Abstract
The binding of amphiphilic molecules to lipid bilayers is followed by 19F NMR using chemical shift and line shape differences between the solution and membrane-tethered states of –CF3 and –CHF2 groups. A chemical shift separation of 1.6 ppm combined with a high natural abundance and high sensitivity of 19F nuclei offers an advantage of using 19F NMR spectroscopy as an efficient tool for rapid time-resolved screening of pharmaceuticals for membrane binding. We illustrate the approach with molecules containing both fluorinated tails and an acrylate moiety, resolving the signals of molecules in solution from those bound to synthetic dimyristoylphosphatidylcholine bilayers both with and without magic angle sample spinning. The potential in vitro and in vivo biomedical applications are outlined. The presented method is applicable with the conventional NMR equipment, magnetic fields of several Tesla, stationary samples, and natural abundance isotopes.
Introduction
Fluorinated pharmaceuticals have a wide range of applications including anesthesiology,1 cancer therapy,2 amyloid plaque therapy,3,4 anti-inflammatory drugs,5 and others. Due to the unique properties of the di- and trifluoromethyl groups, including high electronegativity, high lipophilicity and high steric demand, they may improve the profile of bioactive compounds considerably, by enhancing absorption and permeability. While mass spectrometry (MS) and liquid chromatography (LC) are typically used in vitro and 18F-PET in vivo, 19F NMR spectroscopy is a rapidly emerging tool for in vitro studies of drug binding and structure in lipid membranes6–8 and in vivo MR research9 primarily because of high 19F NMR sensitivity and very low background signal. The 100% natural abundance and high magnetic moment of 19F (γ(19F) = 0.94*γ(1H) result in 19F NMR sensitivity similar to that of 1H.8 Here, we report on using both magic angle spinning (MAS) and static 19F NMR spectroscopy to study the transfer of fluorinated amphiphilic molecules from aqueous solution into a model membrane environment. The choice of the amphiphilic molecules studied here is motivated by their acrylate moiety. As demonstrated with 2-hydroxyethyl acrylate, molecular addition of parahydrogen followed by spin order transfer to the ether 13C allows NMR sensitivity enhancement by 104–105 decaying with a time constant of tens of seconds.10 Specifically, the long fluorocarbon chain of 4,4,5,5,6,6,7,7,8,8,9,9,9-tridecafluoro-2-hydroxynonyl acrylate (TDHA) ensures a strong binding affinity to the lipid membranes, allowing for investigation of the effect of the lipid membrane environment on the 19F chemical shift and CSA of the –CF3 group. A second example is 2,2,3,3-tetrafluoropropyl acrylate (TFPA). While TFPA has fewer fluorines in the fluorocarbon moiety, it is more soluble in aqueous solvents, an important prerequisite for most in vivo applications. Therefore, TFPA is a good representative of modern pharmaceuticals in terms of solubility, molecular weight, and number of fluorines,1–4,7–9 and the findings presented here would also be relevant to a large number of potential pharmaceuticals.
Experimental Details
We mixed TDHA (Sigma-Aldrich/Isotec, Miamisburg, OH) with 1,2-dimyristoylphosphatidylcholine (DMPC) (Avanti Polar Lipids, Inc., Alabaster, AL) in a 1:20 molar ratio in chloroform followed by solvent evaporation and incubated the product in warm deionized water above the phase transition temperature of DMPC of ~24 °C to produce hydrated DMPC bilayers. The hydrated lipid pellet containing TDHA was packed in a 4 mm zirconium magic angle spinning (MAS) rotor for NMR. The MAS technique modulates anisotropic spin interactions, narrowing the line width and revealing the isotropic chemical shift in solid and partially ordered molecules, for which spectral resolution is typically limited by the powder distribution of dipolar couplings and chemical shift anisotropy.
Solubility screening by NMR (not shown here) of several potential acrylate-based agents demonstrated that TDHA is very poorly soluble in aqueous buffers, presumably due to the long hydrophobic fluorocarbon chain. Thus, TDHA and close analogues are not optimal as a water-soluble agent for in vivo MR research due to difficulties of agent delivery. The shorter fluorocarbon chain of TFPA (Sigma-Aldrich) results in a solubility of ~20 mmol/L in room temperature water. TFPA binding to synthetic lipid bilayers was tested by addition of the saturated aqueous solution of the molecular precursor to hydrated DMPC bilayers, which were prepared by solvent evaporation of DMPC solution in chloroform followed by incubation in warm deionized water. The final TFPA concentration after mixing with the DMPC (100 mg) suspension was 10 mmol/L in 5 mL aqueous solution.
