Abstract
Apurinic/apyrimidinic endonuclease 1 (Ape1/Ref-1) dysregulation has been identified in several human tumors and in patients with a variety of neurodegenerative diseases. However, the function of Ape1/Ref-1 is unclear. We show here that Ape1/Ref-1 increases the expression of glial cell-derived neurotropic factor (GDNF) receptor α1 (GFRα1), a key receptor for GDNF. Expression of Ape1/Ref-1 led to an increase in the GDNF responsiveness in human fibroblast. Ape1/Ref-1 induced GFRα1 transcription through enhanced binding of NF-κB complexes to the GFRα1 promoter. GFRα1 levels correlate proportionally with Ape1/Ref-1 in cancer cells. The knockdown of endogenous Ape1/Ref-1 in pancreatic cancer cells markedly suppressed GFRα1 expression and invasion in response to GNDF, while overexpression of GFRα1 restored invasion. In neuronal cells, the Ape1/Ref-1-mediated increase in GDNF responsiveness not only stimulated neurite outgrowth but also protected the cells from β-amyloid peptide and oxidative stress. Our results show that Ape1/Ref-1 is a novel physiological regulator of GDNF responsiveness, and they also suggest that Ape1/Ref-1-induced GFRα1 expression may play important roles in pancreatic cancer progression and neuronal cell survival.
The major human apurinic and apyrimidinic (AP) endonuclease 1 Ape1 (also known as Redox factor 1 [Ref-1]), which is homologous to Escherichia coli exonuclease III, plays a key role in both short-patch and long-patch base excision repair (20, 40). It cleaves the AP sites in DNA and allows them to be repaired by other enzymes involved in base excision repair (16, 23, 24). The AP sites can be formed by chemical hydrolysis, the oxygen metabolism, ionizing radiation, UV irradiation, alkylating agents, or oxidizing agents (16, 53). In addition, AP sites can also arise spontaneously, where it has been estimated that 20,000 purine samples and 500 pyrimidines are lost in each 24-h cell cycle in human cells (52). The presence of AP sites blocks DNA replication, leading to DNA breakage, mutagenicity, and cytotoxicity. Ape1/Ref-1 contributes to more than 95% of the total cellular AP site-specific activity (12), which is consistent with Ape1/Ref-1 being essential for maintaining the genomic stability.
Ape1/Ref-1 is a multifunctional protein that is not only responsible for the repair of AP sites but also stimulates the DNA-binding activity of the AP-1 family of transcription factors via a redox-dependent mechanism (1, 96). This effect is mediated via the reduction of a conserved cysteine residue located at the DNA-binding domains of c-fos and c-jun (97). Ape1/Ref-1 is also capable of modulating or activating other classes of transcription factors, including NF-κB, p53, Egr-1, c-Myb, HLF, and Pax-8, via a similar reducing action (20). The ability of Ape1/Ref-1 to activate the transcription factors involved in the cellular response to various stresses suggests that Ape1/Ref-1 may play an important role in various cellular processes.
Ape1/Ref-1 has been implicated in the protection against cell death resulting from various toxic stimuli. The reduction of Ape1/Ref-1 has been reported to sensitizing the cells against oxidative DNA damage (54, 91). Consistently, Ape1/Ref-1 overexpression provokes an increase in resistance to some alkylating agents and oxidative stress (32, 35, 73). Studies have reported elevated Ape1/Ref-1 levels or altered subcellular localization in various types of cancers, such as epithelial ovarian cancers, cervical cancers, prostate cell tumors, melanoma, gliomas, rhabdomyosarcoma, and germ cell tumors, which are associated with tumor resistance and progression (7, 21, 22, 43, 74, 99, 100). Ape1/Ref-1 is highly expressed in selected regions of the central nervous system (68, 95). A reduction in Ape1/Ref-1 expression occurs in the hippocampus after hypoxic-ischemic injury (93), in the cortex after compression injury (49), and in the spinal cord after ischemia (76). In addition, alterations in Ape1/Ref-1 expression and mutations in the Ape1/Ref-1 gene have been detected in patients with a variety of neurodegenerative diseases (17, 66, 83). Thus, Ape1/Ref-1 has been implicated in tumor progression, and Ape1/Ref-1 dysfunction may contribute to development of neurodegenerative disease. However, the molecular mechanisms underlying these effects are unclear.
In the present study, we sought to determine which genes are regulated by Ape1/Ref-1, particularly those that might be involved in cancer progression and neuronal survival, using annealing control primer (ACP)-based reverse transcription-PCR (RT-PCR) analysis. Here, we report that an Ape1/Ref-1 target gene, glial cell-derived neurotropic factor (GDNF) receptor α1 (GFRα1), which were identified through this screening, contributes to the Ape1/Ref-1-mediated increase in cancer cell invasion, neuronal differentiation, and survival.
MATERIALS AND METHODS
Reagents and cell culture.
H2O2 and GDNF were purchased from Sigma (St. Louis, MO). β-Amyloid peptide (peptide 1-42) was kindly provided by I. S. Park (Chosun University). GM00637 (human fibroblast), Neuro2a (mouse neuroblastoma), and SK-N-SH (human neuroblastoma) cells were cultured in Eagle minimum essential medium containing 10% fetal bovine serum (FBS). MIA PaCa-2 and PANC-1 (human pancreatic cancer) cells were grown in Dulbecco modified Eagle medium containing 10% FBS. PC-3 (human prostatic cancer), BxPC3, and CAPAN-2 (human pancreatic cancer) were maintained in RPMI 1640 (Gibco, Grand Island, NY) containing 10% FBS, as were KM12SM (human colon cancer) cells. DMS53 (human small-cell lung cancer) cells were grown in Waymouth's medium (Life Technologies, Rockville, MD) supplemented with 10% FBS. All cells were maintained in cell-specific media at 37°C in a humidified atmosphere of 5% CO2. All cell lines except GM00637 was obtained from the American Type Culture Collection (ATCC; Rockville, MD). The GM00637 cell line was obtained from the Cornell Institute for Medical Research. SN4741 was provided by H. S. Jeon (Chosun University).
Western blotting and immunoprecipitation.
Western blot analysis was performed as described previously (103) using the following antibodies: Ret (sc-167G), c-Src (sc-8056), Ape1/Ref-1 (sc-13104), Pro MM9 (sc-6840), and β-actin (sc-47778; Santa Cruz Biotechnology, Santa Cruz, CA); Ret (Tyr905) (catalog no. 3221) and p-Src (Tyr416) (catalog no. 2101; Cell Signaling Technology, Danvers, MA); and human GFRα-1 (AF714) and rat GFRα1 (MAB560; R&D Systems, Minneapolis, MN). For immunoprecipitation, cells were harvested 10 min after GDNF treatment (30 ng/ml) and washed with ice-cold phosphate-buffered saline before being lysed with lysis buffer (50 mM Tris-HCl [pH 8.0], 1 mM EDTA, 150 mM NaCl, 0.5% sodium deoxycholate, 0.02% sodium azide, 1 mM NaF, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 1% Nonidet P-40, 1 mM dithiothreitol, 0.1% sodium dodecyl sulfate [SDS], 2 μg of pepstatin/ml, 2 μg of leupeptin/ml, 2 μg of aprotinin/ml). After incubation for 1 h at 4°C, cellular debris was removed by centrifugation at 14,000 × g for 30 min. The cell lysates (1 mg of protein) were immunoprecipitated using anti-Ret antibodies and protein G-agarose beads (Santa Cruz Biotechnology) for 6 h at 4°C. The beads were then washed extensively with lysis buffer, boiled in SDS sample buffer, fractionated by SDS-polyacrylamide gel electrophoresis (PAGE), and analyzed by Western blotting with anti-phospho-Ret, anti-GFRα1, anti-c-Src, or anti-Ret antibodies.
Plasmid construction.
Human Ape1/Ref-1 cDNA was amplified by RT-PCR using Ape1/Ref-1-specific primers (5′-TCTAGAATGCCGAAGCGTGGGAAAAAGG-3′ and 5′-GGTACCTCACAGTG CTAGGTATAGGGTG-3′) from human GM00637 fibroblasts. The AP endonuclease domain deletion mutant ΔAPD was amplified by RT-PCR using specific primers (forward [5′-GGATCCATGCCGAAGCGTGGGAAAAAG-3′], reverse [5′-GAATTCTCATGAACATTTGGTCTCTTGAAGGCAC-3′]) from full-length human Ape1/Ref-1 cDNA. Redox domain deletion mutant ΔRD was amplified by RT-PCR using specific primers (forward [5′-GGATCCATGGAGAACAAACTACCAGCTGAACT-3′], reverse [5′-GAATTCTCACAGTGCTAGGTATAGGGTGA-3′]) from full-length human Ape1/Ref-1 cDNA. The sequence of each construct was confirmed by cycle sequencing using a Genomelab GeXP system (Beckman Coulter, Inc., Fullerton, CA). The amplified cDNA was then cloned into a pcDNA3 mammalian expression vector (Invitrogen, Carlsbad, CA) and a pShuttle vector (Invitrogen). The pShuttle-hApe1/Ref-11 construct was then doubly digested with PI-SceI/I-CeuI, and the purified product was ligated by using Adeno-X DNA. The DNA was subsequently linearized with PacI and purified before transfection into HEK293 cells by using Lipofectamine (Invitrogen). After transduction, HEK293 cell layers were overlaid with agarose and assessed for viral plaque formation 10 days later. For virus collection, the cells were lysed by three consecutive freeze-thaw cycles, and virus particles were collected from the supernatant. The virus titer was ∼107 PFU/ml, which was determined by using an endpoint dilution assay. Adeno-X-LacZ adenovirus (Clontech, Mountain View, CA) was used as a control. The transduction efficiency was tested by in situ X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining, and infection with Ad-LacZ at a multiplicity of infection of 50 to 100 resulted in 90 to 100% of the cells testing positive in GM00637 human fibroblast cells.
Human GFRα1 cDNA was amplified by RT-PCR using GFRα1-specific primers (5′-AAGGAAATAACCACCATGTTCCTGGCGACCCTGTAC-3′ and 5′-TGATGTTTCTGTTAAAGATAATAGGGTGGA-3′) from Ape1/Ref-1-transfected GM00637 cells. Green fluorescent protein (GFP) cDNA was amplified by RT-PCR using GFP-specific primers (forward [5′-ATG GTG AGC AAG GGC GAG GAG]-3′, reverse [5′-CTT GTA CAG CTC GTC CAT GCC G-3′]) from pEGFP-N3 (Clontech). The GFRα1 and GFP cDNAs were subcloned into pCR8GW/TOPO (K2500-20; Invitrogen) after sequencing. LR recombination reactions using pLenti6/UbC/V5-DEST (V499-10; Invitrogen), viral packaging using 293FT cells, and titration of the full lentiviral vector were performed by using the Invitrogen Gateway System and ViraPower Lentiviral Expression System. The presence of GFP and GFRα1 was confirmed by PCR, and correct insertion of the clone was further confirmed by sequencing. Lentiviral transduction, expression, and titration were performed as specified by Invitrogen using HT-1080 cells (CCL-121; ATCC). The packed virus was concentrated by ultracentrifugation (20,000 × g for 2 h at 4°C) using Centricon filters (YM-50000; Millipore, Billerica, MA).
GFRα1 promoter constructs.
