Abstract
Background: Muscular instability is an important risk factor for lumbar spine injury and chronic low-back pain. Although the lumbar multifidus muscle is considered an important paraspinal muscle, its design features are not completely understood. The purpose of the present study was to determine the architectural properties, in vivo sarcomere length operating range, and passive mechanical properties of the human multifidus muscle. We hypothesized that its architecture would be characterized by short fibers and a large physiological cross-sectional area and that it would operate over a relatively wide range of sarcomere lengths but would have very stiff passive material properties.
Methods: The lumbar spines of eight cadaver specimens were excised en bloc from T12 to the sacrum. Multifidus muscles were isolated from each vertebral level, permitting the architectural measurements of mass, sarcomere length, normalized fiber length, physiological cross-sectional area, and fiber length-to-muscle length ratio. To determine the sarcomere length operating range of the muscle, sarcomere lengths were measured from intraoperative biopsy specimens that were obtained with the spine in the flexed and extended positions. The material properties of single muscle fibers were obtained from passive stress-strain tests of excised biopsy specimens.
Results: The average muscle mass (and standard error) was 146 ± 8.7 g, and the average sarcomere length was 2.27 ± 0.06 μm, yielding an average normalized fiber length of 5.66 ± 0.65 cm, an average physiological cross-sectional area of 23.9 ± 3.0 cm2, and an average fiber length-to-muscle length ratio of 0.21 ± 0.03. Intraoperative sarcomere length measurements revealed that the muscle operates from 1.98 ± 0.15 μm in extension to 2.70 ± 0.11 μm in flexion. Passive mechanical data suggested that the material properties of the muscle are comparable with those of muscles of the arm or leg.
Conclusions: The architectural design (a high cross-sectional area and a low fiber length-to-muscle length ratio) demonstrates that the multifidus muscle is uniquely designed as a stabilizer to produce large forces. Furthermore, multifidus sarcomeres are positioned on the ascending portion of the length-tension curve, allowing the muscle to become stronger as the spine assumes a forward-leaning posture.
Clinical Relevance: These findings demonstrate that the human multifidus muscle is designed to function as a dynamic stabilizer of the lumbar spine.
The posterior paraspinal muscles provide motion and dynamic stability to the multisegmented, multi-articular spinal column1. Numerous studies have investigated the anatomical2-5 and histochemical6 properties of many of these muscles with the goal of providing surgical guidelines or understanding normal muscle function. Similarly, imaging studies of the multifidus and other muscles have demonstrated pathological changes that are associated with other spinal abnormalities such as chronic low-back pain7,8, disc herniation9, scoliosis10, and degenerative lumbar kyphosis11. Paradoxically, some operations designed to treat these various spinal disorders actually disrupt these muscles and, in turn, may lead to substantial functional deficits12-15 or various pain syndromes16-18. Minimally invasive spine surgery techniques strive to minimize surgical trauma to these muscles, thereby preserving their function19.
The structure, function, and design of skeletal muscles have been investigated primarily in studies of the upper20,21 and lower extremities22,23, not the spine. One universal finding in studies of extremity muscles is that skeletal muscle architecture, defined as the number and orientation of muscle fibers within a muscle, is the only accurate predictor of muscle function24,25. Without architectural data, it is nearly impossible to predict a muscle's function, as evidenced by the fact that all high-resolution functional musculoskeletal models rely heavily on architecture to make functional predictions3,5,26. It is therefore unfortunate that studies of muscle fiber type6, anatomy, and morphology2,3 have not rigorously investigated the architectural properties of the multifidus muscle. Stokes and Gardner-Morse5 attempted to quantify the physiological cross-sectional area and fiber length of this muscle with stereoradiography. However, the muscle tissue must be studied directly to accurately measure these architectural features24. The architectural properties of other spinal muscles were studied by Delp et al.4. Those investigators demonstrated that lumbar spinal muscles generally had relatively short fibers (approximately 10 cm) with moderate physiological cross-sectional areas (approximately 10 cm2), which suggests that these muscles probably function as stabilizers.
A second major lesson learned from extremity muscles is that the range over which muscle sarcomeres operate and their passive mechanical properties are stereotypical and functionally relevant. For example, it has been demonstrated that wrist flexors and extensors operate on opposite sides of the sarcomere length-tension curve and have similar elastic moduli, which causes a precise mechanical balance between flexion and extension moments throughout the range of wrist motion27. Whether any such design is present in spinal muscles is unknown as no sarcomere length-joint angle measurements have been reported for spinal muscles, to our knowledge.
The purpose of the present study was to combine quantitative anatomical studies with patient-based intraoperative sarcomere length measurements and single-cell passive mechanics. We hypothesized that the architecture of the multifidus muscle would be characterized by short fibers and a large physiological cross-sectional area. Knowing that the spine is a relatively mobile system in the sagittal plane and that the multifidus may have relatively short fibers, we also hypothesized that it would operate over a relatively wide range of sarcomere lengths but would have very stiff passive material properties.
