Abstract
Infection with antibiotic-resistant bacteria, such as vancomycin-resistant Enterococcus (VRE), is a dangerous and costly complication of broad-spectrum antibiotic therapy1,2. How antibiotic-mediated elimination of commensal bacteria promotes infection by antibiotic-resistant bacteria is a fertile area for speculation with few defined mechanisms. Here we demonstrate that antibiotic treatment of mice notably downregulates intestinal expression of RegIIIγ (also known as Reg3g), a secreted C-type lectin that kills Gram-positive bacteria, including VRE. Downregulation of RegIIIγ markedly decreases in vivo killing of VRE in the intestine of antibiotic-treated mice. Stimulation of intestinal Toll-like receptor 4 by oral administration of lipopolysaccharide re-induces RegIIIγ, thereby boosting innate immune resistance of antibiotic-treated mice against VRE. Compromised mucosal innate immune defence, as induced by broad-spectrum antibiotic therapy, can be corrected by selectively stimulating mucosal epithelial Toll-like receptors, providing a potential therapeutic approach to reduce colonization and infection by antibiotic-resistant microbes.
Infections caused by highly antibiotic-resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) and VRE, are an increasing menace in hospitalized patients3,4. Treatment of serious VRE infections is limited by the paucity of effective antibiotics5. VRE colonizes the gastrointestinal tract and it is likely that systemic bloodstream infections are the result of dissemination from the intestine6. It has been widely assumed that antibiotic treatment, by eliminating commensal flora, opens intestinal niches and provides increased access to nutrients, thereby enhancing VRE survival and proliferation. Recent studies, however, have demonstrated that commensal microbes in the intestine induce expression of proteins that restrict bacterial survival and growth7,8. Thus, whereas commensal microbes may directly restrict VRE proliferation, an alternative hypothesis is that commensal microbes inhibit VRE indirectly by activating mucosal innate immune defenses.
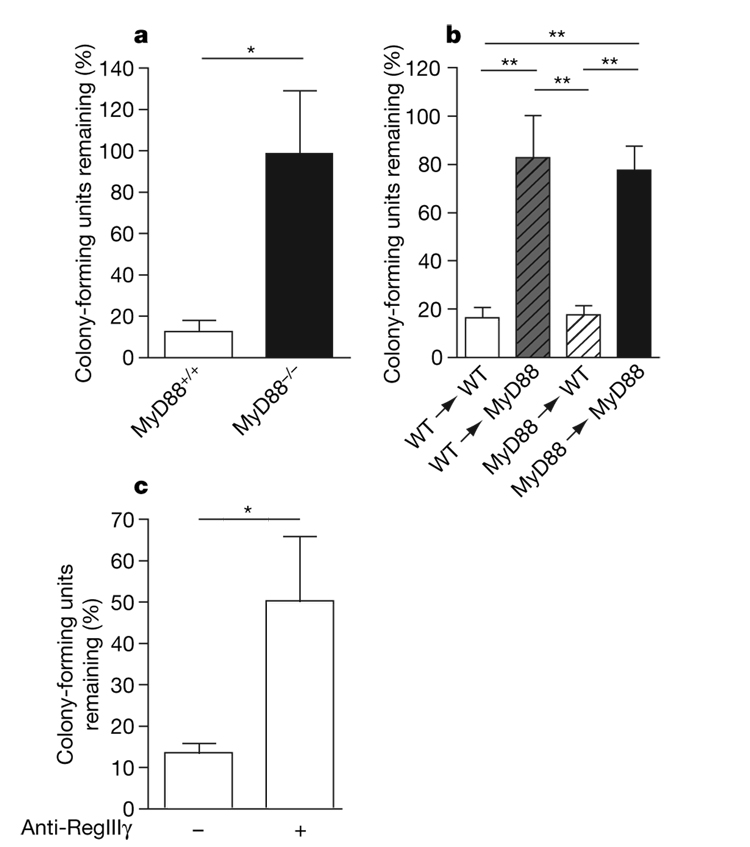
To determine, as a first step, whether innate immune signalling by means of the Toll-like receptor (TLR)–MyD88 pathway restricts VRE colonization of the intestinal tract in vivo, we compared VRE killing in ligated ileal loops of wild-type and MyD88-deficient mice. Figure 1a demonstrates that VRE was killed much more effectively in ileal loops of wild-type than MyD88-deficient mice. Intestinal inhibition of VRE did not require MyD88-mediated signals in bone-marrow-derived cells, but instead was required in non-bone-marrow-derived, presumably epithelial, cells (Fig. 1b). RegIIIγ is a secreted lectin with potent bactericidal activity against Gram-positive bacteria that is expressed by intestinal epithelial and Paneth cells7. Expression of RegIIIγ is dependent on TLR–MyD88-mediated signals in intestinal epithelial cells and is induced by commensal microbes7,9. To determine whether RegIIIγ mediates in vivo killing of VRE in the intestine, we injected a blocking polyclonal antiserum10 against RegIIIγ into ileal loops of wild-type mice before inoculation of VRE. The number of surviving VRE bacteria was increased by over 400% in intestines treated with RegIIIγ-specific antiserum (Fig. 1c), indicating that RegIIIγ mediates in vivo VRE killing.