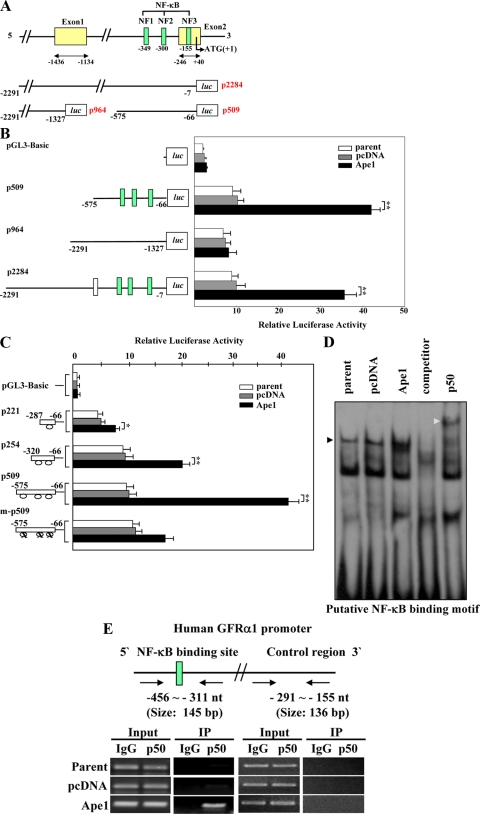
Genomic DNA from human fibroblast GM00637 cells was prepared by using a Puregene DNA purification system (Gentra Systems, Inc., Minneapolis, MN) for a GFRα1 promoter region search (National Center for Biotechnology Information, AC005872). Fragments corresponding to the GFRα1 upstream sequence were amplified by PCR using the above DNA as a template. Each amplified fragment was then cloned into KpnI/XhoI-digested pGL3-Basic (Promega) for construction of the luciferase reporter vector. The p2284 GFRα1 promoter construct encompassed nucleotides −2291 to −7. The p964 GFRα1 promoter construct contained nucleotides −2291 to −1,327, which includes the GAGA box and part of Exon 2. The p509 GFRα1 promoter construct contained nucleotides −575 to −66, which includes three putative NF-κB-binding sites and part of exon 1. The p221 GFRα1 promoter construct contained nucleotides −287 to −66, which includes one putative NF-κB-binding sites. The p254 GFRα1 promoter construct contained nucleotides −320 to −66, which includes two putative NF-κB-binding site. The m-p509 GFRα1 promoter construct contained three mutated NF-κB-binding sites, which were generated using the p509 GFRα1 promoter construct as a template with a Muta-Direct site-directed mutagenesis kit (Intron Biotechnology, Suwon, South Korea). The mutated nucleotide sequences (indicated in capital letters) were as follows: mutated NF1, 5′-gttggaaatCGcc-3′; mutated NF2, 5′-ggTAgagtctccg-3′; and mutated NF3, 5′-cccggagttGGct-3′.
Promoter luciferase activity assays.
The assays were performed as previously described (103). Luciferase activity was normalized based on the β-galactosidase activity and adjusted using empty pGL3-Basic vector to determine the relative luciferase activity. Each experiment was repeated at least three times.
EMSA.
An electrophoretic mobility shift assay (EMSA) was carried out according to the procedure as described previously (48). NF1 (5′-CCTCACCCCGGTGTTGGAAATTCCCCAAAGGCGGGA-3′) was used as a probe. Double-stranded NF1 was biotin labeled by using a Biotin 3′-End DNA labeling kit (Pierce, Rockford, IL). Antibodies to p50 (sc-114) or p52 (sc-848; Santa Cruz Biotechnology) were incubated in reaction buffer for 20 min at room temperature prior to the binding reaction.
ChIP.
Chromatin immunoprecipitation (ChIP) was performed by using an EZ ChIP kit (Upstate, Temecula, CA) according to the manufacturer's instructions. The cell lysates were sonicated with five 5-s pulses using an ultrasonic processor (VCX130; Sonics & Materials, Inc., Newtown, CT) equipped with a 3-mm tip and set to 70% of maximum power. Immunoprecipitation was performed using 100 μl of sheared cross-linked chromatin (corresponding to 2 × 106 cell equivalents), 5 μg of anti-p50 antibody, and 60 μl of protein G-agarose. To reverse the DNA-protein cross-links, 8 μl of 5 M NaCl was added to 200 μl of the eluted protein-DNA complexes, followed by incubation at 65°C for 4 h. The DNA was then purified using a spin column. PCR was used to detect the p50-protected DNA fragments. The region amplified was the promoter region of human GFRα1 (positions −456 to −311 from ATG), which contains a putative NF-κB (NF1) binding site. The DNA sequence of the 5′ primer was 5′-AACCCTCTTCAGACCT-3′, and that of the 3′ primer was 5′-CTCCCCTCCCCCGTTG-3′. The region amplified was also the promoter region of human GFRα1 (positions −291 to −155 from ATG), which does not contain the NF-κB (NF1) binding site. The DNA sequence of the 5′ primer was 5′-CTCCGGCGCTCTCCGC-3′, and that of the 3′ primer was 5′-CTGGCAGCAGCCACC-3′. Anti-rabbit immunoglobulin G was purchased from Jackson Immunoresearch Laboratories (West Grove, PA).
Neurite outgrowth assay.
Vector/Neuro2a cells and mock, control small interfering RNA (siRNA)-, or GFRα1 siRNA-transfected Neuro2a/Ape1 cells were seeded in 60-mm plates at a density of 105 cells/well and maintained at 37°C in a humidified atmosphere of 5% CO2 for 24 h. The cells were then transfected with pCMV-DsRed-Express (Clontech) and incubated in serum-free medium for 24 h. The cells were then treated with or without 30 ng of GDNF/ml for an additional 24 h to allow neurite elongation to proceed, and the length and number of neurites was recorded for the DsRed-positive cells by fluorescence microscopy (excitation, 540 nm; Carl Zeiss) and quantified using Axiovision 4.6 image analysis software. At least 200 cells from 9 to 12 random fields were analyzed per experiment; each experiment was performed in triplicate. The data were analyzed by factorial analysis of variance, and each value is given as the mean ± the standard deviation (SD) of three independent experiments.
RT-PCR.
RNA extraction was carried out by using RNA-STAT-60 according to the manufacturer's instructions (Tel-Test, Inc., Friendswood, TX). RT was performed by using the M-MLV cDNA synthesis system (Promega, Madison, WI). The primers used were as follows: Ape1/Ref-1 forward (5′-ATGCCGAAGCGTGGGAAAAA-3′) and Ape1/Ref-1 reverse (5′-TCACAGTGCTAGGTATAGGGTGATAGG-3′) (designed to amplify a 957-bp region); Redox Ape1/Ref-1 forward (5′-ATGCCGAAGCGTGGGAAAAAG-3′) and Redox Ape1/Ref-1 reverse (5′-TCATGAACATTTGGTCTCTTG-3′) (designed to amplify a 300-bp region); Repair Ape1/Ref-1 forward (5′-GAGAACAAACTACCAGCTGAA-3′) and Repair Ape1/Ref-1 reverse (5′-TCACAGTGCTAGGTATAGGGT-3′) (designed to amplify a 656-bp region); GFRα1 forward (5′-AAGGAGACCAACTTCAGCCT-3′) and GFRα1 reverse (5′-TTGCAGACATCGTTGGACAC-3′) (designed to amplify a 397-bp region); and GAPDH forward (5′-TGACCACAGTCCATGCCATC-3′) and GAPDH reverse (5′-TTACTCCTTGGAGGCCATGT-3′) (designed to amplify a 492-bp region). To avoid overamplification, aliquots of each reaction mixture were retrieved after 25, 30, and 35 cycles for analysis by agarose gel electrophoresis and ethidium bromide staining.
siRNA knockdown of Ape1/Ref-1 and GFRα1.
The siRNA target sites within the human Ape1/Ref-1 and GFRα1 genes were chosen by using Ambion's siRNA Target Finder program (Austin, TX): Ape1/Ref-1 siRNA (534 bp from the ATG), 5′-GUCUGGUACGACUGGAGUAtt-3′ (sense) and 5′-UACUCCAGUCGUACCAGACtt-3′ (antisense); and human GFRα1 siRNA (1,216 bp from the ATG), 5′-UGUGUCGGGCAAUACACACtt-3′ (sense) and 5′-GUGUGUAUUGCCCGACACAtt-3′ (antisense). The control siRNA (AM4611) was purchased from Ambion. The control siRNA served as a negative control. The siRNAs were prepared by using a transcription-based method with a Silencer siRNA construction kit (Ambion). Mouse GFRα1 siRNA (sc-35470) and scramble siRNA (sc-37007) were purchased from Santa Cruz. The cells were transfected with the siRNA duplexes using Lipofectamine RNAiMAX (Invitrogen).
Proliferation assays.
Cells were cultured in serum free medium for 12 h. The cells were then treated with or without 10 or 30 ng of GDNF/ml in serum-free medium for the indicated times. For our siRNA studies, cells were transfected with control siRNA, Ape1/Ref-1 siRNA, or GFRα1 siRNA 24 h prior to the addition of GDNF. In some experiments, the cells were infected with LacZ or Ape1/Ref-1 adenovirus or with GFP or GFRα1 lentivirus 24 h before transfection with the siRNA. Cellular proliferation was determined either by MTT assay according to the manufacturer's instructions (Roche Applied Science, Mannheim, Germany) or by counting the cells under a light microscope.
Neuronal cell survival assay.
Vector/Neuro2a cells and mock-, control siRNA-, or GFRα1 siRNA-transfected Neuro2a/Ape1cells were cultured in serum-free medium for 24 h. The cells were then incubated with or without 30 ng of GDNF/ml for 30 min and treated with 15 μM β-amyloid peptide (peptide 1-42) or 20 μM H2O2 for an additional 24 h, and WST-1 tetrazolium salt (Roche Applied Science) was added. After 2 h, the absorbance values at 450 nm were determined by using a Multiskan Spectrum apparatus (Thermo Labsystems, Stockholm, Sweden). The reduction of WST in the treated cells is expressed as the percentage of GDNF in the untreated vector/Neuro2a cells. All experiments were performed in triplicate.
Invasion assay.
Cellular invasion was assayed in triplicate using an BioCoat Matrigel invasion chamber (BD Biosciences Discovery Labware, Bedford, MA) according to the manufacturer's instructions. The basic invasion assay was done as follows: cells were transfected with control siRNA, Ape1/Ref-1 siRNA, or GFRα1 siRNA and allowed to recover. In some experiments, cells were infected with GFP or GFRα1 lentivirus 24 h before siRNA transfection. At 48 h posttransfection, cells were treated with trypsin, pelleted, and resuspended in serum-free media. Approximately 2 × 105 pancreatic cancer cells were plated in a single well of a six-well invasion chamber plate in serum-free medium containing 30 ng of GDNF/ml. A total volume of 2 ml of complete medium (10% FBS) containing 30 ng of GDNF/ml was placed in the bottom chamber to serve as a chemoattractant in the lower chamber. After 24 h (MIA PaCa-2) or 36 h (BXPC3), the cells that had invaded the lower surface of the membranes were fixed with methanol and stained with hematoxylin and eosin. The number of invading cells was determined by counting the cells that invaded to the lower side of the filter using a light microscopy at ×40. Ten random fields were counted for each filter, and each sample was assayed in triplicate.
Statistical analysis.
All values are expressed as means ± the SD. Where indicated, we performed analyses of significance by the two-tailed Student t test. We considered P < 0.05 as significant and P < 0.01 as highly significant (indicated by “*” and “**,” respectively, in the figures).
RESULTS
Ape1/Ref-1 mediates an increase in GFRα1 expression.
