Abstract
The response of healthy and diseased cartilage of the knee to the mechanics of walking is examined, with the goal of providing insight into the relationship between the kinematics and kinetics of the knee during walking and the maintenance of cartilage health. The combination of information from three-dimensional thickness models of cartilage derived from magnetic resonance imaging and the analysis of the interaction between load at the knee and kinematic changes during walking associated with loss of the anterior cruciate ligament demonstrated the importance of considering walking mechanics as an important factor in the initiation and progression of osteoarthritis. In particular, this material suggests that knee cartilage becomes conditioned to loading and to the large number of repetitive cycles of loading that occur during walking and that healthy cartilage homeostasis is maintained as long as there are no changes to the normal patterns of locomotion, the structure of the knee joint, or cartilage biology. Thus, there is the potential for a degenerative pathway to be initiated when a condition such as anterior cruciate ligament injury causes the repetitive loading during walking to shift to a new location. The sensitivity of cartilage to the kinematic changes is illustrated with the anterior cruciate ligament-deficient knee and the regional variations in cartilage morphology. The material presented here supports the conclusion that individual variations in the range of loading and kinematics at the knee during walking can have a profound influence on the initiation and progression of osteoarthritis of the knee.
It has been suggested that the mechanical environment of the knee during walking can influence both the health and breakdown of articular cartilage of the knee1. The importance of walking in the context of cartilage homeostasis suggests that the knee cartilage becomes conditioned2 to both the load and the number of load cycles associated with walking. Thus, an activity like walking, the most common activity of daily living as well as one that produces a large cyclically reproducible pattern of loading, should be an important consideration in the analysis of the maintenance of the structure, organization, and biology of healthy knee cartilage3. Likewise, the mechanics of walking can influence the initial breakdown of cartilage4 if the normal patterns of loading are changed due to injury or other conditions that would alter the normal balance between loading and the biological maintenance of healthy cartilage5-7.
The purpose of this review is to consolidate the results of several studies on the response of healthy and diseased knee cartilage to the mechanics of walking, with the goal of providing new insight into the relationship between the kinematics and kinetics of the knee during walking and the maintenance of cartilage health.
Cartilage Morphology and Walking Mechanics
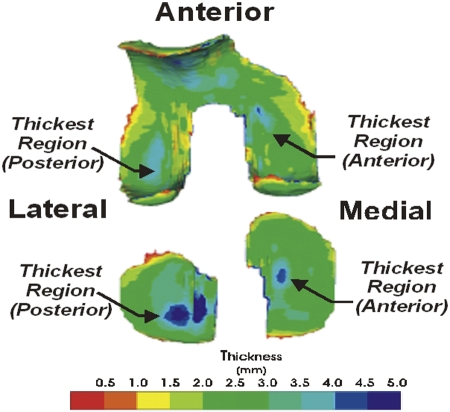
As noted above, it has been suggested that knee cartilage adapts to the cyclic loading that is specific to walking1,3. An examination of the regional variation of healthy cartilage thickness indicates that the thickest cartilage is in the load-bearing areas of the tibiofemoral articulation, which are in contact during the stance phase of walking, when the knee is near full extension. With use of three-dimensional cartilage models (Fig. 1) segmented from magnetic resonance images8 of healthy knees, researchers have identified several common features of articular cartilage thickness variation3,9. The cartilage of the tibial and femoral condyles is thicker in the posterior weight-bearing regions of the lateral compartment, which are the areas that are in contact during walking. In the medial compartment, the tibial cartilage and femoral cartilage were thicker in the anterior weight-bearing regions. The thickness variation on the tibia mirrored the thickness variation on the femoral condyles in each compartment in regions where the knee is in contact during walking. Furthermore, the anterior-to-posterior asymmetry in cartilage thickness between the lateral and medial compartments is consistent with typical patterns of internal-external rotation motion of the knee during walking. Thus, the pattern of loading during walking has a substantial influence on the general characteristic of the regional variation of cartilage thickness in the thickest weight-bearing areas of the knee joint.
Fig. 1.
A typical three-dimensional color thickness map of femoral and tibial cartilage. The thickest regions of the tibial and femoral weight-bearing regions (the primary weight-bearing regions during walking) are aligned when the knee is near full extension.
