Abstract
Background: Titanium implants that have been grit-blasted and acid-etched to produce a rough microtopography support more bone integration than do smooth-surfaced implants. In vitro studies have suggested that this is due to a stimulatory effect on osteoblasts. It is not known if grit-blasted and acid-etched Ti6Al4V implants also stimulate osteoblasts and increase bone formation clinically. In this study, we examined the effects of micrometer-scale-structured Ti6Al4V surfaces on cell responses in vitro and on tissue responses in vivo.
Methods: Ti6Al4V disks were either machined to produce smooth surfaces with an average roughness (Ra) of 0.2 μm or grit-blasted, resulting in an Ra of 2.0, 3.0, or 3.3 μm. Human osteoblast-like cells were cultured on the disks and on tissue culture polystyrene. The cell number, markers of osteoblast differentiation, and levels of local factors in the conditioned media were determined at confluence. In addition, Ti6Al4V pedicle screws with smooth or rough surfaces were implanted into the L4 and L5 vertebrae of fifteen two-year-old sheep. Osteointegration was evaluated at twelve weeks with histomorphometry and on the basis of removal torque.
Results: The cell numbers on the Ti6Al4V surfaces were lower than those on the tissue culture polystyrene; the effect was greatest on the roughest surface. The alkaline-phosphatase-specific activity of cell lysates was decreased in a surface-dependent manner, whereas osteocalcin, prostaglandin E2, transforming growth factor-β1, and osteoprotegerin levels were higher on the rough surfaces. Bone-implant contact was greater around the rough-surfaced Ti6Al4V screws, and the torque needed to remove the rough screws from the bone was more than twice that required to remove the smooth screws.
Conclusions: Increased micrometer-scale surface roughness increases osteoblast differentiation and local factor production in vitro, which may contribute to increased bone formation and osteointegration in vivo. There was a correlation between in vitro and in vivo observations, indicating that the use of screws with rough surfaces will result in better bone-implant contact and implant stability.
Clinical Relevance: The osteointegration of screws with rough microtopographies is likely to be better than that of screws with smoother surfaces.
In vitro studies have suggested that successful osteointegration of bone growth around an implant is dependent on the surface properties of the implant material, including surface morphology, chemical composition, and surface energy1. Surface microtopography is an important determinant of biological interactions between tissue and biomaterials. Numerous physical and chemical methods have been applied to titanium-based biomaterials to produce nanometer to micrometer-scale surface structures. The common surface treatments include machining, grit-blasting, acid-etching, alkaline attack, electrochemical methods, and deposition of ions2. In vivo studies have demonstrated that bone tends to form on surfaces with micrometer-scale roughness, whereas fibrous connective tissue forms on smooth surfaces3-5.
In vitro cell-culture studies have established that certain surface structures regulate osteoblast attachment, proliferation, differentiation, and mineralization6-9. When human osteoblast cells are cultured on titanium surfaces with micrometer-scale roughness, they differentiate into secretory osteoblasts that exhibit increased levels of alkaline phosphatase and osteocalcin1. These cells also produce increased levels of growth factors and cytokines, such as transforming growth factor beta-1 (TGF-β1), prostaglandin E2, and osteoprotegerin, which promote osteoblastic activity and inhibit osteoclastic activity. Moreover, osteoblasts are modulated by local or systemic hormones in a way that is synergistic with surface roughness10,11.
In vivo studies have indicated that microstructured implant surfaces support bone responses that are better than those supported by implants of the same type but with smooth surfaces. For example, titanium implants with surfaces that have micrometer-scale roughness exhibit improved contact with bone in comparison with that of smooth-surfaced implants, and this finding was correlated with greater removal torque strength12-14. Comparison of dental implants that had microstructured surfaces with implants of the same type but with smooth surfaces showed that osteointegration occurs more quickly with the microstructured surfaces and the peri-implant bone provides increased mechanical support5,15-17.
Titanium alloys are used in orthopaedics because their mechanical properties are stronger than those of metallic titanium. However, relatively less is known about the biological response to titanium-alloy surfaces with micrometer-scale morphological features. Although both metallic titanium and titanium alloys form oxide layers on their surfaces, they are not chemically identical. A few in vitro studies have shown that osteoblasts are sensitive to the microarchitecture of the titanium-aluminum-vanadium (Ti6Al4V) surfaces18-20. As was noted on the pure titanium materials, osteoblast morphology, proliferation, differentiation, and gene expression are influenced by rough Ti6Al4V surfaces.
These observations suggested that microrough surfaces might improve fixation of orthopaedic screws, particularly in applications where micromotion is a problem. The load that occurs on the human spine during normal daily life transfers a variety of forces and moments to the pedicle screws21. This process can result in micromotion at the bone-screw interface, potentially delaying or preventing fixation of the screw to the bone and ultimately resulting in loosening of the screw. Surface treatments that produce micrometer-scale structure may increase osteointegration and reduce implant failure.
In this study, we tested the hypothesis that micrometer-scale roughness achieved by grit-blasting Ti6Al4V would improve osteoblast differentiation and enhance osteointegration and mechanical strength of the bone-implant interface compared with those characteristics of smooth Ti6Al4V surfaces. The study was designed with three objectives: to study the osteoblast response to a rough Ti6Al4V surface microstructure produced by grit-blasting, to assess whether microstructured surfaces produced by grit-blasting can increase osteointegration of pedicle screws, and to determine if the results from the in vitro assessment correlated with the clinical performance in an orthopaedic application.
Materials and Methods
Disk Preparation and Characterization
Ti6Al4V disks were supplied by Impliant (Ramat Poleg, Israel) and 15-mm-diameter specimens were punched out to fit a well of a twenty-four-well culture plate. Surfaces either were machined or were grit-blasted with 40 to 80-mesh hydroxyapatite/beta-tricalcium phosphate particles to result in micrometer-scale roughness. The disk surfaces were then cleaned and passivated according to ASTM (American Society for Testing and Materials) F86-01 guidelines. One disk of each type was randomly selected to be used for surface characterization. Surface morphology was determined with scanning electron microscopy (LEO 1530 Electron Microscope; Zeiss, Thornwood, New York), and the average peak-to-valley distance (Ra) for each disk surface was determined with contact profilometry (Surtronic 3P Profilometer; Taylor Hobson, Leicester, England). The Ra of the smooth surface was 0.2 μm, and the Ra values for the grit-blasted surfaces were 2.0, 3.0, and 3.3 μm. The chemical composition of each surface was determined with x-ray photon spectroscopy (AXIS Ultra; Kratos Analytical, Manchester, United Kingdom), which is used to examine the superficial 10 nm of the substrate, and by energy-dispersive analysis of x-rays (Tecnai F20 G2; FEI, Hillsboro, Oregon), which provides information at a depth of 1 μm. Prior to cell culture, all titanium-alloy disks were washed in distilled water in an ultrasonic cleaner and were sterilized with oxygen plasma (PDC-32G; Harrick Plasma, Ithaca, New York).
