Abstract
Background: Degeneration of the tibiofemoral articular cartilage often develops in patients with posterior cruciate ligament deficiency, yet little research has focused on the etiology of this specific type of cartilage degeneration. In this study, we hypothesized that posterior cruciate ligament deficiency changes the location and magnitude of cartilage deformation in the tibiofemoral joint.
Methods: Fourteen patients with a posterior cruciate ligament injury in one knee and the contralateral side intact participated in the study. First, both knees were imaged with use of a specific magnetic resonance imaging sequence to create three-dimensional knee models of the surfaces of the bone and cartilage. Next, each patient performed a single leg lunge as images were recorded with a dual fluoroscopic system at 0°, 30°, 60°, 75°, 90°, 105°, and 120° of knee flexion. Finally, the three-dimensional knee models and fluoroscopic images were used to reproduce the in vivo knee position at each flexion angle with use of a previously described image-matching method. With use of these series of knee models, the location and magnitude of peak tibiofemoral cartilage deformation at each flexion angle were compared between the intact contralateral and posterior cruciate ligament-deficient knees.
Results: In the medial compartment of the posterior cruciate ligament-deficient knees, the location and magnitude of peak cartilage deformation were significantly changed, compared with those in the intact contralateral knees, between 75° and 120° of flexion, with a more anterior and medial location of peak cartilage deformation on the tibial plateau as well as increased deformation of the cartilage. In the lateral compartment, no significant differences in the location or magnitude of peak cartilage deformation were found between the intact and posterior cruciate ligament-deficient knees.
Conclusions: The altered kinematics associated with posterior cruciate ligament deficiency resulted in a shift of the tibiofemoral contact location and an increase in cartilage deformation in the medial compartment beyond 75° of knee flexion. The magnitude of the medial contact shift in the posterior cruciate ligament-deficient knee was on the same order as that of the anterior contact shift.
Clinical Relevance: The observed changes in the location and magnitude of cartilage deformation in the tibiofemoral joint provide insight about the development of degeneration of the tibiofemoral joint cartilage in patients with posterior cruciate ligament deficiency. Our data also suggest that recreating mediolateral stability of posterior cruciate ligament-deficient knees might be of importance in addition to surgically improving anteroposterior translation.
Clinically, rupture of the posterior cruciate ligament is associated with posterior instability, patellar pain, and an increased prevalence of knee osteoarthritis1-6. These complications have led some clinicians and researchers in the past to advocate surgical reconstruction of the posterior cruciate ligament3,7,8. However, many studies have documented articular cartilage lesions and premature degenerative changes after posterior cruciate ligament reconstruction, even though posterior stability was successfully restored8-12. As a result, a substantial amount of research on intact, posterior cruciate ligament-deficient, and surgically reconstructed knees has been performed, in the hopes of developing new and improved strategies for the treatment of posterior cruciate ligament deficiency.
The posterior cruciate ligament is thought to act as the primary restraint to posterior tibial translation of the knee at higher flexion angles13-22. Apparently, the posterior cruciate ligament is not only oriented anteriorly with respect to the tibia to constrain posterior tibial translation, it is also oriented medially from its tibial to femoral attachment region so that it also constrains lateral translation of the tibia23,24. These findings could explain why posterior cruciate ligament deficiency not only increased posterior tibial translation and external tibial rotation16,25-27 but also increased lateral translation in our in vivo study of eight patients with posterior cruciate ligament deficiency24.
Despite the progress in our understanding of the anatomy and biomechanics of the posterior cruciate ligament, it remains unclear how and to what extent the subtle changes in in vivo joint kinematics that have been observed in posterior cruciate ligament-deficient knees contribute to the initiation of osteoarthritis. If a goal of the treatment of posterior cruciate ligament deficiency is the prevention of destruction of the tibiofemoral cartilage, then the impact of posterior cruciate ligament deficiency on the contact biomechanics of the cartilage needs to be understood.
The objective of this study was to investigate, with the use of a combined dual orthogonal fluoroscopic and magnetic resonance imaging technique, the effect of posterior cruciate ligament deficiency on the location of tibiofemoral cartilage contact as well as the magnitude of in vivo cartilage contact deformation during weight-bearing knee flexion28-30. We hypothesized that posterior cruciate ligament deficiency changes the location and magnitude of peak cartilage deformation in the tibiofemoral joint.
