Abstract
Purpose: To describe the presentation and clinical course of patients with nephrogenic systemic fibrosis (NSF) at a large acute-care hospital, to evaluate the overall incidence of NSF, and to assess the effect of a hospital-wide policy regarding gadolinium-based contrast agent (GBCA) use on NSF incidence.
Materials and Methods: A review of all cases of NSF observed at an institution from 2003 to 2008 was conducted. This HIPAA-compliant study was approved by the institutional review board. The informed consent requirement was waived. Demographics, medical history, and associated conditions were recorded. Radiologic procedures were evaluated if they were performed within 1 year prior to NSF onset. GBCA use was assessed by checking the electronic database for each procedure. The incidence of NSF was compared before and after implementation of an institutional policy designed to assess risk of NSF prior to GBCA use.
Results: All 33 patients with NSF (mean age, 49 years; age range, 15–78 years) had advanced renal failure (estimated glomerular filtration rate < 15 mL/min/1.73 m2) when the GBCA was injected. Twenty-six patients had severe chronic or end-stage renal disease, and seven had acute renal failure. The mean interval between contrast material injection and NSF onset was 29 days ± 25 (standard deviation) (range, 4–112 days). The overall incidence of NSF was 36.5 cases per 100 000 gadolinium-enhanced magnetic resonance (MR) examinations between 2003 and 2006 and four cases per 100 000 gadolinium-enhanced MR examinations between 2007 and 2008 after screening for NSF risk was instituted (Fisher exact test, P = .001). Five patients developed NSF in the peritransplant period, and four underwent a catheter-based radiographic procedure with administration of a GBCA.
Conclusion: Common associations of GBCA MR imaging and NSF were acute and severe chronic renal failure and liver or renal transplantation. Screening procedures performed before MR imaging to determine which patients were at risk of developing NSF appear to reduce the incidence of this complication and further support the belief that NSF is associated with GBCA administration.
© RSNA, 2009
To our knowledge, the first cases of nephrogenic systemic fibrosis (NSF) were seen in 1997 by LeBoit at a renal transplantation center in southern California (1). These cases were described as a scleroderma-like fibrosing entity of the skin associated with renal insufficiency. It is now recognized that NSF is a systemic condition that may involve the pleura, pericardium, lungs, joints, and striated muscle, including the diaphragm and myocardium (2–4). In 2006, Grobner suggested that gadolinium-based contrast agents (GBCAs) might be implicated in the pathogenesis of this disease (5). This hypothesis has gained general acceptance with the publication of case series in which the authors confirmed the association between GBCA administration and NSF (6–11) and recent studies in which the authors reported gadolinium deposits in the tissue of patients with NSF (12–14).
Reported associations with NSF include severe renal failure (glomerular filtration rate < 30 mL/min/1.73 m2) and injection of a GBCA for magnetic resonance (MR) imaging. Other conditions associated with an increased risk of disease in the early literature are hypercoagulability, deep vein thrombosis, and tissue injury secondary to surgical procedures (15). The 1-year incidence of NSF in the presence of all recognized risk factors (end-stage renal disease, GBCA administration, dialysis, and proinflammatory event) has been estimated to be 4.6% (10). Since this condition occurs infrequently and is less likely to be seen in the future because of the recognition of the association with GBCA, additional information and reports of the disease continue to be necessary to expand current knowledge.
We present a review of the cases of NSF diagnosed in the Johns Hopkins Hospital system from 2003 to 2008. The purpose of this review was to describe the presentation and clinical course of patients with NSF at a large acute-care hospital, to evaluate the overall incidence of NSF, and to assess the effect of a hospital-wide policy regarding GBCA use on NSF incidence.
MATERIALS AND METHODS
This Health Insurance Portability and Accountability Act–compliant study was approved by the Johns Hopkins Hospital institutional review board. The requirement for patient informed consent was waived. Clinical reports, laboratory tests, and pathology summaries were retrospectively reviewed for 33 patients in whom NSF was diagnosed between 2003 and 2008. Six of these patients were referred to our institution from other facilities. Thirty-one patients had a definitive histopathologic diagnosis of NSF, while two patients had a clinical diagnosis that was deemed sufficient for inclusion in this study. Patients were identified by reviewing the records of the Departments of Dermatopathology and Pathology at Johns Hopkins Hospital. Attending staff physicians who specialized in nephrology, dermatology, and rheumatology were also contacted to identify patients with NSF, and their records were correlated with the Departments of Dermatopathology and Pathology databases.