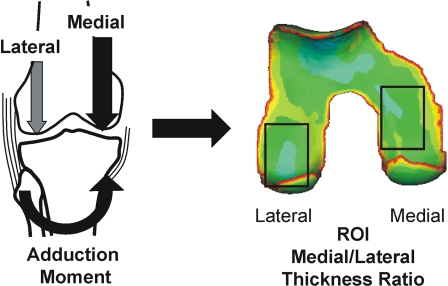
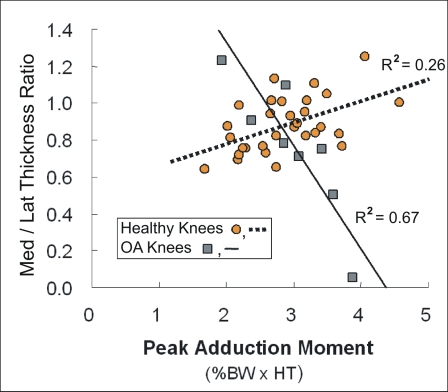
A closer look at the mechanics of walking indicates that individual differences in the local load magnitude can influence variations of cartilage thickness within a specific weight-bearing region of the joint. In particular, it has been shown that the knee adduction moment during walking influences the distribution of the force between the medial and lateral compartments10 of the knee (Fig. 2-A), thus providing a useful measure to evaluate medial-to-lateral variations in cartilage thickness while controlling for intersubject variations. As such, it has been shown that the magnitude of the peak adduction moment during normal walking has been associated with the ratio of medial-to-lateral cartilage thickness in the load-bearing regions of the knee that are common to walking3,9. In particular, for healthy cartilage, the relative thickness of the medial cartilage increases with the magnitude of the adduction moment (Fig. 2-B), suggesting that healthy cartilage adapts to higher repetitive loads during walking by increasing thickness on a regional basis.
Fig. 2-A.
The adduction moment at the knee is associated with the medial-to-lateral load distribution at the knee. The corresponding medial and lateral femoral cartilage load-bearing regions of interest (ROI) are designated by rectangles on the color thickness map of the femoral cartilage.
Fig. 2-B.
The ratio of the medial thickness to lateral thickness in the load-bearing regions of healthy knees (represented by orange circles and dashed linear regression line, R2 = 0.26) during walking is greater for individuals who walk with a higher adduction moment (normalized by subject body weight × height [BW × HT]), while the relative medial thickness is less in patients who have osteoarthritis of the knee (OA) (represented by grey squares and solid linear regression line, R2 = 0.67) and a higher adduction moment.
In contrast to healthy cartilage, patients with osteoarthritis have a relative decrease in thickness3 in the load-bearing regions of the medial compartment with a higher adduction moment (Fig. 2-B). The relationship between the high adduction moments and thinner cartilage in patients with osteoarthritis of the knee is consistent with the findings of clinical studies, which suggest that the adduction moment during walking can be predictive of the clinical outcome of treatment11, disease severity12,13, and disease progression14 for medial-compartment osteoarthritis of the knee. The contrasting results for the healthy knee and the osteoarthritic knee suggest that healthy cartilage responds positively to load, whereas degraded cartilage in osteoarthritic knees responds negatively to load. The response of healthy cartilage to load is consistent with the findings of experimental and theoretical studies, which suggest that load can produce an adaptive response (thickening and enhanced mechanical properties)5,14-16. The negative response of osteoarthritic cartilage suggests that cellular response in degraded cartilage cannot adapt to the repetitive loads during walking and degrades at a higher rate with higher loading during walking17.
Cartilage Health and Kinematic Changes
The variation in cartilage thickness due to the local mechanical environment associated with walking provides an interesting basis to analyze the sensitivity of cartilage health to kinematic changes of the knee during walking. Anterior cruciate ligament injury is associated with premature osteoarthritis of the knee18-22. Kinematic changes at the knee following anterior cruciate ligament injury have been implicated as a factor in the cause of premature osteoarthritis in this population23-25. The normal external rotation and anterior translation of the tibia that occurs as the knee extends during terminal swing is reduced in the absence of the anterior cruciate ligament, and the tibia maintains this offset relative to normal throughout the stance phase of the walking cycle23.
