Abstract
LR11 is an ApoE receptor that is enriched in the brain. We have shown that LR11 is markedly downregulated in patients with sporadic Alzheimer disease (AD). This finding led us to explore whether reduced LR11 expression reflects a primary mechanism of disease or merely a secondary consequence of other AD-associated changes. Therefore, LR11 expression was assessed in a transgenic mouse model of AD and familial AD (FAD) brains. Immunohistochemistry and immunoblotting of LR11 in PS1/APP transgenic and wild-type mice indicated that LR11 levels are not affected by genotype or accumulation of amyloid pathology. LR11 expression was also evaluated based on immunoblotting and LR11 immunostaining intensity in human frontal cortex in controls, sporadic AD, and FAD, including cases with presenilin-1 (PS1) and presenilin-2 (PS2) mutations. Although LR11 was reduced in sporadic AD, there was no difference in protein level or staining intensity between control and FAD cases. The finding that LR11 expression is unaffected in both a mouse model of AD and autosomal-dominant forms of AD suggests that LR11 is not regulated by amyloid accumulation or other AD neuropathologic changes. We hypothesize that LR11 loss may be specific to sporadic AD and influence amyloid pathology through mechanisms independent of substrate–enzyme interactions regulated by FAD mutations.
Keywords: Alzheimer disease, ApoE receptor, Familial Alzheimer disease, LR11, Mouse model of AD
INTRODUCTION
Alzheimer disease (AD) is a profoundly debilitating condition that is characterized by the progressive degeneration of neural systems involved in regulating mood, cognition, and memory. Neuropathologically, AD is characterized by the formation of 2 pathologic hallmarks, neurofibrillary tangles rich in hyperphosphorylated tau and plaques that are composed primarily of beta-amyloid (Aβ) (1). The mechanisms underlying the pathogenesis of AD are incompletely understood, but the amyloid cascade hypothesis has become the predominant view of AD pathogenesis (2). In the amyloid model, a shift in the processing of the amyloid precursor protein (APP) from nonpathologic metabolites to the increased generation and/or the reduced clearance of Aβ is believed to be the seminal event in AD pathogenesis.
The vast majority of patients with AD have late-onset sporadic AD. In a small number of patients, however, genetic mutations have been discovered that result in more aggressive pathology with earlier disease onset. Mutations in APP and presenilin-1 and -2, components of the γ-secretase complex that cleaves APP and yields Aβ, cause familial forms of AD (FAD) (3–7). Importantly, all known FAD mutations result in the increased production and deposition of Aβ in brain, lending support to the amyloid hypothesis. In sporadic AD, the mechanism of Aβ accumulation in the brain is likely a result of a complex molecular cascade that includes additional factors distinct from those produced by FAD mutations.
In brain tissue of individuals with sporadic AD, we have found a striking reduction in the ApoE receptor LR11/SorLA, particularly in the vulnerable areas of the cortex and hippocampus (8). LR11 is a member of the low-density lipoprotein receptor (LDL-R) family (9–11). The best-studied role of LDL-Rs is in cholesterol regulation through the binding and uptake of lipid-rich particles (12). Recent evidence has highlighted the involvement of lipoprotein receptors and cholesterol metabolism in the regulation of amyloid generation and is beginning to clarify the connection between cholesterol and neurodegeneration (13–15). All members of the LDL-R family can bind the lipid carrier molecule ApoE, and individuals with one or 2 APOE ε4 alleles have an increased risk of developing AD and earlier disease onset. LR11 has additional functional domains and, as a mosaic receptor, may serve many physiological functions, including cargo transport, chaperone-like activity, signaling, and intracellular sorting (16–21). Recently, we and others have shown that LR11 can interact with APP, influence the trafficking of APP, and regulate Aβ levels in vitro and in vivo (22, 23).
It is not clear, however, whether LR11 loss observed in sporadic AD occurs downstream of the AD pathologic cascade or if reduction in LR11 expression could be a proximal contributor to the disease process. To establish whether LR11 can be regulated by amyloid accumulation in vivo, we examined LR11 expression in a transgenic animal model of amyloidosis as well as brains of individuals carrying FAD mutations. The transgenic mice used in this study were engineered to express the human presenilin-1 (PS1) gene with exon 9 deletion as well as the Swedish APP mutation, both of which were identified in familial forms of the disease (24). Individuals with FAD and the PS1/APP mice are similar in the underlying mechanism of amyloid pathology, one that is believed to be different in origin from that found in sporadic AD.