The Ape1/Ref-1 was expressed in the human fibroblast cells using the replication-deficient adenoviral vector harboring the human Ape1/Ref-1 (Ad-Ape1/Ref-1) genes. In an attempt to identify the specific targets regulated by Ape1/Ref-1, ACP-based RT-PCR analysis was performed, and the expression patterns in a human fibroblast cells GM00637 transfected separately with Ad-Ape1/Ref-1 and Ad-LacZ were compared. We identified several partial cDNAs that were only expressed by Ad-Ape1/Ref-1 but not by Ad-LacZ, including a 324-bp amplicon that displayed complete homology to GFRα1 mRNA (see Fig. S1 in the supplemental material). GFRα1, which is a key receptor for GDNF family proteins (42, 86), promotes neuronal survival (2, 29, 44, 85) and is involved in tumor cell proliferation and invasion (79, 90).
To confirm this ACP-based RT-PCR result, semiquantitative RT-PCR analyses of Ad-LacZ- and Ad-Ape1/Ref-1-infected fibroblast cells were performed. Semiquantitative RT-PCR analysis using the GFRα1 primers showed that the expression level of the GFRα1 genes was increased dramatically by infecting them with Ad-Ape1/Ref-1 but not when they were infected with Ad-LacZ (Fig. 1A). In order to determine whether this increase in the GFRα1 mRNA levels correspond to an increase in the GFRα1 protein level, Western blots analyses were carried out with an antibody to GFRα1. SDS-PAGE was used to separate the whole-cell extracts of the protein from the Ape1/Ref-1-transfected cells, as well as to separate the protein from the LacZ-transfected cells. Western blot analysis with the GFRα1 antibody showed that the GFRα1 protein levels were higher in the Ape1/Ref-1-transfected cells than in the LacZ-transfected cells (Fig. 1B).
FIG. 1.

GFRα1 expression after the adenovirus-mediated transfer of Ape1/Ref-1 in GM00637 cells. (A) The GM00637 cells were transfected with Ad-LacZ or Ad-Ape1/Ref-1 at a multiplicity of infection of 50, and the cells were harvested 48 h after the infection. The total RNA was extracted and subjected to semiquantitative RT-PCR using the Ape1/Ref-1-, GFRα-, and GAPDH-specific primers. (B) Protein extracts prepared 48 h after the infection with Ad-LacZ or Ad-Ape1/Ref-1. A 20-μg portion of the total protein was loaded onto an SDS-polyacrylamide gel for Western blot analysis. Antibodies to Ape1/Ref-1 and GFRα1 were used. The detection of α-tubulin was used as the loading control. (C) The GM00637 cells were transfected with control pcDNA3 vector (control), repair Ape1/Ref-1 mutant expression vector (ΔRD), or redox Ape1/Ref-1 mutant expression vector (ΔAPD), and the total RNA was then extracted and subjected to semiquantitative RT-PCR using the Redox Ape1/Ref-1, Repair Ape1/Ref-1, GFRα, and GAPDH primers.
Ape1/Ref-1 has been found to exhibit distinct functions that facilitate the DNA-binding activities of several transcription factors through redox-dependent and redox-independent mechanisms (20). The highly conserved C-terminal region of Ape1/Ref-1 is involved in the repair of apyrimidic/apurinic nucleotides. The N-terminal region of Ape1/Ref-1 contains the nuclear localization sequence and the redox regulatory domain. To investigate whether the redox function of Ape/ref-1 contributes to the Ape1/Ref-1-mediated increase in the GFRα1 transcription, two deletion constructs were made from a full-length Ape1/Ref-1 cDNA. A repair Ape1/Ref-1 mutant (ΔRD: deletion of redox domain) lacked codons 1 to 100, including the redox domain; a redox Ape1/Ref-1 mutant (ΔAPD: deletion of AP endonuclease) lacked codons 101 to 318, containing the C-terminal region proposed to AP endonuclease domain. GM00637 cells were transfected with full-length Ape1/Ref-1, ΔRD, or ΔAPD expressing plasmid, and then the GFRα1 mRNA was measured. As shown in Fig. 1C, the ΔAPD-transfected cells still exhibited high expression of the GFRα1 mRNA. However, ΔRD-transfected cells showed no induction of GFRα1 mRNA. In the control experiment, the empty vector transfected cells showed no induction of GFRα1 mRNA. This indicates that redox function of Ape1/Ref-1 is an important role in the Ape1/Ref-1-mediated increase in GFRα1 transcript.
Involvement of the p50 NF-κB transcription factor in the upregulation of GFRα1 by Ape1/Ref-1.
These results suggest that Ape1/Ref-1 induces GFRα1 transcription. Therefore, the relationship between Ape1/Ref-1 generation and GFRα1 expression was next analyzed. To identify the regulatory elements of GFRα1 that are activated in Ape1/Ref-1-expressing GM00637 cells, we made reporter constructs composed of p2284 (positions −2291 to −7), p964 (positions −2291 to −1327), and p509 (positions −575 to −66) fused to a luciferase reporter gene (Fig. 2A). The full-length promoter construct p2284 displayed an ∼3.7-fold increase in luciferase activity after transfection into Ape1/Ref-1-expressing cells, as did the truncated promoter construct p509 (Fig. 2B). Since Ape1/Ref-1 is an important mediator of NF-κB activity (57, 63, 80), and given that the p509 GFRα1 promoter contains three putative NF-κB-binding sites, it is possible that Ape1/Ref-1-induced NF-κB activity may contribute to the increase in GFRα1 promoter activity. To investigate this possibility, we transfected control and Ape1/Ref-1-expressing cells with one of four GFRα1 promoter-luciferase constructs: p221 (one NF-κB-binding motif), p254 (two NF-κB-binding motifs), p509 (three NF-κB-binding motifs), and m-p509 (three mutated NF-κB-binding motifs) and found that the Ape1/Ref-1-mediated increase in luciferase activity was dependent on the number of NF-κB-binding motifs, and transfection with the NF-κB mutant construct m-p509 resulted in a striking decrease in Ape1/Ref-1-induced promoter activity (Fig. 2C).
FIG. 2.
Ape1/Ref-1 increases GFRα1 promoter activity by enhancing p50 NF-κB activation. (A) The upper panel shows a schematic representation of the human GFRα1 promoter region. The three putative NF-κB-binding sites spanning positions −349 to −335 (NF1), −300 to −287 (NF2), and −155 to −143 (NF3) are shown in green. The middle and lower panels show the GFRα1 promoter region of the promoter-reporter constructs p2284 (positions −2291 to −7), p964 (positions −2291 to −1327), and p509 (positions −575 to −66). (B) Parent cells and pcDNA3- and Ape1/Ref-11-expressing cells were transfected with pGL3-Basic, p2284, p964, or p509. Representations of the promoters are shown. The values reported are means ± the SD from six separate experiments. **, P < 0.01. (C) Cells were transfected with pGL3-Basic, p509 (positions −575 to −66), p221 (positions −287 to −66 of p509), p254 (positions −320 to −66 of p509), or m-p509 (p509 containing three mutated NF-κB-binding motifs). Schematic representations of the promoters are shown; ovals represent the consensus sites for the NF-κB-binding motifs. The values shown are means ± the SD from six separate experiments. **, P < 0.01. (D) Biotin-labeled probes oligonucleotides containing the putative NF-κB-binding site (NF1) from the GFRα1 promoter region were incubated with nuclear extracts prepared from parent cells and pcDNA3- and Ape1/Ref-1-transfected cells. Unlabeled oligonucleotides were used as a competitor. For supershift assays, anti-p50 or anti-p52 antibodies were added to the reaction mixtures, followed by incubation for 30 min prior to separation of the DNA-protein complexes. Black and gray triangles indicate DNA-protein complexes and antibody-supershifted complexes, respectively. (E) At the top of the panel is a schematic representation of the human GFRα1 promoter region. Chromatin from parent cells and pcDNA3- and Ape1/Ref-1-expressing GM00637 cells were fixed with formaldehyde and fragmented by sonication. The fragmented chromatin was immunoprecipitated using anti-p50 antibody and anti-rabbit IgG and then analyzed by PCR using primer sets specific for the promoter region containing putative NF-κB-binding site (green box: NF1) or control region, as indicated by the arrow.
We next used an EMSA to investigate the possibility that Ape1/Ref-1 expression enhances the binding of NF-κB to DNA. The binding activity of NF-κB was analyzed in GM00637 cells after transfection with Ape1/Ref-1 using oligonucleotides corresponding to the putative NF-κB-binding site in the GFRα1 promoter (Fig. 2D). The DNA-binding ability of NF-κB in the Ape1/Ref-1-expressing cells was significantly increased; the band was efficiently removed by an excess of unlabeled NF-κB oligonucleotides. Antibody against p50 NF-κB completely inhibited the formation of NF-κB-DNA complexes in Ape1/Ref-1-expressing cells, resulting in a supershift. We also confirmed Ape1/Ref-1-expressing cells treated with Ape1/Ref-1 siRNA showed significant suppression of the formation of NF-κB-DNA complexes (data not shown). Accordingly, we performed ChIP assays in parent cells and in pcDNA3- and Ape1/Ref-1 expression vector-transfected cells for direct confirmation of the binding of NF-κB to the endogenous GFRα1 promoter in vivo. In the Ape1/Ref-1-expressing cells, but not in the parent or pcDNA3-transfected cells, the GFRα1 promoter region adjacent to the NF-κB site was precipitated by anti-p50 antibody (Fig. 2E). Taken together, the results in Fig. 2 indicate that p50 NF-κB binds specifically to putative NF-κB sites present in the GFRα1 promoter region, thus enhancing GFRα1 expression in Ape1/Ref-1-transfected cells.
Ape1/Ref-1-mediated increase in GFRα1 expression is contributed to the enhancement of the GDNF responsiveness in GM00637 cells.
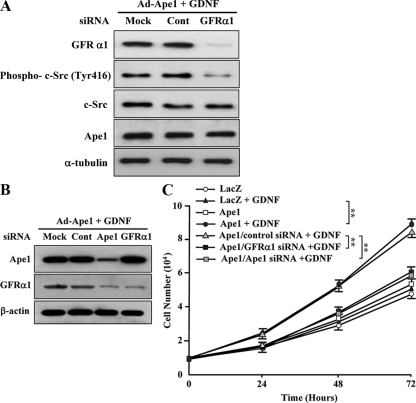
GFRα1 interacts with the GDNF family, resulting in the activation of the intracellular pathway, which contributes to cell proliferation, survival, and differentiation (2, 78). Therefore, we investigated whether the GFRα1 induction mediated by Ape1/Ref-1 could promote a functional interaction with GDNF. The receptor tyrosine kinase, Ret, is a major component in the signaling cascade activated by members of the GDNF family (42, 86). However, Ret was not detected in the parental GM00637 cells or in Ad-LacZ- and Ad-Ape1/Ref-1-infected cells (data not shown). Recent in vitro studies have shown that exogenously applied GDNF interacts with cells expressing GFRα1, leading to the activation of the Ret-dependent and Ret-independent signal pathways (71, 72, 88). Because Src-family kinase has been reported to be the direct downstream target of the GDNF/GFRα signal pathway in the Ret-deficient cell lines (71, 72, 88), we examined whether GDNF induced Src activation by investigating the phosphorylation status of Src. We found that the Ape1/Ref-1-infected cells treated with the control siRNA showed c-Src phosphorylation at Tyr418 in response to GDNF (Fig. 3A). In order to determine whether GFRα1 contributes to Ape1/Ref-1-induced c-Src phosphorylation in response to GDNF, siRNA in the form of 21-bp RNA duplexes that target GFRα1 was used in an attempt to inhibit its expression level. Western blot analysis revealed that the cells transfected with GFRα1 siRNA showed >90% decreased GFRα1 expression compared to control siRNA-transfected cells (Fig. 3A). Indeed, the Ape1/Ref-1-infected cells treated with the GFRα1 siRNA showed the attenuation of the GDNF-induced c-Src phosphorylation. These results suggested that Ape1/Ref-1-induced GFRα1 expression triggered the GDNF-mediated Src phosphorylation in human fibroblast cells.
