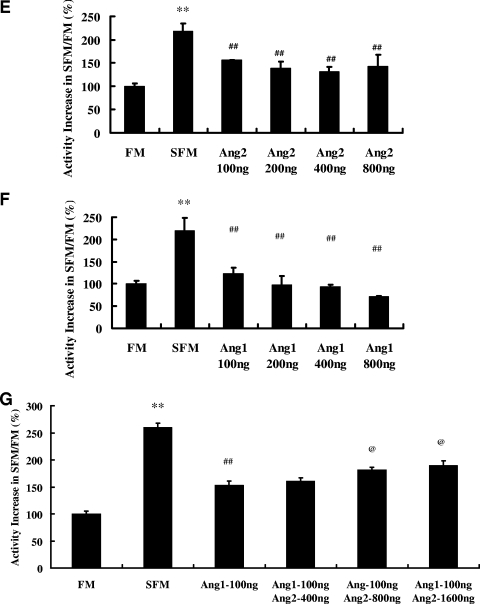
Having established the agonist activity of exogenous and endogenous Ang2 on Tie2 in ECs, we next tested whether this activity would be additive to the known agonist Ang1. One hundred ng/ml of Ang1 induced strong phosphorylation of Tie2, and the addition of increasing concentrations of Ang2 reduced Tie2 phosphorylation in a dose-dependent manner (Fig. 6A and B). Similarly, Ang2 dose-dependently inhibited the Ang1-dependent activation of Akt (Fig. 6C and D). Next, we wished to compare the protection conferred by Tie2 ligands alone or in combination against serum deprivation-induced apoptosis. In SFM, 100, 200, 400, and 800 ng/ml of Ang2 reduced caspase-3 activity 29%, 37%, 39% and 34%, respectively (P < 0.01 for each versus results with SFM without Ang2) (Fig. 6E). By comparison, equal amounts of Ang1 reduced caspase-3 activity 44%, 55%, 57%, and 67% (P < 0.01 for each versus results with SFM without Ang1) (Fig. 6F). Finally, when we treated SFM-exposed HUVECs with 100 ng/ml of Ang1 in combination with increasing concentrations of Ang2, the protective effect of Ang1 in reducing caspase-3 activity was reduced by 20%, 28%, and 45% by 400, 800, and 1,600 ng/ml of Ang2 (P < 0.05 for 800 and 1,600 ng/ml of Ang2 versus results for Ang1 alone) (Fig. 6G). Our data indicate that Ang2 attenuates Ang1-induced Tie2 signaling and that Ang2 dose-dependently antagonizes Ang1-mediated protection from apoptosis.
FIG. 6.
Ang2 antagonizes the activity of Ang1 on HUVECs. (A and B) Ang2 antagonizes Ang1-induced Tie2 phosphorylation. HUVECs in six-well plates were stimulated for 30 min with 100 ng/ml of Ang1 in the absence or presence of increasing concentrations of Ang2. Phospho- and total Tie2 levels were analyzed with IP (anti-Tie2) and Western blotting (pTyro and Tie2). Representative blots of three independent experiments (A) are depicted, along with the P/T ratio (B) of Tie2 (means ± standard deviations; n = 3). (C and D) Ang2-antagonized Ang1 induced Akt phosphorylation. HUVECs in six-well plates were stimulated for 30 min with 100 ng/ml of Ang1 in the absence or presence of increasing concentrations of Ang2. Phospho- and total Akt were compared. Representative blots of three independent experiments (C) are depicted, along with the P/T ratio (D) of Akt (means ± standard deviations; n = 3). (E and F) Ang2 or Ang1 alone attenuates serum deprivation-induced caspase-3 elevation. Forty-eight hours after HUVECs were inoculated into six-well plates, the cells were exposed to FM or SFM for eight further hours in the presence of Ang2 (E) or Ang1 (F) alone at the indicated concentrations before cell lysates were prepared and caspase-3 activities were analyzed (n = 10). **, P value is <0.01 for comparison of results between SFM and FM. #, P value is <0.05; ##, P value is <0.01 for comparison of results between SFM and SFM plus Ang2 or Ang1 conditions. (G) Ang2 antagonizes the activity of Ang1 in reducing caspase-3 activity of HUVECs subjected to serum deprivation. Forty-eight hours after HUVECs were inoculated into six-well plates, the cells were exposed to FM or SFM for eight further hours in the presence of 100 ng/ml of Ang1 and indicated concentrations of Ang2 before lysates were prepared and caspase-3 activities were analyzed (n = 10). **, P value of <0.01 for comparison of results between SFM and FM. ##, P value of <0.01 for comparison of results for cells treated with or without Ang1 alone. @, P value of <0.05 for comparison of results for cells treated with Ang1 plus Ang2 versus those treated with Ang1 alone.