In this study, we show that unlike sporadic AD, LR11 protein expression is unchanged in PS1/APP mice and FAD brain tissue, suggesting that LR11 is not simply regulated by amyloid or downstream molecular events that follow amyloidosis. These observations provide support for the hypothesis that LR11 loss is a proximal event in sporadic AD and potentially a key component of the complex pathologic cascade that causes sporadic AD. Given the continually growing body of evidence concerning ApoE receptors and amyloidosis, LR11 may play a role in the development of sporadic AD. Taken together with the recent evidence that LR11 regulates Aβ production both in vitro and in vivo, LR11 may prove to be an important therapeutic target for AD.
MATERIALS AND METHODS
Presenilin-1/Amyloid Precursor Protein Mice
Double transgenic PS1ΔEx9/APPswe mice were acquired from David Borchelt (Johns Hopkins University). Amyloid accumulation in these mice has been previously characterized with diffuse and neuritic plaque pathology occurring at or before 6 months of age (24). Brains from 3-, 6-, 9-, and 18-month-old mice were hemisected. One hemisphere was prepared for immunohistochemistry as described subsequently, and the cortex was dissected from the remaining hemisphere and homogenized using a Konte tissue grinder for biochemical measurements.
Immunohistochemical Analysis of Amyloid and LR11 in Presenilin-1/Amyloid Precursor Protein Transgenic Mouse Brain
Hemibrains of PS1/APP transgenic mice and control mice were immersion-fixed with 4% paraformaldehyde for 3 hours at 4°C, then cryoprotected in 30% sucrose before sectioning on a freezing-sliding microtome to obtain 50-μm-thick coronal sections. Immunohistochemical processing was performed using free-floating sections and immunoperoxidase methods. Sections were treated with hydrogen peroxide, washed in Tris buffer, blocked with normal serum, and incubated with anti-LR11 C-terminal antibody, the specificity of which has already been established (25). On day 2, sections were incubated with biotinylated secondary antibody followed by avidin–biotin–peroxidase complex for 1 hour at 4°C (Vector Elite ABC kit; Vector Laboratories, Burlingame, CA). Immunoreactivity was visualized with fluorescent-conjugated tyramide reagent (TSA Cyanine 3 System; PerkinElmer Life Sciences, Boston, MA). Mounted tissue sections were dehydrated briefly, hydrated, then submerged in 1% thioflavine-S solution for 8 minutes, and rinsed in 80% ethanol. Confocal images were captured on a Zeiss LSM510 microscope.
Western Blotting of LR11 Protein in Presenilin-1/Amyloid Precursor Protein Mice and Human Tissue
Immunoblotting was performed according to standard procedures. Protein concentrations were measured with a protein assay kit (BCA; Pierce, Rockford, IL). Samples were solubilized in Laemmli sample buffer, separated on 7.5% SDS-polyacrylamide gels, and transferred electrophoretically to PVDF membranes (Immobilon-P; Millipore, Billerica, MA). Blots were blocked for 1 hour at room temperature and probed with either a monoclonal LR11 primary antibody (26) or polyclonal LR11 C-terminal antibody. Loading consistency was established with a monoclonal elongation factor-1α (EF1-α) antibody (Upstate Cell Signaling Solutions, Charlottesville, VA) (27) or a monoclonal actin antibody (Abcam, Cambridge, MA) (28). Sections were then incubated with fluorophore-conjugated goat anti-mouse and goat anti-rabbit secondary antibodies (Molecular Probes, Eugene, OR) at room temperature for 1 hour. Images were captured and band intensities quantified using an Odyssey Image Station (LiCor, Lincoln, NE).