Materials and Methods
Cadaveric Specimens
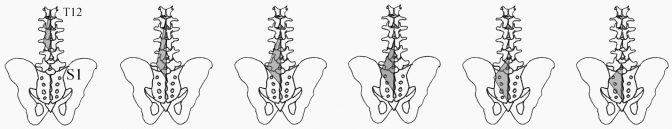
Muscle architecture was determined according to the method of Sacks and Roy28 as described by Lieber et al.20 for muscles of the upper extremity. Eight cadaver lumbar spines were harvested en bloc from T12 to the sacrum, were stripped of superficial soft tissue, and were immersion-fixed in 10% formalin for seventy-two hours. Demographic data on the donors are shown in Table I. The specimens were positioned in the supine posture at the time of fixation to maintain a neutral lumbar spine position. After fixation, the superficial lumbar fascia was excised and the longissimus and iliocostalis lumborum muscles were reflected to reveal the multifidus muscle (Fig. 1). After mapping of the locations for muscle fascicle harvesting, one side of the muscle was dissected free of its osseous attachments and was placed in phosphate-buffered saline solution for storage in order to remove residual fixative. The remaining side was stored in phosphate-buffered saline solution intact on the osseous vertebral column from T12 to the sacrum. The excised half then was removed from storage buffer, was gently blotted dry, and was weighed. After whole-muscle mass measurements, the muscle was divided into thirds (including the T12 and L1 origins, L2 and L3 origins, and L4 and L5 origins) to determine its regional mass distribution. Muscle length (Lm) was defined as the distance from the origin of the most cephalad fibers to the insertion of the most caudad fibers. For each origin level ranging from T12 to L5, the surface pennation angle was measured as the orientation of the fibers in each predefined region (Fig. 1) relative to the line of action of the distal tendon of each muscle.
TABLE I.
Demographic Data on Donors of Cadaveric Specimens
| Characteristic | Value |
|---|---|
| Age at time of death*(yr) | 84 ± 3 |
| Male:female ratio (no. of donors) | 5:3 |
| Height*(cm) | 170.5 ± 11.1 |
| Weight*(kg) | 81.1 ± 15.3 |
| Vertebral body height*(cm) | 2.67 ± 0.27 |
| Vertebral body width*(cm) | 5.19 ± 1.28 |
The values are given as the mean and the standard deviation (n = 8).
Fig. 1.
Posterior schematic of the lumbosacral region. In the repeated images (left to right), the shaded areas depict the regions sampled for each segmental level (from T12 to L5).
On the intact half of each specimen, muscle fiber bundles were carefully dissected from the proximal tendon to the distal tendon of each muscle region (as defined by osseous origin) (Fig. 1). Fiber bundle length was measured with use of a digital caliper (accuracy, ±0.01 mm). The fiber bundles were then placed in mild sulfuric acid solution (15% volume per volume) for thirty minutes to partially digest surrounding connective tissue and then were rinsed in phosphate-buffered saline solution. Our intention in using this approach was to sample fibers randomly across the entire muscle in order to reflect the architectural properties of the muscle accurately and to identify region-specific architectural differences. Under light microscopy (model MZ12.5; Leica Microsystems, Wetzlar, Germany), three small muscle fiber bundles (consisting of five to fifty individual fibers) were isolated from each muscle region and were mounted on slides. Sarcomere length (Ls) for each of the dissected bundles was determined by means of laser diffraction with use of the zeroth to first order diffraction angles as previously described29. To compensate for variations in raw fiber length that occur because of the position of the spine during fixation, muscle fiber lengths were normalized (Lf) by scaling to the optimal sarcomere length of human muscle (2.7 μm)27. This approach allowed for greater sampling of normalized fiber length values. Approximately 2400 independent Ls measurements (∼24 bundles per muscle × ∼25 fibers per bundle × ∼4 sarcomere length values per bundle) were made.
In addition to the above measurements, the physiological cross-sectional area (PCSA) was calculated according to the equation24
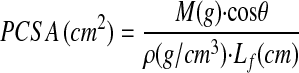 |
where θ is the pennation angle, M is muscle mass, and ρ is muscle density (1.112 g/cm3)30. The Lf/Lm ratio, which is an index of the excursion design, was also calculated. For example, muscles that contain fibers that span the entire length of the muscle (Lf/Lm ratio = 1.0) are designed more for excursion in comparison with muscles that have fibers spanning half of the length of the muscle (Lf/Lm ratio = 0.5). This ratio is a useful parameter to consider because it is independent of the absolute magnitude of muscle fiber length. The physiological cross-sectional area was determined because it is the only muscle structural parameter known to accurately predict the maximum force produced by a muscle24.