Cell Culture Studies
Human osteoblast-like MG63 cells were obtained from the American Type Culture Collection (Rockville, Maryland). The cells were cultured in Dulbecco's modified Eagle medium containing 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in an atmosphere of 5% CO2 and 100% humidity. Cells were plated at a density of 10,000 cells/cm2 on tissue culture polystyrene and on all titanium-alloy surfaces. Media were exchanged at twenty-four hours and then every forty-eight hours until the cells reached confluence on the tissue culture polystyrene.
Cell morphology on the test surfaces was examined with scanning electron microscopy (LEO Electron Microscope) of six-day cultures. The cells were fixed with 2.5% glutaraldehyde in cacodylate buffer, dehydrated in a graded series of ethanols followed by hexamethyldisilazane, and then coated with gold.
Cells typically reached confluence on the tissue culture polystyrene on the sixth day. Cell number was determined in all cultures twenty-four hours later, on the seventh day. Cells were released from the surfaces by two sequential incubations in 0.25% trypsin for ten minutes at 37°C, in order to ensure that any remaining cells were removed from the rough titanium surfaces. Released cells were counted with use of an automatic cell counter (Z1 Cell and Particle Counter; Beckman Coulter, Fullerton, California).
We used two determinants of osteoblast differentiation in seven-day cultures: the alkaline-phosphatase-specific activity (orthophosphoric monoester phosphohydrolase [alkaline], E.C. 3.1.3.1) of the cell lysates and the osteocalcin content of the conditioned media of the same cells. Alkaline phosphatase is an early marker of differentiation and reaches its highest levels as mineralization is initiated. Osteocalcin is a late marker of differentiation and increases as mineral is deposited. Lysates were prepared with use of isolated cells collected by centrifugation after counting. Enzyme activity was assayed by measuring the release of para-nitrophenol from para-nitrophenylphosphate at pH 10.2, and the results were normalized to the protein content of the cell lysates22. The levels of osteocalcin in the conditioned media were measured with use of a commercially available radioimmunoassay kit (Human Osteocalcin RIA Kit; Biomedical Technologies, Stoughton, Massachusetts) and normalized to the cell number, as described previously11.
The conditioned media from the seven-day cultures were also assayed for growth factors and cytokines. Osteoprotegerin was measured with use of an enzyme-linked immunosorbent assay (ELISA) kit (DY805 Osteoprotegerin DuoSet; R&D Systems, Minneapolis, Minnesota). Prostaglandin E2 was assessed with use of a commercially available competitive binding radioimmunoassay kit (NEK020A Prostaglandin E2 [125I] RIA kit; PerkinElmer, Waltham, Massachusetts). Active TGF-β1 was measured prior to acidification of the conditioned media, with use of an enzyme-linked immunosorbent assay kit specific for human TGF-β1 (G7591 TGF-β1 Emax ImmunoAssay System; Promega, Madison, Wisconsin). Total TGF-β1 was measured after acidifying the media, and latent TGF-β1 was defined as the total TGF-β1 minus the active TGF-β111.
Pedicle Screws
Pedicle screws were fabricated by Impliant to be 5.5 × 25 mm and were cleaned and sterilized by the company prior to surgery. The pedicle screw surfaces were produced in the same way as the titanium-alloy disks used in the in vitro experiments, with some differences. The disks were initially cut from titanium-alloy bars and then machine-polished, resulting in machine marks as described below. The screws were roll-formed to produce threads, resulting in smooth surfaces with an Ra of 0.1 μm; these screws were identical to commercial pedicle screws marketed by Impliant in Germany. Twenty-eight of the screws were grit-blasted with hydroxyapatite/beta-tricalcium phosphate to reach a micrometer-scale roughness of 3.0 μm (Fig. 1, a). One screw of each type was randomly selected for use for surface characterization. The morphology of the smooth and rough screw surfaces was examined with scanning electron microscopy, and the surface chemical composition was analyzed with x-ray photon spectroscopy to assess the top 10 nm and with energy-dispersive analysis of x-rays to assess the top 1 μm, as described above.
Fig. 1.
In vivo assessment of osteointegration. a: The macrostructure of a grit-blasted pedicle screw as demonstrated by scanning electron microscopy (×20). b: The positions of the pedicle screws were confirmed radiographically after implantation. The pedicle screws were inserted into the pedicular notch of L4 and L5 within the vertebral body. Fusion rods were connected vertically in order to achieve fixation and load-bearing.
The sheep-spine model was chosen because of its similarities with a human spine in terms of anatomy, size, material characteristics, and biomechanical characteristics23. The study was approved by the Committee for the Supervision of Animal Experiments at the Hebrew University Hadassah (Jerusalem, Israel). Fifteen healthy female adult Assaf sheep (Sharona, Israel) weighing between 59 and 75 kg were randomly assigned to one of two treatment groups. Eight ewes received smooth screws, and seven ewes received rough screws. These numbers of sheep were based on a power analysis, which indicated that this was the least number of animals that would allow detection of significant differences. The animals were premedicated with an intramuscular injection of ketamine and xylazine (11 and 0.22 mg/kg). Anesthesia was induced with an intramuscular injection of ketamine and diazepam (10 and 0.2 to 0.5 mg/kg), and the animals were connected to a general anesthesia machine that provided isoflurane (1% to 3%) and oxygen (up to 100%). The operations were done with the sheep under general anesthesia and positioned on their abdomen. Pedicle screws were inserted bilaterally into the pedicular notch of L4 and L5 within the vertebral body (Fig. 1, b). Thus, each ewe received two screws per vertebra, or four screws in total, and all screws in each ewe were of the same type. Fusion rods were connected vertically to the screws in order to achieve fixation and load-bearing. Bone chips of the lamina were used as graft as needed laterally, after which the muscles and skin were closed. Radiographs were made with the animals under general anesthesia to evaluate the placement of the pedicle screws. Postoperatively, the animals were treated with the broad-spectrum antibiotic Ceporex (cephalexin; 7 mL/day for ten days). The nonsteroidal anti-inflammatory drug Tolfine (tolfenamic acid; 2 mg/kg, 1.5 mL/day) was given once a day for three days for postoperative pain relief. The animals were able to walk undisturbed after recovery from the anesthesia.