LR11 Immunostaining in Human Tissue
Paraffin sections of cognitively normal controls, sporadic AD, and FAD subjects from the Emory University ADRC, University of Washington ADRC, and Drexel University brain banks were used in this study. Specifically, there were 11 control 868 cases from individuals who had been identified as cognitively normal and free of AD-related neuropathology, 17 sporadic AD cases, and 14 FAD cases (9 PS1 cases and 5 PS2 cases; see Table for details). Blocks of frontal cortex were fixed for 24 to 48 hours in 4% paraformaldehyde or for 2 weeks in 10% neutral-buffered formalin and then embedded in paraffin. Paraffin-embedded blocks were cut into 8-μm sections, deparaffinized, and pretreated with pepsin (Biomeda, Foster City, CA). Sections were incubated with a polyclonal LR11 C-terminal primary and biotinylated goat anti-rabbit secondary antibodies as described previously. Immunoreactivity was visualized with 3,3-diaminobenzidine tetrahydrochloride (DAB). Sections were also counterstained with hematoxylin. Control sections incubated without primary antibody showed negligible staining.
TABLE.
Human Cases
| Human Tissue | Number of Cases | Mean Age (years) Standard Deviation | Gender (Male/Female) | PMI (hours) Standard Deviation | Mean LR11 Staining Score Standard Error of Mean | APOE* |
|---|---|---|---|---|---|---|
| Control | 11 | 66.4 ± 12.4 | 7/4 | 10.4 ± 8.6 | 3.2 ± 0.27 | ε2/ε3 (1) |
| ε2/ε4 (1) | ||||||
| ε3/ε3 (6) | ||||||
| ε3/ε4 (2) | ||||||
| FAD PS1 | 9 | 51.2 ± 12.8 | 5/4 | 9.0 ± 6.5 | 3.1 ± 0.39 | ε2/ε3 (1) |
| ε3/ε3 (8) | ||||||
| FAD PS2 | 5 | 68.9 ± 9.8 | 2/3 | 10.0 ± 7.6 | 3.2 ± 0.37 | ε2/ε3 (1) |
| ε3/ε3 (2) | ||||||
| ε3/ε4 (2) | ||||||
| Sporadic AD | 17 | 75.7 ± 7.4 | 8/9 | 11.5 ± 8.8 | 2.1 ± 0.17 | ε2/ε3 (3) |
| ε3/ε3 (5) | ||||||
| ε3/ε4 (5) | ||||||
| ε4/ε4 (3) |
, APOE genotypes from one control and one sporadic AD case were not available.
PMI, postmortem interval, APOE, APOE genotype (APOE genotype is reported as a combination of any 2 alleles from 3 possible [ε2, ε3, ε4]); numbers in parentheses indicate number of cases that exhibited a specific genotype; FAD, familial Alzheimer Disease; PS, presenilin; AD, Alzheimer disease.
Semiquantitative Scoring of LR11 Immunostaining Intensity
A 5-point scale of immunostaining intensity was used to quantify LR11 expression levels with the following parameters: 1 = little to no staining; 2 = light staining; 3 = moderate staining; 4 = moderate to strong staining; and 5 = very strong staining. Scoring was conducted in blinded fashion by one observer.
RESULTS
Amyloid Accumulation and LR11 Expression in PS1/APP Mouse Tissue
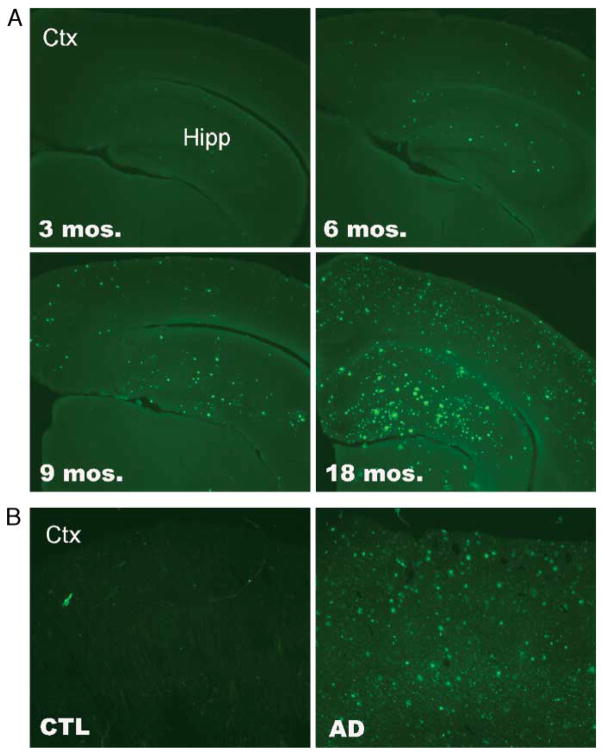
To evaluate whether extensive amyloidosis can lead to a reduction in LR11 expression in vivo, we qualitatively and quantitatively assessed LR11 protein expression in 3-, 6-, 9-, and 18-month-old PS1/APP and wild-type littermate control mice. PS1/APP mice develop amyloid accumulation early and show evidence of thioflavine-S-positive plaques by 3 months of age. Amyloid burden increases progressively with age (Fig. 1A), and by 18 months of age, PS1/APP mice exhibit extensive plaque pathology similar that observed in human AD brain (Fig. 1B).