DISCUSSION
Our results strongly support the hypothesis that Ang2 can be either an agonist or an antagonist of the Tie2 receptor, depending upon the context. Specifically, we found that cultured ECs respond to exogenous Ang2 alone by activating the Tie2 receptor. We observed that ECs secrete Ang2 in the conditioned medium and that blockade of this autocrine loop lowers the basal activation of Tie2 and its downstream pathway, PI3K/Akt. Phenotypically, inhibition of this loop results in less resistance to apoptosis and a reduced migratory response. We asked how the agonistic effect of Ang2 compares to that of the more widely accepted naturally occurring Tie2 agonist, Ang1. We found that Ang2 is a less potent activator of Tie2 and that it binds Tie2 with lower affinity than Ang1. Either Ang1 or Ang2 can render ECs resistant to serum deprivation-induced apoptosis, but as expected, Ang1's effect is more potent. Finally, we explored what happens when Ang1 and Ang2 are applied together to ECs. Rather than observing an additive stimulation of Tie2, we found that Ang2 inhibited Ang1-induced Tie2 activation and reduced Ang1-mediated protection against EC apoptosis in a dose-dependent fashion. We conclude, therefore, that cultured ECs rely on self-produced Ang2 for trophic effects but that in the presence of the stronger Tie2 activator Ang1, Ang2 can actually dampen Tie2 signaling. Thus, we propose that Ang2 is a partial agonist/antagonist of Tie2.
The original report describing Ang2 found that it was a naturally occurring antagonist of Tie2 in ECs (28). In contrast, more-recent reports have shown that excess Ang2 can induce Tie2 activation (8, 19, 22, 41). Kim et al. first reported that a high dose of Ang2 (800 ng/ml) could induce Tie2 phosphorylation in HUVECs in vitro and protect HUVECs from serum deprivation-induced apoptosis, probably via the PI3K/Akt pathway. However, no obvious effect was noted when less than 400 ng/ml of Ang2 was applied (22). Ang2-induced Tie2 phosphorylation was then confirmed in HUVECs (>300 ng/ml of Ang2) and in murine brain capillary cells (31, 41). In those studies, Ang2 was reported to promote capillary-like endothelial tube formation in three-dimensional culture systems. Ang2 was found to have less potency than Ang1 in HUVECs (41). Again, the PI3K/Akt pathway was reported to be involved. Our results confirm that Ang2 can activate Tie2 and extend our knowledge about this activity by showing that such activation can occur with lower doses of Ang2 (200 ng/ml) than previously reported and that the activation of Tie2 by Ang2 is not significantly dose responsive above 800 ng/ml.
While several groups have reported that exogenous Ang2 can activate Tie2, we also found that endogenous production of Ang2 is vital to the health of ECs. ECs are known to synthesize, store, and secrete Ang2 but not Ang1 (12). We found that disruption of this autocrine loop by “extracellular” (addition of sTie2 or Ang-2-specific neutralizing antibody to culture medium) or “intracellular” (two different siRNAs against Ang2 transcript) methods reduced Tie2 phosphorylation by 60 to 80%. In addition, the levels of pTie2 in those cells show a close correlation with Ang2 levels in either culture medium or cell lysates (data not shown). ECs unable to engage in this tonic autoactivation developed reduced signaling along the critical PI3K/Akt pathway, were less resistant to apoptosis, and were unable to migrate as well. Furthermore, such cells were markedly impaired in their ability to form tubes (see Fig. S3 in the supplemental material). A similar situation arises in vivo in lymphatic vessels, where Ang1 is lacking due to the absence of periendothelial cells. Lymphatic vessel formation is highly dependent on Ang2/Tie2 signaling, as illustrated by gross lymphatic patterning defects in Ang2 null mice (15).