In Vivo Assessment of Pedicle Screw Fixation
The animals were killed twelve weeks after screw implantation, and radiographs were made. The L4 and L5 vertebrae were removed with the screws en bloc. Left and right vertebrae were randomized for histological, microcomputed tomography, and mechanical testing. One L4 vertebra and one L5 vertebra from each animal were fixed in 4% buffered formalin and processed for undecalcified bone histomorphometry, as described below. The other L4 and L5 vertebrae were frozen in saline solution and stored at −80°C; after thawing, they were examined with microcomputed tomography and then used for analysis of removal torque. Histomorphometry and mechanical testing were performed only on the osteointegrated screws, including the malpositioned screws that had contact with bone, as determined with radiographic analysis. However, malpositioned implants that failed to become at least partially integrated in host bone were not included in the statistical analysis, on the assumption that the failure to support bone formation was a surgical failure or was specific to a particular animal and not due to a surface property.
Radiographic and Microcomputed Tomography Analysis
An orthopaedic surgeon who had not performed the surgery evaluated all radiographs and histological sections to detect relevant malpositioning of the pedicle screws. One of each type of screw was determined to be malpositioned. In addition, five screws had failed to be osteointegrated; three of these were rough-surfaced screws (two in one animal and one in another animal), and two were smooth-surfaced screws (both in the same animal).
After thawing, each tissue block was scanned with a voxel spacing of 16 μm (μCT 40; Scanco Medical, Basserdorf, Switzerland). Although computer software is available for subtracting the metallic implant from the bone, the scatter caused by the implants was too great to permit the resolution needed to determine the bone-implant contact at the interface with use of microcomputed tomography. However, the images were useful for a general assessment of osteointegration.
Histological and Histomorphometric Analyses
Tissue sections were prepared from plastic-embedded formalin-fixed tissue blocks with the implant intact. These sections were ground to a final thickness of 10 to 20 μm and stained with hematoxylin and eosin for qualitative histological analysis. The non-osteointegrated screws were not included in the histomorphometric assessment described below because bone-implant contact was not present and therefore could not be measured. The percentage of bone-implant contact of the osteointegrated screws was measured as the fractional, linear extent of bone apposed to the screw surface divided by the total surface perimeter of the screw24. We also calculated the expected bone-implant contact percentage, which is the theoretical amount of bone-implant contact for the specific bone volume analyzed25. To do this, an image of the screw profile was superimposed onto the surrounding bone at three locations: 150, 500, and 1000 μm lateral to the original screw surface. The actual percentage of bone-implant contact was measured at each site, and the mean percentage of bone-implant contact was calculated as well. On the basis of the assumption that the area of bone away from the screw represents the bone replaced by the screw, the percentage of bone-implant contact measured on each superimposed image should represent the amount of bone with which the screw could potentially have come into contact. In addition, we measured the bone-volume percentage, which is the percentage of mineralized bone in the total tissue volume25. Histomorphometric findings were recorded for individual screws and then tabulated and summarized with use of a histomorphometric data management program (Image-Pro, version 5; Media Cybernetics, Bethesda, Maryland).
Mechanical Testing
Removal torque was measured with use of a calibrated MTS 858 Mini Bionix II testing system (MTS, Eden Prairie, Minnesota) (Fig. 2). Tissue blocks for sixteen smooth and twelve rough pedicle screws were tested. Following microcomputed tomography analysis, the specimens were wrapped in latex to prevent cement from penetrating the bone, embedded in Wood's metal, and fixed into a metal cup. The exposed end of the screw was attached with an adapter fixed to the axial-torsional load transducer with a universal joint, with optimal alignment achieved. The screws were rotated 60° counterclockwise at a rate of 0.5°/sec. The torque angle and moments were recorded automatically with a TestStar data acquisition system (MTS). From the torsion tests, the maximum torque and angle stiffness were calculated. The maximum torque was defined as the removal torque.
Fig. 2.
Experimental setup for assessing removal torque of pedicle screws in sheep spine. Left: A representative example. Right: The apparatus.
Statistical Analysis
The in vitro data presented here were derived from one of two separate sets of experiments. Both sets of experiments yielded comparable observations. For the in vitro cell culture experiments, each data point represents the mean and standard error of the mean for six individual cultures. Data were first tested with analysis of variance; when statistical differences were detected, a Student t test for multiple comparisons with the Bonferroni modification was used.
In general, in vivo results are presented as the mean and standard error of the mean for the number of samples indicated. Each implant was considered to be a separate n value. Because the animals received four screws of each type, we also calculated the data per animal and found that the observed differences were consistent with the findings of the analysis based on individual screws. The bone-implant-contact data are presented as the mean and standard deviation for the osteointegrated screws. Data were tested with analysis of variance, and significance between groups was determined with use of a one-tailed Student t test. For both the in vitro and the in vivo results, p values of <0.05 were considered to be significant.
Results
Scanning electron microscopy of the smooth disk surfaces showed a concentric microgroove pattern because of the mechanical machining, with an Ra of 0.2 μm (Fig. 3, a). The rough surfaces produced by grit-blasting had Ra values of 2.0 μm (Fig. 3, b), 3.0 μm (Fig. 3, c), and 3.3 μm (Fig. 3, d). The blasted surfaces had irregular geometries with large tubercles and cracks.
Fig. 3.
Scanning electron micrographs showing the surface morphology of machined and grit-blasted Ti6Al4V substrates (×1200). The surfaces were machine-polished (a) or grit-blasted to an Ra of 2.0, 3.0, or 3.3 μm (b, c, and d, respectively).