Recent studies have reported that kinematic changes in the anterior cruciate ligament-deficient knee were associated with the spatial pattern of articular cartilage thinning in the knee26,27. One study examined the possibility that early patterns of cartilage thinning in the anterior cruciate ligament-deficient population are related to changes in knee kinematics after anterior cruciate ligament rupture26. Average anterior-posterior translation and internal-external rotation between the femur and tibia during the stance phase of walking were calculated for both anterior cruciate ligament-intact and deficient knees. The anterior-posterior translational and internal-external rotational offsets were calculated by subtracting the average anterior-posterior translation and internal-external rotation of the contralateral anterior cruciate ligament-intact knee from those of the anterior cruciate ligament-deficient knee, respectively, for each anterior cruciate ligament-deficient subject. As a group, the anterior cruciate ligament-deficient knees had an average shift toward internal tibial rotation23 during walking. However, when the kinematic changes could be classified into an anterior (femur on tibia) shift group and a posterior (femur on tibia) shift group, the kinematic changes could be related to region-specific cartilage thinning26.
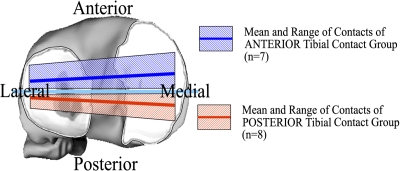
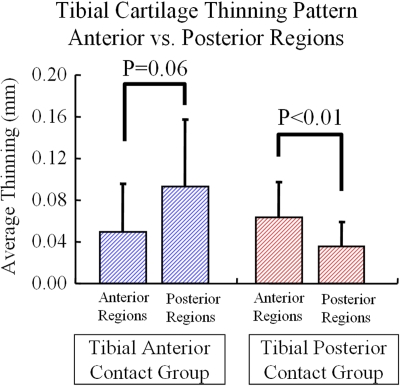
Tibial articular cartilage thinning was determined from the thickness maps of the three-dimensional tibial cartilage models from knee magnetic resonance images26. Cartilage thickness maps from the injured knee were mirrored and registered to the cartilage models from the uninjured knee with use of surface-matching techniques. The difference between the healthy and injured knees shows the amount of thinning or thickening in the cartilage from the anterior cruciate ligament-deficient knee relative to the uninjured knee. The amount of tibial cartilage thinning was associated with the specific kinematic changes that caused a shift of contact to either the posterior or anterior aspect of the tibia (Figs. 3-A and 3-B) during walking. The subjects with a posterior tibial shift in the anterior cruciate ligament-deficient knee relative to the anterior cruciate ligament-intact knee had significantly more tibial cartilage thinning in anterior regions than in posterior regions (p < 0.01), and the subjects with anterior tibial shift had more thinning in posterior regions than in anterior regions.
Fig. 3-A Fig. 3-B.
Figs. 3-A and 3-B Patients with an anterior cruciate ligament deficiency had thinning of tibial cartilage that was associated with specific kinematic changes26. The patients who had a posterior tibial shift in the anterior cruciate ligament-deficient knee relative to the intact knee had significantly greater tibial cartilage thinning in anterior regions than in posterior regions (p < 0.01), and the subjects with anterior tibial shift had more thinning in posterior regions than in anterior regions. The error bars in Figure 3-B represent one standard deviation.
Interestingly, the thinning was prevalent in the region that was unloaded after anterior cruciate ligament rupture. This study implies that the subtle change in ambulatory knee kinematics after knee injury may cause early cartilage thinning by changing the load distribution. These patterns of early thinning may be markers of events leading to the initiation of premature osteoarthritis in patients following anterior cruciate ligament injury.
The change in the rotational characteristics at the knee could cause specific regions of the cartilage to be loaded that were not loaded prior to the anterior cruciate ligament injury. It has been suggested that the altered contact mechanics in the newly loaded regions could produce local degenerative changes in the articular cartilage3,4,27,28. As previously reported, cartilage in highly loaded areas has mechanically adapted relative to underused areas in which signs of fibrillation can be observed in healthy knees in relatively young subjects29. Thus, a spatial shift in the contact region could place loads on a region of cartilage that may not adapt to the rapidly increased load, thereby initiating degenerative changes.