Demographic information, medical history, surgical procedures performed within 3 months of NSF onset, and symptoms and signs at the onset of NSF and associated conditions (defined as any conditions that manifested concomitantly with the symptoms of NSF, regardless of cause) were abstracted from the medical records. Given the extensive list of comorbidities in these patients, the determination of initial symptoms and signs required use of a prefixed method to prevent erroneous association of NSF with symptoms related to a concomitant disease. NSF symptoms were recorded when they were present for at least 3 consecutive days and later led to a biopsy that resulted in the diagnosis of NSF. Dialysis history was also extracted from the patient records; however, blood flow rates were unavailable.
Data regarding radiologic procedures performed up to 1 year before the diagnosis of NSF were extracted from our radiology information system. We defined the index radiologic procedure as the last procedure in which a GBCA was used before the onset of NSF. The 1-week, 1-month, and 1-year cumulative doses of GBCA were calculated. The type of GBCA and the dose used were determined from data in the radiology information system for each procedure and compared with the digitally scanned documents at the time of the procedure for reliability.
Full-thickness skin biopsy results were analyzed by members of the Department of Dermatology, Division of Dermatopathology. The estimated glomerular filtration rate (GFR) was calculated with the four-variable Modification of Diet in Renal Disease equation, as follows: GFR = 186 · Cr–1.154 · A–0.203 · 0.742 (if female) · 1.212 (if black), where Cr is serum creatinine level and A is patient age (16,17). Patients were classified as having (a) end-stage renal disease if they were undergoing permanent renal replacement therapy, (b) stage 5 chronic kidney disease if they were not undergoing dialysis but their estimated glomerular filtration rate was less than 15 mL/min/1.73 m2, and (c) acute renal failure if their renal function had deteriorated acutely before the radiologic index procedure was performed (medical record documentation was required).
Statistical Analysis
All continuous parameters were described as means ± standard deviations, and all categorical parameters were summarized as proportions. The Fisher exact test was used to examine the differences between two proportions (18). A multiple logistic regression model was used to identify factors that were associated with cumulative gadolinium dose. The incidence of NSF was calculated by dividing the number of cases of NSF that were detected with an index MR procedure by the number of contrast material–enhanced MR examinations performed. The mean doses for the two contrast agents were compared with the Student t test. All P values reported are two sided. P < .05 indicated a significant difference.
RESULTS
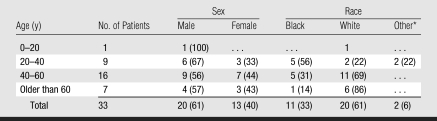
The demographic characteristics of the cohort are summarized in Table 1. Of the 33 patients, 20 (61%) were white, 11 (33%) were black, one (3%) was of Hispanic descent, and one (3%) was of Middle Eastern descent. The mean age of all patients was 49 years (range, 15–78 years). The mean age of men was 51 years (range, 28–73 years), and the mean age of female patients was 47 years (range, 15–78 years). Twenty-five (76%) of the 33 patients were aged 20 to 60 years. One (3%) patient was aged 15 years at presentation, while seven (21%) patients were older than 60 years. Tables 2 and 3 contain data regarding GBCA use and associated comorbidities.
Table 1.
Patient Demographics
Note.—Data are numbers of patients. Data in parentheses are percentages.
One patient was of Hispanic descent, and one was of Middle Eastern descent.
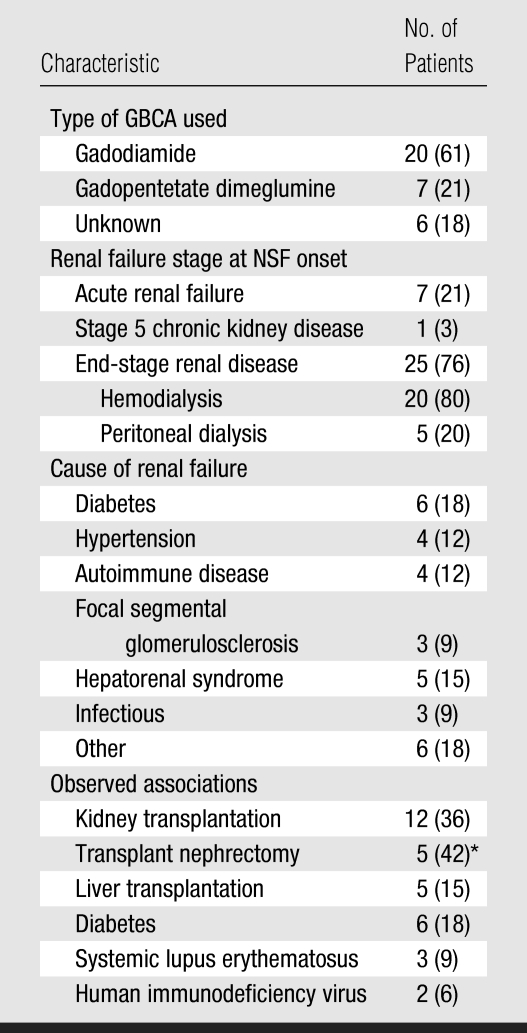
Table 2.