The surface morphologies of the smooth pedicle screws differed from those of the smooth titanium-alloy disks. The smooth pedicle screws had small undulations on the surfaces produced by the mechanical process (Fig. 4, a and b). In contrast, the rough pedicle screws had larger irregularities because of the grit-blasting (Fig. 4, c and d), similar to the grit-blasted disk surfaces. The Ra of the smooth pedicle screws was <0.1 μm, and the Ra of the grit-blasted screws was 3.0 μm.
Fig. 4.
Scanning electron micrographs showing the surface morphology of machined pedicle screws (a [×240] and b [×1000]) and grit-blasted pedicle screws with an Ra of 3 μm (c [×240] and d [×1000]).
X-ray photon spectroscopy showed that the top 10 nm of all disk surfaces were contaminated with carbon to a similar extent (38.4% to 51.2%) (see Appendix). Besides carbon, the major chemical components on the surfaces at the nanoscale level were oxygen and titanium. This finding is consistent with the fact that titanium spontaneously forms a layer of TiO2 when exposed to air and this layer is quickly contaminated with atmospheric hydrocarbons. In addition, silicon (4.1%) was found on the smooth titanium-alloy surfaces because of the process used by Impliant to polish the disks. Failure to detect calcium and phosphorus on the grit-blasted disks indicates that the cleaning protocol effectively removed them from the surface.
X-ray photon spectroscopy showed that the screw surfaces were also contaminated with carbon (see Appendix). On the smooth screws, this contamination was extensive enough to obscure the underlying metal. A TiO2 layer forms on titanium alloys, accounting for the 2:1 ratio of titanium and oxygen noted on the grit-blasted screw surface. Silicon was present at greater levels on the smooth screw surfaces as a result of the polishing involved in the fabrication process, and calcium and phosphorus were present at greater levels on the grit-blasted surfaces as a result of residual calcium phosphate following the grit-blasting process.
Subsurface chemical analysis at a depth of 1 μm by energy-dispersive analysis of x-rays confirmed that the titanium-alloy disks were composed of titanium, aluminum, and vanadium (see Appendix). The presence of an oxide layer was still evident. The greater depth of penetration afforded by the rougher surfaces exposed more vanadium and showed that zirconium was present. The screw surfaces were more contaminated with carbon than were the disks. The calcium and phosphorus shown to be present on the screw surfaces by the x-ray photon spectroscopy were not detected by the energy-dispersive analysis of x-rays, indicating that they were only on the most superficial layer.
In Vitro Assessment of Cell Response
Cellular morphology was sensitive to the disk surface properties. When osteoblast-like MG63 cells were grown on tissue culture polystyrene (data not shown) or smooth titanium-alloy surfaces, the cells were spindle-shaped and elongated. In addition, they were aligned with the microgrooved structure of the machined disks (Fig. 5, a). On the rougher, grit-blasted surfaces, most cells appeared triangular, polygonal, or rounded with cytoplasmic extensions into the pits of the blasted topography (Fig. 5, b, c, and d).
Fig. 5.
Scanning electron micrographs showing the morphology of MG63 cells cultured for six days on a machined Ti6Al4V substrate (a) or grit-blasted Ti6Al4V substrates with Ra values of 2.0, 3.0, and 3.3 μm (b, c, and d, respectively) (×1000).
The cell number was reduced on all titanium-alloy disk surfaces compared with the number on the tissue culture polystyrene (p < 0.05) (Fig. 6-A). The effect was more prominent on the grit-blasted surfaces. Compared with the cell number on the smooth titanium-alloy surfaces, the cell numbers on the surfaces with 2.0, 3.0, and 3.3-μm roughnesses were decreased by 83%, 88%, and 94%, respectively.
Fig. 6-AFig. 6-C Fig. 6-D.
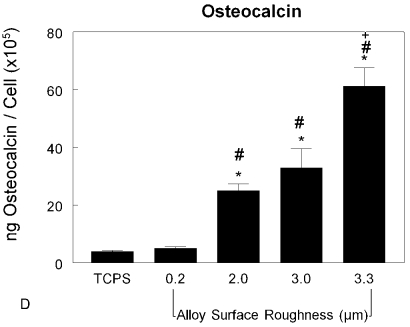
Figs. 6-A through 6-D Effects of surface structure on osteoblast cell number and differentiation. MG63 cells were cultured on tissue culture polystyrene (TCPS), machined Ti6Al4V substrates with an Ra of 0.2 μm, or grit-blasted Ti6Al4V substrates with an Ra of 2.0, 3.0, or 3.3 μm. Values are expressed as the mean and standard error of the mean of six independent cultures. Data were derived from one of two separate experiments, both of which had comparable results. Data were analyzed with analysis of variance, and significant differences between groups were determined with use of the Bonferroni modification of the Student t test. *p < 0.05 as compared with the tissue culture polystyrene, #p < 0.05 as compared with the 0.2-μm-Ra machined surface, and +p < 0.05 as compared with the 3.0-μm-Ra grit-blasted surface. Fig. 6-A The cells were harvested at six days, and the cell numbers were counted. Fig. 6-B Alkaline-phosphatase-specific activity was measured in isolated cell lysates. Fig. 6-C Osteocalcin levels were measured in the conditioned media of confluent cultures. Fig. 6-D Osteocalcin levels normalized by cell number were calculated.
Although there were fewer cells on the rougher disk surfaces, those that were present were more differentiated. Alkaline-phosphatase-specific activity is an early marker of osteoblast differentiation; it declines in late differentiation. The enzyme activity exhibited by the MG63 cells cultured on the titanium-alloy disk surfaces was lower than that exhibited on the tissue culture polystyrene (Fig. 6-B) (p < 0.05). The enzyme activities of cells on the grit-blasted disk surfaces were lower than those on the machined surface (p < 0.05). There was no difference among the cultures on the rough surfaces. Osteocalcin is a late differentiation marker of osteoblasts. Osteocalcin concentrations in the conditioned media were increased on the grit-blasted titanium-alloy surfaces (Fig. 6-C), with the greatest amounts in the conditioned media of the cells grown on the 3.3-μm-Ra titanium-alloy disks (p < 0.05). This surface-dependent effect was more pronounced when it was calculated on a per-cell basis (Fig. 6-D). The osteocalcin levels on the rough surfaces were increased four, six, and elevenfold compared with those on the smooth surfaces.