Discussion
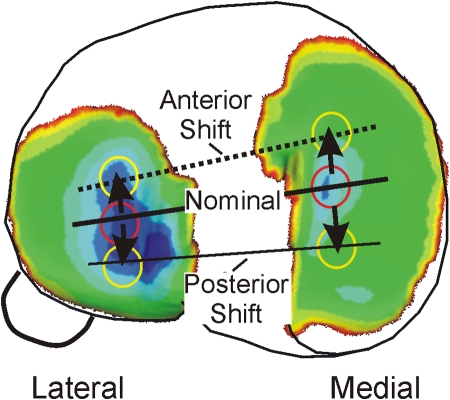
Taken together, the observations described above suggest that the knee cartilage becomes conditioned to loading and to the large number of repetitive cycles of loading that occur during walking and that healthy cartilage homeostasis is maintained as long as there are no changes to the normal patterns of locomotion, the structure of the knee joint, or cartilage biology. However, when a condition such as anterior cruciate ligament injury causes the repetitive loading during walking to shift to a new location, there is potential for a degenerative pathway to be initiated. The sensitivity of cartilage to the kinematic changes associated with the anterior cruciate ligament deficiency of the joint (Figs. 3-A and 3-B) can be analyzed by combining the kinematic shifts with phenotypic variation in the cartilage thickness map (Fig. 4). The kinematic changes shift the nominal contact to regions of thinner cartilage in both the medial and lateral compartments. The more conforming contact of the medial compartment makes this compartment more sensitive to a kinematic change in comparison with the lateral compartment27.
Fig. 4.
The anterior and posterior shifts shown in Figure 3-A move general contact regions to areas of thinner cartilage in the medial compartment.
The regional differences in cartilage thickness have recently been related to the collagen organization and chondrocyte morphology that are present in the central (not covered by the meniscus) and peripheral (covered by the meniscus) regions of the superficial zone of the tibial plateau of the articular cartilage from porcine stifle joints30,31. The collagen organization and chondrocyte morphology were significantly different between regions. The superficial zone in the peripheral region contained smaller, flatter chondrocytes and a thicker zone of larger-diameter tangential fibers, while the central region contained larger, rounder cells and a thinner tangential fiber zone. While not specific to local thickness variations or spatial variations in cartilage morphology, biological and mechanical properties have been related to specific regions of cartilage32-38. For healthy cartilage, it has been shown that highly loaded regions show increased thickness and enhanced mechanical properties, while infrequently loaded regions show cartilage with degraded properties, including fibrillation, relative to loaded regions, even in relatively young subjects29.
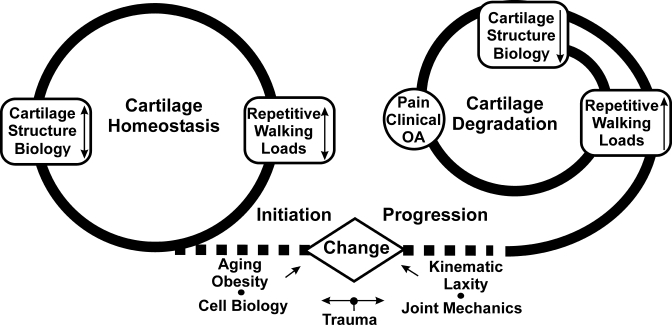
The causes of degenerative changes in articular cartilage are complex and involve interrelated biological, mechanical, and structural pathways. The interrelationship or coupling of these pathways converges at an in vivo systems level in humans. The analysis of in vivo ambulatory mechanics presented here supports a basis for analyzing the influence of the mechanics of walking on the initiation and progression of osteoarthritis of the knee. The initiation of osteoarthritis can be associated with a kinematic change in the patterns of walking of sufficient magnitude (due to injury, increased laxity, neuromuscular changes, or obesity) to shift load to regions of knee cartilage that are not conditioned to chronic ambulatory loading. At some point, cartilage can no longer adapt to the altered chronic ambulatory loading and may begin to degrade. Once cartilage starts to degrade, it responds negatively to load and the rate of progression of osteoarthritis increases with loading (Fig. 5). The initiation phase might be caused by abnormal kinematics, while loading drives the progression phase. The material presented here supports the conclusion that individual variations in the range of loading and kinematics of the knee during walking can have a profound influence on the initiation and progression of osteoarthritis of the knee. Thus, the mechanics of walking can play an important role in the consideration of new methods for prevention and treatment of osteoarthritis. 
Fig. 5.