Figure 1. MyD88-mediated signalling in non-haematopoietic cells is required for RegIIIγ-mediated clearance of VRE.
a, b, One-thousand VRE were injected into ileal loops of MyD88+/+, MyD88−/− (a) and MyD88 bone-marrow-chimaeric (b) mice. Two hours after injection the isolated section of the intestine was harvested and colony-forming units were determined; n=5–6 each group. Bacterial numbers are expressed as the percentage of recovered VRE. For a, *P=0.02, unpaired t-test; for b, **P < 0.01, one-way ANOVA with Bonferroni correction. WT, wild type. c, Antiserum against RegIIIγ or pre-immune serum was injected into ileal loops of wild-type mice before injection of 1,000 VRE. After 2 h, bacterial growth in the luminal fluid of the isolated section of the small intestine was determined, Bacterial numbers are expressed as the percentage of recovered VRE; n=5 each group. *P=0.049, unpaired t-test. Error bars denote s.e.m.
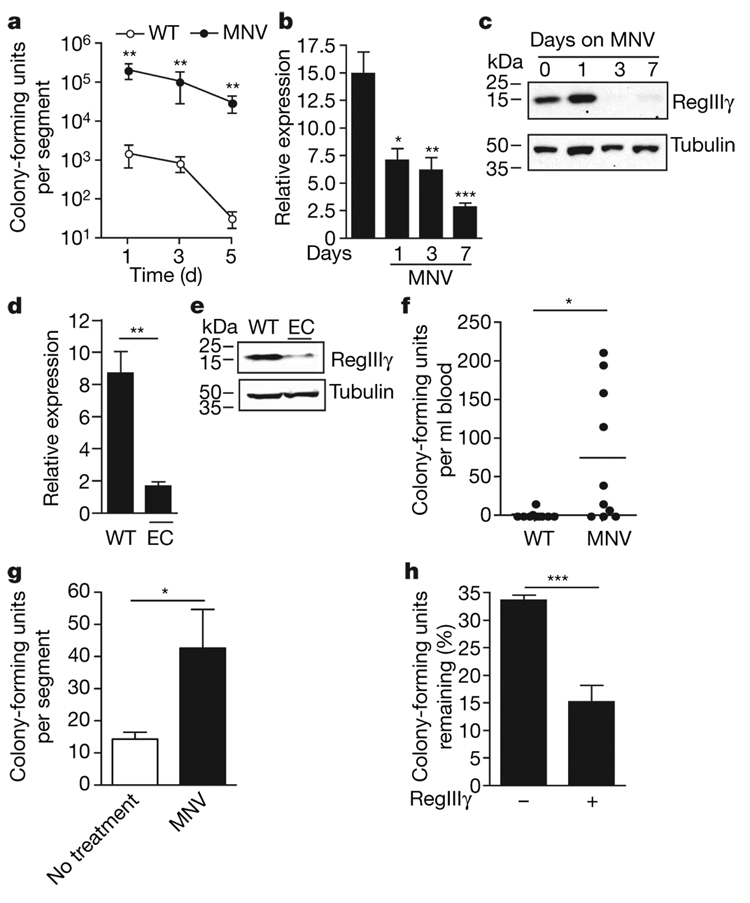
Administration of the broad-spectrum antibiotic combination metronidazole, neomycin and vancomycin (MNV), to which VRE is resistant, markedly increases colonization of the small intestine, caecum and colon with VRE (Fig. 2a and Supplementary Fig. 1), confirming previously published studies11–15. It remains unclear, however, why elimination of the relatively sparse microbial flora of the small intestine increases the number of VRE by over 100–1,000-fold. Because VRE is acquired by the oral route and passes through the small intestine before systemic dissemination, we determined the impact of antibiotic administration on the expression of RegIIIγ messenger RNA and protein levels in the distal ileum in wild-type mice and in mice treated with MNV for 1, 3 and 7 days. RegIIIγ messenger RNA levels decreased within 1 day of antibiotic administration and continued to decrease over the subsequent 7 days, whereas RegIIIγ protein expression was markedly diminished within 3 days of antibiotic initiation (Fig. 2b, c). Another antibiotic regimen consisting of enrofloxacin and clindamycin, which, like MNV, eliminates Gram-positive, Gram-negative and anaerobic commensal bacteria, similarly reduced RegIIIγ mRNA and protein expression (Fig. 2d, e). In contrast, other mediators of antimicrobial defence—cryptdins and angiogenin-4—were not significantly downregulated by antibiotic administration (Supplementary Fig. 2).
Figure 2. RegIIIγ expression is downregulated in antibiotic-treated mice, correlating with decreased clearance of VRE.