Two other groups have reported autocrine actions of Ang2 on ECs expressing Tie2 (8, 38). Sharpfenecker et al. (38) found that extracellular inhibition of Ang2 with sTie2 was ineffective at blunting autocrine Ang-2 signaling, leading them to propose an “intracrine” effect of Ang2. However, the results are not directly comparable to ours for two reasons: (a) they never knocked down endogenous Ang2 expression or used Ang2 null ECs, and (b) they were using a coculture system of ECs grown atop vascular smooth muscle cells, a point to which we will return below. On the other hand, Daly et al. (8) found that Ang-2 blocking antibody and Ang2 siRNA were equally capable of disrupting autocrine Ang2-induced Tie2 activation. However, they observed this autocrine Ang2/Tie2 loop only in ECs that were “stressed” either by chemical inhibition of PI3K or by virally mediated overexpression of the transcription factor FOXO1. Our results clearly demonstrate that this loop also exists in and is vitally important to “unstressed” ECs under more typical culture conditions.
In comparing Ang1 and Ang2, we found that both ligands can activate Tie2 but that Ang2's signaling effect is less potent (e.g., 11.5-fold Tie2 activation with 200 ng/ml of Ang1 versus 7.5-fold with 400 ng/ml Ang2). We also observed that Ang2 binds Tie2 with ∼20-fold-lower affinity than does Ang1. Though perhaps not the sole explanation, the lower binding affinity of Ang2 for Tie2 probably does contribute to its diminished potency at the receptor. Mixing experiments did not show an additive effect on Tie2 activity but rather showed that Ang2 dose-dependently inhibited Ang1 signaling. The reduction in Tie2 phosphorylation was biologically relevant, as evidenced by the progressive loss of Ang1-mediated protection from apoptosis as the Ang2 dose was increased. The mixing study is consistent with the results reported for ECs from the original Ang2 article (28). Finally, we found that addition of either Ang1 or Ang2 inhibits the binding of the other ligand to Tie2, suggesting competition between the angiopoietins for the same binding pocket on Tie2. When excess Ang-2 is partially unseated by Ang1, Tie2 phosphorylation rises because Ang1 is the more potent agonist; when Ang1 is unseated by Ang2, Tie2 phosphorylation falls for the same reason. Figure S4 in the supplemental material shows that depletion of endogenous Ang2 by two different siRNAs results in increased Ang1-dependent Tie2 phosphorylation. This finding further supports our chief conclusion that Ang2 acts as a Tie2 antagonist when Ang1 is present.
To our knowledge, this is the first description of an endogenous partial agonist/antagonist for a receptor tyrosine kinase. Small-molecule partial agonists have been described for opioid, adrenergic, among others (11). One non-G-protein-coupled-receptor example of partial agonist compounds is the selective estrogen receptor modulator tamoxifen (2). However, we are aware of no other endogenous multiligand-single-receptor system that provides a precedent for the actions mediated by Ang2. As such, the Ang/Tie2 system offers investigators a novel opportunity to study the endogenous mechanisms that modulate signal transduction through receptor tyrosine kinases.
Ang2 may be a “dummy” ligand that weakly activates Tie2, but in high enough concentrations, it can “unseat” local Ang1 from its receptor. Others have repeatedly shown that Ang1 is highly multimerized in its native form whereas Ang2 is less aggregated (4, 25). The multimerization of Ang1 may account for its ability to strongly activate Tie2 (25). Ang1 aggregates would theoretically have reduced entropy for binding Tie2 monomers, since the binding of any one Ang1 molecule to any one Tie2 favors the binding of additional noncovalently tethered Ang1 molecules to nearby Tie2 monomers. In this way, Ang1 aggregates would favor the clustering and cross-phosphorylation of cell surface Tie2. An excess of Ang2 in the presence of Ang1 could disrupt Ang1-Tie2 clusters and favor the formation of lower-order multimers—e.g., dimers and trimers—of Ang2-Tie2, resulting in some degree of receptor activation but significantly less than that achieved by large clusters of Tie2 induced by comparable concentrations of Ang1. In this fashion, Ang2 could antagonize Ang1 signaling. However, in a system devoid of Ang1, Ang2 would weakly activate Tie2.