The high-resolution 19F MAS solid-state NMR of TDHA was performed in a 11.7 T Bruker Avance spectrometer equipped with an H/X/Y MAS triple resonance probe with the 1H channel tuned to the 19F frequency. A 4.7 T Bruker Avance small-animal MRI scanner equipped with a dual-tuned custom-made probe11 was used for the 19F spectroscopy of TFPA. A NaF aqueous solution was used as an external chemical shift reference at −121.5 ppm.
Results and Discussion
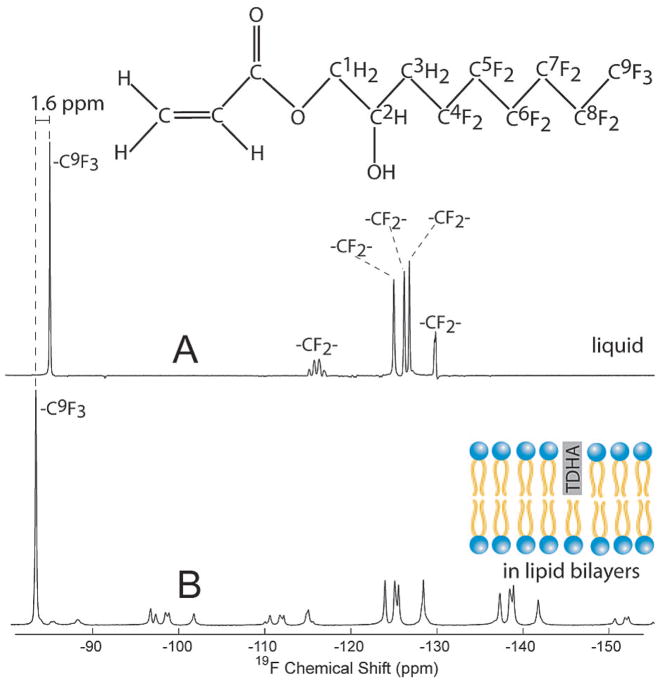
The 19F MAS spectra of TDHA in solution and in DMPC bilayers (Figure 1) demonstrate the effect of molecular binding of the reagent to lipid membranes. The signal from the –CF3 group at −91 ppm in solution (Figure 1A) shifts almost entirely to −93 ppm (Figure 1B) when the lipid phase is present. This difference of 1.6 ppm is interpreted as the change in the isotropic average of the chemical shift in a membrane environment compared to that for its solution spectrum. While resonances from other fluorines are shifted as well, the –CF3 resonance has very good chemical shift separation from others and thus can be assigned unambiguously. Note that the smaller lines at ~−100, ~−112, ~−139, and −153 ppm are spinning side bands of a group of peaks from −123 to −129 ppm. This suggests that residual chemical shift anisotropy (CSA) and dipolar interactions, which are averaged in solution to isotropic values by rapid molecular reorientation, are only partially averaged for TDHA in the lipid environment.
Figure 1.
Magic angle spinning (MAS) 1H coupled 19F spectra of TDHA in the liquid state and bound to DMPC bilayers. The spectra are acquired with a 11.7 T Bruker Avance solid-state NMR spectrometer using an H/X/Y triple resonance MAS variable temperature probe with the 1H channel tuned to the 19F frequency. The liquid-state spectrum (A) is that of a mixture of 1 mg of TDHA in 80 mg of water, 16 scans at 25 °C. The lipid-phase spectrum (B) is that of TDHA in a sample also containing DMPC (1:20 molar ratio), 64 scans at 25 °C. The binding of TDHA to an anisotropic lipid phase is clear from the onset of spinning side bands and shifts of the isotropic chemical shifts (B).