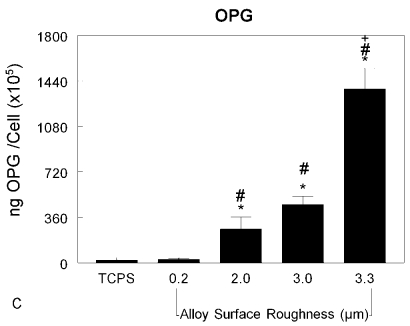
The MG63 cells also responded to the surface by altering their local environment. The content of prostaglandin E2 in the media was elevated in the cultures grown on all of the titanium-alloy disks and was further elevated in the cultures on the grit-blasted disks (Fig. 7-A) (p < 0.05). To eliminate the possibility that this was an artifact of the experimental design, we also determined the amount of each factor per cell and found that the increase was indeed due to increased production per cell (Fig. 7-B) (p < 0.05). Similar effects were observed when we assessed osteoprotegerin content (Fig. 7-C) (p < 0.05). Also, the amount of TGF-β1 in the conditioned media was increased in a surface-dependent manner, with the greatest amounts in the media conditioned by cells grown on the roughest surfaces (p < 0.05) (Fig. 7-D).
Fig. 7-AFig. 7-C Fig. 7-D.
Figs. 7-A through 7-D Effects of surface structure on local factor production. MG63 cells were cultured on tissue culture polystyrene (TCPS), machined Ti6Al4V substrates with an Ra of 0.2 μm, or grit-blasted Ti6Al4V substrates with an Ra of 2.0, 3.0, or 3.3 μm. Values are expressed as the mean and standard error of the mean of six independent cultures. Data were derived from one of two separate experiments, both of which had comparable results. Data were analyzed with analysis of variance, and significant differences between groups were determined with use of the Bonferroni modification of the Student t test. *p < 0.05 as compared with the tissue culture polystyrene, #p < 0.05 as compared with the 0.2-μm-Ra machined surface, and +p < 0.05 as compared with the 3.0-μm-Ra grit-blasted surface. Fig. 7-A The total content of prostaglandin E2 (PGE2) in the conditioned media was determined with a radioimmunoassay kit. Fig. 7-B The amount of prostaglandin E2 normalized by cell number was determined with a radioimmunoassay kit. Fig. 7-C Osteoprotegerin (OPG) in the conditioned media was measured with use of an ELISA kit. Fig. 7-D Transforming growth factor beta-1 (TGF-β1) in the conditioned media was measured with use of an ELISA kit.
In Vivo Assessments
At the onset of the study, the average ages (and standard error of the mean) of the sheep in the smooth and rough-pedicle-screw groups were 2.4 ± 0.5 and 2.1 ± 0.4 years old, respectively. The average weights of the two groups were 66.2 ± 12.0 and 64.7 ± 2.9 kg. There were no apparent differences between the two groups in terms of age or weight (p > 0.05). The screws were well positioned in the L4 and L5 vertebral segments. At the time of screw retrieval, none of the sheep showed any signs of substantial weight loss or weight gain. Radiographs showed that the screws remained within the bone of the L4 and L5 vertebrae (Fig. 1, b). This was confirmed by microcomputed tomography. Peri-implant bone formation was seen with both smooth screws (Fig. 8, A, B, and C) and grit-blasted screws (Fig. 8, D, E, and F). However, the limitations of the resolution of the implant-bone interface with use of this imaging technology precluded an assessment of the quality of the tissue at the interface.
Fig. 8.
Microcomputed tomography scans of machined (A, B, and C) and grit-blasted (D, E, and F) Ti6Al4V pedicle screws in the L4 vertebral bone of sheep.
Histological analysis demonstrated that bone formation was not continuous around the screws, but the degree of bone formation was surface-dependent. Although some bone contact was seen on the smooth surfaces, areas were also covered by fibrous tissue (Fig. 9, a, b, and c). During histological processing, the fibrous tissue contracted and detached from the surface. Fibrous tissues were also seen adjacent to grit-blasted surfaces, but the fibrous layer was thinner and there was less breakage due to processing dehydration. Moreover, when breakage was found, it was within the bone and not at the bone-implant interface. On most rough surfaces, bone cells were directly attached to the surface and had produced mineralized matrix (Fig. 9, d, e, and f).
Fig. 9.
Undecalcified histological sections of peri-implant bone formation following osteointegration of smooth (a, b, and c) and rough (d, e, and f) Ti6Al4V pedicle screws in the L4 vertebra of sheep (hematoxylin and eosin; no magnification).
These histological observations were supported by histomorphometric measurements. Within the group of tissue blocks processed for histological analysis, fourteen of the sixteen smooth screws were osteointegrated at twelve weeks and eleven of the fourteen grit-blasted pedicle screws were integrated and were used for analysis. The average bone-implant contact was greater for the grit-blasted screws: 73.5% compared with 59.6% for the smooth screws (Table I) (p < 0.05). No significant differences in bone volume or the expected bone-implant contact were found.
TABLE I.
Percent Bone-Implant Contact of Osteointegrated Ti6Al4V Pedicle Screws Twelve Weeks After Implantation in the L4 and L5 Vertebrae of Sheep
| Smooth Screws | Grit-Blasted Screws | |
|---|---|---|
| Osteointegrated/total no. of screws | 14/16 | 11/14 |
| Bone-implant contact*(%) | 59.6 ± 25.8† | 73.5 ± 28.5† |
| Expected bone-implant contact*‡(%) | 83.7 ± 6.4 | 81.4 ± 9.9 |
| Bone volume*§(%) | 78.1 ± 5.6 | 79.5 ± 5.3 |
The data are given as the mean and standard deviation.
P < 0.05.
Expected bone-implant contact was defined as the theoretical amount of bone-implant contact for the specific bone volume analyzed.
The bone volume is the percentage of mineralized bone in the total tissue volume.
Removal torque measurements also showed a significant surface-dependent difference in the mechanical strength of the osteointegration. Grit-blasted pedicle screws (n = 12) required 5.3 ± 0.4 Nm for displacement, whereas machined screws (n = 16) required 2.3 ± 0.3 Nm (p < 0.0001).