In Vivo Sarcomere Lengths
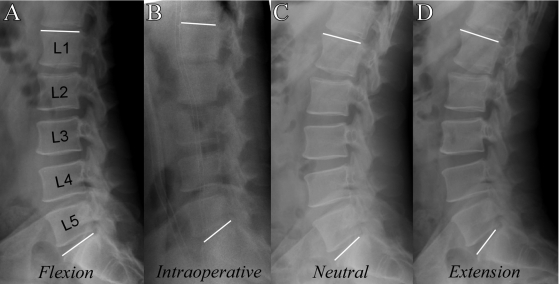
Under a University of California at San Diego Human Research Protections Program-approved protocol, multifidus muscle specimens were obtained from sixteen patients undergoing spinal surgery (Table II). After skin incision, the dorsolumbar fascia was incised and the multifidus muscle was identified by its position adjacent to the spinous process and the cranial/medial-to-caudal/lateral projection of its fibers. A small segment of the multifidus on the posterolateral region of the muscle belly (between L4 and S1) (Table II) was isolated by means of blunt dissection along natural fascicular planes with Metzenbaum scissors. A specialized clamp was then slipped over the bundle, with care being taken to avoid undue manipulation or tension on the muscle. The clamp was deployed, and the section of muscle within the jaws of the clamp was resected and immediately was placed in Formalin to fix the biopsy specimen in its in vivo configuration. Laser diffraction was then used (as described above) to measure sarcomere lengths27. This method was used because the depth of the muscle within the surgical field precluded intraoperative laser diffraction measurements. Pilot experiments comparing in situ laser diffraction with this clamping method demonstrated that clamped sarcomere lengths were within 8% of their in vivo lengths (unpublished data). Depending on the surgical procedure to be performed, either a Jackson Spinal Table (Mizuhosi, Union City, California) was used to position the spine in prone (near-extension) (n = 8) or a Wilson Frame (Mizuhosi) was used to position the spine in flexion (n = 8). The intraoperative lumbar spine position was quantified and was interpreted by measuring the intraoperative L1-S1 angle and comparing it with that on the preoperative flexion, neutral, and extension lateral plain radiographs (Fig. 2).
TABLE II.
Patient Data and Biopsy Findings
| Characteristic | In Vivo Sarcomere Length Study | Passive Mechanics Study (Subcohort) |
|---|---|---|
| Age (yr) | 58 ± 5 | 56 ± 4 |
| Male:female ratio (no. of patients) | 7:9 | 6:8 |
| Biopsy level (L4-L5/L5-S1) (no. of patients) | 11/5 | 10/4 |
| Diagnosis | ||
| Osseous | 9 | 10 |
| Disc | 6 | 3 |
| Trauma | 1 | 1 |
| Fiber diameter*(mm) | — | 0.105 ± 0.003 |
| Slack sarcomere length*(μm) | — | 2.15 ± 0.05 |
| Failure sarcomere length*(μm) | — | 6.74 ± 0.33 |
| Elastic modulus*(kPa) | — | 36.87 ± 1.89 |
The values are given as the mean and the standard error based on three fibers per biopsy specimen (n = 14 specimens).
Fig. 2.
Representative preoperative and intraoperative lateral radiographs of the spine, made in the various conditions measured. Note that these conditions were used to determine the physiological conditions under which biopsy specimens were obtained for the in vivo data plotted in Fig. 5. A: Preoperative standing flexion lateral radiograph. B: Intraoperative lateral radiograph. C: Preoperative standing neutral lateral radiograph. D: Preoperative standing extension lateral radiograph.
Passive Single Cell Mechanics
A second biopsy specimen was obtained from a subcohort of fourteen of the sixteen patients and was immediately placed in a muscle “relaxing solution” composed of ethylene glycol bis (2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) (7.5 mmol/L), potassium propionate (170 mmol/L), magnesium acetate (2 mmol/L), imidazole (5 mmol/L), creatine phosphate (10 mmol/L), adenosine triphosphate (ATP) (4 mmol/L), leupeptin (a protease inhibitor) (17 mg/mL), and E-64 (a protease inhibitor) (4 mg/mL)31. This solution prevented depolarization across any site of disrupted membrane and proteolytic degradation, either of which can destroy the specimen. Muscle fibers were either immediately dissected from the fresh biopsy specimen or were placed into a storage solution composed of relaxing solution mixed with 50% glycerol and stored at −20°C. Fibers stored in this manner have been shown to have stable mechanical properties for as long as three months31,32, but all fibers in the present study were tested within twenty-one days. Stored fibers showed no signs of deterioration such as alterations in translucency or structural abnormalities.
The fiber-testing protocol was designed to measure the muscle fiber's elastic properties apart from any velocity-dependent properties, as previously described33. Briefly, the dissected single-fiber segment was secured on either side to 125-μm titanium wires with use of 10-0 silk suture loops. One wire was secured to an ultrasensitive force transducer (Model 405 A [sensitivity, 10 V/g]; Aurora Scientific, Aurora, Ontario, Canada), and the other was secured to a micromanipulator. The fiber was transilluminated with a 7-mW helium-neon laser to permit sarcomere length measurement by means of laser diffraction34. The resolution of this method is approximately 5 nm35. The system was calibrated with a 2.50-μm plastic blazed diffraction grating before experimentation (Diffraction Gratings, Nashville, Tennessee). Following calibration and mounting, fibers were lengthened until a force registered on a load cell that defined baseline load and slack sarcomere length. To define fiber elastic modulus, mounted fibers were lengthened in 250-μm increments, after which stress-relaxation was permitted for two minutes and both sarcomere length and tension were again recorded. Segments were elongated until mechanical failure. The slope of the stress-strain curve between 2.0 and 4.25 μm was defined as the elastic modulus, and the sarcomere length that was recorded prior to the lengthening that resulted in failure was defined as peak sarcomere length. Fibers were discarded if they did not produce a clear diffraction pattern, if any irregularities appeared along their length, or if they were severed or slipped at either suture-attachment point during the test.