Materials and Methods
Fourteen patients (ten men and four women ranging in age from nineteen to sixty-four years old) with nine right and five left knees with a posterior cruciate ligament rupture documented by clinical examination (a positive posterior drawer test as measured by the senior author [T.J.G.]) and demonstrated by magnetic resonance imaging were included in this study (see Appendix). All subjects had a healthy contralateral knee. Injury to other ligaments, noticeable cartilage lesions, and injury to the underlying bone were reasons for exclusion from the study. Patients with a meniscal injury requiring removal of <50% of the meniscus were included in this study, since patients with an isolated posterior cruciate ligament injury and no damage to the meniscus are relatively rare and it is difficult to precisely quantify the extent to which the meniscus is damaged without arthroscopic examination. The purpose of the study was explained in detail to all of the subjects at the time of recruitment. Each subject signed a consent form that had been approved by our institutional review board. Eight of these fourteen subjects had been included in our previous study of the six-degrees-of-freedom tibiofemoral kinematics in patients with posterior cruciate ligament deficiency24.
Magnetic resonance and dual orthogonal fluoroscopic imaging techniques have been described in detail and validated in previous studies28,31-33. In brief, a protocol established in our laboratory was used to image both the left and the right knee with a magnetic resonance imaging scanner to create three-dimensional meshed models of the knees31. Each anatomic knee model included the osseous geometry of the femur, tibia, and fibula as well as the tibial and femoral cartilage layers. After the magnetic resonance image-based computer models were constructed, both knees of each subject were simultaneously imaged with use of two orthogonally placed fluoroscopes as the patient performed a single leg quasistatic lunge at 0°, 30°, 60°, 75°, 90°, 105°, and 120° of flexion while the upper body remained upright. Next, the fluoroscopic images were imported into solid-modeling software and placed in the orthogonal planes on the basis of the position of the fluoroscopes during the imaging of the patient. In the following step, the three-dimensional magnetic resonance image-based knee model of the patient was imported into the same software, viewed from the two orthogonal directions corresponding to the orthogonal fluoroscopic setup used to acquire the images, and independently manipulated in six degrees of freedom inside the software until the projections of the model matched the outlines of the fluoroscopic images. When the projections match the outlines of the images made during in vivo knee flexion, the model reproduces the in vivo position of the knee. Finally, the relative positions of the cartilage layers on the femur and tibia were determined from the series of models used to reproduce knee motion. When cartilage contact occurred during knee flexion, the articular surface meshes of the tibia and femur overlapped.
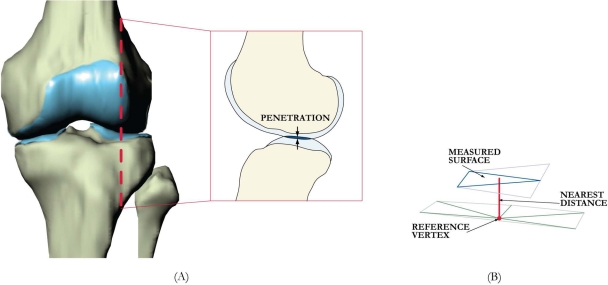
In this study, cartilage deformation was defined for each vertex of the articular surface mesh as the amount of penetration (Fig. 1, A) divided by the sum of the tibial and femoral cartilage surface thicknesses33,34. The cartilage thickness was calculated by finding the smallest Euclidian distance connecting a vertex of the articular surface to the cartilage-bone interface (Fig. 1, B).
Fig. 1.
A: Sagittal section of a left knee, illustrating cartilage penetration (defined in this study as cartilage deformation). B: Method of measuring cartilage thickness and penetration depth from meshed surfaces.
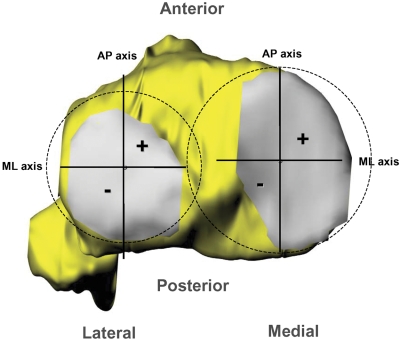
The locations of peak cartilage deformation were referenced to Cartesian coordinate systems on the tibial plateaus, as has been described in detail in previous publications (Fig. 2)30,32,35. The origin of each coordinate system was located by the center of a circle, which was fit to the posterior edge of each tibial compartment. The anteroposterior and mediolateral axes split each tibial plateau into quadrants. In the anteroposterior direction, a location anterior to the mediolateral axis was considered positive. In the mediolateral direction, a location medial to the anteroposterior axis was considered positive.