FIGURE 1.
Amyloid pathology in brain. (A) Coronal sections from 3-, 6-, 9-, and 18-month-old PS1/APP mice. Thioflavine-S stain reveals increasing accumulation of amyloid plaques with age in cortex (Ctx) and hippocampus (Hipp). (B) Coronal sections of frontal cortex from non-diseased control (CTL) and sporadic Alzheimer disease (AD) human brain. Thioflavine-S staining reveals no plaque pathology in control brain and extensive plaque accumulation in human AD brain. Original magnification: 5×.
Immunohistochemical analysis of LR11 protein expression reveals similar LR11 immunoreactivity between PS1/APP mice and wild-type littermates, even in older mice that exhibit robust amyloidosis (Fig. 2). Quantitative Western blotting confirms that LR11 protein expression levels are not altered in PS1/APP mice compared with age-matched wild-type littermates (Fig. 3). The finding that LR11 expression is unaltered in a transgenic mouse model of aggressive amyloidosis is an important observation and supports the hypothesis that loss of LR11 protein expression in sporadic AD brain is not simply a byproduct of amyloid accumulation. However, these mice do not fully recapitulate the pathogenic cascade of AD. Most notably, transgenic PS1/APP mice do not exhibit tangle pathology or extensive neurodegeneration. Therefore, it was important to further assess LR11 expression in human tissue, specifically comparing sporadic AD and genetically based FAD variants.
FIGURE 2.
LR11 immunofluorescence in presenilin-1/amyloid precursor protein (PS1/APP) mouse brain. Representative coronal sections from one-month-old (left) and 18-month-old (right) wild-type (WT) and PS1/APP mice were processed for LR11 immunostaining. (Top panels) Low-magnification view of LR11 staining in cortex (ctx) and CA1 region of hippocampus (ca1). Corpus callosum is also labeled (cc). Scale bar = 100 μm. (Middle panels) High-magnification view of LR11 staining in cortical pyramidal neurons. Scale bar = 10 μm. (Bottom panels) High-magnification view of LR11 staining in CA1 hippocampal pyramidal neurons. Scale bar = 10 μm.
FIGURE 3.
Immunoblots of LR11 protein from PS1/APP mouse brain. Cortical tissue from wild-type (WT) and PS1/APP mice at 3, 6, 9, and 18 months of age were immunoblotted for LR11 and EF1α protein expression to establish loading consistency. Each band is representative of one animal. Densitometric quantitation of LR11 expression is graphed on the right side (relative intensity). LR11 expression in PS1/APP mice was not significantly different from control at any of the time points tested.
LR11 Expression in Sporadic Alzheimer Disease, Familial Alzheimer Disease, and Control Tissue
To assess LR11 protein expression in cognitively normal, sporadic AD, and FAD tissue, we evaluated the intensity of LR11 immunoreactivity in frontal cortex of 42 individuals (Table). Gender and postmortem interval did not differ among the 3 groups (χ2 = 0.783, p = 0.676; analysis of variance [ANOVA], F = 0.244, df = 2,27, p = 0.785). Statistical analysis of age among groups was significantly different (ANOVA, F = 8.43, df = 3,37, p < 0.001), and a Bonferroni posttest found that the PS1 cases were younger than the sporadic AD group (t = 4.99, p < 0.001); however, there was no difference in age among the control, sporadic AD, and PS2 cases. Although control, sporadic AD, and FAD cases did not significantly vary with regard to the presence of the ApoE ε4 allele (χ2 = 4.379, p = 0.112), there was a higher frequency of ε4 allele presence in the sporadic AD group (8 of 16) compared with the control (3 of 11) and FAD groups (2 of 9), as expected. Fixation conditions varied somewhat among cases, which were either fixed for 2 weeks in formalin or for 24 to 48 hours in 4% paraformaldehyde; however, we observed no consistent trends in staining intensity scores between the formalin and paraformaldehyde-fixed tissue sections.