a, Persistence and density of VRE intestinal colonization in mice. Mice were treated with MNV in drinking water starting 2 days before oral infection with 109 VRE by gavage. Bacterial counts within the distal small intestine were determined 1, 3 and 5 days after VRE infection (n=5 each group and time point; **P=0.008, Mann–Whitney test). b, Messenger RNA was extracted from the terminal ileum of wild-type mice (first bar) and mice receiving antibiotics (MNV) for 1, 3 and 7 days. RegIIIγ expression was normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh; n=4–10 mice per group; *P=0.02, **P=0.0011, ***P=0.0001, each compared to untreated, unpaired t-test). c, Protein extracts from the distal small intestine of untreated and MNV-treated mice for 1, 3 and 7 days were analysed by western blotting with RegIIIγ-specific antiserum. Tubulin was used as a loading control. d, Messenger RNA was extracted from the terminal ileum of untreated or enrofloxacin and clindamycin (EC)-treated mice, and RegIIIγ expression was determined (n=2 for wild-type and n=4 for EC-treated mice; **P=0.0017, unpaired t-test). e, Protein extracts from the distal small intestine of wild-type mice and mice receiving antibiotics (EC) for 7 days were analysed by western blotting with RegIIIγ-specific antiserum. f, Wild-type mice and mice administered antibiotics (MNV) in the drinking water for 7 days were infected with 1010 VRE by gastric gavage, and the number of bacteria in the blood was determined 24 h after infection (n = 10 each group; *P = 0.013, Mann–Whitney test). g, Ileal loops of wild-type mice and mice receiving antibiotics for 7 days (MNV) were injected with 1,000 VRE, and bacterial survival was measured 2 h later. Bacterial numbers are expressed as percentage of recovered VRE (n = 8 each group; *P = 0.04, unpaired t-test). h, RegIIIγ or control protein (β2-microglobulin) was inoculated into ileal loops, and VRE survival was assessed (n = 5 each group; ***P = 0.0004, unpaired t-test). Error bars denote s.e.m.
To determine whether diminished RegIIIγ levels correlate with increased susceptibility to VRE, wild-type mice receiving MNV were orally infected with VRE and dissemination to the blood stream was determined 24 h later. Whereas VRE bacteremia was uncommon in untreated mice, viable VRE was cultured from the blood of most antibiotic-treated mice (Fig. 2f). Moreover, assays for in vivo luminal killing demonstrated markedly impaired inactivation of VRE in the distal small bowel of antibiotic-treated mice (Fig. 2g). To determine whether reconstitution of RegIIIγ in the intestine of antibiotic-treated mice corrects defective VRE killing, recombinant RegIIIγ was injected into ileal loops of antibiotic-treated mice before VRE inoculation and bacterial killing were measured. Administration of RegIIIγ markedly diminished VRE survival in the gut of antibiotic-treated mice (Fig. 2h), demonstrating that exogenous RegIIIγ complements the innate immune defect induced by broad-spectrum antibiotic therapy.