Discussion
This study demonstrates that osteoblasts are sensitive to small variations in the surface microstructure of Ti6Al4V substrates in a manner that is similar to their behavior on commercially pure titanium1,6-9,26. As the micrometer-scale roughness is increased, the cells become more differentiated and produce higher levels of factors associated with bone formation and inhibition of bone-remodeling. In previous studies examining the responses of MG63 cells to titanium microstructures, the variations in topography presented to the cells were of a greater magnitude than those used here, but, as the present data indicate, an Ra of 3.0 μm appears to be an important determinant of osteogenic differentiation.
Part of the difference seen between cells grown on the machined substrates and those grown on the grit-blasted substrates is due to the chemistry of the surface. The machining process left a greater residue of carbon and silicon on the screws. How this may have contributed to the overall effect is not known, but differences in adsorption of serum components are likely to have played a role27-29.
Most importantly, the behavior of the MG63 cells in the culture model was predictive of the quality of osteointegration in vivo. To our knowledge, this is the first demonstration of this correlation in an orthopaedic application, although there have been numerous demonstrations of this relationship with respect to dental implants18-20. MG63 cells, while human-derived, are an osteosarcoma cell line and as such lack cell-cycle controls typical of nontransformed cells. They are immature osteoblasts, which makes them useful for studying factors that promote osteoblastic differentiation. The behavior of MG63 cells on titanium substrates is comparable with that of normal human osteoblasts, rat bone-marrow cells, neonatal mouse calvarial osteoblasts, and fetal rat calvarial osteoblasts6,30-32, and their value as a model system for studying cell responses to titanium was recently described in a review33. The present study indicates that MG63 cells can be used to compare titanium-alloy materials as well. Grit-blasted titanium-alloy substrates with an Ra of ≥3.0 μm supported osteoblastic differentiation of the cells in vitro and yielded significantly greater bone-implant contact and removal torque strength in vivo when compared with the machined screws.
All screws were retrieved at twelve weeks, so we do not know whether the rate of bone formation was affected or if the rougher surfaces caused more sites to support osteogenesis, although at the same rate. As noted above, surface chemistry is likely to have contributed to the outcome, as adsorption of serum components might impact the types of cells that attach to screws and their production of local regulatory factors as well34.
Grit-blasting is a surface treatment that is widely used to create micrometer-scale surface roughness. Studies have shown that the effects of submicrometer surface structure on osteoblasts are prominent only in the presence of micrometer-scale roughness35. A similar phenomenon was observed in this study. The machined (control) titanium-alloy disks used in this experiment had a microgroove pattern with an Ra of 0.2 μm. The MG63 cells formed a parallel alignment along the direction of the grooves, as has been observed by other researchers36,37. Despite this characteristic alignment of the cells with the topography, cell differentiation and local factor production were only slightly, if at all, different from what is typically seen when cells are grown on tissue culture polystyrene in the absence of any specific physical orientation. This result confirmed our previous finding that submicrometer roughness has minor effects on osteoblasts, but in combination with micrometer-scale roughness there is a synergistic increase in cell differentiation35. Grit-blasting produces a complex surface with both micrometer-scale and submicrometer-scale structural elements and thus is an efficient method for modifying surface structure and regulating cell phenotype.
The titanium-alloy surface roughness modulated the ability of MG63 cells to synthesize and secrete autocrine and paracrine mediators associated with an osteogenic microenvironment characterized by promotion of bone formation and inhibition of bone resorption. As surface roughness increased, prostaglandin E2, TGF-β1, and osteoprotegerin levels increased. Prostaglandin E2 is required for osteoblast activity38, and prostaglandins mediate cell responses to surface structure39-41. Most of the TGF-β1 secreted by MG63 cells on microstructured titanium surfaces is stored in latent form, and the amount is sensitive to surface structure42. This growth factor stimulates matrix synthesis and differentiation of osteoblasts43 and also inhibits osteoclasts44. Finally, osteoprotegerin is produced by osteoblasts as a decoy receptor, preventing binding of receptor activator of nuclear factor kappa-B ligand on the osteoblast to its receptor on osteoclasts and thereby preventing contact-dependent activation of osteoclast maturation. The elevated level of osteoprotegerin on rough surfaces plays an important role in coordinating the sequence of osteoclast maturation in the bone-remodeling cycle26.
Previous studies have demonstrated that carbon is a common contaminant on titanium and alloy surfaces exposed to air as a result of adsorption of atmospheric hydrocarbons45,46, and this was noted in our study as well. Recent research has shown that preventing titanium surfaces from coming into contact with the atmosphere could efficiently reduce carbon contamination and increase bone cell response47,48. It is of interest that the grit-blasted screw surfaces in our study had lower carbon levels and supported greater bone-implant contact and removal torque. Because of limitations in the materials supply, we examined the surface chemistry of only one sample in each group. Therefore, we were unable to determine statistically whether surface chemistry contributed to the difference in the observed cell behavior.
At twelve weeks after implantation, more bone had formed around the pedicle screws with the rough microarchitecture, leading to a stronger removal torque force. In our study, the high interference due to the titanium alloy limited use of microcomputed tomography for quantitative analysis of the bone-implant interface. However, the findings on the microcomputed tomography images supported an interpretation that there was greater bone-implant contact when microstructured pedicle screws had been used.
By definition, measurements of bone-implant contact can be performed only with osteointegrated implants. By excluding the nonintegrated screws from this analysis, a potential bias was introduced into the study. Three of the nonintegrated screws had a rough surface, and two of those rough screws were in the same animal. Two of the nonintegrated screws had a smooth surface, and they were in a different animal. It is likely that the implant failures were due to difficulties related to the surgery, particularly in the sheep that had two failures of the same screw type, and not to a specific feature or features of the screws themselves. It is also possible that the thickness of the sections obscured some of the detail at the bone-implant interface, potentially leading to an overestimation of the bone apposition. All sections were of uniform thickness, however, so differences in peri-implant bone formation between the rough and smooth screws are valid in the context of the present study.
In summary, we investigated the effects of Ti6Al4V surface structure on osteoblast behavior in vitro and on bone formation in vivo. The effects of the titanium-alloy surface on bone formation in vivo were consistent with the results of the in vitro study. Our in vitro data support the hypothesis that the grit-blasted titanium-alloy surface promoted osteointegration by stimulating osteoblast activity, with a potential to reduce osteoclast activity. Our results suggest that surface structure should be considered in the design of titanium-alloy implants to improve the outcomes associated with the medical devices.