In control brains, LR11 expression is characterized by strong punctuate staining, especially obvious in larger pyramidal neurons in frontal cortex and hippocampus (Fig. 4). LR11 expression in neurons remains restricted to the somatodendritic compartment of the cells and is typically cytoplasmic, consistent with its known localization to endosomal compartments and Golgi membrane (22, 23). In contrast to the strong staining in controls, there is a striking loss of LR11 staining in frontal cortex of individuals with sporadic AD (8).
FIGURE 4.
LR11 immunohistochemistry in human brain. Paraffin sections from frontal cortex of cognitively normal (control), sporadic Alzheimer disease, presenilin (PS) -1 variants of familial Alzheimer disease (FAD) (PS1), and PS2 variants of FAD (PS2) were stained for LR11 expression and visualized with DAB (brown deposits). Low-magnification images captured at 20× and high-magnification insets were captured at 100×. Cell bodies were counterstained with hematoxylin (blue).
LR11 staining in frontal cortex from FAD, sporadic AD, and cognitively normal individuals revealed a different pattern of LR11 expression in individuals with FAD and individuals with sporadic AD (Fig. 4). On the 5-tiered scale used here, LR11 staining in control tissue was generally moderate (mean score = 3.2 [n = 11]), whereas staining in sporadic AD tissue typically fell into the light-staining group (mean score = 2.11 [n = 17]). The FAD staining mean was 3.14 (n = 14), corresponding to moderate staining intensity. A Kruskal-Wallis statistical analysis revealed a significant difference in staining intensity among the 3 experimental groups (Fig. 5; F = 12.13, p = 0.002). As expected, semi-quantitative scoring of LR11 immunointensity level showed reduced staining intensity in individuals with sporadic AD compared with control tissue (p < 0.05; Dunn multiple comparison test). Within the sporadic AD group, the presence of an ApoE ε4 allele did not affect LR11 staining intensity in this study (Mann-Whitney, F = 19.5, p = 0.19); however, we did observe a weak trend toward increased LR11 immunostaining intensity in ε4-negative compared with ε4-positive sporadic AD cases that may warrant future studies using larger cohorts.
FIGURE 5.
Results from semiquantitative LR11 staining intensity measures in human brain. LR11-immunolabeled paraffin sections were evaluated on a 5-point scale for intensity of staining. Scale definition: 1 = little to no staining; 2 = light staining; 3 = moderate staining; 4 = moderate–strong staining; and 5 = strong staining. **, Mean LR11 immunostaining intensity was significantly less in sporadic Alzheimer disease than both control and familial Alzheimer disease (FAD). The FAD group consists of presenilin (PS) -1 and PS2 cases grouped together for statistical comparison. Refer to the Table for staining means and standard error of mean. Each point represents the intensity score of a single case. The mean staining intensity of each group is plotted as a horizontal line.
In contrast to the sporadic AD cases, individuals carrying either PS1 or PS2 familial mutations showed no reduction in LR11 intensity compared with control tissue. Because LR11 staining intensity was not significantly different in the PS1 FAD variants compared with PS2 variants, these individuals were pooled for statistical analyses. LR11 staining intensity in FAD cases was significantly higher than that in sporadic AD tissue (p < 0.01, Dunn multiple comparison test).
In addition to LR11 immunostaining, quantitative immunoblotting was used to measure total protein levels in a representative subset of control (n = 5), sporadic AD (n = 6), and FAD (n = 8) cases for which frozen tissues were available (Fig. 6). Compared with controls, LR11 was decreased in sporadic AD cases by 30% (t = 2.165, df = 9, p = 0.058). Whereas the difference between LR11 expression levels in control (n = 5) and sporadic AD (n = 6) cases did not quite reach statistical significance in this small cohort, these results are consistent with our previous published findings, in which we found a 25% reduction in LR11 protein level (8). Conversely, LR11 protein level was unchanged in FAD frontal cortex compared with controls (t = 0.504, df = 10, p = 0.625). It is worth noting that the ages of control, sporadic AD, and PS2 cases were not significantly different. Therefore, the loss of LR11 seen in individuals with sporadic AD cannot simply be attributed to age-associated changes in brain. Extending the previous observation that LR11 expression is unchanged in PS1/APP transgenic mice, these results indicate that LR11 expression and distribution are unaltered in individuals carrying familial forms of AD.