Close inspection of our results also suggests that differences between Ang1/Tie2 and Ang2/Tie2 signaling may exist not only at the level of the receptor but also in the strength of the downstream signal. Whereas 400 ng/ml of Ang2 induces Tie2 phosphorylation to ∼65% of 200 ng/ml of Ang1 (Fig. 1B), the effect of the same doses on Akt phosphorylation is significantly less (Fig. 3D). This suggests not only that Ang2 is a lower-affinity binder of Tie2 with a less-potent phosphorylation effect at the receptor but that postreceptor signaling is also attenuated compared to Ang1. While the reason for this is unclear and merits further investigation, several possibilities exist. First, our results do not exclude the possibility that Ang2 and Ang1 promote phosphorylation of different sets of tyrosine residues in the cytoplasmic tail of Tie2. For example, tyrosine 1100 is required for the recruitment of the SH2-domain-containing p85 subunit of PI3K (21, 26). Interestingly, treatment with PI3K inhibitors only partially abrogates the Ang1-signaled migratory response (21), suggesting the involvement of other tyrosines in this response, such as tyrosine 1106, which may recruit Dok-R and activate intracellular GTPases that favor migration (20). A second possibility is that the enhanced clustering of Tie2 monomers by Ang1 versus Ang2 may increase the cell surface localization of PI3K (10). The enzymatic components of this cascade will tend to amplify small upstream differences as signals are transduced downstream, which could explain how a small difference in Tie2 activation leads to progressively larger differences in PI3K and Akt activation. Last, Ang1 is known to have at least one non-Tie2 receptor (6, 7). We cannot rule out that coreceptors on HUVECs are partly responsible for differences in signal transduction efficiency.
Ang1 is made primarily by periendothelial cells (i.e., pericytes and vascular smooth muscle cells), whereas Ang2 is synthesized by ECs. Given that Ang1 is more matrix bound than Ang2 and that circulating Ang2 concentrations are generally low (<2 ng/ml) in unperturbed individuals (17, 36), one could expect a high degree of basal Tie2 activation. This is indeed the case in organs throughout the body (44, 46). In the Ang1-rich in vivo setting of blood vessels, local increases in Ang2 could precisely attenuate the tonic activity of Tie2. The ability to carefully decrease Tie2 activation would be beneficial in certain settings—i.e., to destabilize formed vessels, to promote local inflammation, or to permit a local increase in vascular permeability.
The finely tuned balance of endogenous Tie2 ligands may be upset in diseases like cancer and sepsis. Solid tumors can strongly upregulate the production of Ang2, in part through the induction of hypoxia-inducible factors in the tumor cells (32, 37, 39). Extra Ang2 may contribute to local downregulation of Tie2 activity. In turn, this could enable the necessary destabilization of blood vessels that initiates tumor angiogenesis. In fact, a compound related to the Ang2 neutralizing antibody used in this report also possesses potent antiangiogenic and antitumor effects in murine xenograft models (34).
In sepsis—a syndrome of disseminated infection that results in several vascular changes, including increased permeability and endothelial inflammation—we and others have found high levels (20 to 200 ng/ml) of circulating Ang2 (17, 35, 36) and have observed relative Tie2 inhibition in animal models of sepsis (unpublished results), implying that the rise in Ang2 is antagonizing local Ang1/Tie2 signaling. The observation that heavily supraphysiological circulating levels of Ang2 in vivo (∼10 μg/ml) can also activate Tie2 (8) highlights the importance of regulating Ang1 and Ang2 within a narrow range. One implication of the present work is that reduction of circulating Ang2 (e.g., by a neutralizing antibody) in a disease such as sepsis would not be expected to have as positive an impact on Tie2 activity as administration of excess Ang1.