Data Analysis
Whole-muscle comparisons between the multifidus and the other lumbar spine muscles were made with independent-samples t tests with use of the means, standard deviations, and sample sizes reported by Delp et al.4. After screening of the data for normality and homogeneity of variances, regional comparisons within the muscle were made with use of one-way analyses of variance with repeated measures. Post hoc t tests with Sidak corrections were used to identify specific regional differences when main effects were identified. The Sidak correction was used because it corrects for multiple comparisons, yet it is not overly conservative as compared with the Bonferroni correction.
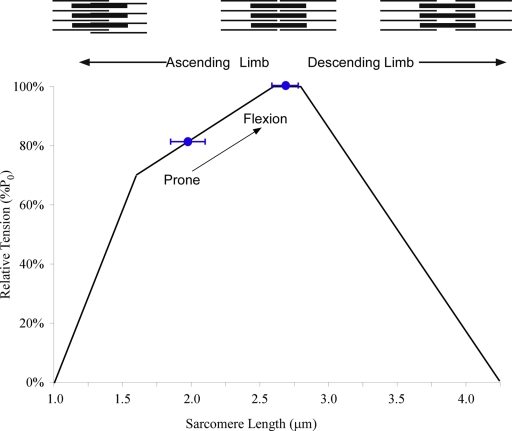
In vivo sarcomere lengths are reported for both prone (near-extended) and flexed lumbar spine positions on a graphical representation of the human sarcomere length-tension curve27. For passive mechanical testing, fiber diameter, slack sarcomere length, failure (peak) sarcomere length, and elastic modulus were determined. Modulus was defined by the slope of a least-squares fit of the stress-strain curve between sarcomere lengths of 2.0 and 4.25 μm, which represents the physiological upper limit of actin and myosin filament overlap in humans. To provide context for the modulus value, these data were compared (with use of an independent-samples t test) with previously published data for the vastus lateralis36 and antebrachial muscles37.
All values are reported as the mean and the standard error unless otherwise noted. P values were set to 0.05 except for post hoc tests, for which the experimentwise p value of 0.05 was adjusted according to the Sidak correction for multiple comparisons.
Source of Funding
DePuy provided material support for cadaveric specimens. The National Institutes of Health provided salary support.
Results
Cadaver Data
The average mass of the multifidus muscle (146.1 ± 8.7 g) was similar to those that have been reported for other lumbar spine muscles4, including the rectus abdominis (185.0 ± 13.6 g), quadratus lumborum (41.2 ± 1.7 g), spinalis thoracis (20.4 ± 2.7 g), longissimus thoracis (146.8 ± 13.9 g), and iliocostalis lumborum (121.8 ± 13.4 g). In this context, the multifidus was only larger than the spinalis thoracis and quadratus lumborum muscles. When the multifidus was subdivided into thirds (including the T12 and L1 origins, L2 and L3 origins, and L4 and L5 origins), the middle third of the muscle encompassed the bulk (60.6% ± 2.6%) of the muscle's mass, which was significantly greater than either the proximal third (10.1% ± 0.7%; p < 0.05) or the distal third (29.3% ± 3.1%; p < 0.05).
Physiological Cross-Sectional Area
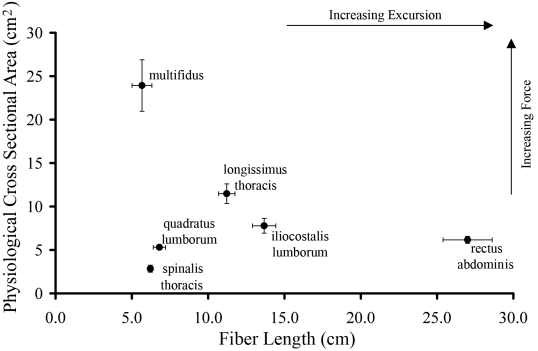
In contrast to the mass data, the physiological cross-sectional area was impressively large in the multifidus muscle (23.9 ± 3.0 cm2). This value was more than twice as large as that for any other muscle in the lumbar region (p < 0.05) (Fig. 3) in spite of the fact that its mass was similar to that of the longissimus thoracis and smaller than that of the rectus abdominis.
Fig. 3.
Scatterplot showing the relationship between physiological cross-sectional area and fiber length. As physiological cross-sectional area is proportional to muscle force and fiber length is proportional to muscle excursion, this type of plot illustrates the functional design of a muscle. These data illustrate that the multifidus has the largest force-generating capacity in the lumbar spine and is designed for stability. (The data on muscles other than the multifidus were adapted from the article by Delp et al.4.)