FIGURE 6.
Representative immunoblots for LR11 protein expression in human brain. Immunoblots were probed with an LR11 C-terminus antibody and N-terminus β-actin antibody as a loading control. Graphic representation of LR11 protein is plotted as percent of control protein level. LR11 protein expression is decreased by approximately 30% in sporadic Alzheimer disease (AD) cases (p = 0.058) compared with control. Familial AD cases have equivalent LR11 protein compared with controls (p = 0.699). Error bars represent standard error of the mean.
DISCUSSION
Recently, we identified the striking loss of LR11 transcript and protein in brains of individuals with sporadic AD (8). Like with many biologic changes that occur as a consequence of the pathologic cascade in AD, this loss could simply be explained by downstream events associated with amyloidosis and neurodegeneration. However, we report here that in both a transgenic mouse model of amyloidosis and in brain tissue of individuals harboring similar genetic mutations that cause familial AD, there is no loss of LR11 protein expression. These results suggest that reduced expression of LR11 is not simply an effect of amyloid accumulation in sporadic AD brain.
Although neuropathologic features of FAD and sporadic AD are very similar, the trigger to pathogenesis is distinct. In the case of FAD, development of disease is directly linked to genetic mutations. Mutations in 3 genes, PS1, PS2, and APP, result in increased production of Aβ, particularly the longer Aβ1–42 species, by modifying the substrate (APP) or by modulating the activity of the γ-secretase enzyme that releases Aβ from full-length APP (3–7). Although amyloid accumulation is often considered a primary event in sporadic AD, the cause of increased amyloid production or deposition is unclear. This difference in underlying mechanism of disease may explain the marked difference in LR11 expression in FAD compared with sporadic AD.
Because the initial identification of APOE polymorphism and disease susceptibility, it has become increasingly clear that there is a strong association between cholesterol metabolism and amyloid accumulation (14, 29). Although the number of APOE ε4 allele carriers between our 3 experimental groups was not significantly different, we cannot rule out a modest effect of ApoE genotype on LR11 expression, particularly among sporadic AD cases. It is unclear if there is a differential effect of ApoE isoforms on the activity of LDL-Rs in the brain. The affinity of LR11 for different isoforms of ApoE has not been directly examined; however, low-density lipoprotein receptor family members, LDL-R, and the low-density lipoprotein receptor-related protein (LRP), have similar affinities for apoE3 and apoE4. Whether apoE3 and apoE4 can differentially influence LR11 expression and/or trafficking is an intriguing question for future studies.
In addition to APOE, a number of other genes involved in cholesterol transport and catabolism have been implicated as putative risk factors for sporadic AD, including LRP (30). Epidemiologic evidence has suggested protection from development of AD in individuals treated with drugs that lower cholesterol levels by inhibiting HMG CoA reductase, the rate-limiting enzyme in cholesterol synthesis (31, 32). A number of studies have shown that statin treatment in vitro and in vivo can regulate levels of Aβ (33, 34). It is also becoming clear that altered cholesterol trafficking can influence APP processing and Aβ production (14). For example, mutation of the Niemann-Pick protein (NPC1) is characterized by aberrant cholesterol storage in endosomal and lysosomal compartments and leads to elevated levels of Aβ as well as the formation of neurofibrillary tangles in brain (35–37).
Our results indicate that LR11 loss is specific to the more common sporadic form of AD and may represent a proximal component of the pathogenic cascade. We hypothesize that LR11 may protect against amyloidogenesis in brain. Therefore, loss of LR11, which is seen in sporadic AD, may exacerbate amyloidosis and potentiate early events in the disease process. Recent studies suggest that LR11 can interact with APP and BACE in Golgi and endosomal compartments and influence the trafficking and processing of APP (22, 23, 38). Increased LR11 expression in cell culture increases the colocalization of APP with endocytic markers and reduces the generation of Aβ. Mechanistically, LR11 may protect from amyloidogenesis by shuttling full-length APP through the degradative endosomal pathway and altering substrate–enzyme interactions between APP and its amyloidogenic enzymatic partners, BACE and γ-secretase. The search for molecules that play a primary role in amyloidosis is of great interest to medical science because a major goal of AD research is to identify therapeutic targets that may stem the onset and progression of disease. Pharmacologic treatments that can increase LR11 expression or function may prove to be an effective, novel therapeutic strategy.