FIG. 3.
Ape1/Ref-1 increases c-Src phosphorylation and cellular proliferation in response to GDNF through GFRα1. (A) Ad-Ape1/Ref-1-infected GM00637 cells were transfected with the mock, control siRNA (Cont), or GFRα1 siRNA. At 48 h after transfection, cells were incubated with GDNF (10 ng/ml) for 1 h. Equal amounts (20 μg of proteins) of the cell lysates were separated by SDS-10% PAGE and then transferred onto a nitrocellulose membrane. The membrane was immunoblotted with anti-Ape1/Ref-1, anti-GFRα1, or anti-phospho c-Src (Tyr418) antibodies. To control for equal loading, the membrane was stripped and reprobed against nonphosphorylated c-Src and α-tubulin. (B) Ad-Ape1/Ref-1-infected GM00637 cells were transfected with the mock, control-siRNA (Cont), Ape1/Ref-1-siRNA (Ape1), or GFRα1-siRNA. At 48 h after transfection, cells were then incubated with GDNF (10 ng/ml) for an additional 24 h. The total protein was extracted from the cells, and equal amounts (20 μg of proteins) of the cell lysates were separated by SDS-10% PAGE and then transferred onto a nitrocellulose membrane. The membrane was immunoblotted with anti-Ape1/Ref-1, anti-GFRα1, or anti-β-actin antibodies. (C) Ad-LacZ (LacZ)- or Ad-Ape1/Ref-1 (Ape1)-infected GM00637 cells were transfected with control siRNA or GFRα1 siRNA and then incubated with or without GDNF (10 ng/ml) for up to 72 h. The number of cells was determined by counting the cells every 24 h after GDNF treatment. Each value is the mean ± the SD from three separate experiments. **, P < 0.01.
It is known that the GDNF/GFRα system regulates cell survival and proliferation (2, 78). Therefore, we next examined the effect of GDNF on the proliferation of Ad-Ape1/Ref-1- and Ad-LacZ-infected cells. At 24 h after the cells were infected with either Ad-Ape1/Ref-1 or Ad-LacZ, the cells were either left untreated or incubated with GDNF, and the number of cells was counted after a period of 1 to 3 days. As shown in Fig. 3B and C, the Ad-Ape1-infected cells treated with GDNF showed a more rapid increase in the number of cells on days 1, 2, and 3 than the Ad-LacZ-infected cells treated with GDNF. To ensure that GFRα1 is required for the GDNF-induced cell proliferation in Ape1/Ref-1-expressing cells, the Ad-Ape1/Ref-1-transfected cells treated with the GFRα1 siRNA showed the significant suppression of the level of cell proliferation in response to GDNF compared to that of control siRNA-transfected cells. These results suggest that the Ape1/Ref-1-mediated increase in GFRα1 expression results in the stimulation of cell proliferation in response to GDNF.
Expression of Ape1/Ref-1 and GFRα1 in various cancer cells.
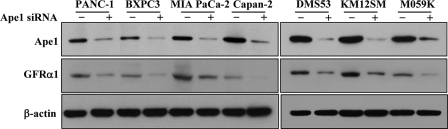
To test whether GFRα1 expression could be attributed to Ape1/Ref-1 in cancer cells, we examined endogenous levels of Ape1/Ref-1 and GFRα1 in tumor cell lines. We observed that, although some cell lines have low Ape1/Ref-1 and high GFRα1, there is a proportional correlation between Ape1/Ref-1 and GFRα1 levels in most cell lines (data not shown). In addition, transfection with Ape1/Ref-1 siRNA suppressed GFRα1 expression in PANC-1, BXPC3, MIA PaCa-2, Capan-2, DMS53, KM12SM, and M059K cancer cells compared to transfection with control siRNA (Fig. 4). Thus, Ape1/Ref-1 expression induces GFRα1 expression in human cancer cell lines.
FIG. 4.
Effect of Ape1/Ref-1 siRNA on GFRα1 expression in human cancer cell lines. The indicated human cancer cells were transfected with control siRNA or Ape1/Ref-1 siRNA. Whole-cell lysates were prepared and subjected to immunoblotting with anti-Ape1/Ref-1, anti-GFRα1, or anti-β-actin antibodies.
Ape1/Ref-1 knockdown reduces pancreatic cancer invasion.
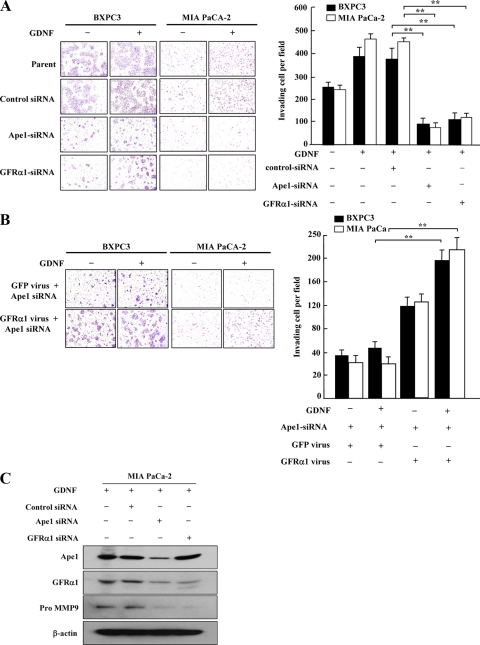
We chose BXPC3 and MIA PaCa-2 cells to test the effects of Ape1/Ref-1-mediated GFRα1 expression on tumor cell growth and invasion, since GDNF increases proliferation and invasion in both cell lines (79). We examined the effect of GDNF on the invasion of BXPC3 and MIA PaCa-2 pancreatic cancer cells in vitro. We observed that GDNF clearly stimulated the invasive activity of the parent and control siRNA-transfected cells but not of the GFRα1 siRNA- or Ape1/Ref-1 siRNA-transfected cells (Fig. 5A). To confirm whether the effect of GDNF is related to the Ape1/Ref-1-mediated expression of GFRα1 in these cells, BXPC3 and MIA PaCa-2 cells were transduced by a lentivirus encoding GFRα1 and then transfected with Ape1/Ref-1 siRNA. The GFRα1 lentivirus rescued GDNF-induced invasion in Ape1/Ref-1 knockdown cells (Fig. 5B), implying that Ape1/Ref-1-mediated GFRα1 expression is involved in pancreatic cancer cell invasion. Matrix metalloproteinases (MMPs) represent a family of proteolytic components of the extracellular matrix and play a critical role during cancer cell invasion (81). MMP-2 and MMP-9 were overexpressed in pancreatic cancer, and this expression strongly correlated with the presence of a desmoplastic reaction and lymphoid invasion (30, 31). Moreover, GDNF upregulates the expression and enzymatic activity of MMP-9 in pancreatic cancer MIA PaCa-2 cells (64). Therefore, the effect of knockdown of Ape1/Ref-1 and GFRα1 on the GDNF-induced pro-MMP-9 expression in MIA PaCa-2 cells was next examined. At 24 h after the transfection of MIA PaCa-2 cells with control siRNA, Ape1/Ref-1 siRNA, or GFRα1 siRNA, the cells were then incubated with GDNF for 24 h. The results showed that the MIA PaCa-2 cells transfected with the Ape1/Ref-1 siRNA had significantly lower pro-MMP-9 levels than the control siRNA-transfected cells (Fig. 5C). We also observed that the transfection of GFRα1 siRNA markedly reduced the level of pro-MMP-9 expression in response to GDNF compared to the control siRNA transfection. These results suggest that the Ape1/Ref-1-mediated increase in GFRα1 expression contributes to GDNF-induced MMP-9 expression in pancreatic cancer MIA PaCa-2 cells.
FIG. 5.
Knockdown of Ape1/Ref-1 decreased pancreatic tumor cell invasion and pro-MMP-9 expression in response to GDNF. (A) Photomicrographs showing the effect of Ape1/Ref-1 siRNA and GFRα1 siRNA on the GDNF-induced increase in BXPC3 and MIA PaCa-2 invasion. The histograms show the average number of invading cells. (B) Photomicrographs showing the influence of the GFRα1 lentivirus on the GDNF-induced invasion of Ape1/Ref-1 siRNA-transfected cells. The histograms show the ability of the GFRα1 lentivirus to rescue the GDNF-induced invasion of cells expressing Ape1/Ref-1 siRNA. The experiments for panels A and B were repeated three times, each in duplicate. The data shown are means ± the SD. **, P < 0.01. (C) MIA PACa-2 cells were transfected with mock, control, Ape1/Ref-1, or GFRα1 siRNA. At 48 h after transfection, cells were incubated with GDNF (30 ng/ml) for an additional 24 h. Whole-cell lysates were prepared and subjected to immunoblotting with anti-Ape1/Ref-1, anti-GFRα1, anti-pro MMP9, or anti-β-actin antibodies.
Ape1/Ref-1-mediated neurite outgrowth and neuronal survival in Neuro2a cells.
GDNF plays an important role in neuronal proliferation, differentiation, and survival (2, 29, 44, 85, 86); thus, we next studied the effect of Ape1/Ref-1 on GFRα1 expression in neuronal cells. To investigate the biological response of Ape1/Ref-1-expressing neuronal cells to GDNF, we established Neuro2a cells that stably expressed Ape1/Ref-1 (Neuro2a/Ape1) or vector alone (vector/Neuro2a) because Neuro2a cells express endogenous Ret but not GFRα1 (15). Immunohistochemical analysis indicated that the vector/Neuro2a cells did not express GFRα1, whereas receptor clusters were observed in the Neuro2a/Ape1 cells (data not shown).
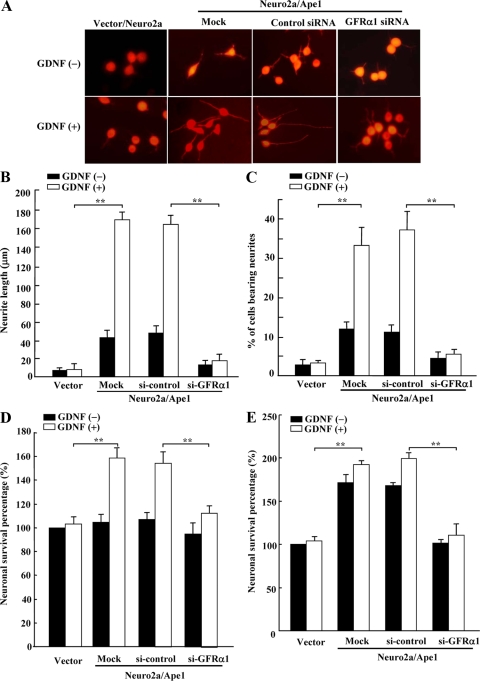
To determine whether increased activation of downstream signaling pathways by GDNF in Neuro2a/Ape1cells affects the outcome of GFRα1/Ret-mediated biological responses, the effect of GDNF on neuronal differentiation and survival (50) was evaluated. In Neuro2a/Ape1cells treated with GDNF, neurite length averaged 172 ± 12 μm (n = 200) after 24 h, which was 94% longer than the average neurite length in control neurons treated with GDNF (11 ± 6 μm, n = 200; Fig. 6A and B). Approximately 32% of the GDNF-treated Neuro2a/Ape1cells had four or more branches compared to 3% for the GDNF-treated controls (Fig. 6C). Moreover, compared to the control siRNA-transfected cells, GFRα1 siRNA-transfected Neuro2a/Ape1cells developed short neurites in response to GDNF. These data indicate that the Ape1/Ref-1-mediated induction of GFRα1 leads to GDNF-induced neuroblastoma differentiation.