Healthy cartilage homeostasis is maintained by the magnitude of the repetitive cyclic loads during walking, and cartilage is thicker in regions with higher loads during walking. The initiation of osteoarthritis (OA) is associated with a change (due to injury, increased laxity, neuromuscular changes, aging, or increased obesity) in the normal balance between the mechanics of walking and the cartilage biology or structure. Once cartilage starts to degrade, it responds negatively to load and the rate of progression of osteoarthritis increases with loading.
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health (NIH) and from the Veterans Administration. Neither they nor a member of their immediate families received payments or other benefits or a commitment or agreement to provide such benefits from a commercial entity. No commercial entity paid or directed, or agreed to pay or direct, any benefits to any research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which the authors, or a member of their immediate families, are affiliated or associated.
References
- 1.Andriacchi TP, Mündermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514-8. [DOI] [PubMed] [Google Scholar]
- 2.Seedhom BB. Conditioning of cartilage during normal activities is an important factor in the development of osteoarthritis. Rheumatology (Oxford). 2006;45:146-9. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi TP, Mündermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447-57. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40:215-22. [DOI] [PubMed] [Google Scholar]
- 5.Smith RL, Donlon BS, Gupta MK, Mohtai M, Das P, Carter DR, Cooke J, Gibbons G, Hutchinson N, Schurman DJ. Effects of fluid-induced shear on articular chondrocyte morphology and metabolism in vitro. J Orthop Res. 1995;13:824-31. [DOI] [PubMed] [Google Scholar]
- 6.Lane Smith R, Trindade MC, Ikenoue T, Mohtai M, Das P, Carter DR, Goodman SB, Schurman DJ. Effects of shear stress on articular chondrocyte metabolism. Biorheology. 2000;37:95-107. [PubMed] [Google Scholar]
- 7.Wong M, Wuethrich P, Buschmann MD, Eggli P, Hunziker E. Chondrocyte biosynthesis correlates with local tissue strain in statically compressed adult articular cartilage. J Orthop Res. 1997;15:189-96. [DOI] [PubMed] [Google Scholar]
- 8.Koo S, Gold GE, Andriacchi TP. Considerations in measuring cartilage thickness using MRI: factors influencing reproducibility and accuracy. Osteoarthritis Cartilage. 2005;13:782-9. [DOI] [PubMed] [Google Scholar]
- 9.Koo S, Andriacchi TP. A comparison of the influence of global functional loads vs. local contact anatomy on articular cartilage thickness at the knee. J Biomech. 2007;40:2961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113-9. [DOI] [PubMed] [Google Scholar]
- 11.Prodromos CC, Andriacchi TP, Galante JO. A relationship between gait and clinical changes following high tibial osteotomy. J Bone Joint Surg Am. 1985;67:1188-94. [PubMed] [Google Scholar]
- 12.Sharma L, Hurwitz DE, Thonar EJ, Sum JA, Lenz ME, Dunlop DD, Schnitzer TJ, Kirwan-Mellis G, Andriacchi TP. Knee adduction moment, serum hyaluronan level, and disease severity in medial tibiofemoral osteoarthritis. Arthritis Rheum. 1998;41:1233-40. [DOI] [PubMed] [Google Scholar]
- 13.Mündermann A, Dyrby CO, Hurwitz DE, Sharma L, Andriacchi TP. Potential strategies to reduce medial compartment loading in patients with knee osteoarthritis of varying severity: reduced walking speed. Arthritis Rheum. 2004;50:1172-78. Erratum in: Arthritis Rheum. 2004;50:4073. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter DR, Beaupré GS, Wong M, Smith RL, Andriacchi TP, Schurman DJ. The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res. 2004;427 Suppl:S69-77. [DOI] [PubMed] [Google Scholar]
- 16.Ikenoue T, Trindade MC, Lee MS, Lin EY, Schurman DJ, Goodman SB, Smith RL. Mechanoregulation of human articular chondrocyte aggrecan and type II collagen expression by intermittent hydrostatic pressure in vitro. J Orthop Res. 2003;21:110-6. [DOI] [PubMed] [Google Scholar]
- 17.Fujisawa T, Hattori T, Takahashi K, Kuboki T, Yamashita A, Takigawa M. Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J Biochem. 1999;125:966-75. [DOI] [PubMed] [Google Scholar]
- 18.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22:632-44. [DOI] [PubMed] [Google Scholar]
- 19.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50:3145-52. [DOI] [PubMed] [Google Scholar]
- 20.Roos EM. Joint injury causes knee osteoarthritis in young adults. Curr Opin Rheumatol. 2005;17:195-200. [DOI] [PubMed] [Google Scholar]
- 21.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3:261-7. [DOI] [PubMed] [Google Scholar]
- 22.Scarvell JM, Smith PN, Refshauge KM, Galloway HR, Woods KR. Association between abnormal kinematics and degenerative change in knees of people with chronic anterior cruciate ligament deficiency: a magnetic resonance imaging study. Aust J Physiother. 2005;51:233-40. [DOI] [PubMed] [Google Scholar]
- 23.Andriacchi TP, Dyrby CO. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech. 2005;38:293-8. [DOI] [PubMed] [Google Scholar]
- 24.Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31:75-9. [DOI] [PubMed] [Google Scholar]
- 25.Tashman S, Collon D, Anderson K, Kolowich P, Anderst W. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975-83. [DOI] [PubMed] [Google Scholar]
- 26.Koo S, Dyrby CO, Andriacchi TP. Abnormal ambulation after ACL rupture affects knee articular cartilage spatial thinning pattern. Presented at the Annual Meeting of the Orthopaedic Research Society; 2007. Feb 11-14; San Diego, CA.