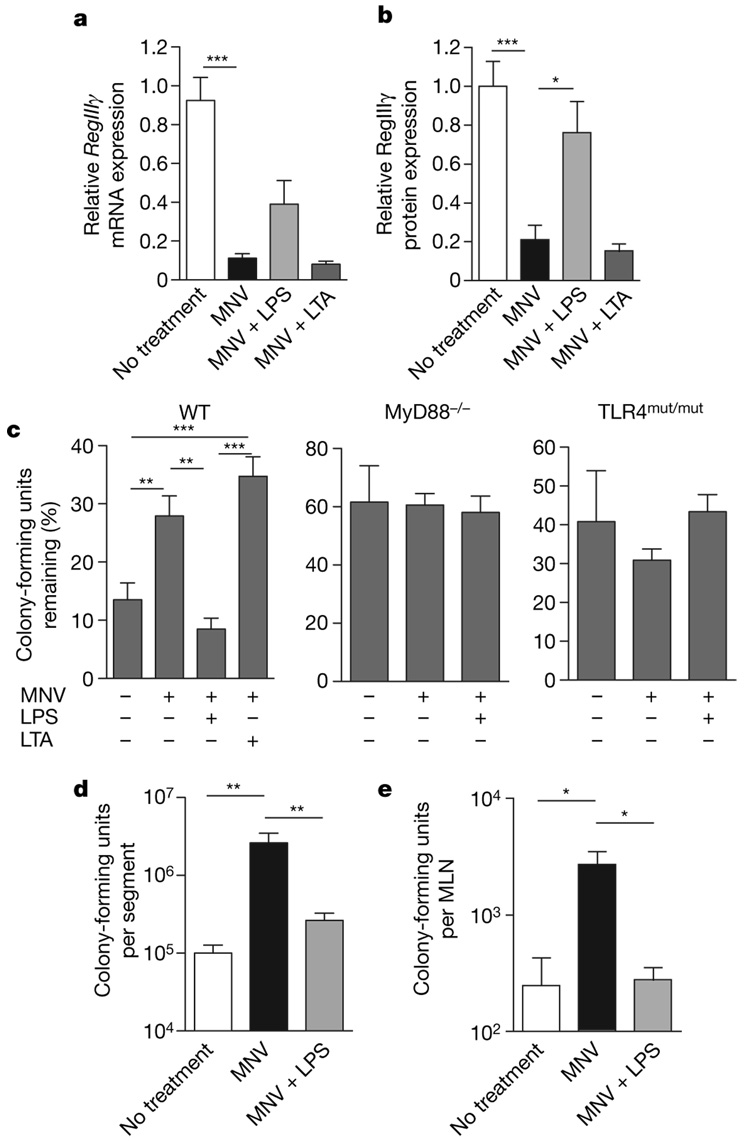
RegIIIγ is induced in the mouse small intestine by the Gram-negative commensal bacterial species Bacteroides thetaiotaomicron16. In contrast, RegIIIγ expression is downregulated by the Gram-positive, probiotic bacterium Bifidobacterium longum16. To determine whether TLR ligands associated with Gram-negative or Gram-positive bacteria can induce RegIIIγ in antibiotic-treated mice, we added lipopolysaccharide (LPS) or lipoteichoic acid (LTA) to the drinking water of antibiotic-treated mice. Quantitative analysis of RegIIIγ protein levels in distal ileum revealed significant upregulation after oral LPS treatment (Fig. 3b and Supplementary Fig. 3), and analysis of RegIIIγ mRNA revealed a similar, albeit not statistically significant, trend (Fig. 3a). In contrast, oral administration of LTA to MNV-treated mice did not increase RegIIIγ mRNA or protein (Fig. 3a, b and Supplementary Fig. 3).