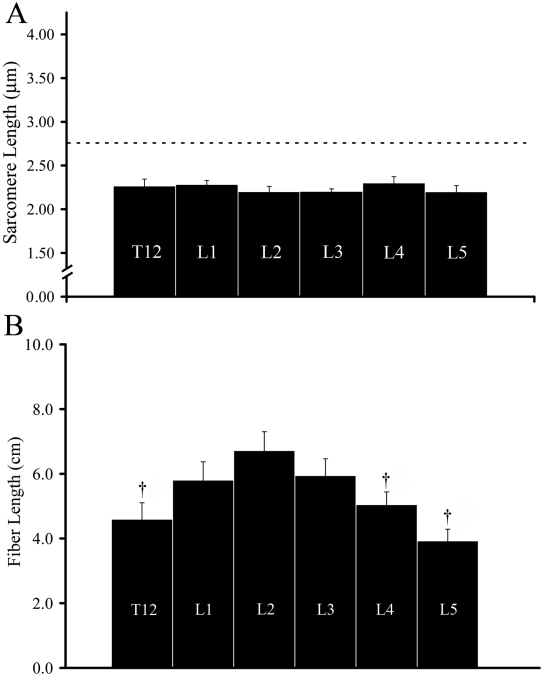
Multifidus Sarcomere and Fiber Length
Sarcomere lengths, as measured in cadaver muscle fascicles with the specimen in a supine position, were very short (average, 2.27 ± 0.06 μm) (Fig. 4, A), suggesting that this muscle operates on the ascending limb of the sarcomere length-tension curve in this position. When the muscle was divided by segmental level of origin, all fascicles had nearly identical sarcomere lengths (coefficient of variation, 4.2%) (Fig. 4, A). Muscle fiber lengths were very short throughout the length of the muscle in terms of absolute values (average, 5.66 ± 0.65 cm) and relative values (Lf/Lm = 0.21 ± 0.03), and both of these parameters varied significantly and systematically among segmental origin levels (p < 0.05) (Fig. 4, B). Post hoc analysis demonstrated that fibers originating from the L1, L2, and L3 spinal levels were significantly longer than fibers originating from the T12, L4, and L5 spinal levels (Fig. 4, B).
Fig. 4.
Bar graphs showing muscle characteristics as a function of segmental level of origin. A: Sarcomere length. B: Fiber length. The dotted line in A indicates optimal sarcomere length in human skeletal muscle. Sarcomere length did not vary among segmental levels, but muscle fibers originating from T12, L4, and L5 were significantly shorter compared with those arising from L1, L2, and L3. †p < 0.05.
In Vivo Data
For the in vivo clamped muscle biopsy specimens, sarcomere lengths were also short (average, 1.98 ± 0.15 μm) with the spine in the prone position, supporting our observations in cadavers. When the spine was flexed (average, 41.4° ± 3.5°), thereby lengthening the muscle, significantly longer sarcomere lengths were observed (average, 2.70 ± 0.11 μm; p < 0.05). However, throughout the range of motion that could be achieved intraoperatively, the muscle operated exclusively on the ascending and plateau regions of the length-tension curve (Fig. 5).
Fig. 5.
Sarcomere length operating range of the multifidus plotted on the human skeletal muscle sarcomere length-tension curve (black line). Blue circles represent average sarcomere length obtained by means of biopsy with the patient in the prone (n = 8) or lumbar flexion (n = 5) position. These data demonstrate that the multifidus muscle operates on the ascending limb of the length-tension curve and becomes intrinsically stronger as the spine is flexed (arrow). Schematic sarcomeres are shown on the ascending and descending limb to scale, based on the quantification of actin and myosin filament lengths reported previously27.
Passive Single-Fiber Mechanics
In contrast to the very unique intraoperative sarcomere lengths, multifidus fibers were similar to other limb muscles located throughout the body with respect to mechanical properties (Table II)36,37. Specifically, slack sarcomere length in the multifidus (2.15 ± 0.05 μm) was similar (p > 0.05) to those in the vastus lateralis (2.39 ± 0.28 μm)36 and a variety of antebrachial muscles, including the extensor digitorum, extensor pollicis, and brachioradialis (2.20 ± 0.04 μm)37. Similarly, the elastic modulus of the multifidus (36.87 ± 1.89 kPa) was similar to those of the vastus lateralis (31.9 ± 4.3 kPa)36 and a variety of upper extremity muscles (28.25 ± 3.31 kPa)37.
Discussion
The data from the present study confirmed our architectural hypothesis that the multifidus muscle has a large physiological cross-sectional area and short muscle fibers. In terms of physiology, our original hypotheses were not supported as the multifidus muscle operates on a relatively narrow portion (the ascending and plateau regions) of the length-tension curve and has passive mechanical properties that are similar to those of many other muscles. However, taken together, these data confirm the unique stabilizing function of the muscle.
The present study demonstrates that the multifidus muscle stands out among all other lumbar muscles, and indeed many extremity muscles, as a most extreme example of a muscle designed to stabilize the lumbar spine against flexion. This functional design was elucidated by means of intraoperative laser diffraction and quantitative architecture measurements and demonstrated (1) an extremely high physiological cross-sectional area, greater than that of any other lumbar spine muscle, and (2) a sarcomere length range exclusively on the ascending portion of the length-tension curve. The large physiological cross-sectional area and relatively short fibers indicate that the multifidus muscle is architecturally designed to produce very large forces over a narrow range of lengths. This design allows the multifidus muscle to function more to stabilize the spine and less to provide motion of the spine. As a stabilizer, it acts to maintain optimal joint forces throughout the spine as the body assumes various positions requiring prolonged flexion (such as assembly line work) or extension (such as standing).
In terms of upper extremity muscles, studies of human muscle design20,29,38 have relied on physiological cross-sectional area to determine which muscles are most appropriate for surgical tendon transfer20,39. Until now, the unique capacity of the multifidus has been missed in previous studies precisely because its physiological cross-sectional area was not measured. Our architectural analysis reveals the obvious basis for its extreme physiological cross-sectional area: high mass combined with very short fibers. This design permits packing of a very large number of muscle fibers into the fairly constrained space of the lumbar spine. Previous studies of this muscle have only characterized its mass2,3,5, length2,3,5, or a slice of its cross-sectional area12, all of which have marginal functional relevance40.