- 27.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006;442:39-44. [DOI] [PubMed] [Google Scholar]
- 28.Herzog W, Clark A, Longino D. Joint mechanics in osteoarthritis. Novartis Found Symp. 2004;260:79-104, 277-9. [PubMed] [Google Scholar]
- 29.Bullough PG. The pathology of osteoarthritis. In: Moskowitz RW, Howell DS, Goldberg VM, Mankin HJ, editors. Osteoarthritis: diagnosis and medical/surgical management. 2nd ed. Philadelphia: Saunders; 1992. p 39-70.
- 30.Bevill SL, Briant PL, Andriacchi TP. Regional variations in chondrocyte morphology as a cause for cartilage degradation following kinematic changes to normal joint function. Presented at the Annual Meeting of the Orthopaedic Research Society; 2007. Feb 11-14; San Diego, CA.
- 31.Briant PL, Bevill SL, Andriacchi TP. Topological differences in the cartilage superficial layer of the porcine knee. Presented at the Annual Meeting of the Orthopaedic Research Society; 2006. Mar 19-22; Chicago, IL.
- 32.Appleyard RC, Burkhardt D, Ghosh P, Read R, Cake M, Swain MV, Murrell GA. Topographical analysis of the structural, biochemical and dynamic biomechanical properties of cartilage in an ovine model of osteoarthritis. Osteoarthritis Cartilage. 2003;11:65-77. [DOI] [PubMed] [Google Scholar]
- 33.Buschmann MD, Kim YJ, Wong M, Frank E, Hunziker EB, Grodzinsky AJ. Stimulation of aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid flow. Arch Biochem Biophys. 1999;366:1-7. [DOI] [PubMed] [Google Scholar]
- 34.Clark JM. Variation of collagen fiber alignment in a joint surface: a scanning electron microscope study of the tibial plateau in dog, rabbit, and man. J Orthop Res. 1991;9:246-57. [DOI] [PubMed] [Google Scholar]
- 35.Eckstein F, Müller S, Faber SC, Englmeier KH, Reiser M, Putz R. Side differences of knee joint cartilage volume, thickness, and surface area, and correlation with lower limb dominance—an MRI-based study. Osteoarthritis Cartilage. 2002;10:914-21. [DOI] [PubMed] [Google Scholar]
- 36.Eggli PS, Hunziker EB, Schenk RK. Quantitation of structural features characterizing weight and less-weight-bearing regions in articular cartilage: a stereological analysis of medial femoral condyles in young adult rabbits. Anat Rec. 1988;222:217-27. [DOI] [PubMed] [Google Scholar]
- 37.Guilak F, Ratcliffe A, Lane N, Rosenwasser MP, Mow VC. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J Orthop Res. 1994;12:474-84. [DOI] [PubMed] [Google Scholar]
- 38.Little CB, Ghosh P. Variation in proteoglycan metabolism by articular chondrocytes in different joint regions is determined by post-natal mechanical loading. Osteoarthritis Cartilage. 1997;5:49-62. [DOI] [PubMed] [Google Scholar]