Figure 3. Administration of LPS to antibiotic-treated mice restores RegIIIγ levels and enhances luminal VRE killing.
a, Mice were treated with MNV alone, MNV plus 2–4 µg µl−1 LPS, MNV plus 0.25 µg µl−1 LTA, or were left untreated, and RegIIIγ mRNA expression was determined and is expressed relative to untreated mice (n=5–12 each group; ***P < 0.001, one-way ANOVA with Bonferroni correction). b, Quantitative western blot analysis with RegIIIγ-specific antiserum was performed on protein extracts from the distal small intestine of untreated mice as well as mice receiving MNV,MNV plus LPS, or MNV plus LTA (n = 5–12; * P < 0.05, *** P < 0.001, one-way ANOVA with Bonferroni correction). c, VRE survival was determined in ileal loops of wild type, MyD88−/− or TLR4mut/mut mice treated with MNV, MNV plus LPS, MNV plus LTA, or left untreated. Bacterial numbers are expressed as the percentage of recovered VRE (n = 6–11 for wild type, n = 7–8 for MyD88−/−, n = 6–8 for TLR4mut/mut (C3HeJ); **P < 0.01, ***P < 0.001). d, Mice were treated for 7 days with MNV,MNV plus LPS, or left untreated, and then were orally infected with 1010 VRE. Twenty-four hours later, bacterial counts in the distal small intestine were determined (n = 14–15; **P < 0.01, one-way ANOVA with Bonferroni correction). e, Mice were treated as in d and, 24 h later, bacterial counts within MLNs were determined (n = 4–5;*P < 0.05, one-way ANOVA with Bonferroni correction). Error bars denote s.e.m.
To determine whether LPS-induced restoration of RegIIIγ corrects the VRE clearance defect in the intestine of antibiotic-treated mice, luminal killing assays were performed in mice receiving MNV, in mice receiving MNV together with LPS or LTA, and in untreated mice. Whereas mice receiving only antibiotics manifested defective clearance of VRE from small intestine, bowel loops of mice receiving antibiotics together with LPS cleared VRE as efficiently as ileal loops of wild-type, untreated mice (Fig. 3c). Luminal killing of VRE in antibiotic-treated mice receiving LTA, in contrast, was not enhanced (Fig. 3c). The specificity and TLR4–MyD88-dependence of LPS-mediated rescue was investigated with MyD88- or TLR4-mutant mice. Antibiotic administration to MyD88-deficient mice did not increase VRE survival in the ileal lumen, and LPS administration did not increase VRE killing (Fig. 3c). LPS administration to TLR4-mutant mice also did not increase VRE killing in the intestinal lumen, demonstrating that the LPS effect in wild-type mice was TLR4-mediated and that LPS did not directly inhibit VRE survival (Fig. 3c and Supplementary Fig. 4). Antibiotic-treated mice had significantly higher numbers of VRE in the distal small intestine and mesenteric lymph nodes 24 h after oral infection compared to antibiotic-untreated mice, whereas no effect of LPS was found in TLR4-mutant mice (Supplementary Fig. 5a, b). LPS treatment reduced VRE colony-forming units in small intestines and mesenteric lymph nodes (MLNs) to levels observed in wild-type mice (Fig. 3d, e), demonstrating that LPS-mediated enhancement of innate immune defence decreased VRE colonization and dissemination. To exclude systemic LPS effects during oral LPS treatment, serum tumour necrosis factor (TNF) levels were determined in mice treated either with antibiotics or with antibiotics plus LPS for 7 days. TNF was not detected in either group, whereas mice receiving LPS intraperitoneally had increased serum TNF levels (Supplementary Fig. 6). To determine whether LPS-treated mice ingested equivalent amounts of antibiotic, vancomycin levels in the colons of mice receiving or not receiving LPS were determined. No significant difference was found between the groups, indicating that LPS administration did not reduce the intake of antibiotics in drinking water (Supplementary Fig. 7).