FIG. 6.
Ape1/Ref-1 expression increases the GDNF-induced differentiation and survival of Neuro2a cells. (A) Vector/Neuro2a cells and mock-, control-, or GFRα1 siRNA-transfected Neuro2a/Ape1 cells were serum starved for 24 h and then incubated with or without 30 ng of GDNF/ml for an additional 24 h and visualized by microscopy. (B and C) The mean of the longest neurite (B) and the percentage of neurite-bearing cells (C) were determined for each culture based on measurements taken from 200 neurons in three different experiments. Each data point represents the mean ± the SD (**, P < 0.01). (D and E) Vector/Neuro2a (vector) and Neuro2a/Ape1 cells were cultured with 15 μM β-amyloid peptide (D) or 20 μM H2O2 (E) in the presence or absence of 30 ng of GDNF/ml, and cell survival was calculated as the percentage of untreated vector/Neuro2a cells (adjusted to 100%). Each value is the mean ± the SD from three separate experiments (**, P < 0.01).
Finally, we examined whether APE1-induced GFRα1 expression contributes to neuronal survival using Neuro2a/APE1 and vector/Neuro2a cells cultured with or without GDNF. GDNF-treated Neuro2a/APE1 cells showed a more rapid increase in cell number than did GDNF-treated vector/Neuro2a cells. Transfection with GFRα1 siRNA significantly reduced cell growth in Neuro2a/APE1 cells in response to GDNF compared to control-transfected cells (see Fig. S2 in the supplemental material). In addition, treatment of Neuro2a/APE1 cells with β-amyloid peptide (peptide 1-42) increased cell survival compared to vector/Neuro2a cells (Fig. 6D). Moreover, these effects were abolished by treatment with siRNA against GFRα1. Similar results were obtained in experiments where cell death was induced by H2O2 oxidative stress (Fig. 6E). These results suggest that, as with neuroblastoma differentiation, the APE1-mediated increase in GFRα1 expression promotes GDNF-induced neuronal survival.
DISCUSSION
The GDNF was originally characterized as a potent neurotropic factor specific for the survival and differentiation of the midbrain dopaminergic neurons (50). Subsequently, the biological effects of GDNF on uterine branching in kidney morphogenesis, spermatogenesis, and survival, as well as the differentiation of several other neuronal populations, have considerably extended the range of activities of this polypeptide (60, 70, 77). Currently, four GFRα proteins, GFRα1, GFRα2, GFRα3, and GFRα4 have been identified. GFRα1 mainly binds GNDF, and GFRα2, GFRα3, and GFRα4 bind neurturin, artemin, and persephin, respectively, which are the GDNF family of growth factors (5, 9, 19, 46, 51). The GDNF protein signals through a multicomponent receptor complex, which consists of a glycosylphosphatidylinositol (GPI) binding subunit, which is known as the GDNF family receptor α, and the transmembrane receptor tyrosine kinase (Ret) (2, 78). In the present study, we showed that Ape1 increased GFRα1 expression in human fibroblast GM00637 cells (Fig. 1). It was further found that c-Src, a downstream target protein of GFRα1, was functionally activated by GDNF in Ape1/Ref-1-expressing GM00637 cells (Fig. 3A) and that Ape1/Ref-1-induced GFRα1 expression contributed to the GDNF-mediated increase of cell proliferation in GM00637 cells (Fig. 3C). Thus, these results suggest that Ape1 might influence multiple cellular processes by triggering the GDNF/GFRα signal pathway.
Ape1/Ref-1 is important in mediating DNA binding of the AP-1 protein complex (1, 36, 92, 96). This occurs via a posttranslational mechanism in which conserved cysteine residues in the DNA-binding domains of Fos and Jun proteins are reduced, allowing DNA binding to occur. In addition, Ape1/Ref-1 phosphorylation was observed in vivo, but the acceptor residues have not been identified (25, 37). Upregulation of Ape1/Ref-1 significantly potentiates the hypoxia-induced expression of a reporter construct containing the HIF-1-binding site (38), and Ape1/Ref-1 is thought to be critical in the linking of two coactivator proteins, CBP/p300 and SRC-1, to HIF-1α (10, 18). Furthermore, Ape1/Ref-1 is found to be a component of protein complexes that binds to negative calcium response element (nCaRE) (65), Ku70(Ku86) (14), and heterogeneous nuclear ribonucleoprotein L (47) in the promoter of the parathyroid hormone (PTH) gene, the renin gene, and the Ape1/Ref-1 gene itself, where it may downregulate the expression of these genes (26, 41). Recent work suggests that Ape1/Ref-1 is acetylated by CBP/p300 both in vivo and in vitro, and acetylation stimulates binding to nCaRE in the PTH promoter, leading to downregulation of the PTH gene (6). In addition, the presence of Ape1/Ref-1 in the hypoxia-inducible transcriptional complex is required for the apparent high-affinity association between HIF-1 and its DNA recognition sequence (104). Moreover, Ape1/Ref-1 stably interacts with Y-box-binding protein (YB-1) and enhance its binding to the Y-box element, leading to the activation of the multidrug resistance gene MDR1 (11). Therefore, it appears that Ape1/Ref-1 stimulates the transcriptional activation by redox-dependent and redox-independent mechanisms. The results showed that transfecting the GM00637 cells with the redox domain of Ape1/Ref-1 (ΔAPD), but not the repair domain of Ape1/Ref-1 (ΔRD), still led to a significantly increase in the GFRα1 transcript (Fig. 1C). Thus, Ape1/Ref-1 may regulate the GFRα1 transcription through the activation of redox-dependent transcription factors. Ape1/Ref-1 is involved in the reduction of the Cys-62 residues of p50, which is essential for DNA-binding activity of NF-κB, suggesting Ape1/Ref-1 may act as a redox-sensitive regulator of NF-κB (33, 34, 55, 57). In the present study, we found that the important of NF-κB on Ape1/Ref-1-induced GFRα1 expression using a reporter gene assay (Fig. 2B and C). EMSA (Fig. 2D) and ChIP (Fig. 2E) analyses using the GFRα1 promoter region as a probe showed that p50 NF-κB binding of GFRα1 promoter was significantly increased in the Ape1/Ref-1-expressing cells. Previous in vitro studies using recombinant human Ape1/Ref-1 proteins suggest that cysteine 65 of human Ape1/Ref-1 is the redox-active site of Ape1/Ref-1 (92). However, others found that this cysteine residue is not involved in redox regulation (58, 69). More recently, Ando et al. (4) suggest that Ape1/Ref-1 acts as a “redox chaperone” that facilitates the reduction of redox-sensitive transcription factors by other reducing molecules such as glutathione and thioredoxin. These authors also demonstrated that redox chaperone activity of Ape1/Ref-1 is critical to NF-κB-mediated gene expression in human cells. We observed that mutant Ape1/Ref-1 (APEC65)-transfected cells exhibited still high expression of the GFRα1 transcript (data not shown). Thus, although Ape1/Ref-1 may be involved in the regulation of GFRα1 expression through direct or indirect activation of p50 NF-κB transcription factors, cysteine 65 in Ape/ref-1 does not contributed to the Ape1/Ref-1-mediated induction of GFRα1 expression. However, because the redox domain of Ape1/Ref-1 (ΔAPD) contributes to the Ape1/Ref-1-induced GFRα1 expression, a possibility still remains that other cysteine residues of this domain rather than Cys65 is required for activation of NF-κB.
GDNF-mediated activation of the GFRα/Ret system induces the subsequent signal transduction pathway and transactivation of its target genes, which leads to cell survival and proliferation (2, 78). Although the biological effects in non-neuronal cells are still unclear, several studies have indicated that GDNF/GFRα/Ret system might be involved in tumor cell proliferation and invasion. For example, older mice overexpressing GDNF develop testicular carcinoma after 1 year of age as a result of an invasion of undifferentiating spermatogonia to the interstitium, suggesting that the GDNF/Ret/GFRα signal pathway might be implicated in human germ cell carcinogenesis (56). In addition, the pancreatic cancer cell line contained both GFRα1 and Ret, and GDNF increased the invasive capacity of human pancreatic cancer cell lines (90). Despite the finding of no GFRα1 expression in the normal bile duct, it was expressed clearly in a bile duct carcinoma, indicating that carcinogenesis leads to the aberrant expression of GFRα1 (39). Interestingly, significant increase in Ape1/Ref-1 expression has been demonstrated in malignant tissues, such as epithelial ovarian cancers, cervical cancers, prostate cell tumors, gliomas, rhabdomyosarcoma, and germ cell tumors (7, 43, 59, 84, 98), and a higher Ape1/Ref-1 expression level was reported to be associated with tumor progression (23). Moreover, induction of Ape1/Ref-1 plays a critical role in reactive oxygen species-mediated malignant transformation and protects cells from excess reactive oxygen species stresses. Depletion of Ape1/Ref-1 rendered JB6 mouse epidermal cells more sensitive to apoptosis and inhibited cell transformation (100). In human colon, breast, and ovarian cancer cells, as well as human lymphoblastoid cells, knocking out Ape1/Ref-1 led to a decrease in proliferation (21, 89, 94).
In the present study, we found that both Ape1/Ref-1 and GFRα1 proteins were highly expressed in PNAC-1, BXPC3 MIA PaCa-2, and Capan-2 cells, and the downregulation of GFRα1 expression by Ape1/Ref-1 siRNA was observed in all of these pancreatic cancer cells (Fig. 4). Recently, Zou et al. (105) indicated that redox domain function of Ape1 is involved in the pancreatic cancer progression. Using E330, a small-molecule inhibitor of Ape1 redox domain function, these researchers show that selective blockade of the Ape1 redox function can inhibit pancreatic cancer growth and the migratory ability of pancreatic cancer cells in vitro. We found that the redox domain of Ape1 was essential for Ape1-mediated increase in GFRα1 transcription (Fig. 2). Thus, these results, combined with those of previous studies, indicate that the GFRα1 expression or function induced by Ape1 might be involved in pancreatic cancer cell progression.
Pancreatic adenocarcinoma is a very aggressive and destructive type of cancer that is characterized by an extremely poor prognosis, pronounced invasiveness, and rapid progression (102). A common finding in pancreatic adenocarcinoma is invasion of the intrapancreatic perineural space and the extrapancreatic retroperitoneal nerve plexus, and perineural invasion is an important prognosis factor in pancreatic cancer (8, 62). Several lines of evidence have recently suggested that GDNF signaling may be associated with regulation of the invasive potential in human pancreatic cancer cells (27, 28, 64, 82). The expression of MMP-2 and MMP-9 correlates with the degree of pancreatic cancer cell and lymphoid invasion (61, 101), and GDNF stimulates both the expression and the enzymatic activity of MMP-9 in pancreatic cancer cells (64). However, it is currently unclear which molecular mechanisms are involved in GDNF-mediated pancreatic tumor cell invasion. In the present study, we found that downregulation of Ape1 by siRNA caused a marked reduction in the GFRα1 expression in pancreatic caner cells, as well as the diminished ability of GDNF to stimulate pancreatic cancer BXPC3 and MIA PaCa-3 invasion (Fig. 5A). Moreover, the expression of GFRα1 by viral vector in Ape1-depleted cells led to rescue of the GDNF-induced invasion (Fig. 5B). Furthermore, siRNA knockdown of Ape1 or GFRα1 in MIA PaCa-2 cells resulted in a decrease in GDNF-induced pro-MMP-9 expression (Fig. 5C). Therefore, we postulated here that the Ape1-induced GFRα1 expression might be an underlying mechanism of the invasive behavior of pancreatic cancer cells.