Supplementary Material
Appendix
A table showing the surface chemical compositions of the implants as determined with x-ray photon spectroscopy and energy-dispersive analysis of x-rays is available with the electronic versions of this article, on our web site at jbjs.org (go to the article citation and click on “Supplementary Material”) and on our quarterly CD/DVD (call our subscription department, at 781-449-9780, to order the CD or DVD). 
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from Impliant, Inc., the National Science Foundation (EEC 9731645), and the National Institutes of Health (AR052102). In addition, one or more of the authors or a member of his or her immediate family received, in any one year, payments or other benefits in excess of $10,000 or a commitment or agreement to provide such benefits from a commercial entity (Impliant, Inc.). No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
Investigation performed at Georgia Institute of Technology, Atlanta, Georgia, and Hebrew University Hadassah, Jerusalem, Israel
References
- 1.Boyan BD, Lossdörfer S, Wang L, Zhao G, Lohmann CH, Cochran DL, Schwartz Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Eur Cell Mater. 2003;6:22-7. [DOI] [PubMed] [Google Scholar]
- 2.Bagno A, Di Bello C. Surface treatments and roughness properties of Ti-based biomaterials. J Mater Sci Mater Med. 2004;15:935-49. [DOI] [PubMed] [Google Scholar]
- 3.Brunette DM. Spreading and orientation of epithelial cells on grooved substrata. Exp Cell Res. 1986;167:203-17. [DOI] [PubMed] [Google Scholar]
- 4.Thomas KA, Cook SD. An evaluation of variables influencing implant fixation by direct bone apposition. J Biomed Mater Res. 1985;19:875-901. [DOI] [PubMed] [Google Scholar]
- 5.Cochran DL. A comparison of endosseous dental implant surfaces. J Periodontol. 1999;70:1523-39. [DOI] [PubMed] [Google Scholar]
- 6.Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J Jr, Dean DD, Cochran DL, Boyan BD. Effect of titanium surface-roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995;29:389-401. [DOI] [PubMed] [Google Scholar]
- 7.Brett PM, Harle J, Salih V, Mihoc R, Olsen I, Jones FH, Tonetti M. Roughness response genes in osteoblasts. Bone. 2004;35:124-33. [DOI] [PubMed] [Google Scholar]
- 8.Guizzardi S, Galli C, Martini D, Belletti S, Tinti A, Raspanti M, Taddei P, Ruggeri A, Scandroglio R. Different titanium surface treatment influences human mandibular osteoblast response. J Periodontol. 2004;75:273-82. [DOI] [PubMed] [Google Scholar]
- 9.Keller JC, Schneider GB, Stanford CM, Kellogg B. Effects of implant microtopography on osteoblast cell attachment. Implant Dent. 2003;12:175-81. [DOI] [PubMed] [Google Scholar]
- 10.Ong JL, Carnes DL, Cardenas HL, Cavin R. Surface roughness of titanium on bone morphogenetic protein-2 treated osteoblast cells in vitro. Implant Dent. 1997;6:19-24. [DOI] [PubMed] [Google Scholar]
- 11.Boyan BD, Batzer R, Kieswetter K, Liu Y, Cochran DL, Szmuckler-Moncler S, Dean DD, Schwartz Z. Titanium surface roughness alters responsiveness of MG63 osteoblast-like cells to 1 alpha,25-(OH)2D3. J Biomed Mater Res. 1998;39:77-85. [DOI] [PubMed] [Google Scholar]
- 12.Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J Biomed Mater Res. 1998;40:1-11. [DOI] [PubMed] [Google Scholar]
- 13.Gotfredsen K, Wennerberg A, Johansson C, Skovgaard LT, Hjørting-Hansen E. Anchorage of TiO2-blasted, HA-coated, and machined implants: an experimental study with rabbits. J Biomed Mater Res. 1995;29:1223-31. [DOI] [PubMed] [Google Scholar]
- 14.Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25:889-902. [DOI] [PubMed] [Google Scholar]
- 15.Khang W, Feldman S, Hawley CE, Gunsolley J. A multi-center study comparing dual acid-etched and machined-surfaced implants in various bone qualities. J Periodontol. 2001;72:1384-90. [DOI] [PubMed] [Google Scholar]
- 16.Albrektsson T, Wennerberg A. Oral implant surfaces: part 2—review focusing on clinical knowledge of different surfaces. Int J Prosthodont. 2004;17:544-64. [PubMed] [Google Scholar]
- 17.Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004;83:529-33. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Kim SH, Kim MS, Lee EJ, Oh HG, Oh WM, Park SW, Kim WJ, Lee GJ, Choi NG, Koh JT, Dinh DB, Hardin RR, Johnson K, Sylvia VL, Schmitz JP, Dean DD. Varying Ti-6Al-4V surface roughness induces different early morphologic and molecular responses in MG63 osteoblast-like cells. J Biomed Mater Res A. 2005;74:366-73. [DOI] [PubMed] [Google Scholar]
- 19.Anselme K, Bigerelle M, Noel B, Dufresne E, Judas D, Iost A, Hardouin P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J Biomed Mater Res. 2000;49:155-66. [DOI] [PubMed] [Google Scholar]
- 20.Lincks J, Boyan BD, Blanchard CR, Lohmann CH, Liu Y, Cochran DL, Dean DD, Schwartz Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998;19:2219-32. [DOI] [PubMed] [Google Scholar]
- 21.Gaines RW Jr. The use of pedicle-screw internal fixation for the operative treatment of spinal disorders. J Bone Joint Surg Am. 2000;82:1458-76. [DOI] [PubMed] [Google Scholar]
- 22.Bretaudiere JP, Spillman T. Alkaline phosphatases. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Weinheim, Germany: Verlag Chemica; 1984. p 75-92.
- 23.Fini M, Giavaresi G, Greggi T, Martini L, Aldini NN, Parisini P, Giardino R. Biological assessment of the bone-screw interface after insertion of uncoated and hydroxyapatite-coated pedicular screws in the osteopenic sheep. J Biomed Mater Res A. 2003;66:176-83. [DOI] [PubMed] [Google Scholar]
- 24.Recker RR, editor. Bone histomorphometry: techniques and interpretation. Boca Raton, FL: CRC Press; 1983.