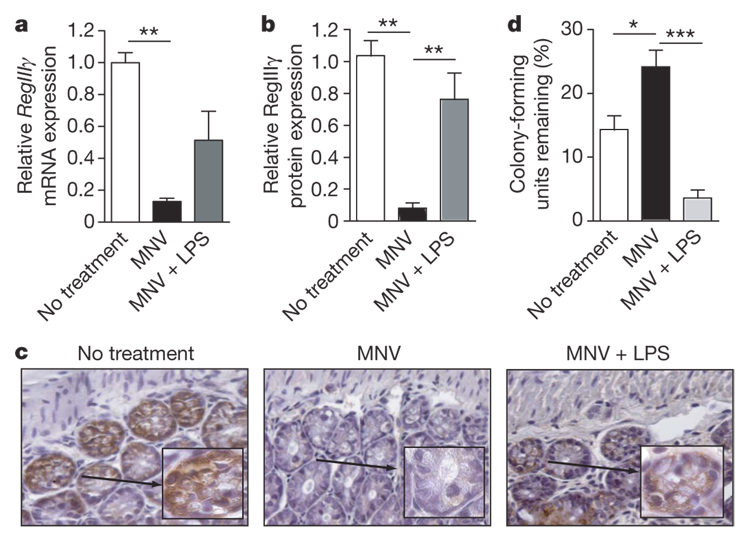
Whereas the preceding experiments demonstrated that administration of LPS concurrent with antibiotic treatment ameliorates the innate immune defect associated with elimination of the commensal intestinal flora, in clinical situations it might be important to enhance resistance after antibiotics have already been administered. Therefore, mice that had received antibiotics for 4 days were treated with oral LPS. RegIIIγ protein levels were significantly upregulated in mice receiving LPS after 4 days of antibiotic treatment and RegIIIγ mRNA levels were also increased to levels that, however, did not achieve statistical significance (Fig. 4b and Supplementary Fig. 8). Immunohistochemistry demonstrated RegIIIγ protein expression in Paneth cells and epithelial cells of the distal small intestine in wild-type mice and an absence of RegIIIγ in mice treated with antibiotics. In contrast, mice treated with LPS in addition to antibiotics expressed readily detectable amounts of RegIIIγ on immunohistochemical analysis (Fig. 4c). In line with these data, luminal killing of VRE was also significantly enhanced in mice receiving LPS to rescue innate immune defenses (Fig. 4d).
Figure 4. Delayed LPS treatment of mice receiving antibiotics restores RegIIIγ levels and enhances luminal VRE killing.
a, Mice were treated with MNV for 4 days before the addition of LPS (1–4 µg µl−1) to the drinking water. RegIIIγ mRNA induction was determined after 7 days LPS treatment and is expressed relative to untreated mice (n = 9–15 in each group; **P < 0.01, one-way ANOVA with Bonferroni correction). b, RegIIIγ protein levels in the distal small intestine of mice treated as in a were determined (n = 5–8; **P < 0.01, one-way ANOVA with Bonferroni correction). c, Immunohistochemical detection of RegIIIγ in paraffin-embedded distal small intestine sections in untreated mice, mice receiving MNV and mice receiving MNV plus LPS (magnification 400-fold, inset 1,000-fold). Representative samples from one of three mice is shown. d, VRE killing in ileal loops of mice treated as in a was determined and expressed as percentage of recovered VRE (n = 5–6 each group; *P < 0.05, ***P < 0.001, one-way ANOVA with Bonferroni correction). Error bars denote s.e.m.
Our findings demonstrate that administration of broad-spectrum antibiotics compromises intestinal innate immune defenses by eliminating commensal microbes, which diminishes expression of antimicrobial molecules in the intestinal mucosa. Compromised intestinal innate immune defence increases susceptibility to anti-biotic-resistant microbes, including the Gram-positive bacterial pathogen VRE. Our experiments show that the innate immune effector molecule RegIIIγ has an important role in host defence against VRE by killing bacteria in the small intestinal lumen. It is likely, however, that other MyD88-induced factors also inhibit intestinal colonization by VRE because MyD88-deficient mice were less effective at inhibiting VRE growth than wild-type mice treated with RegIIIγ antiserum. Along similar lines, oral administration of LPS to antibiotic-treated mice, while inducing RegIIIγ, may also increase intestinal resistance to VRE colonization by inducing other antimicrobial factors.