The findings of the present study support the concept that the uniqueness of the multifidus muscle does not lie in the uniqueness of its fibers but in the uniqueness of its fiber arrangement. From a biomechanical and structural standpoint, multifidus fibers are similar to other muscle fibers in terms of slack sarcomere lengths, elastic modulus, and whether fibers express the fast or slow myosin heavy chain isoform6,36. Compared with upper extremity muscles41 and more recent data on the quadriceps muscle36, the multifidus yields slack sarcomere lengths of about 2.2 μm and elastic moduli of about 35 kPa. This indicates that the fibers themselves have biomechanical properties comparable with those of other muscles in the body. One might have expected that the slack sarcomere length would be much shorter and the modulus much greater in comparison with those of other muscles because of its sarcomere length operating range, but this was not the case. Thus, previous studies that emphasized intrinsic features of the paraspinal muscles (such as fiber type or fiber size6) and did not measure architecture likely overlooked the principal attribute that makes the multifidus muscle a powerful spine stabilizer.
The measurement of sarcomere lengths in the present study permitted the discovery of a second important design feature of the multifidus muscle, specifically, that it is designed to operate on the ascending portion and plateau region of the sarcomere length-tension curve (Fig. 5). The sarcomere length-tension relationship is one of the classic structure-function relationships in all of biology. The anatomical basis of this relationship is the changing interdigitation of actin and myosin filaments as sarcomere length is changed. Thus, at very short lengths (the ascending portion), as sarcomere length increases, muscle force increases, whereas at longer lengths (the descending portion), as sarcomere length increases, muscle force decreases. Muscles in the upper extremities operate on both the ascending and descending limbs of the length-tension relationship27. However, the present study demonstrates that, unlike that of many limb muscles, the sarcomere length operating range of the multifidus muscle remains on the ascending portion of the length-tension curve as the spine flexes. In the present study, we were able to achieve a mean intraoperative flexion angle of 41.4° ± 3.5°, which compares favorably with maximum flexion values reported in the literature42. Operating on the ascending limb allows the multifidus muscle to become intrinsically stronger as the spine flexes until it would theoretically produce maximum force. This design is especially appealing as it creates a “proportional feedback” system in which the greater the deflection from neutral, the greater the restoring force. Clinically, operating on the ascending limb provides the necessary stabilizing force as the body leans forward, a position known to elevate intradiscal pressure and to cause increased low-back pain in patients with spinal disorders43,44. Secondarily, the data also demonstrate that the muscle does not lengthen sufficiently to operate onto the descending portion of the length tension curve, which would impair force production. Given this design feature, one could hypothesize that if the muscle operated at longer lengths, stability would be compromised. Whether chronic changes in sarcomere length operating range could result from disc disease, deformity, chronic disuse, or surgical trauma is not yet known. Current studies are underway in our laboratory to investigate such effects.
An important consideration in spine surgery is the trauma caused to the multifidus muscle during standard posterior midline approaches. Some of these open surgical approaches remove the spinous processes to which the paraspinal muscles attach, disrupt the neurovascular supply, and compress the muscle with prolonged retraction, leading to adverse histologic and biochemical changes45,46. Current minimally invasive surgical procedures that have been developed for spine surgery offer the unique opportunity to perform many of the same surgical procedures with less disruption to the major spine extensor musculature. These results provide a theoretical backdrop that argues for protection of the multifidus muscle during surgery, but more data are needed to determine whether muscle protection is causally related to the short-term benefits associated with minimally invasive surgical techniques.
The findings of the present study have implications for orthopaedic clinical practice. The importance of the multifidus muscle to spine function (in terms of force production) must be considered. While we have not explicitly measured force production, the high-resolution physiological cross-sectional area measurements, such as performed here, are directly related to force-producing capacity, as demonstrated by Edgerton and colleagues24. Those investigators demonstrated that mammalian muscle generates approximately 250 kPa of stress under conditions of optimal sarcomere length and maximum activation. Using this value, we predict that the multifidus muscle as a whole could direct approximately 60 N of extension force to the spine (250 N/m2 × 0.24 m2), which is more than twice the amount that could be generated by any other lumbar extensor muscle.
Future studies are needed to determine the extent to which some of these design issues of the multifidus muscle apply to other lumbar extensors. In addition, the extent to which pathological changes can alter these properties is not clear. Finally, the underlying developmental processes that lead to such a unique muscle design as that of the multifidus are unknown but are currently under study47-49. 