Fig. 2.
The Cartesian coordinate system for the tibial plateau. In the anteroposterior (AP) direction, a location anterior to the mediolateral axis was considered positive. In the mediolateral (ML) direction, a location medial to the anteroposterior axis was considered positive.
A one-way repeated-measures analysis of variance and the Student-Newman-Keuls test were used to compare the location and magnitude of peak cartilage deformation of the intact contralateral knees with those of the posterior cruciate ligament-deficient knees. Differences were considered significant at the level of p < 0.05.
Source of Funding
The funding sources had no involvement in the study design; in the collection, analysis, or interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
Results
Location of Peak Cartilage Deformation
Healthy Contralateral Knees
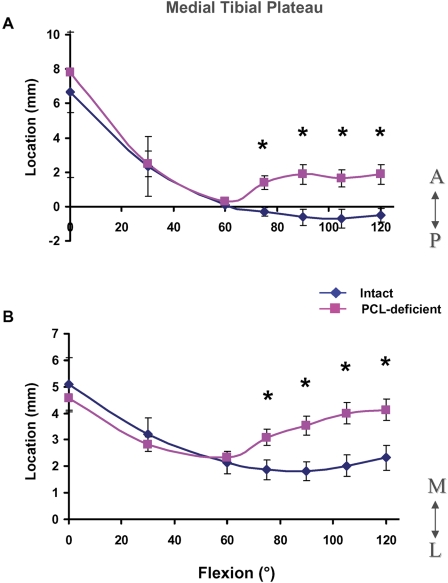
In the medial tibial plateau, peak deformation moved posteromedially with flexion, from a mean (and standard deviation) of 6.7 ± 2.1 mm anterior to the mediolateral axis and 5.1 ± 1.4 mm medial to the anteroposterior axis at 0° to a mean of −0.5 ± 0.2 mm posterior to the mediolateral axis (Fig. 3, A) and 2.0 ± 0.3 mm medial to the anteroposterior axis (Fig. 3, B) at 60°, where it remained for the rest of flexion.
Fig. 3.
Location of peak cartilage deformation on the medial tibial plateau in the anteroposterior (A) and mediolateral (B) directions in the intact and posterior cruciate ligament (PCL)-deficient knees as a function of knee flexion angle. A = anterior to mediolateral axis, P = posterior to mediolateral axis, M = medial to anteroposterior axis, and L = lateral to anteroposterior axis. The values are given as the mean and standard deviation. *P < 0.05 as determined with one-way repeated-measures analysis of variance.
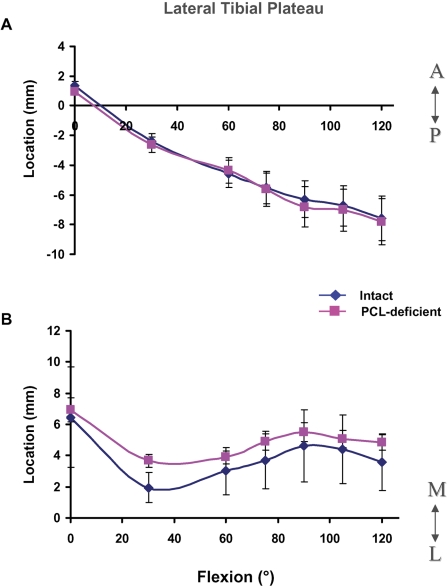
In the lateral tibial plateau, peak deformation moved posteriorly throughout flexion, from a mean of 1.4 ± 2.3 mm anterior to the mediolateral axis at 0° to a mean of −7.6 ± 2.1 mm posterior to the mediolateral axis at 120° (Fig. 4, A). Peak deformation moved laterally in the lateral tibial plateau until 30°, at which point it was 1.9 ± 4.4 mm from the anteroposterior axis; peak deformation then moved medially until 90°, reaching a value of 4.6 ± 2.2 mm from the anteroposterior axis (Fig. 4, B).
Fig. 4.