Altering the complexity of the intestinal commensal flora with broad-spectrum antibiotics provides an opportunity for otherwise innocuous microbes to cause systemic disease11,17,18. Specific TLR signalling can substitute for signals induced by commensal microbes and restore RegIIIγ, thereby enhancing defence against VRE. It is interesting that LPS, the principal component of the outer membrane of Gram-negative bacteria, induces RegIIIγ, which acts selectively against Gram-positive bacteria by binding surface-exposed peptide-glycan. In contrast, LTA, a predominant surface glycolipid of Gram-positive bacteria, does not induce RegIIIγ, nor does it enhance clearance of VRE. Our results, therefore, support the previous findings16 demonstrating that a commensal Gram-negative bacterium, but not a probiotic Gram-positive bacterium, induces RegIIIγ, suggesting that bacteria that belong to different classes can wage battles against each other by inducing innate antimicrobial mechanisms in the mammalian mucosa that selectively inactive bacteria belonging to different classes.
Therapy with TLR ligands, though frequently contemplated, is complicated by the induction of inflammatory cytokines that can, at the extreme, result in septic shock and death19. This dire outcome occurs after systemic administration of some TLR ligands and thus profoundly limits enthusiasm for this form of therapy. In contrast, the gastrointestinal tract is exposed to TLR ligands with great regularity, if not continuously20,21. Recent studies, however, indicate that the intestinal mucosa is not blind to TLR ligands22–24, but that responses, while tempered, contribute to epithelial homeostasis25,26 and enhance innate immune defence against intestinal bacteria9. Thus, although systemic treatment with TLR ligands is fraught with difficulties, administration of TLR ligands to mucosal surfaces with the goal of enhancing innate immune defence may, in certain clinical situations, provide salutary effects.
Although it is presumed that depletion of commensal microbes has the direct effect of providing space and nutrients for pathogenic organisms, our findings indicate that broad-spectrum antibiotics may provide more important indirect advantages to pathogens by impairing mucosal innate immune defenses. Specific induction of antimicrobial molecules, for example, RegIIIγ by the oral administration of LPS, could therefore be of potential therapeutic use in patients receiving broad-spectrum antibiotic therapy.
METHODS SUMMARY
Mice, bacteria and infection
Mice were infected by means of gavage with 109 or 1010 colony-forming units (as indicated) of the vancomycin-resistant E. faecium strain purchased from ATCC (stock number 700221). Bacterial counts within various organs were determined by homogenizing different organs in PBS containing 0.1% Triton X-100. Bacterial counts within luminal contents were determined by flushing the lumen with PBS containing 0.1% Triton X-100 and homogenizing the intestinal wall. For VRE counts in the blood, colony-forming units were determined by directly plating the blood. Plating was performed on EnterococcoseI agar plates (Difco) with vancomycin (8 µgml−1, Sigma). VRE colonies were identified by appearance and confirmed by Gram staining.
Gut commensal depletion and reconstitution with LPS or LTA
Animals were provided with vancomycin (1 gl−1, Sigma), neomycin sulphate (500mg l−1, Sigma) and metronidazole (1 g l−1, Baxter) in drinking water for at least 7 days unless otherwise indicated. Alternatively, animals were given enrofloxacin (Baytril, 400 mg l−1) and clindamycin (400 mg l−1, Sigma) in the drinking water for 7 days. For some experiments, animals were treated for 4 days with vancomycin, neomycin and metronidazole before LPS from Escherichia coli 0127:B8 (1–4 µg µl−1, Sigma; purified by phenol extraction) was added to the drinking water for additional 7 days. Alternatively, for prophylactic administration, drinking water was supplemented from the beginning of the experiment on with vancomycin, neomycin and metronidazole together with LPS from E. coli 0127:B8 (2–4 µg µl−1) or LTA from Bacillus subtilis (0.25 µg µl−1, Sigma).
In vivo VRE killing
Ligated ileal loop experiments were performed as described previously9. PBS (250 µl) containing 1,000 VRE were injected into the ligated loop. Luminal fluid was plated after 2 h.