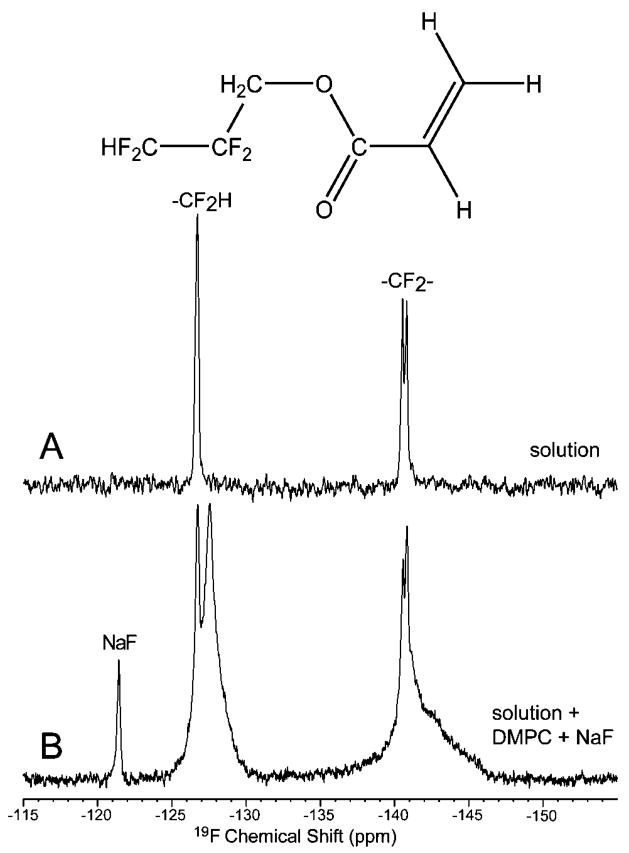
The demonstrated affinity of TDHA for DMPC lipid bilayers is accompanied by a low solubility in water of <0.1 mmol/L. Furthermore, for biomedical application, binding to lipid membranes must be readily apparent under nonspinning conditions, preferably in a small-animal or clinical MR scanner. For these reasons, additional studies were performed using TFPA, which, by virtue of its shorter fluorinated tail, was anticipated to have greater water solubility and less complete partitioning into the lipid. This is demonstrated in Figure 2. The spectrum acquired from an aqueous TFPA solution (Figure 2A) shows only resonances corresponding to the solution, while the line shapes of the binding assay spectrum (Figure 2B) consist of two components, narrow (liquid TFPA) and broad (bound TFPA to DMPC bilayers). The chemical shift separation between ordered and free TFPA signals of the –CHF2 group of 1.6 ppm provides sufficient resolution at 4.7 T between the membrane-bound and liquid-state TFPA without either proton decoupling or MAS. The fraction bound was 93 ± 5% (n = 3) based on integral measurements. As already observed for TDHA, residual CSA and dipolar couplings are responsible for line broadening in the bound state. Note that these sources of broadening have been dramatically reduced in TFPA (<4 ppm for –CF2–, peak at ~140.5 ppm, Figure 2B) compared to that for TDHA (>25 ppm for –CF2–, group of peaks at 124–129 ppm, Figure 1B). This is understood as being indicative of weakened interactions with the hydrophobic core of DMPC bilayers because TFPA has a much shorter hydrofluorocarbon chain. Nevertheless, we find that the 2,2,3,3-tetrafluoropropyl moiety provided sufficient anchoring of the agent to lipid membranes to provide a readily resolved NMR diagnostic of the extent of binding.
Figure 2.
Nonspinning 19F NMR spectra at 4.7 T demonstrating binding of TFPA to the DMPC membrane. NaF was used as an external (A) and internal (B) reference at −121.5 ppm. Spectrum A was recorded from a 20 mmol/L TFPA solution in water, while spectrum (B) was acquired after the TFPA solution was added to prepared DMPC lipid bilayers in water solution. Neither sample spinning nor proton decoupling was employed; yet, spectral resolution suffices to quantify partitioning of TFPA between phases.
An upper limit on the rate of the TFPA binding can be estimated from the observation that partitioning between phases was already complete at 45 s. This limited time resolution was due to the ~30 s time interval necessary to mix TFPA solution with lipid membranes, followed by the several second 19F acquisition time. We found no significant difference in the bound spectra acquired after 45 s or after several minutes and several hours (not shown).
The short fluorinated tail of TFPA suffices to induce predominant partitioning into the lipid and enables the use of 19F spectroscopy as a noninvasive tool for quantitation of the binding. The change of the 19F chemical shift of the –C19F2H group by 1.6 ppm provides partial spectral resolution, and in addition, the signal of the membrane-bound TFPA is broadened by chemical shift anisotropy and dipolar couplings. However, the residual mobility of the bound form limits the broadening by CSA and dipolar coupling interactions to only a few ppm, allowing a convenient assignment and quantification of “free” and membrane-bound fractions. Moreover, since both the spectral separation and the broadening in Hz due to CSA scale linearly with magnetic field, the ability to deconvolute the signal in the two phases is expected to depend only weakly on the magnetic field strength, offering similar resolution and sensitivity in the lower fields typical of clinical MR scanners (usually less than 3 T) and potentially allowing in vivo studies of drug binding with in vivo animal models and in humans.