Location of peak cartilage deformation on the lateral tibial plateau in the anteroposterior (A) and mediolateral (B) directions in the intact and posterior cruciate ligament (PCL)-deficient knees as a function of knee flexion angle. A = anterior to mediolateral axis, P = posterior to mediolateral axis, M = medial to anteroposterior axis, and L = lateral to anteroposterior axis. The values are given as the mean and standard deviation.
Posterior Cruciate Ligament-Deficient Knees
In the medial compartment, posterior cruciate ligament deficiency significantly changed the location of peak cartilage deformation between 75° and 120° of flexion, with the posterior cruciate ligament-deficient knee having a more anterior and medial cartilage contact location as compared with the intact knee. During flexion of ≥75°, contact in the medial compartment shifted anteriorly by an average of 2.2 ± 0.4 mm (Fig. 3, A) and medially by an average of 1.9 ± 0.4 mm (Fig. 3, B) as compared with the location in the intact knees. These values include the data of the four subjects with a partial meniscectomy. Contact in the medial compartments of the four posterior cruciate ligament-deficient knees with partial meniscectomy shifted anteriorly by an average of 2.1 ± 0.5 mm and medially by an average of 2.0 ± 0.5 mm between 75° and 120° of flexion.
In the lateral compartment, no significant differences in the anteroposterior and mediolateral motion of cartilage contact were observed between the intact and posterior cruciate ligament-deficient knees (Fig. 4, A and B).
Magnitude of Peak Cartilage Deformation
Healthy Contralateral Knees
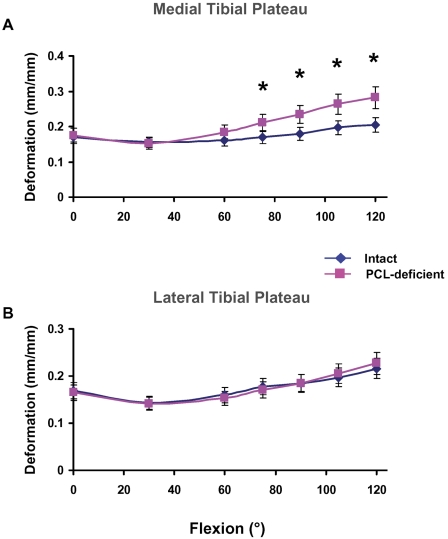
In the medial compartment, peak deformation increased with flexion, from a mean of 0.17 ± 0.09 mm/mm at full extension to a mean of 0.20 ± 0.07 mm/mm at 120° of flexion (Fig. 5, A). In the lateral compartment, a similar increase in peak deformation was observed, with mean values of 0.17 ± 0.08 mm/mm at full extension and 0.22 ± 0.07 mm/mm at 120° of flexion (Fig. 5, B).
Fig. 5.
Magnitude of peak cartilage deformation on the medial (A) and lateral (B) tibial plateaus in the intact and posterior cruciate ligament (PCL)-deficient knees. *P < 0.05 as determined with one-way repeated-measures analysis of variance.
Posterior Cruciate Ligament-Deficient Knees
In the medial compartment, rupture of the posterior cruciate ligament caused a significant gradual increase in cartilage deformation, as compared with that in the intact knees, from 75° to 120° of flexion (Fig. 5, A). The maximum increase in cartilage deformation after posterior cruciate ligament rupture occurred at 120° of flexion (0.20 ± 0.07 mm/mm in the intact knee compared with 0.28 ± 0.08 mm/mm in the posterior cruciate ligament-deficient knee). We did not detect significant differences in cartilage deformation from 0° to 60° of flexion between the healthy and posterior cruciate ligament-deficient knees.
In the lateral compartment, we did not detect any significant differences in cartilage deformation between the intact and posterior cruciate ligament-deficient knees throughout the range of flexion (Fig. 5, B).
Discussion
In this study, we investigated the location and magnitude of tibiofemoral cartilage deformation in posterior cruciate ligament-deficient knees. In the medial compartment of these knees, the location and magnitude of peak cartilage deformation were significantly changed, as compared with the findings in the intact contralateral knees, between 75° and 120° of flexion, with a more anterior and medial location as well as an increased magnitude. In the lateral compartment, no significant differences were found in the location or magnitude of peak tibiofemoral cartilage deformation between the intact and posterior cruciate ligament-deficient knees.