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from DePuy Spine, the Department of Veterans Affairs Rehabilitation Research and Development, and NIH grants HD048501 and HD050837. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
Investigation performed at the Departments of Orthopaedic Surgery, Radiology, and Bioengineering, University of California at San Diego and Veterans Administration Medical Centers, San Diego, California
References
- 1.Bogduk N, Macintosh JE, Pearcy MJ. A universal model of the lumbar back muscles in the upright position. Spine. 1992;17:897-913. [DOI] [PubMed] [Google Scholar]
- 2.Macintosh JE, Bogduk N. The biomechanics of the lumbar multifidus. Clin Biomech (Bristol, Avon). 1986;1:205-13. [DOI] [PubMed] [Google Scholar]
- 3.Macintosh JE, Pearcy MJ, Bogduk N. The axial torque of the lumbar back muscles: torsion strength of the back muscles. Aust N Z J Surg. 1993;63:205-12. [DOI] [PubMed] [Google Scholar]
- 4.Delp SL, Suryanarayanan S, Murray WM, Uhlir J, Triolo RJ. Architecture of the rectus abdominis, quadratus lumborum, and erector spinae. J Biomech. 2001;34:371-5. [DOI] [PubMed] [Google Scholar]
- 5.Stokes IA, Gardner-Morse M. Quantitative anatomy of the lumbar musculature. J Biomech. 1999;32:11-6. [DOI] [PubMed] [Google Scholar]
- 6.Rantanen J, Rissanen A, Kalimo H. Lumbar muscle fiber size and type distribution in normal subjects. Eur Spine J. 1994;3:331-5. [DOI] [PubMed] [Google Scholar]
- 7.Flicker PL, Fleckenstein JL, Ferry K, Payne J, Ward C, Mayer T, Parkey RW, Peshock RM. Lumbar muscle usage in chronic low back pain. Magnetic resonance image evaluation. Spine. 1993;18:582-6. [DOI] [PubMed] [Google Scholar]
- 8.Parkkola R, Rytoköski U, Kormano M. Magnetic resonance imaging of the discs and trunk muscles in patients with chronic low back pain and healthy control subjects. Spine. 1993;18:830-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhao WP, Kawaguchi Y, Matsui H, Kanamori M, Kimura T. Histochemistry and morphology of the multifidus muscle in lumbar disc herniation: comparative study between diseased and normal sides. Spine. 2000;25:2191-9. [DOI] [PubMed] [Google Scholar]
- 10.Chan YL, Cheng JC, Guo X, King AD, Griffith JF, Metreweli C. MRI evaluation of multifidus muscles in adolescent idiopathic scoliosis. Pediatr Radiol. 1999;29:360-3. [DOI] [PubMed] [Google Scholar]
- 11.Kang CH, Shin MJ, Kim SM, Lee SH, Lee CS. MRI of paraspinal muscles in lumbar degenerative kyphosis patients and control patients with chronic low back pain. Clin Radiol. 2007;62:479-86. [DOI] [PubMed] [Google Scholar]
- 12.Gille O, Jolivet E, Dousset V, Degrise C, Obeid I, Vital JM, Skalli W. Erector spinae muscle changes on magnetic resonance imaging following lumbar surgery through a posterior approach. Spine. 2007;32:1236-41. [DOI] [PubMed] [Google Scholar]
- 13.Sasaoka R, Nakamura H, Konishi S, Nagayama R, Suzuki E, Terai H, Takaoka K. Objective assessment of reduced invasiveness in MED. Compared with conventional one-level laminotomy. Eur Spine J. 2006;15:577-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazard RG. Failed back surgery syndrome: surgical and nonsurgical approaches. Clin Orthop Relat Res. 2006;443:228-32. [DOI] [PubMed] [Google Scholar]
- 15.Skaf G, Bouclaous C, Alaraj A, Chamoun R. Clinical outcome of surgical treatment of failed back surgery syndrome. Surg Neurol. 2005;64:483-9. [DOI] [PubMed] [Google Scholar]
- 16.Boswell MV, Trescot AM, Datta S, Schultz DM, Hansen HC, Abdi S, Sehgal N, Shah RV, Singh V, Benyamin RM, Patel VB, Buenaventura RM, Colson JD, Cordner HJ, Epter RS, Jasper JF, Dunbar EE, Atluri SL, Bowman RC, Deer TR, Swicegood JR, Staats PS, Smith HS, Burton AW, Kloth DS, Giordano J, Manchikanti L; American Society of Interventional Pain Physicians. Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician. 2007;10:7-111. [PubMed] [Google Scholar]
- 17.Taylor RS. Spinal cord stimulation in complex regional pain syndrome and refractory neuropathic back and leg pain/failed back surgery syndrome: results of a systematic review and meta-analysis. J Pain Symptom Manage. 2006;31(4 Suppl):S13-9. [DOI] [PubMed] [Google Scholar]
- 18.Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137-47. [DOI] [PubMed] [Google Scholar]
- 19.Kim KT, Lee SH, Suk KS, Bae SC. The quantitative analysis of tissue injury markers after mini-open lumbar fusion. Spine. 2006;31:712-6. [DOI] [PubMed] [Google Scholar]
- 20.Lieber RL, Jacobson MD, Fazeli BM, Abrams RA, Botte MJ. Architecture of selected muscles of the arm and forearm: anatomy and implications for tendon transfer. J Hand Surg [Am]. 1992;17:787-98. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson MD, Raab R, Fazeli BM, Abrams RA, Botte MJ, Lieber RL. Architectural design of the human intrinsic hand muscles. J Hand Surg [Am]. 1992;17A:804-9. [DOI] [PubMed] [Google Scholar]