This study has several limitations. Our data suggesting an agonist role for Ang2 are derived from in vitro studies in which Ang1 expression was absent or barely detectable. Future studies using coculture of ECs with vascular smooth muscle cells or an endothelial cell-specific knockout of Ang2 in mice should further clarify the role of Ang2 in the maintenance of vascular homeostasis. Second, the biochemistry underlying the ability of a ligand to act as both an agonist and an antagonist merits further investigation. Third, additional cell biological responses in Ang1-Ang2 mixing experiments should be measured, such as changes in monolayer permeability or surface expression of inflammatory adhesion molecules. Related to this, Ang1 and Ang2 may activate distinct postreceptor signaling pathways within ECs. Finally, coreceptors or other angiopoietin receptors, such as Tie1, may be involved in the modulation of signals through Tie2 (48).
In conclusion, using one model system, we have found that Ang2 can act as either a weak Tie2 agonist or a dose-dependent Tie2 antagonist. To our knowledge, this is the first description of an endogenous partial agonist/antagonist system targeted to one receptor. Ang2 is an agonist only in the absence of Ang1, and Ang1-deprived ECs rely on autocrine Ang2 stimulation for cell survival and an appropriate migratory response. Ang2 binds Tie2 with less affinity than Ang1, and the latter is a more potent activator of Tie2. When they are combined, Ang2 inhibits Ang1 signaling and downstream effects, thus becoming an antagonist. These findings help reconcile divergent reports on the biological actions of Ang2 and have significant implications for the design of therapies targeted to the angiopoietin-Tie2 pathway.
Supplementary Material
Acknowledgments
H.T.Y. is funded by an Alaska Kidney Foundation-ASN research grant, and S.M.P. is funded by an NIH K08, DK069316, and R01 HL093234. S.A.K. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 17 February 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abdel-Malak, N. A., C. B. Srikant, A. S. Kristof, S. A. Magder, J. A. Di Battista, and S. N. Hussain. 2008. Angiopoietin-1 promotes endothelial cell proliferation and migration through AP-1-dependent autocrine production of interleukin-8. Blood 1114145-4154. [DOI] [PubMed] [Google Scholar]
- 2.Barkhem, T., B. Carlsson, Y. Nilsson, E. Enmark, J. Gustafsson, and S. Nilsson. 1998. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol. Pharmacol. 54105-112. [DOI] [PubMed] [Google Scholar]
- 3.Chen, J. X., M. L. Lawrence, G. Cunningham, B. W. Christman, and B. Meyrick. 2004. HSP90 and Akt modulate Ang-1-induced angiogenesis via NO in coronary artery endothelium. J. Appl. Physiol. 96612-620. [DOI] [PubMed] [Google Scholar]
- 4.Cho, C. H., K. E. Kim, J. Byun, H. S. Jang, D. K. Kim, P. Baluk, F. Baffert, G. M. Lee, N. Mochizuki, J. Kim, B. H. Jeon, D. M. McDonald, and G. Y. Koh. 2005. Long-term and sustained COMP-Ang1 induces long-lasting vascular enlargement and enhanced blood flow. Circ. Res. 9786-94. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, B., D. Barkan, Y. Levy, I. Goldberg, E. Fridman, J. Kopolovic, and M. Rubinstein. 2001. Leptin induces angiopoietin-2 expression in adipose tissues. J. Biol. Chem. 2767697-7700. [DOI] [PubMed] [Google Scholar]
- 6.Dallabrida, S. M., N. Ismail, J. R. Oberle, B. E. Himes, and M. A. Rupnick. 2005. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ. Res. 96e8-e24. [DOI] [PubMed] [Google Scholar]