Recently, several lines evidence have suggested that dysfunction of Ape1/Ref-1 may contribute to the development of neurodegenerative disease. For example, alteration in Ape1/Ref-1 expression and mutations in the Ape1/Ref-1 gene have been found in patients with a variety of neurodegenerative diseases (45, 66, 83). Reducing Ape1/Ref-1 sensitized neuronal cells to a variety of DNA-damaging agents, such as H2O2 and methyl methanesulfonate (67). In addition, young rats exhibited transient increase in Ape1/Ref-1 protein expression in the brain, including hippocampus, in response to oxidative stress, whereas aged rats showed no response (17), suggesting that adaptation to oxidative stress is compromised in older rats. Moreover, overexpressing Ape1/Ref-1 in hippocampal and sensory cells results in a significant increase in cell viability after exposure to hydrogen peroxide (89). Preventing the loss of Ape1/Ref-1 by inhibiting protein synthesis rescued neurons from experimentally induced cell death (13). We have shown here that Ape1/Ref-1 expression led to enhancement of neuronal differentiation and survival (Fig. 6), and the expression of GFRα1 induced by Ape1/Ref-1 is required for these effects. The GDNF/GFRα signaling pathway promotes the survival of various neurons, including peripheral autonomic and sensory neurons, as well as central motor and dopamine neurons (3). Moreover, in various animal models of Parkinson's disease, GDNF can prevent the neurotoxin-induced death of dopamine neurons and can promote functional recovery (29, 85). The ability of GDNF to rescue dopaminergic neurons supported idea that GDNF might ameliorate the degeneration of dopaminergic neurons in patients with Parkinson's disease. Therefore, GDNF has been considered as a therapeutic candidate for the treatment of Parkinson's disease (44). GDNF is also a good candidate molecule for studies and possible treatment of motor neuron diseases, such as amyotrophic lateral sclerosis or acute neuronal trauma (75). Indeed, GFRα is upregulated in axotomized motor neurons and in regions distal to axotomized sciatic nerves (87). This further supports the search for a role for a GDNF/GFRα signaling pathway in studies of motor neuronal disease. Therefore, it is possible that Ape1/Ref-1-induced GFRα expression is involved in neuronal function and survival, which might suggest a protective role against the development of neurodegenerative diseases.
In summary, we show that Ape1/Ref-1 is a novel key regulator of GFRα1. Our results indicate that an Ape1/Ref-1-mediated increase in GFRα1 contributes to human fibroblast cell proliferation, as well as to pancreatic tumor proliferation and invasion. The connection between Ape1/Ref-1 and GFRα1 may not be restricted to pancreatic cancer progression; GFRα1 is also regulated by Ape1/Ref-1 in various other cancers. The relationship with GFRα1 suggests that Ape1/Ref-1 is also directly linked to the regulation of neuronal differentiation and survival, which may be important for promoting stress resistance and regulating the cell life span under normal conditions. The connection between Ape1/Ref-1 and the GDNF/GFRα1 pathway may be a double-edged sword, since it may affect both neuronal survival and cancer progression, depending on the site of Ape1/Ref-1 expression and on the target cell. Thus, the Ape1/Ref-1 may be a potential therapeutic target for pancreatic cancer and neurodegenerative disorders.
Supplementary Material
Acknowledgments
This research was supported by M1063901 and M20706000032 from the Korean Ministry of Education, Science and Technology. H.-M.S. was supported by the Chosun University Fund 2002.
Footnotes
Published ahead of print on 2 February 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abate, C., L. Patel, F. J. Rauscher III, and T. Curran. 1990. Redox regulation of fos and jun DNA-binding activity in vitro. Science 2491157-1161. [DOI] [PubMed] [Google Scholar]
- 2.Airaksinen, M. S., and M. Saarma. 2002. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 3383-394. [DOI] [PubMed] [Google Scholar]
- 3.Airaksinen, M. S., A. Titievsky, and M. Saarma. 1999. GDNF family neurotrophic factor signaling: four masters, one servant? Mol. Cell Neurosci. 13313-325. [DOI] [PubMed] [Google Scholar]
- 4.Ando, K., S. Hirao, Y. Kabe, Y. Ogura, I. Sato, Y. Yamaguchi, T. Wada, and H. Handa. 2008. A new APE1/Ref-1-dependent pathway leading to reduction of NF-κB and AP-1, and activation of their DNA-binding activity. Nucleic Acids Res. 364327-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baloh, R. H., M. G. Tansey, P. A. Lampe, T. J. Fahrner, H. Enomoto, K. S. Simburger, M. L. Leitner, T. Araki, E. M. Johnson, Jr., and J. Milbrandt. 1998. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRα3-RET receptor complex. Neuron 211291-1302. [DOI] [PubMed] [Google Scholar]
- 6.Bhakat, K. K., T. Izumi, S. H. Yang, T. K. Hazra, and S. Mitra. 2003. Role of acetylated human AP-endonuclease (APE1/Ref-1) in regulation of the parathyroid hormone gene. EMBO J. 226299-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobola, M. S., A. Blank, M. S. Berger, B. A. Stevens, and J. R. Silber. 2001. Apurinic/apyrimidinic endonuclease activity is elevated in human adult gliomas. Clin. Cancer Res. 73510-3518. [PubMed] [Google Scholar]
- 8.Bockman, D. E., M. Buchler, and H. G. Beger. 1994. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology 107219-230. [DOI] [PubMed] [Google Scholar]
- 9.Buj-Bello, A., J. Adu, L. G. Pinon, A. Horton, J. Thompson, A. Rosenthal, M. Chinchetru, V. L. Buchman, and A. M. Davies. 1997. Neurturin responsiveness requires a GPI-linked receptor and the Ret receptor tyrosine kinase. Nature 387721-724. [DOI] [PubMed] [Google Scholar]
- 10.Carrero, P., K. Okamoto, P. Coumailleau, S. O'Brien, H. Tanaka, and L. Poellinger. 2000. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1alpha. Mol. Cell. Biol. 20402-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chattopadhyay, R., S. Das, A. K. Maiti, I. Boldogh, J. Xie, T. K. Hazra, K. Kohno, S. Mitra, and K. K. Bhakat. 2008. Regulatory role of human AP-endonuclease (APE1/Ref-1) in YB-1-mediated activation of the multidrug resistance gene MDR1. Mol. Cell. Biol. 287066-7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, D. S., T. Herman, and B. Demple. 1991. Two distinct human DNA diesterases that hydrolyze 3′-blocking deoxyribose fragments from oxidized DNA. Nucleic Acids Res. 195907-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiarini, L. B., F. G. Freitas, H. Petrs-Silva, and R. Linden. 2000. Evidence that the bifunctional redox factor / AP endonuclease Ref-1 is an antiapoptotic protein associated with differentiation in the developing retina. Cell Death Differ. 7272-281. [DOI] [PubMed] [Google Scholar]
- 14.Chung, U., T. Igarashi, T. Nishishita, H. Iwanari, A. Iwamatsu, A. Suwa, T. Mimori, K. Hata, S. Ebisu, E. Ogata, T. Fujita, and T. Okazaki. 1996. The interaction between Ku antigen and REF1 protein mediates negative gene regulation by extracellular calcium. J. Biol. Chem. 2718593-8598. [DOI] [PubMed] [Google Scholar]
- 15.Coulpier, M., J. Anders, and C. F. Ibanez. 2002. Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J. Biol. Chem. 2771991-1999. [DOI] [PubMed] [Google Scholar]
- 16.Demple, B., and L. Harrison. 1994. Repair of oxidative damage to DNA: enzymology and biology. Annu. Rev. Biochem. 63915-948. [DOI] [PubMed] [Google Scholar]
- 17.Edwards, M., D. K. Rassin, T. Izumi, S. Mitra, and J. R. Perez-Polo. 1998. APE/Ref-1 responses to oxidative stress in aged rats. J. Neurosci. Res. 54635-638. [DOI] [PubMed] [Google Scholar]
- 18.Ema, M., K. Hirota, J. Mimura, H. Abe, J. Yodoi, K. Sogawa, L. Poellinger, and Y. Fujii-Kuriyama. 1999. Molecular mechanisms of transcription activation by HLF and HIF1α in response to hypoxia: their stabilization and redox signal-induced interaction with CBP/p300. EMBO J. 181905-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enokido, Y., F. de Sauvage, J. A. Hongo, N. Ninkina, A. Rosenthal, V. L. Buchman, and A. M. Davies. 1998. GFRα-4 and the tyrosine kinase Ret form a functional receptor complex for persephin. Curr. Biol. 81019-1022. [DOI] [PubMed] [Google Scholar]
- 20.Evans, A. R., M. Limp-Foster, and M. R. Kelley. 2000. Going APE over ref-1. Mutat. Res. 46183-108. [DOI] [PubMed] [Google Scholar]
- 21.Fishel, M. L., Y. He, A. M. Reed, H. Chin-Sinex, G. D. Hutchins, M. S. Mendonca, and M. R. Kelley. 2008. Knockdown of the DNA repair and redox signaling protein Ape1/Ref-1 blocks ovarian cancer cell and tumor growth. DNA Repair 7177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freitas, S., D. H. Moore, H. Michael, and M. R. Kelley. 2003. Studies of apurinic/apyrimidinic endonuclease/ref-1 expression in epithelial ovarian cancer: correlations with tumor progression and platinum resistance. Clin. Cancer Res. 94689-4694. [PubMed] [Google Scholar]
- 23.Fritz, G. 2000. Human APE/Ref-1 protein. Int. J. Biochem. Cell Biol. 32925-929. [DOI] [PubMed] [Google Scholar]
- 24.Fritz, G., S. Grosch, M. Tomicic, and B. Kaina. 2003. APE/Ref-1 and the mammalian response to genotoxic stress. Toxicology 19367-78. [DOI] [PubMed] [Google Scholar]
- 25.Fritz, G., and B. Kaina. 1999. Phosphorylation of the DNA repair protein APE/REF-1 by CKII affects redox regulation of AP-1. Oncogene 181033-1040. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs, S., J. Philippe, P. Corvol, and F. Pinet. 2003. Implication of Ref-1 in the repression of renin gene transcription by intracellular calcium. J. Hypertens. 21327-335. [DOI] [PubMed] [Google Scholar]
- 27.Funahashi, H., Y. Okada, H. Sawai, H. Takahashi, Y. Matsuo, H. Takeyama, and T. Manabe. 2005. The role of glial cell line-derived neurotrophic factor (GDNF) and integrins for invasion and metastasis in human pancreatic cancer cells. J. Surg. Oncol. 9177-83. [DOI] [PubMed] [Google Scholar]