Acknowledgments
The authors thank Veronica Walker, Howard D. Rees, and Stephanie Carter for excellent technical assistance.
This work was supported by NIH-NIA grants AG024214, AG025688, and AG05136.
References
- 1.Selkoe DJ. Alzheimer’s disease: Genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer disease: Mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140:627–38. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- 3.Sherrington R, Rogaev E, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–60. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 4.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 5.Murrell J, Farlow M, Ghetti B, et al. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science. 1991;254:97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- 6.Rogaev E, Sherrington R, Rogaeva E, et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376:775–78. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 7.Levy-Lahad E, Wasco W, Poorkaj P, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–77. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 8.Scherzer CR, Offe K, Gearing M, et al. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch Neurol. 2004;61:1200–05. doi: 10.1001/archneur.61.8.1200. [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen L, Madsen P, Jacobsen C, et al. Activation and functional characterization of the mosaic receptor SorLA/LR11. J Biol Chem. 2001;276:22788–96. doi: 10.1074/jbc.M100857200. [DOI] [PubMed] [Google Scholar]
- 10.Herz J, Beffert U. Apolipoprotein E receptors: Linking brain development and Alzheimer’s disease. Nat Rev Neurosci. 2000;1:51–58. doi: 10.1038/35036221. [DOI] [PubMed] [Google Scholar]
- 11.Taira K, Bujo H, Hirayama S, et al. LR11, a mosaic LDL receptor family member, mediates the uptake of ApoE-rich lipoproteins in vitro. Arterioscler Thromb Vasc Biol. 2001;21:1501–506. doi: 10.1161/hq0901.094500. [DOI] [PubMed] [Google Scholar]
- 12.Herz J, Bock HH. Lipoprotein receptors in the nervous system. Ann Rev Biochem. 2002;71:405–34. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- 13.Howland DS, Trusko SP, Savage MJ, et al. Modulation of secreted beta-amyloid precursor protein and amyloid beta-peptide in brain by cholesterol. J Biol Chem. 1998;273:16576–82. doi: 10.1074/jbc.273.26.16576. [DOI] [PubMed] [Google Scholar]
- 14.Chauhan NB. Membrane dynamics, cholesterol homeostasis, and Alzheimer’s disease. J Lipid Res. 2003;44:2019–29. doi: 10.1194/jlr.R300010-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Wang SS, Rymer DL, Good TA. Reduction in cholesterol and sialic acid content protects cells from the toxic effects of beta-amyloid peptides. J Biol Chem. 2001;276:42027–34. doi: 10.1074/jbc.M102834200. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen L, Madsen P, Nielsen MS, et al. The sorLA cytoplasmic domain interacts with GGA1 and -2 and defines minimum requirements for GGA binding. FEBS Lett. 2002;511:155–58. doi: 10.1016/s0014-5793(01)03299-9. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen L, Madsen P, Jacobsen C, et al. Activation and functional characterization of the mosaic receptor SorLA/LR11. J Biol Chem. 2001;276:22788–96. doi: 10.1074/jbc.M100857200. [DOI] [PubMed] [Google Scholar]
- 18.Herz J. The LDL receptor gene family: (Un)expected signal transducers in the brain. Neuron. 2001;29:571–81. doi: 10.1016/s0896-6273(01)00234-3. [DOI] [PubMed] [Google Scholar]
- 19.Nykjaer A, Willnow TE. The low-density lipoprotein receptor gene family: A cellular Swiss army knife? Trends Cell Biol. 2002;12:273–80. doi: 10.1016/s0962-8924(02)02282-1. [DOI] [PubMed] [Google Scholar]
- 20.Yamazaki H, Bujo H, Saito Y. A novel member of the LDL receptor gene family with eleven binding repeats is structurally related to neural adhesion molecules and a yeast vacuolar protein sorting receptor. J Atheroscler Thromb. 1997;4:20–26. doi: 10.5551/jat1994.4.20. [DOI] [PubMed] [Google Scholar]