- 7.Dallabrida, S. M., N. S. Ismail, E. A. Pravda, E. M. Parodi, R. Dickie, E. M. Durand, J. Lai, F. Cassiola, R. A. Rogers, and M. A. Rupnick. 2008. Integrin binding angiopoietin-1 monomers reduce cardiac hypertrophy. FASEB J. 223010-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly, C., E. Pasnikowski, E. Burova, V. Wong, T. H. Aldrich, J. Griffiths, E. Ioffe, T. J. Daly, J. P. Fandl, N. Papadopoulos, D. M. McDonald, G. Thurston, G. D. Yancopoulos, and J. S. Rudge. 2006. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc. Natl. Acad. Sci. USA 10315491-15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, S., T. H. Aldrich, P. F. Jones, A. Acheson, D. L. Compton, V. Jain, T. E. Ryan, J. Bruno, C. Radziejewski, P. C. Maisonpierre, and G. D. Yancopoulos. 1996. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 871161-1169. [DOI] [PubMed] [Google Scholar]
- 10.Davis, S., N. Papadopoulos, T. H. Aldrich, P. C. Maisonpierre, T. Huang, L. Kovac, A. Xu, R. Leidich, E. Radziejewska, A. Rafique, J. Goldberg, V. Jain, K. Bailey, M. Karow, J. Fandl, S. J. Samuelsson, E. Ioffe, J. S. Rudge, T. J. Daly, C. Radziejewski, and G. D. Yancopoulos. 2003. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat. Struct. Biol. 1038-44. [DOI] [PubMed] [Google Scholar]
- 11.Dum, J. E., and A. Herz. 1981. In vivo receptor binding of the opiate partial agonist, buprenorphine, correlated with its agonistic and antagonistic actions. Br. J. Pharmacol. 74627-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiedler, U., M. Scharpfenecker, S. Koidl, A. Hegen, V. Grunow, J. M. Schmidt, W. Kriz, G. Thurston, and H. G. Augustin. 2004. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 1034150-4156. [DOI] [PubMed] [Google Scholar]
- 13.Fujikawa, K., I. de Aos Scherpenseel, S. K. Jain, E. Presman, R. A. Christensen, and L. Varticovski. 1999. Role of PI 3-kinase in angiopoietin-1-mediated migration and attachment-dependent survival of endothelial cells. Exp. Cell Res. 253663-672. [DOI] [PubMed] [Google Scholar]
- 14.Gale, N. W., G. Thurston, S. Davis, S. J. Wiegand, J. Holash, J. S. Rudge, and G. D. Yancopoulos. 2002. Complementary and coordinated roles of the VEGFs and angiopoietins during normal and pathologic vascular formation. Cold Spring Harb. Symp. Quant. Biol. 67267-273. [DOI] [PubMed] [Google Scholar]
- 15.Gale, N. W., G. Thurston, S. F. Hackett, R. Renard, Q. Wang, J. McClain, C. Martin, C. Witte, M. H. Witte, D. Jackson, C. Suri, P. A. Campochiaro, S. J. Wiegand, and G. D. Yancopoulos. 2002. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell 3411-423. [DOI] [PubMed] [Google Scholar]
- 16.Gamble, J. R., J. Drew, L. Trezise, A. Underwood, M. Parsons, L. Kasminkas, J. Rudge, G. Yancopoulos, and M. A. Vadas. 2000. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ. Res. 87603-607. [DOI] [PubMed] [Google Scholar]
- 17.Giuliano, J. S., Jr., P. M. Lahni, M. T. Bigham, P. B. Manning, D. P. Nelson, H. R. Wong, and D. S. Wheeler. 2008. Plasma angiopoietin-2 levels increase in children following cardiopulmonary bypass. Intensive Care Med. 341851-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammes, H. P., J. Lin, P. Wagner, Y. Feng, F. Vom Hagen, T. Krzizok, O. Renner, G. Breier, M. Brownlee, and U. Deutsch. 2004. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 531104-1110. [DOI] [PubMed] [Google Scholar]
- 19.Harfouche, R., and S. N. Hussain. 2006. Signaling and regulation of endothelial cell survival by angiopoietin-2. Am. J. Physiol. Heart Circ. Physiol. 291H1635-H1645. [DOI] [PubMed] [Google Scholar]