In our previous in vivo analysis of tibiofemoral joint kinematics, posterior cruciate ligament-deficient knees displayed increased posterior tibial translation beyond 30° of flexion compared with that in healthy control knees24. Similar findings have been well documented in the literature16,25-27,36. However, posterior cruciate ligament deficiency also resulted in an average 1.1-mm increase in lateral tibial translation at 90° of flexion. The findings of the present analysis of cartilage deformation in posterior cruciate ligament-deficient knees are consistent with these altered tibiofemoral joint kinematics, as the increased posterior and lateral tibial translation could be related, respectively, to the increased anterior and medial locations of cartilage deformation on the medial tibial plateau.
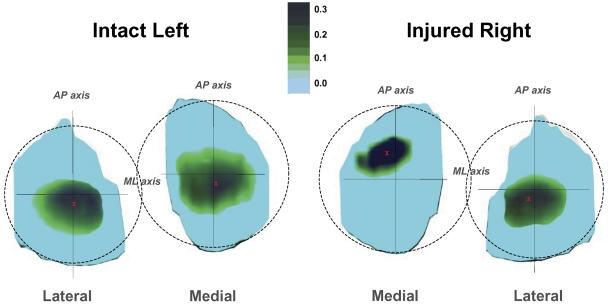
In normal knees, cartilage is up to 50% thicker in regions where cartilage-to-cartilage contact is present35. Healthy cartilage is believed to adapt to mechanical stimuli37,38 and ultimately become dependent on the maintenance of the mechanical stimulus for normal tissue function39. The thicker cartilage within the cartilage-to-cartilage contact area may result in a reduced contact stress, as was demonstrated by a three-dimensional finite-element analysis suggesting that thicker cartilage bears a lower peak contact stress than thinner cartilage under the same loading conditions40. In the present study, we found that posterior cruciate ligament deficiency significantly altered tibiofemoral cartilage contact in both the anteroposterior and the mediolateral direction in the medial compartment of the knee at higher flexion angles. In the presence of posterior cruciate ligament injury, cartilage contact not only shifted anteriorly, as was expected on the basis of increased posterior tibial translation, but also medially on the surface of the tibial plateau in flexion positions of ≥75°, forcing the femur to ride up the upslope of the medial tibial plateau, a region of thinner cartilage34,41. This medial shift of the location of cartilage contact after posterior cruciate ligament rupture to incongruent, thinner articular regions increased the cartilage deformation in those regions (Fig. 6). Increased cartilage deformation is associated with increased mechanical loading of the articular contact region within the knee, which in turn has been linked to higher rates of progression of osteoarthritis42. The relatively greater increase of cartilage deformation in the medial compartment compared with that in the lateral compartment observed in the posterior cruciate ligament-deficient knees in the present study is consistent with the reported increased development of osteoarthritis in the medial compartment of the knee joint following posterior cruciate ligament injury2,4.
Fig. 6.
Color map of the cartilage deformation in the intact (left) and posterior cruciate ligament-deficient (right) knees of a typical subject with the knees in 105° of flexion, illustrating the effect of posterior cruciate ligament deficiency on the location and magnitude of cartilage deformation at flexion angles beyond 75°. The red X on the cartilage surfaces indicates the location of peak cartilage deformation. Note the more anterior (relative to the mediolateral axis) and medial (relative to the anteroposterior axis) contact location of cartilage deformation in the medial compartment of the posterior cruciate ligament-deficient knee. The darker color depicts a higher magnitude of peak cartilage deformation (in millimeters). The illustrative color map was created by assigning an increasingly darker color value to each increased value of cartilage deformation for each vertex of the articular surface mesh. AP = anteroposterior, and ML = mediolateral.