- 28.Funahashi, H., H. Takeyama, H. Sawai, A. Furuta, M. Sato, Y. Okada, T. Hayakawa, M. Tanaka, and T. Manabe. 2003. Alteration of integrin expression by glial cell line-derived neurotrophic factor (GDNF) in human pancreatic cancer cells. Pancreas 27190-196. [DOI] [PubMed] [Google Scholar]
- 29.Gash, D. M., Z. Zhang, A. Ovadia, W. A. Cass, A. Yi, L. Simmerman, D. Russell, D. Martin, P. A. Lapchak, F. Collins, B. J. Hoffer, and G. A. Gerhardt. 1996. Functional recovery in Parkinsonian monkeys treated with GDNF. Nature 380252-255. [DOI] [PubMed] [Google Scholar]
- 30.Gong, Y. L., G. M. Xu, W. D. Huang, and L. B. Chen. 2000. Expression of matrix metalloproteinases and the tissue inhibitors of metalloproteinases and their local invasiveness and metastasis in Chinese human pancreatic cancer. J. Surg. Oncol. 7395-99. [DOI] [PubMed] [Google Scholar]
- 31.Gress, T. M., F. Muller-Pillasch, M. M. Lerch, H. Friess, M. Buchler, and G. Adler. 1995. Expression and in-situ localization of genes coding for extracellular matrix proteins and extracellular matrix degrading proteases in pancreatic cancer. Int. J. Cancer 62407-413. [DOI] [PubMed] [Google Scholar]
- 32.Grosch, S., G. Fritz, and B. Kaina. 1998. Apurinic endonuclease (Ref-1) is induced in mammalian cells by oxidative stress and involved in clastogenic adaptation. Cancer Res. 584410-4416. [PubMed] [Google Scholar]
- 33.Guan, Z., D. Basi, Q. Li, A. Mariash, Y. F. Xia, J. G. Geng, E. Kao, and J. L. Hall. 2005. Loss of redox factor 1 decreases NF-κB activity and increases susceptibility of endothelial cells to apoptosis. Arterioscler. Thromb. Vasc. Biol. 2596-101. [DOI] [PubMed] [Google Scholar]
- 34.Hall, J. L., X. Wang, A. Van, Y. Zhao, and G. H. Gibbons. 2001. Overexpression of Ref-1 inhibits hypoxia and tumor necrosis factor-induced endothelial cell apoptosis through nuclear factor-κB-independent and -dependent pathways. Circ. Res. 881247-1253. [DOI] [PubMed] [Google Scholar]
- 35.Herring, C. J., C. M. West, D. P. Wilks, S. E. Davidson, R. D. Hunter, P. Berry, G. Forster, J. MacKinnon, J. A. Rafferty, R. H. Elder, J. H. Hendry, and G. P. Margison. 1998. Levels of the DNA repair enzyme human apurinic/apyrimidinic endonuclease (APE1, APEX, Ref-1) are associated with the intrinsic radiosensitivity of cervical cancers. Br. J. Cancer 781128-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirota, K., M. Matsui, S. Iwata, A. Nishiyama, K. Mori, and J. Yodoi. 1997. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc. Natl. Acad. Sci. USA 943633-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh, M. M., V. Hegde, M. R. Kelley, and W. A. Deutsch. 2001. Activation of APE/Ref-1 redox activity is mediated by reactive oxygen species and PKC phosphorylation. Nucleic Acids Res. 293116-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang, L. E., Z. Arany, D. M. Livingston, and H. F. Bunn. 1996. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J. Biol. Chem. 27132253-32259. [DOI] [PubMed] [Google Scholar]
- 39.Iwahashi, N., T. Nagasaka, G. Tezel, T. Iwashita, N. Asai, Y. Murakumo, K. Kiuchi, K. Sakata, Y. Nimura, and M. Takahashi. 2002. Expression of glial cell line-derived neurotrophic factor correlates with perineural invasion of bile duct carcinoma. Cancer 94167-174. [DOI] [PubMed] [Google Scholar]
- 40.Izumi, T., T. K. Hazra, I. Boldogh, A. E. Tomkinson, M. S. Park, S. Ikeda, and S. Mitra. 2000. Requirement for human AP endonuclease 1 for repair of 3′-blocking damage at DNA single-strand breaks induced by reactive oxygen species. Carcinogenesis 211329-1334. [PubMed] [Google Scholar]
- 41.Izumi, T., W. D. Henner, and S. Mitra. 1996. Negative regulation of the major human AP-endonuclease, a multifunctional protein. Biochemistry 3514679-14683. [DOI] [PubMed] [Google Scholar]
- 42.Jing, S., D. Wen, Y. Yu, P. L. Holst, Y. Luo, M. Fang, R. Tamir, L. Antonio, Z. Hu, R. Cupples, J. C. Louis, S. Hu, B. W. Altrock, and G. M. Fox. 1996. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-α, a novel receptor for GDNF. Cell 851113-1124. [DOI] [PubMed] [Google Scholar]
- 43.Kelley, M. R., L. Cheng, R. Foster, R. Tritt, J. Jiang, J. Broshears, and M. Koch. 2001. Elevated and altered expression of the multifunctional DNA base excision repair and redox enzyme Ape1/ref-1 in prostate cancer. Clin. Cancer Res. 7824-830. [PubMed] [Google Scholar]
- 44.Kirik, D., B. Georgievska, and A. Bjorklund. 2004. Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat. Neurosci. 7105-110. [DOI] [PubMed] [Google Scholar]
- 45.Kisby, G. E., J. Milne, and C. Sweatt. 1997. Evidence of reduced DNA repair in amyotrophic lateral sclerosis brain tissue. Neuroreport 81337-1340. [DOI] [PubMed] [Google Scholar]
- 46.Klein, R. D., D. Sherman, W. H. Ho, D. Stone, G. L. Bennett, B. Moffat, R. Vandlen, L. Simmons, Q. Gu, J. A. Hongo, B. Devaux, K. Poulsen, M. Armanini, C. Nozaki, N. Asai, A. Goddard, H. Phillips, C. E. Henderson, M. Takahashi, and A. Rosenthal. 1997. A GPI-linked protein that interacts with Ret to form a candidate neurturin receptor. Nature 387717-721. [DOI] [PubMed] [Google Scholar]
- 47.Kuninger, D. T., T. Izumi, J. Papaconstantinou, and S. Mitra. 2002. Human AP-endonuclease 1 and hnRNP-L interact with a nCaRE-like repressor element in the AP-endonuclease 1 promoter. Nucleic Acids Res. 30823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee, M. R., S. H. Kim, H. J. Cho, K. Y. Lee, A. R. Moon, H. G. Jeong, J. S. Lee, J. W. Hyun, M. H. Chung, and H. J. You. 2004. Transcription factors NF-YA regulate the induction of human OGG1 following DNA-alkylating agent methylmethane sulfonate (MMS) treatment. J. Biol. Chem. 2799857-9866. [DOI] [PubMed] [Google Scholar]
- 49.Lewen, A., T. Sugawara, Y. Gasche, M. Fujimura, and P. H. Chan. 2001. Oxidative cellular damage and the reduction of APE/Ref-1 expression after experimental traumatic brain injury. Neurobiol. Dis. 8380-390. [DOI] [PubMed] [Google Scholar]
- 50.Lin, L. F., D. H. Doherty, J. D. Lile, S. Bektesh, and F. Collins. 1993. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 2601130-1132. [DOI] [PubMed] [Google Scholar]
- 51.Lindahl, M., T. Timmusk, J. Rossi, M. Saarma, and M. S. Airaksinen. 2000. Expression and alternative splicing of mouse Gfra4 suggest roles in endocrine cell development. Mol. Cell Neurosci. 15522-533. [DOI] [PubMed] [Google Scholar]
- 52.Lindahl, T., and B. Nyberg. 1972. Rate of depurination of native deoxyribonucleic acid. Biochemistry 113610-3618. [DOI] [PubMed] [Google Scholar]
- 53.Loeb, L. A., and B. D. Preston. 1986. Mutagenesis by apurinic/apyrimidinic sites. Annu. Rev. Genet. 20201-230. [DOI] [PubMed] [Google Scholar]
- 54.Ludwig, D. L., M. A. MacInnes, Y. Takiguchi, P. E. Purtymun, M. Henrie, M. Flannery, J. Meneses, R. A. Pedersen, and D. J. Chen. 1998. A murine AP-endonuclease gene-targeted deficiency with post-implantation embryonic progression and ionizing radiation sensitivity. Mutat. Res. 40917-29. [DOI] [PubMed] [Google Scholar]
- 55.Matthews, J. R., N. Wakasugi, J. L. Virelizier, J. Yodoi, and R. T. Hay. 1992. Thioredoxin regulates the DNA binding activity of NF-κB by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 203821-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meng, X., M. Lindahl, M. E. Hyvonen, M. Parvinen, D. G. de Rooij, M. W. Hess, A. Raatikainen-Ahokas, K. Sainio, H. Rauvala, M. Lakso, J. G. Pichel, H. Westphal, M. Saarma, and H. Sariola. 2000. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2871489-1493. [DOI] [PubMed] [Google Scholar]
- 57.Mitomo, K., K. Nakayama, K. Fujimoto, X. Sun, S. Seki, and K. Yamamoto. 1994. Two different cellular redox systems regulate the DNA-binding activity of the p50 subunit of NF-κB in vitro. Gene 145197-203. [DOI] [PubMed] [Google Scholar]
- 58.Mol, C. D., T. Izumi, S. Mitra, and J. A. Tainer. 2000. DNA-bound structures and mutants reveal abasic DNA binding by APE1 and DNA repair coordination. Nature 403451-456. [DOI] [PubMed] [Google Scholar]
- 59.Moore, D. H., H. Michael, R. Tritt, S. H. Parsons, and M. R. Kelley. 2000. Alterations in the expression of the DNA repair/redox enzyme APE/Ref-1 in epithelial ovarian cancers. Clin. Cancer Res. 6602-609. [PubMed] [Google Scholar]
- 60.Moore, M. W., R. D. Klein, I. Farinas, H. Sauer, M. Armanini, H. Phillips, L. F. Reichardt, A. M. Ryan, K. Carver-Moore, and A. Rosenthal. 1996. Renal and neuronal abnormalities in mice lacking GDNF. Nature 38276-79. [DOI] [PubMed] [Google Scholar]
- 61.Nagakawa, Y., T. Aoki, K. Kasuya, A. Tsuchida, and Y. Koyanagi. 2002. Histologic features of venous invasion, expression of vascular endothelial growth factor and matrix metalloproteinase-2 and matrix metalloproteinase-9, and the relation with liver metastasis in pancreatic cancer. Pancreas 24169-178. [DOI] [PubMed] [Google Scholar]
- 62.Nakao, A., A. Harada, T. Nonami, T. Kaneko, and H. Takagi. 1996. Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas 12357-361. [DOI] [PubMed] [Google Scholar]
- 63.Nishi, T., N. Shimizu, M. Hiramoto, I. Sato, Y. Yamaguchi, M. Hasegawa, S. Aizawa, H. Tanaka, K. Kataoka, H. Watanabe, and H. Handa. 2002. Spatial redox regulation of a critical cysteine residue of NF-κB in vivo. J. Biol. Chem. 27744548-44556. [DOI] [PubMed] [Google Scholar]
- 64.Okada, Y., G. Eibl, J. P. Duffy, H. A. Reber, and O. J. Hines. 2003. Glial cell-derived neurotrophic factor upregulates the expression and activation of matrix metalloproteinase-9 in human pancreatic cancer. Surgery 134293-299. [DOI] [PubMed] [Google Scholar]
- 65.Okazaki, T., U. Chung, T. Nishishita, S. Ebisu, S. Usuda, S. Mishiro, S. Xanthoudakis, T. Igarashi, and E. Ogata. 1994. A redox factor protein, Ref1, is involved in negative gene regulation by extracellular calcium. J. Biol. Chem. 26927855-27862. [PubMed] [Google Scholar]