- 20.Jones, N., S. H. Chen, C. Sturk, Z. Master, J. Tran, R. S. Kerbel, and D. J. Dumont. 2003. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function. Mol. Cell. Biol. 232658-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, N., Z. Master, J. Jones, D. Bouchard, Y. Gunji, H. Sasaki, R. Daly, K. Alitalo, and D. J. Dumont. 1999. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J. Biol. Chem. 27430896-30905. [DOI] [PubMed] [Google Scholar]
- 22.Kim, I., J. H. Kim, S. O. Moon, H. J. Kwak, N. G. Kim, and G. Y. Koh. 2000. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene 194549-4552. [DOI] [PubMed] [Google Scholar]
- 23.Kim, I., J. H. Kim, Y. S. Ryu, M. Liu, and G. Y. Koh. 2000. Tumor necrosis factor-alpha upregulates angiopoietin-2 in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 269361-365. [DOI] [PubMed] [Google Scholar]
- 24.Kim, I., S. O. Moon, S. K. Park, S. W. Chae, and G. Y. Koh. 2001. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ. Res. 89477-479. [DOI] [PubMed] [Google Scholar]
- 25.Kim, K. T., H. H. Choi, M. O. Steinmetz, B. Maco, R. A. Kammerer, S. Y. Ahn, H. Z. Kim, G. M. Lee, and G. Y. Koh. 2005. Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. J. Biol. Chem. 28020126-20131. [DOI] [PubMed] [Google Scholar]
- 26.Kontos, C. D., T. P. Stauffer, W. P. Yang, J. D. York, L. Huang, M. A. Blanar, T. Meyer, and K. G. Peters. 1998. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol. Cell. Biol. 184131-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, H. J., S. W. Bae, G. Y. Koh, and Y. S. Lee. 2008. COMP-Ang1, angiopoietin-1 variant protects radiation-induced bone marrow damage in C57BL/6 mice. J. Radiat. Res. (Tokyo) 49313-320. [DOI] [PubMed] [Google Scholar]
- 28.Maisonpierre, P. C., C. Suri, P. F. Jones, S. Bartunkova, S. J. Wiegand, C. Radziejewski, D. Compton, J. McClain, T. H. Aldrich, N. Papadopoulos, T. J. Daly, S. Davis, T. N. Sato, and G. D. Yancopoulos. 1997. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 27755-60. [DOI] [PubMed] [Google Scholar]
- 29.Makinde, T., and D. K. Agrawal. 2008. Intra and extravascular transmembrane signalling of angiopoietin-1-Tie2 receptor in health and disease. J. Cell Mol. Med. 12810-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mammoto, T., S. M. Parikh, A. Mammoto, D. Gallagher, B. Chan, G. Mostoslavsky, D. E. Ingber, and V. P. Sukhatme. 2007. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J. Biol. Chem. 28223910-23918. [DOI] [PubMed] [Google Scholar]
- 31.Mochizuki, Y., T. Nakamura, H. Kanetake, and S. Kanda. 2002. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J. Cell Sci. 115175-183. [DOI] [PubMed] [Google Scholar]
- 32.Moon, W. S., K. H. Rhyu, M. J. Kang, D. G. Lee, H. C. Yu, J. H. Yeum, G. Y. Koh, and A. S. Tarnawski. 2003. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Mod. Pathol. 16552-557. [DOI] [PubMed] [Google Scholar]
- 33.Oh, H., H. Takagi, K. Suzuma, A. Otani, M. Matsumura, and Y. Honda. 1999. Hypoxia and vascular endothelial growth factor selectively up-regulate angiopoietin-2 in bovine microvascular endothelial cells. J. Biol. Chem. 27415732-15739. [DOI] [PubMed] [Google Scholar]