- 25.Trisi P, Lazzara R, Rebaudi A, Rao W, Testori T, Porter SS. Bone-implant contact on machined and dual acid-etched surfaces after 2 months of healing in the human maxilla. J Periodontol. 2003;74:945-56. [DOI] [PubMed] [Google Scholar]
- 26.Lossdörfer S, Schwartz Z, Wang L, Lohmann CH, Turner JD, Wieland M, Cochran DL, Boyan BD. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J Biomed Mater Res A. 2004;70:361-9. [DOI] [PubMed] [Google Scholar]
- 27.Advincula M, Fan X, Lemons J, Advincula R. Surface modification of surface sol-gel derived titanium oxide films by self-assembled monolayers (SAMs) and non-specific protein adsorption studies. Colloids Surf B Biointerfaces. 2005;42:29-43. [DOI] [PubMed] [Google Scholar]
- 28.Serro AP, Bastos M, Pessoa JC, Saramago B. Bovine serum albumin conformational changes upon adsorption on titania and on hydroxyapatite and their relation with biomineralization. J Biomed Mater Res A. 2004;70:420-7. [DOI] [PubMed] [Google Scholar]
- 29.Zeng H, Chittur KK, Lacefield WR. Analysis of bovine serum albumin adsorption on calcium phosphate and titanium surfaces. Biomaterials. 1999;20:377-84. [DOI] [PubMed] [Google Scholar]
- 30.Lohmann CH, Tandy EM, Sylvia VL, Hell-Vocke AK, Cochran DL, Dean DD, Boyan BD, Schwartz Z. Response of normal female human osteoblasts (NHOst) to 17beta-estradiol is modulated by implant surface morphology. J Biomed Mater Res. 2002;62:204-13. [DOI] [PubMed] [Google Scholar]
- 31.Schneider GB, Perinpanayagam H, Clegg M, Zaharias R, Seabold D, Keller J, Stanford C. Implant surface roughness affects osteoblast gene expression. J Dent Res. 2003;82:372-6. [DOI] [PubMed] [Google Scholar]
- 32.Dekker RJ, van Blitterswijk CA, Hofland I, Engelberts PJ, Li J, de Bruijn JD. Studying the effect of different macrostructures on in vitro cell behaviour and in vivo bone formation using a tissue engineering approach. Novartis Found Symp. 2003;249:148-67; discussion 167-9, 170-4, 239-41. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz Z, Nasazky E, Boyan BD. Surface microtopography regulates osteointegration: the role of implant surface microtopography in osteointegration. Alpha Omegan. 2005;98:9-19. [PubMed] [Google Scholar]
- 34.Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003;67:932-49. [PubMed] [Google Scholar]
- 35.Zhao G, Zinger O, Schwartz Z, Wieland M, Landolt D, Boyan BD. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin Oral Implants Res. 2006;17:258-64. [DOI] [PubMed] [Google Scholar]
- 36.Walboomers XF, Croes HJ, Ginsel LA, Jansen JA. Contact guidance of rat fibroblasts on various implant materials. J Biomed Mater Res. 1999;47:204-12. [DOI] [PubMed] [Google Scholar]
- 37.Brunette DM, Ratkay J, Chehroudi B. Behaviour of osteoblasts on micromachined surfaces. In: Davies JE, editor. The bone-biomaterial interface. Toronto, Ontario: University of Toronto Press; 1991. p 170-80.
- 38.Raisz LG, Fall PM. Biphasic effects of prostaglandin E2 on bone formation in cultured fetal rat calvariae: interaction with cortisol. Endocrinology. 1990;126:1654-9. [DOI] [PubMed] [Google Scholar]
- 39.Sisk MA, Lohmann CH, Cochran DL, Sylvia VL, Simpson JP, Dean DD, Boyan BD, Schwartz Z. Inhibition of cyclooxygenase by indomethacin modulates osteoblast response to titanium surface roughness in a time-dependent manner. Clin Oral Implants Res. 2001;12:52-61. [DOI] [PubMed] [Google Scholar]
- 40.Boyan BD, Lohmann CH, Sisk M, Liu Y, Sylvia VL, Cochran DL, Dean DD, Schwartz Z. Both cyclooxygenase-1 and cyclooxygenase-2 mediate osteoblast response to titanium surface roughness. J Biomed Mater Res. 2001;55:350-9. [DOI] [PubMed] [Google Scholar]
- 41.Tang CH, Yang RS, Fu WM. Prostaglandin E2 stimulates fibronectin expression through EP1 receptor, phospholipase C, protein kinase Calpha and c-Src pathway in primary cultured rat osteoblasts. J Biol Chem. 2005;280:22907-16. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz Z, Lohmann CH, Sisk M, Cochran DL, Sylvia VL, Simpson J, Dean DD, Boyan BD. Local factor production by MG63 osteoblast-like cells in response to surface roughness and 1,25-(OH)2D3 is mediated via protein kinase C- and protein kinase A-dependent pathways. Biomaterials. 2001;22:731-41. [DOI] [PubMed] [Google Scholar]
- 43.Bonewald LF, Schwartz Z, Swain LD, Ramirez V, Poser J, Boyan BD. Stimulation of plasma membrane and matrix vesicle enzyme activity by transforming growth factor-beta in osteosarcoma cell cultures. J Cell Physiol. 1990;145:200-6. [DOI] [PubMed] [Google Scholar]
- 44.Mundy GR, Bonewald LF. Role of TGF beta in bone remodeling. Ann N Y Acad Sci. 1990;593:91-7. [DOI] [PubMed] [Google Scholar]
- 45.Leitão E, Barbosa MA, de Groot K. XPS characterization of surface films formed on surface-modified implant materials after cell culture. J Mater Sci Mater Med. 1997;8:423-6. [DOI] [PubMed] [Google Scholar]
- 46.Deligianni DD, Katsala N, Ladas S, Sotiropoulou D, Amedee J, Missirlis YF. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials. 2001;22:1241-51. [DOI] [PubMed] [Google Scholar]
- 47.Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006;76:323-34. [DOI] [PubMed] [Google Scholar]
- 48.Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, Boyan BD. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74:49-58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.