Conclusions
Excellent 19F chemical shift separation between soluble and membrane-bound fractions of TDHA and TFPA is demonstrated even in the presence of residual line broadening due to chemical shift and dipolar coupling interactions. Since the approach presented builds on the exceptional sensitivity of 19F nuclei, the detection of small quantities of molecular pharmaceuticals is possible as well as in vivo studies of molecular binding. Moreover, the method can be extended to in vivo detection, allowing monitoring of the time course of in vivo binding or/and localized detection on the time scale of seconds or longer. Additionally, the method presented requires no expensive and specialized instrumentation, only retuning of the widely available proton MRI coils or NMR probes, and could be used for convenient detection of 19F in the fields typical of MR scanners and wide-bore NMR spectrometers.
We conclude that the 19F NMR spectroscopy of di- and trifluoromethyl moieties is a valuable tool to study drug binding to lipid membranes at the molecular and atomic level with high 19F NMR sensitivity and very low background signal. We also speculate that this methodology can be also extended to other hydrophobic targets such as proteins including β-amyloid and coronary plaques,3,4,12,13 serum albumins, and hydrophobic membrane-associated peptides and receptors and others.
Acknowledgments
We thankfully acknowledge funding from the Tobacco Related Disease Research Program New Investigator Grant 16KT-0044, NIH 1R21 CA118509, NIH R01 CA 122513, the Rudi Schulte Research Institute (RSRI) (B.D.R., E.Y.C.), the James G. Boswell Fellowship (E.Y.C.), the American Heart Association (P.B., S.K.C.), the American Brain Tumor Association (P.B.), the Beckman Institute Pilot Program: “Spin Polarized Molecules for Structural and Systems Biology” (D.P.W.), the Cancer Research and Prevention Foundation (E.Y.C.), and the SURF program at Caltech (D.T.). We thank Dr. Sonjong Hwang for providing convenient access to the 11.7 T Bruker Avance solid-state NMR facility at Caltech.
References and Notes
- 1.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Anesthesiology. 1997;86:836–847. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Procissi D, Claus F, Burgman P, Koziorowski J, Chapman JD, Thakur SB, Matei C, Ling CC, Koutcher JA. Clin Cancer Res. 2007;13:3738–3747. doi: 10.1158/1078-0432.CCR-06-1563. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty DP, Walsh SM, Kiyota T, Dong Y, Ikezu T, Vennerstrom JL. J Med Chem. 2007;50:4986–4992. doi: 10.1021/jm070085f. [DOI] [PubMed] [Google Scholar]
- 4.Higuchi M, Iwata N, Matsuba Y, Sato K, Sasamoto K, Saido TC. Nat Neurosci. 2005;8:527–533. doi: 10.1038/nn1422. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 6.Dalvit C, Mongelli N, Papeo G, Giordano P, Veronesi M, Moskau D, Kummerle R. J Am Chem Soc. 2005;127:13380–13385. doi: 10.1021/ja0542385. [DOI] [PubMed] [Google Scholar]
- 7.Grage SL, Ulrich AS. J Magn Reson. 2000;146:81–88. doi: 10.1006/jmre.2000.2127. [DOI] [PubMed] [Google Scholar]
- 8.Ulrich AS. Prog Nucl Mag Res Spectrosc. 2005;46:1–21. [Google Scholar]
- 9.Eltahtawy A, Wolf W. Cancer Res. 1991;51:5806–5812. [PubMed] [Google Scholar]
- 10.Bhattacharya P, Harris K, Lin AP, Mansson M, Norton VA, Perman WH, Weitekamp DP, Ross BD. Magn Reson Mater Phys Biol Med. 2005;18:245–256. doi: 10.1007/s10334-005-0007-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang QW, Zhang H, Lakshmi KV, Lee DK, Bradley CH, Wittebort RJ. J Magn Reson. 1998;132:167–171. [Google Scholar]
- 12.Felton CV, Crook D, Davies MJ, Oliver MF. Arterioscler Thromb Vasc Biol. 1997;17:1337–1345. doi: 10.1161/01.atv.17.7.1337. [DOI] [PubMed] [Google Scholar]
- 13.Mori M, Itabe H, Higashi Y, Fujimoto Y, Shiomi M, Yoshizumi M, Ouchi Y, Takano T. J Lipid Res. 2001;42:1771–1781. [PubMed] [Google Scholar]