Generation of bone marrow chimaeras, RT–PCR, western blot, immunohisto-chemistry and determination of vancomycin levels were performed in accordance with standard protocols and as described previously9.
Supplementary Material
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Acknowledgements
The authors thank L.V. Hooper for providing polyclonal RegIIIγ antiserum, I. Leiner for technical assistance, and W. Falk, B. Salzberger and all members of the Pamer laboratory for discussions. This research was supported by the Alexander von Humboldt Foundation through a Feodor Lynen postdoctoral fellowship to KB and NIH grant AI39031 and AI42135 to EGP.
References
- 1.Rice LB. Emergence of vancomycin-resistant enterococci. Emerg. Infect. Dis. 2001;7:183–187. doi: 10.3201/eid0702.010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice LB. Antimicrobial resistance in gram-positive bacteria. Am. J. Infect. Control. 2006;34:S11–S19. doi: 10.1016/j.ajic.2006.05.220. [DOI] [PubMed] [Google Scholar]
- 3.Murray BE. Vancomycin-resistant enterococcal infections. N. Engl. J. Med. 2000;342:710–721. doi: 10.1056/NEJM200003093421007. [DOI] [PubMed] [Google Scholar]
- 4.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 2000;21:510–515. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel RP, Edmond MB. Managing antibiotic resistance. N. Engl. J. Med. 2000;343:1961–1963. doi: 10.1056/NEJM200012283432610. [DOI] [PubMed] [Google Scholar]
- 6.Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 2004;39:219–226. doi: 10.1086/422002. [DOI] [PubMed] [Google Scholar]
- 7.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nature Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 9.Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J. Exp. Med. 2007;204:1891–1900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cash HL, Whitham CV, Hooper LV. Refolding, purification, and characterization of human and murine RegIII proteins expressed in Escherichia coli. Protein Expr. Purif. 2006;48:151–159. doi: 10.1016/j.pep.2006.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donskey CJ, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N. Engl. J. Med. 2000;343:1925–1932. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazaki S, et al. Development of systemic bacteraemia after oral inoculation of vancomycin-resistant enterococci inmice. J. Med. Microbiol. 2001;50:695–701. doi: 10.1099/0022-1317-50-8-695. [DOI] [PubMed] [Google Scholar]
- 13.Stiefel U, Pultz NJ, Helfand MS, Donskey CJ. Increased susceptibility to vancomycin-resistant Enterococcus intestinal colonization persists after completion of anti-anaerobic antibiotic treatment in mice. Infect. Control Hosp. Epidemiol. 2004;25:373–379. doi: 10.1086/502408. [DOI] [PubMed] [Google Scholar]
- 14.Wells CL, Jechorek RP, Erlandsen SL. Evidence for the translocation of Enterococcus faecalis across the mouse intestinal tract. J. Infect. Dis. 1990;162:82–90. doi: 10.1093/infdis/162.1.82. [DOI] [PubMed] [Google Scholar]
- 15.Wells CL, Jechorek RP, Maddaus MA, Simmons RL. Effects of clindamycin and metronidazole on the intestinal colonization and translocation of enterococci in mice. Antimicrob. Agents Chemother. 1988;32:1769–1775. doi: 10.1128/aac.32.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol. 2006;4:e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pultz NJ, Stiefel U, Subramanyan S, Helfand MS, Donskey CJ. Mechanisms by which anaerobic microbiota inhibit the establishment in mice of intestinal colonization by vancomycin-resistant Enterococcus. J. Infect. Dis. 2005;191:949–956. doi: 10.1086/428090. [DOI] [PubMed] [Google Scholar]
- 18.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N. Engl. J. Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 19.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nature Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 20.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J. Immunol. 2005;174:4453–4460. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 21.Lotz M, Menard S, Hornef M. Innate immune recognition on the intestinal mucosa. Int. J. Med. Microbiol. 2007;297:379–392. doi: 10.1016/j.ijmm.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Cario E, et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 23.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J. Exp. Med. 2002;195:559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortega-Cava CF, et al. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J. Immunol. 2003;170:3977–3985. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- 25.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl Acad. Sci. USA. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.