- 66.Olkowski, Z. L. 1998. Mutant AP endonuclease in patients with amyotrophic lateral sclerosis. Neuroreport 9239-242. [DOI] [PubMed] [Google Scholar]
- 67.Ono, Y., T. Furuta, T. Ohmoto, K. Akiyama, and S. Seki. 1994. Stable expression in rat glioma cells of sense and antisense nucleic acids to a human multifunctional DNA repair enzyme, APEX nuclease. Mutat. Res. 31555-63. [DOI] [PubMed] [Google Scholar]
- 68.Ono, Y., K. Matsumoto, T. Furuta, T. Ohmoto, K. Akiyama, and S. Seki. 1995. Relationship between expression of a major apurinic/apyrimidinic endonuclease (APEX nuclease) and susceptibility to genotoxic agents in human glioma cell lines. J. Neurooncol. 25183-192. [DOI] [PubMed] [Google Scholar]
- 69.Ordway, J. M., D. Eberhart, and T. Curran. 2003. Cysteine 64 of Ref-1 is not essential for redox regulation of AP-1 DNA binding. Mol. Cell. Biol. 234257-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pichel, J. G., L. Shen, H. Z. Sheng, A. C. Granholm, J. Drago, A. Grinberg, E. J. Lee, S. P. Huang, M. Saarma, B. J. Hoffer, H. Sariola, and H. Westphal. 1996. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 38273-76. [DOI] [PubMed] [Google Scholar]
- 71.Popsueva, A., D. Poteryaev, E. Arighi, X. Meng, A. Angers-Loustau, D. Kaplan, M. Saarma, and H. Sariola. 2003. GDNF promotes tubulogenesis of GFRα1-expressing MDCK cells by Src-mediated phosphorylation of Met receptor tyrosine kinase. J. Cell Biol. 161119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poteryaev, D., A. Titievsky, Y. F. Sun, J. Thomas-Crusells, M. Lindahl, M. Billaud, U. Arumae, and M. Saarma. 1999. GDNF triggers a novel ret-independent Src kinase family-coupled signaling via a GPI-linked GDNF receptor alpha1. FEBS Lett. 46363-66. [DOI] [PubMed] [Google Scholar]
- 73.Ramana, C. V., I. Boldogh, T. Izumi, and S. Mitra. 1998. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc. Natl. Acad. Sci. USA 955061-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robertson, K. A., H. A. Bullock, Y. Xu, R. Tritt, E. Zimmerman, T. M. Ulbright, R. S. Foster, L. H. Einhorn, and M. R. Kelley. 2001. Altered expression of Ape1/ref-1 in germ cell tumors and overexpression in NT2 cells confers resistance to bleomycin and radiation. Cancer Res. 612220-2225. [PubMed] [Google Scholar]
- 75.Saarma, M., and H. Sariola. 1999. Other neurotrophic factors: glial cell line-derived neurotrophic factor (GDNF). Microsc. Res. Tech. 45292-302. [DOI] [PubMed] [Google Scholar]
- 76.Sakurai, M., T. Nagata, K. Abe, T. Horinouchi, Y. Itoyama, and K. Tabayashi. 2003. Oxidative damage and reduction of redox factor-1 expression after transient spinal cord ischemia in rabbits. J. Vasc. Surg. 37446-452. [DOI] [PubMed] [Google Scholar]
- 77.Sanchez, M. P., I. Silos-Santiago, J. Frisen, B. He, S. A. Lira, and M. Barbacid. 1996. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 38270-73. [DOI] [PubMed] [Google Scholar]
- 78.Sariola, H., and M. Saarma. 2003. Novel functions and signalling pathways for GDNF. J. Cell Sci. 1163855-3862. [DOI] [PubMed] [Google Scholar]
- 79.Sawai, H., Y. Okada, K. Kazanjian, J. Kim, S. Hasan, O. J. Hines, H. A. Reber, D. S. Hoon, and G. Eibl. 2005. The G691S RET polymorphism increases glial cell line-derived neurotrophic factor-induced pancreatic cancer cell invasion by amplifying mitogen-activated protein kinase signaling. Cancer Res. 6511536-11544. [DOI] [PubMed] [Google Scholar]
- 80.Shimizu, N., K. Sugimoto, J. Tang, T. Nishi, I. Sato, M. Hiramoto, S. Aizawa, M. Hatakeyama, R. Ohba, H. Hatori, T. Yoshikawa, F. Suzuki, A. Oomori, H. Tanaka, H. Kawaguchi, H. Watanabe, and H. Handa. 2000. High-performance affinity beads for identifying drug receptors. Nat. Biotechnol. 18877-881. [DOI] [PubMed] [Google Scholar]
- 81.Sternlicht, M. D., and Z. Werb. 2001. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 17463-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takahashi, H., H. Funahashi, H. Sawai, M. Sakamoto, Y. Matsuo, M. Yamamoto, Y. Okada, T. Hayakawa, and T. Manabe. 2004. Glial cell line-derived neurotrophic factor enhances nuclear factor-κB activity and invasive potential in human pancreatic cancer cells. Pancreas 2922-27. [DOI] [PubMed] [Google Scholar]
- 83.Tan, Z., N. Sun, and S. S. Schreiber. 1998. Immunohistochemical localization of redox factor-1 (Ref-1) in Alzheimer's hippocampus. Neuroreport 92749-2752. [DOI] [PubMed] [Google Scholar]
- 84.Thomson, B., R. Tritt, M. Davis, and M. R. Kelley. 2001. Histology-specific expression of a DNA repair protein in pediatric rhabdomyosarcomas. J. Pediatr. Hematol. Oncol. 23234-239. [DOI] [PubMed] [Google Scholar]
- 85.Tomac, A., E. Lindqvist, L. F. Lin, S. O. Ogren, D. Young, B. J. Hoffer, and L. Olson. 1995. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373335-339. [DOI] [PubMed] [Google Scholar]
- 86.Treanor, J. J., L. Goodman, F. de Sauvage, D. M. Stone, K. T. Poulsen, C. D. Beck, C. Gray, M. P. Armanini, R. A. Pollock, F. Hefti, H. S. Phillips, A. Goddard, M. W. Moore, A. Buj-Bello, A. M. Davies, N. Asai, M. Takahashi, R. Vandlen, C. E. Henderson, and A. Rosenthal. 1996. Characterization of a multicomponent receptor for GDNF. Nature 38280-83. [DOI] [PubMed] [Google Scholar]
- 87.Trupp, M., N. Belluardo, H. Funakoshi, and C. F. Ibanez. 1997. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J. Neurosci. 173554-3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trupp, M., R. Scott, S. R. Whittemore, and C. F. Ibanez. 1999. Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J. Biol. Chem. 27420885-20894. [DOI] [PubMed] [Google Scholar]
- 89.Vasko, M. R., C. Guo, and M. R. Kelley. 2005. The multifunctional DNA repair/redox enzyme Ape1/Ref-1 promotes survival of neurons after oxidative stress. DNA Repair 4367-379. [DOI] [PubMed] [Google Scholar]
- 90.Veit, C., F. Genze, A. Menke, S. Hoeffert, T. M. Gress, P. Gierschik, and K. Giehl. 2004. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res. 645291-5300. [DOI] [PubMed] [Google Scholar]
- 91.Walker, L. J., R. B. Craig, A. L. Harris, and I. D. Hickson. 1994. A role for the human DNA repair enzyme HAP1 in cellular protection against DNA-damaging agents and hypoxic stress. Nucleic Acids Res. 224884-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Walker, L. J., C. N. Robson, E. Black, D. Gillespie, and I. D. Hickson. 1993. Identification of residues in the human DNA repair enzyme HAP1 (Ref-1) that are essential for redox regulation of Jun DNA binding. Mol. Cell. Biol. 135370-5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walton, M., P. Lawlor, E. Sirimanne, C. Williams, P. Gluckman, and M. Dragunow. 1997. Loss of Ref-1 protein expression precedes DNA fragmentation in apoptotic neurons. Brain Res. Mol. Brain Res. 44167-170. [DOI] [PubMed] [Google Scholar]
- 94.Wang, D., M. Luo, and M. R. Kelley. 2004. Human apurinic endonuclease 1 (APE1) expression and prognostic significance in osteosarcoma: enhanced sensitivity of osteosarcoma to DNA-damaging agents using silencing RNA APE1 expression inhibition. Mol. Cancer Ther. 3679-686. [PubMed] [Google Scholar]
- 95.Wilson, T. M., S. A. Rivkees, W. A. Deutsch, and M. R. Kelley. 1996. Differential expression of the apurinic /apyrimidinic endonuclease (APE/ref-1) multifunctional DNA base excision repair gene during fetal development and in adult rat brain and testis. Mutat. Res. 362237-248. [DOI] [PubMed] [Google Scholar]
- 96.Xanthoudakis, S., and T. Curran. 1992. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 11653-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xanthoudakis, S., G. Miao, F. Wang, Y. C. Pan, and T. Curran. 1992. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 113323-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu, Y., D. H. Moore, J. Broshears, L. Liu, T. M. Wilson, and M. R. Kelley. 1997. The apurinic/apyrimidinic endonuclease (APE/ref-1) DNA repair enzyme is elevated in premalignant and malignant cervical cancer. Anticancer Res. 173713-3719. [PubMed] [Google Scholar]
- 99.Yang, S., K. Irani, S. E. Heffron, F. Jurnak, and F. L. Meyskens, Jr. 2005. Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE/Ref-1) in human melanoma and identification of the therapeutic potential of resveratrol as an APE/Ref-1 inhibitor. Mol. Cancer Ther. 41923-1935. [DOI] [PubMed] [Google Scholar]
- 100.Yang, S., B. J. Misner, R. J. Chiu, and F. L. Meyskens, Jr. 2007. Redox effector factor-1, combined with reactive oxygen species, plays an important role in the transformation of JB6 cells. Carcinogenesis 282382-2390. [DOI] [PubMed] [Google Scholar]
- 101.Yang, X., E. D. Staren, J. M. Howard, T. Iwamura, J. E. Bartsch, and H. E. Appert. 2001. Invasiveness and MMP expression in pancreatic carcinoma. J. Surg. Res. 9833-39. [DOI] [PubMed] [Google Scholar]
- 102.Yeo, T. P., R. H. Hruban, S. D. Leach, R. E. Wilentz, T. A. Sohn, S. E. Kern, C. A. Iacobuzio-Donahue, A. Maitra, M. Goggins, M. I. Canto, R. A. Abrams, D. Laheru, E. M. Jaffee, M. Hidalgo, and C. J. Yeo. 2002. Pancreatic cancer. Curr. Probl. Cancer 26176-275. [DOI] [PubMed] [Google Scholar]
- 103.Youn, C. K., H. J. Cho, S. H. Kim, H. B. Kim, M. H. Kim, I. Y. Chang, J. S. Lee, M. H. Chung, K. S. Hahm, and H. J. You. 2005. Bcl-2 expression suppresses mismatch repair activity through inhibition of E2F transcriptional activity. Nat. Cell Biol. 7137-147. [DOI] [PubMed] [Google Scholar]
- 104.Ziel, K. A., C. C. Campbell, G. L. Wilson, and M. N. Gillespie. 2004. Ref-1/Ape is critical for formation of the hypoxia-inducible transcriptional complex on the hypoxic response element of the rat pulmonary artery endothelial cell VEGF gene. FASEB J. 18986-988. [DOI] [PubMed] [Google Scholar]
- 105.Zou, G. M., and A. Maitra. 2008. Small-molecule inhibitor of the AP endonuclease 1/REF-1 E3330 inhibits pancreatic cancer cell growth and migration. Mol. Cancer Ther. 72012-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.