- 22.Wickiewicz TL, Roy RR, Powell PL, Edgerton VR. Muscle architecture of the human lower limb. Clin Orthop Relat Res. 1983;179:275-83. [PubMed] [Google Scholar]
- 23.Friederich JA, Brand RA. Muscle fiber architecture in the human lower limb. J Biomech. 1990;23:91-5. [DOI] [PubMed] [Google Scholar]
- 24.Powell PL, Roy RR, Kanim P, Bello MA, Edgerton VR. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol. 1984;57:1715-21. [DOI] [PubMed] [Google Scholar]
- 25.Lieber RL, Friden J. Clinical significance of skeletal muscle architecture. Clin Orthop Relat Res. 2001;383:140-51. [DOI] [PubMed] [Google Scholar]
- 26.Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng. 1990;37:757-67. [DOI] [PubMed] [Google Scholar]
- 27.Lieber RL, Loren GJ, Fridén J. In vivo measurement of human wrist extensor muscle sarcomere length changes. J Neurophysiol. 1994;71:874-81. [DOI] [PubMed] [Google Scholar]
- 28.Sacks RD, Roy RR. Architecture of the hindlimb muscles of cats: functional significance. J Morphol. 1982;173:185-95. [DOI] [PubMed] [Google Scholar]
- 29.Lieber RL, Fazeli BM, Botte MJ. Architecture of selected wrist flexor and extensor muscles. J Hand Surg [Am]. 1990;15:244-50. [DOI] [PubMed] [Google Scholar]
- 30.Ward SR, Lieber RL. Density and hydration of fresh and fixed skeletal muscle. J Biomech. 2005;38:2317-20. [DOI] [PubMed] [Google Scholar]
- 31.Wood DS, Zollman J, Reuban JP, Brandt PW. Human skeletal muscle: properties of the “chemically skinned” fiber. Science. 1975;187:1075-6. [DOI] [PubMed] [Google Scholar]
- 32.Moss RL. Sarcomere length-tension relations of frog skinned muscle fibers during calcium activation at short lengths. J Physiol. 1979;292:177-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fung YC. Biomechanics: mechanical properties of living tissues. New York: Springer; 1981. p 433.
- 34.Lieber RL, Yeh Y, Baskin RJ. Sarcomere length determination using laser diffraction. Effect of beam and fiber diameter. Biophys J. 1984;45:1007-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baskin RJ, Roos KP, Yeh Y. Light diffraction study of single skeletal muscle fibers. Biophys J. 1979;28:45-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boakes JL, Foran J, Ward SR, Lieber RL. Muscle adaptation by serial sarcomere addition 1 year after femoral lengthening. Clin Orthop Relat Res. 2007;456:250-3. [DOI] [PubMed] [Google Scholar]
- 37.Fridén J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. 2003;27:157-64. [DOI] [PubMed] [Google Scholar]
- 38.Fridén J, Albrecht D, Lieber RL. Biomechanical analysis of the brachioradialis as a donor in tendon transfer. Clin Orthop Relat Res. 2001;383:152-61. [DOI] [PubMed] [Google Scholar]
- 39.Abrams GD, Ward SR, Fridén J, Lieber RL. Pronator teres is an appropriate donor muscle for restoration of wrist and thumb extension. J Hand Surg [Am]. 2005;30:1068-73. [DOI] [PubMed] [Google Scholar]
- 40.Lieber RL, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647-66. [DOI] [PubMed] [Google Scholar]
- 41.Lieber RL, Runesson E, Einarsson F, Fridén J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve. 2003;28:464-71. [DOI] [PubMed] [Google Scholar]
- 42.Edmondston SJ, Song S, Bricknell RV, Davies PA, Fersum K, Humphries P, Wickenden D, Singer KP. MRI evaluation of lumbar spine flexion and extension in asymptomatic individuals. Man Ther. 2000;5:158-64. [DOI] [PubMed] [Google Scholar]
- 43.Andersson GB, Ortengren R, Nachemson A. Intradiskal pressure, intra-abdominal pressure and myoelectric back muscle activity related to posture and loading. Clin Orthop Relat Res. 1977;129:156-64. [DOI] [PubMed] [Google Scholar]
- 44.Snook SH, Webster BS, McGorry RW, Fogleman MT, McCann KB. The reduction of chronic nonspecific low back pain through the control of early morning lumbar flexion. A randomized controlled trial. Spine. 1998;23:2601-7. [DOI] [PubMed] [Google Scholar]
- 45.Weber BR, Grob D, Dvorák J, Müntener M. Posterior surgical approach to the lumbar spine and its effect on the multifidus muscle. Spine. 1997;22:1765-72. [DOI] [PubMed] [Google Scholar]
- 46.Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. Part 1: histologic and histochemical analyses in rats. Spine. 1994;19:2590-7. [DOI] [PubMed] [Google Scholar]
- 47.Gomez C, David V, Peet NM, Vico L, Chenu C, Malaval L, Skerry TM. Absence of mechanical loading in utero influences bone mass and architecture but not innervation in Myod-Myf5-deficient mice. J Anat. 2007;210:259-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Meulen T, Schipper H, van Leeuwen JL, Kranenbarg S. Effects of decreased muscle activity on developing axial musculature in nicb107 mutant zebrafish (Danio rerio). J Exp Biol. 2005;208(Pt 19):3675-87. [DOI] [PubMed] [Google Scholar]
- 49.Duxson MJ, Sheard PW. Formation of new myotubes occurs exclusively at the multiple innervation zones of an embryonic large muscle. Dev Dyn. 1995;204:391-405. [DOI] [PubMed] [Google Scholar]