- 21.Lintzel J, Franke I, Riedel IB, et al. Characterization of the VPS10 domain of SorLA/LR11 as binding site for the neuropeptide HA. Biol Chem. 2002;383:1727–33. doi: 10.1515/BC.2002.193. [DOI] [PubMed] [Google Scholar]
- 22.Offe K, Dodson SE, Gearing M, et al. The lipoprotein receptor LR11 regulates Abeta production and APP traffic in endosomal compartments. J Neurosci. 2006;26:1596–603. doi: 10.1523/JNEUROSCI.4946-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen OM, Reiche J, Schmidt V, et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102:13461–66. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borchelt DR, Ratovitski T, van Lare J, et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19:939–45. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 25.Hampe W, Riedel IB, Lintzel J, et al. Ectodomain shedding, translocation and synthesis of SorLA are stimulated by its ligand head activator. J Cell Sci. 2000;113:4475–85. doi: 10.1242/jcs.113.24.4475. [DOI] [PubMed] [Google Scholar]
- 26.Hirayama S, Bujo H, Yamazaki H, et al. Differential expression of LR11 during proliferation and differentiation of cultured neuroblastoma cells. Biochem Biophys Res Commun. 2000;275:365–73. doi: 10.1006/bbrc.2000.3312. [DOI] [PubMed] [Google Scholar]
- 27.Kimball SR, Shantz LM, Horetsky RL, et al. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem. 1999;274:11647–52. doi: 10.1074/jbc.274.17.11647. [DOI] [PubMed] [Google Scholar]
- 28.North AJ, Gimona M, Cross RA, et al. Calponin is localised in both the contractile apparatus and the cytoskeleton of smooth muscle cells. J Cell Sci. 1994;107:437–44. doi: 10.1242/jcs.107.3.437. [DOI] [PubMed] [Google Scholar]
- 29.Wolozin B. Cholesterol, statins and dementia. Curr Opin Lipidol. 2004;15:667–72. doi: 10.1097/00041433-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez L, Alvarez V, Gonzalez P, et al. Variation in the LRP-associated protein gene (LRPAP1) is associated with late-onset Alzheimer disease. Am J Med Genet. 001;105:76–78. [PubMed] [Google Scholar]
- 31.Jick H, Zornberg GL, Jick SS, et al. Statins and the risk of dementia. Lancet. 2000;356:1627–31. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 32.Wolozin B, Kellman W, Ruosseau P, et al. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–43. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 33.Simons M, Keller P, De Strooper B, et al. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:6460–64. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kojro E, Gimpl G, Lammich S, et al. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha-secretase ADAM 10. Proc Natl Acad Sci U S A. 2001;98:5815–20. doi: 10.1073/pnas.081612998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito Y, Suzuki K, Nanba E, et al. Niemann-Pick type C disease: Accelerated neurofibrillary tangle formation and amyloid beta deposition associated with apolipoprotein E epsilon 4 homozygosity. Ann Neurol. 2002;52:351–55. doi: 10.1002/ana.10266. [DOI] [PubMed] [Google Scholar]
- 36.Manni ME, Cescato R, Paganetti PA. Lack of beta-amyloid production in M19 cells deficient in site 2 processing of the sterol regulatory element binding proteins. FEBS Lett. 1998;427:367–70. doi: 10.1016/s0014-5793(98)00469-4. [DOI] [PubMed] [Google Scholar]
- 37.Jin LW, Shie FS, Maezawa I, et al. Intracellular accumulation of amyloidogenic fragments of amyloid-beta precursor protein in neurons with Niemann-Pick type C defects is associated with endosomal abnormalities. Am J Pathol. 2004;164:975–85. doi: 10.1016/s0002-9440(10)63185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spoelgen R, von Arnim CA, Thomas AV, et al. Interaction of the cytosolic domains of sorLA/LR11 with the amyloid precursor protein (APP) and beta-secretase beta-site APP-cleaving enzyme. J Neurosci. 2006;26:418–28. doi: 10.1523/JNEUROSCI.3882-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]