As the presented data were obtained during only one functional in vivo activity—namely, the single leg lunge—we advise caution when extrapolating the data to other functional activities. Nevertheless, we believe that these findings might be useful for the design of improved treatment protocols for posterior cruciate ligament deficiency. First, as we did not detect differences in the cartilage biomechanics between the intact and posterior cruciate ligament-deficient knees during the single leg lunge between 0° and 60° of flexion, our findings suggest that rehabilitation exercises might be safely performed in this range of flexion. On the other hand, repetitive deep knee squats should be avoided by subjects with posterior cruciate ligament deficiency, so as not to increase the tibiofemoral cartilage deformation. Second, it is interesting to note that the magnitude of medial contact shift in the posterior cruciate ligament-deficient knees was on the same order as the magnitude of the anterior contact shift. This suggests that, when a posterior cruciate ligament reconstruction is performed with either a single or a double-bundle graft, recreation of the mediolateral stability of posterior cruciate ligament-deficient knees may be as important as the surgical improvement of anteroposterior translation. Finally, in a recent cadaver study, Giffin et al. demonstrated, with a robotic testing system, that increasing the tibial slope with a sagittal osteotomy successfully reduced the abnormal tibial sag in the posterior cruciate ligament-deficient knee, shifting the resting position of the tibia anteriorly43. On the basis of our data, it could be theorized in future studies that the osteotomy should include a varus component, possibly reducing the abnormal medial shift following posterior cruciate ligament injury and thereby possibly reducing the increase in cartilage deformation.
This study had limitations. As a result of the constraints of the imaging technique, motion and deformation of the meniscus were not detectable on the fluoroscopic images. However, on the basis of our previous validation of the imaging technique32, we do not believe that this limitation affected the cartilage-cartilage contact data, as articular surface mesh penetration was recorded only at the location of in vivo tibiofemoral cartilage contact. In addition, as mentioned earlier, we acquired data during only one functional activity, a single leg lunge. Other in vivo activities such as walking, running, and stair climbing should be considered in future studies. Furthermore, it should be noted that no ground reaction forces were measured in this study. In the future, a force-plate will be incorporated into the system. Since isolated posterior cruciate ligament injuries are rare, we included some patients who had a partial tear of one of the menisci. Because there were only fourteen subjects in our study, there was not enough statistical power for us to analyze the effect of partial removal of the meniscus as well. The findings from this study might therefore have been affected by the meniscal tears. Another limitation is that the patients were investigated at different time intervals after the injury. In future studies, patients in whom posterior cruciate ligament deficiency has been treated conservatively should be followed for longer periods with use of a methodology similar to that employed in our study. Tibiofemoral contact and the health of the cartilage could therefore be monitored over time to quantify any possible biomechanical relationships.
In conclusion, the altered kinematics caused by posterior cruciate ligament deficiency resulted in a shift of the tibiofemoral contact location and an increase in the in vivo deformation beyond 75° of flexion in the medial compartment. This injurious “jab-and-cross combo” provides insight about the development of degeneration of tibiofemoral joint cartilage in patients with posterior cruciate ligament deficiency. If the prevention of osteoarthritis in patients with posterior cruciate ligament deficiency is a goal of the treating physician, the function of the injured ligament should be restored as closely to normal as possible, in both the anteroposterior and mediolateral directions, thereby possibly better normalizing the cartilage biomechanics of the knee.
Supplementary Material
Acknowledgments
Note: The authors thank Dr. Louis DeFrate, Dr. Kyung Nha, Dr. Dain Allred, and Mr. Ramprasad Papannagari for their technical assistance.
Appendix
A table presenting clinical details on all fourteen study subjects is available with the electronic versions of this article, on our web site at jbjs.org (go to the article citation and click on “Supplementary Material”) and on our quarterly CD/DVD (call our subscription department, at 781-449-9780, to order the CD or DVD). 