- 34.Oliner, J., H. Min, J. Leal, D. Yu, S. Rao, E. You, X. Tang, H. Kim, S. Meyer, S. J. Han, N. Hawkins, R. Rosenfeld, E. Davy, K. Graham, F. Jacobsen, S. Stevenson, J. Ho, Q. Chen, T. Hartmann, M. Michaels, M. Kelley, L. Li, K. Sitney, F. Martin, J. R. Sun, N. Zhang, J. Lu, J. Estrada, R. Kumar, A. Coxon, S. Kaufman, J. Pretorius, S. Scully, R. Cattley, M. Payton, S. Coats, L. Nguyen, B. Desilva, A. Ndifor, I. Hayward, R. Radinsky, T. Boone, and R. Kendall. 2004. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell 6507-516. [DOI] [PubMed] [Google Scholar]
- 35.Orfanos, S. E., A. Kotanidou, C. Glynos, C. Athanasiou, S. Tsigkos, I. Dimopoulou, C. Sotiropoulou, S. Zakynthinos, A. Armaganidis, A. Papapetropoulos, and C. Roussos. 2007. Angiopoietin-2 is increased in severe sepsis: correlation with inflammatory mediators. Crit. Care Med. 35199-206. [DOI] [PubMed] [Google Scholar]
- 36.Parikh, S. M., T. Mammoto, A. Schultz, H. T. Yuan, D. Christiani, S. A. Karumanchi, and V. P. Sukhatme. 2006. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 3e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pichiule, P., J. C. Chavez, and J. C. LaManna. 2004. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J. Biol. Chem. 27912171-12180. [DOI] [PubMed] [Google Scholar]
- 38.Scharpfenecker, M., U. Fiedler, Y. Reiss, and H. G. Augustin. 2005. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J. Cell Sci. 118771-780. [DOI] [PubMed] [Google Scholar]
- 39.Sfiligoi, C., A. de Luca, I. Cascone, V. Sorbello, L. Fuso, R. Ponzone, N. Biglia, E. Audero, R. Arisio, F. Bussolino, P. Sismondi, and M. De Bortoli. 2003. Angiopoietin-2 expression in breast cancer correlates with lymph node invasion and short survival. Int. J. Cancer 103466-474. [DOI] [PubMed] [Google Scholar]
- 40.Suri, C., P. F. Jones, S. Patan, S. Bartunkova, P. C. Maisonpierre, S. Davis, T. N. Sato, and G. D. Yancopoulos. 1996. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 871171-1180. [DOI] [PubMed] [Google Scholar]
- 41.Teichert-Kuliszewska, K., P. C. Maisonpierre, N. Jones, A. I. Campbell, Z. Master, M. P. Bendeck, K. Alitalo, D. J. Dumont, G. D. Yancopoulos, and D. J. Stewart. 2001. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc. Res. 49659-670. [DOI] [PubMed] [Google Scholar]
- 42.Thurston, G. 2003. Role of angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 31461-68. [DOI] [PubMed] [Google Scholar]
- 43.Thurston, G., C. Suri, K. Smith, J. McClain, T. N. Sato, G. D. Yancopoulos, and D. M. McDonald. 1999. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 2862511-2514. [DOI] [PubMed] [Google Scholar]
- 44.Wong, A. L., Z. A. Haroon, S. Werner, M. W. Dewhirst, C. S. Greenberg, and K. G. Peters. 1997. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ. Res. 81567-574. [DOI] [PubMed] [Google Scholar]
- 45.Yancopoulos, G. D., S. Davis, N. W. Gale, J. S. Rudge, S. J. Wiegand, and J. Holash. 2000. Vascular-specific growth factors and blood vessel formation. Nature 407242-248. [DOI] [PubMed] [Google Scholar]
- 46.Yuan, H. T., C. Suri, G. D. Yancopoulos, and A. S. Woolf. 1999. Expression of angiopoietin-1, angiopoietin-2, and the Tie-2 receptor tyrosine kinase during mouse kidney maturation. J. Am. Soc. Nephrol. 101722-1736. [DOI] [PubMed] [Google Scholar]
- 47.Yuan, H. T., P. G. Tipping, X. Z. Li, D. A. Long, and A. S. Woolf. 2002. Angiopoietin correlates with glomerular capillary loss in anti-glomerular basement membrane glomerulonephritis. Kidney Int. 612078-2089. [DOI] [PubMed] [Google Scholar]
- 48.Yuan, H. T., S. Venkatesha, B. Chan, U. Deutsch, T. Mammoto, V. P. Sukhatme, A. S. Woolf, and S. A. Karumanchi. 2007. Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J. 213171-3183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.