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health (Grant R01 AR052408-02), the National Football League Charities Foundation, and the Belgian American Educational Foundation. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
Investigation performed at the Bioengineering Laboratory, Department of Orthopaedic Surgery, Massachusetts General Hospital/Harvard Medical School, Boston, Massachusetts
References
- 1.Boynton MD, Tietjens BR. Long-term followup of the untreated isolated posterior cruciate ligament-deficient knee. Am J Sports Med. 1996;24:306-10. [DOI] [PubMed] [Google Scholar]
- 2.Clancy WG Jr, Shelbourne KD, Zoellner GB, Keene JS, Reider B, Rosenberg TD. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am. 1983;65:310-22. [PubMed] [Google Scholar]
- 3.Cross MJ, Powell JF. Long-term followup of posterior cruciate ligament rupture: a study of 116 cases. Am J Sports Med. 1984;12:292-7. [DOI] [PubMed] [Google Scholar]
- 4.Keller PM, Shelbourne KD, McCarroll JR, Rettig AC. Nonoperatively treated isolated posterior cruciate ligament injuries. Am J Sports Med. 1993;21:132-6. [DOI] [PubMed] [Google Scholar]
- 5.Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14:35-8. [DOI] [PubMed] [Google Scholar]
- 6.Shelbourne KD, Davis TJ, Patel DV. The natural history of acute, isolated, nonoperatively treated posterior cruciate ligament injuries. A prospective study. Am J Sports Med. 1999;27:276-83. [DOI] [PubMed] [Google Scholar]
- 7.Schulte KR, Chu ET, Fu FH. Arthroscopic posterior cruciate ligament reconstruction. Clin Sports Med. 1997;16:145-56. [DOI] [PubMed] [Google Scholar]
- 8.Moore HA, Larson RL. Posterior cruciate ligament injuries. Results of early surgical repair. Am J Sports Med. 1980;8:68-78. [DOI] [PubMed] [Google Scholar]
- 9.Marks PH, Cameron M, Fu FH. [Reconstruction of the cruciate ligaments with allogeneic transplants. Techniques, results and perspectives]. Orthopade. 1993;22:386-91. German. [PubMed] [Google Scholar]
- 10.Hughston JC, Bowden JA, Andrews JR, Norwood LA. Acute tears of the posterior cruciate ligament. Results of operative treatment. J Bone Joint Surg Am. 1980;62:438-50. [PubMed] [Google Scholar]
- 11.Pournaras J, Symeonides PP, Karkavelas G. The significance of the posterior cruciate ligament in the stability of the knee. An experimental study in dogs. J Bone Joint Surg Br. 1983;65:204-9. [DOI] [PubMed] [Google Scholar]
- 12.Richter M, Kiefer H, Hehl G, Kinzl L. Primary repair for posterior cruciate ligament injuries. An eight-year followup of fifty-three patients. Am J Sports Med. 1996;24:298-305. [DOI] [PubMed] [Google Scholar]
- 13.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62:259-70. [PubMed] [Google Scholar]
- 14.Fanelli GC, Giannotti BF, Edson CJ. The posterior cruciate ligament arthroscopic evaluation and treatment. Arthroscopy. 1994;10:673-88. [DOI] [PubMed] [Google Scholar]
- 15.Fox RJ, Harner CD, Sakane M, Carlin GJ, Woo SL. Determination of the in situ forces in the human posterior cruciate ligament using robotic technology. A cadaveric study. Am J Sports Med. 1998;26:395-401. [DOI] [PubMed] [Google Scholar]
- 16.Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg Am. 1987;69:233-42. [PubMed] [Google Scholar]
- 17.Markolf KL, Slauterbeck JR, Armstrong KL, Shapiro MS, Finerman GA. A biomechanical study of replacement of the posterior cruciate ligament with a graft. Part II: forces in the graft compared with forces in the intact ligament. J Bone Joint Surg Am. 1997;79:381-6. [DOI] [PubMed] [Google Scholar]
- 18.Noyes FR, Stowers SF, Grood ES, Cummings J, VanGinkel LA. Posterior subluxations of the medial and lateral tibiofemoral compartments. An in vitro ligament sectioning study in cadaveric knees. Am J Sports Med. 1993;21:407-14. [DOI] [PubMed] [Google Scholar]
- 19.Fukubayashi T, Torzilli PA, Sherman MF, Warren RF. An in vitro biomechanical evaluation of anterior-posterior motion of the knee. Tibial displacement, rotation, and torque. J Bone Joint Surg Am. 1982;64:258-64. [PubMed] [Google Scholar]
- 20.Race A, Amis AA. The mechanical properties of the two bundles of the human posterior cruciate ligament. J Biomech. 1994;27:13-24. [DOI] [PubMed] [Google Scholar]
- 21.Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clin Orthop Relat Res. 1975;106:216-31. [DOI] [PubMed] [Google Scholar]
- 22.Carlin GJ, Livesay GA, Harner CD, Ishibashi Y, Kim HS, Woo SL. In-situ forces in the human posterior cruciate ligament in response to posterior tibial loading. Ann Biomed Eng. 1996;24:193-7. [DOI] [PubMed] [Google Scholar]
- 23.Papannagari R, Defrate LE, Nha KW, Moses JM, Moussa M, Gill TJ, Li G. Function of posterior cruciate ligament bundles during in vivo knee flexion. Am J Sports Med. 2007;35:1507-12. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Papannagari R, Li M, Bingham JT, Nha KW, Allred D, Gill TJ. Effect of posterior cruciate ligament deficiency on in vivo translation and rotation of the knee during weightbearing flexion. Am J Sports Med. 2008;36:474-9. [DOI] [PubMed] [Google Scholar]
- 25.Gill TJ, DeFrate LE, Wang C, Carey CT, Zayontz S, Zarins B, Li G. The biomechanical effect of posterior cruciate ligament reconstruction on knee joint function. Kinematic response to simulated muscle loads. Am J Sports Med. 2003;31:530-6. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Gill TJ, DeFrate LE, Zayontz S, Glatt V, Zarins B. Biomechanical consequences of PCL deficiency in the knee under simulated muscle loads—an in vitro experimental study. J Orthop Res. 2002;20:887-92. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Most E, DeFrate LE, Suggs JF, Gill TJ, Rubash HE. Effect of the posterior cruciate ligament on posterior stability of the knee in high flexion. J Biomech. 2004;37:779-83. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Wuerz TH, DeFrate LE. Feasibility of using orthogonal fluoroscopic images to measure in vivo joint kinematics. J Biomech Eng. 2004;126:314-8. [DOI] [PubMed] [Google Scholar]
- 29.DeFrate LE, Sun H, Gill TJ, Rubash HE, Li G. In vivo tibiofemoral contact analysis using 3D MRI-based knee models. J Biomech. 2004;37:1499-504. [DOI] [PubMed] [Google Scholar]
- 30.Li G, DeFrate LE, Park SE, Gill TJ, Rubash HE. In vivo articular cartilage contact kinematics of the knee: an investigation using dual-orthogonal fluoroscopy and magnetic resonance image-based computer models. Am J Sports Med. 2005;33:102-7. [DOI] [PubMed] [Google Scholar]
- 31.Defrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med. 2006;34:1240-6. [DOI] [PubMed] [Google Scholar]
- 32.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88:1826-34. [DOI] [PubMed] [Google Scholar]
- 33.Li G, Wan L, Kozanek M. Determination of real-time in-vivo cartilage contact deformation in the ankle joint. J Biomech. 2008;41:128-36. [DOI] [PubMed] [Google Scholar]
- 34.Bingham JT, Papannagari R, Van de Velde SK, Gross C, Gill TJ, Rubash HE, Li G. In-vivo cartilage contact deformation in the healthy human tibiofemoral joint. Rheumatology (Oxford). 2008;47:1622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, Park SE, DeFrate LE, Schutzer ME, Ji L, Gill TJ, Rubash HE. The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-cartilage contact. Clin Biomech (Bristol, Avon). 2005;20:736-44. [DOI] [PubMed] [Google Scholar]
- 36.Logan M, Williams A, Lavelle J, Gedroyc W, Freeman M. The effect of posterior cruciate ligament deficiency on knee kinematics. Am J Sports Med. 2004;32:1915-22. [DOI] [PubMed] [Google Scholar]
- 37.Arokoski JP, Jurvelin JS, Vaatainen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports. 2000;10:186-98. [DOI] [PubMed] [Google Scholar]
- 38.Smith RL, Donlon BS, Gupta MK, Mohtai M, Das P, Carter DR, Cooke J, Gibbons G, Hutchinson N, Schurman DJ. Effects of fluid-induced shear on articular chondrocyte morphology and metabolism in vitro. J Orthop Res. 1995;13:824-31. [DOI] [PubMed] [Google Scholar]
- 39.Carter DR, Beaupré GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998;355 Suppl:S41-55. [DOI] [PubMed] [Google Scholar]
- 40.Li G, Lopez O, Rubash H. Variability of a three-dimensional finite element model constructed using magnetic resonance images of a knee for joint contact stress analysis. J Biomech Eng. 2001;123:341-6. [DOI] [PubMed] [Google Scholar]
- 41.Donahue TL, Hull ML, Rashid MM, Jacobs CR. A finite element model of the human knee joint for the study of tibio-femoral contact. J Biomech Eng. 2002;124:273-80. [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giffin JR, Stabile KJ, Zantop T, Vogrin TM, Woo SL, Harner CD. Importance of tibial slope for stability of the posterior cruciate ligament deficient knee. Am J Sports Med. 2007;35:1443-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.