Type of GBCA Used and Clinical Characteristics of Studied Population
Note.—Data in parentheses are percentages.
Kidney transplantation failed in these patients, and they subsequently underwent nephrectomy of the transplanted organ. The percentage was calculated by using a denominator of 12.
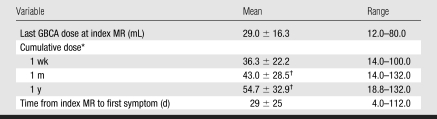
Table 3.
GBCA Exposure and Interval between Exposure and Appearance of NSF Symptoms
Note.—Data on time from index MR to first symptom were available for only 24 patients because records for three patients were incomplete regarding the date of the first symptom. For all other variables, data were available for 27 patients.
The 1-week, 1-month, and 1-year cumulative doses are the sum of all GBCA doses administered to patients in the respective periods.
P < .05 for comparison with the last gadolinium dose.
Gadolinium Exposure
Of the 27 patients with complete records, 23 (85%) underwent gadolinium-enhanced MR imaging as an index procedure, and four (15%) received a GBCA during invasive x-ray–based arteriography. (The dose of the GBCA was not documented in six subjects.) The mean single dose used for temporally related MR imaging was 24.5 mL ± 9.7 (range, 12–40 mL), while the mean single dose used for temporally related x-ray–based angiography was 52.5 mL ± 25 (range, 20–80 mL). For all patients, the mean single dose used in the index procedure was 29.0 mL ± 16.3 (range, 12–80 mL). The average time between GBCA exposure and observation of the first cutaneous symptom was 29 days ± 25.
Eighteen (67%) of 27 patients underwent more than one MR procedure in which a GBCA was used during a 1-year period. For all patients, the 1-week, 1-month, and 1-year cumulative doses were 36.3 mL, 43.0 mL, and 54.7 mL, respectively. None of the patients who underwent multiple procedures that required injection of contrast material during the month prior to the index radiology procedure received both the intravenous and the intraarterial form of GBCA, while five patients received both gadodiamide (Omniscan; GE Healthcare, Princeton, NJ) and gadopentetate dimeglumine (Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, NJ) within 1 year of the index radiology procedure. Gadodiamide was used in the index procedure in four of these patients, while gadopentetate dimeglumine was used in the index procedure in one patient.
Gadodiamide was used in 20 (74%) of the 27 patients, and gadopentetate dimeglumine was used in seven (26%). For MR procedures performed with gadodiamide, the GBCA dose used in the index MR examination was 26.5 mL (mean, 0.15 mmol per kilogram of body weight), the 1-week cumulative dose was 31.4 mL, and the 1-month cumulative dose was 35.0 mL. For MR imaging procedures performed with gadopentetate dimeglumine, the GBCA dose used in the index MR examination was 17.1 mL (mean, 0.1 mmol/kg), the 1-week cumulative dose was 20.0 mL, and the 1-month cumulative dose was 22.9 mL. The mean final dose and the 1-week cumulative dose were greater for examinations performed with gadodiamide than for those performed with gadopentetate dimeglumine (P = .02 and P = .048, respectively). The mean GBCA dose was 59.5 mL for invasive angiography performed with gadodiamide (n = 4); this was significantly greater than the mean GBCA dose for index MR imaging performed with gadodiamide (P < .001).
Renal Failure History
Twenty-six (79%) of 33 patients had chronic renal failure with an estimated glomerular filtration rate of less than 15 mL/min/1.73 m2. Seven patients (21%) had acute renal failure at the time of NSF onset, as evidenced by their clinical presentation and the increase in their serum creatinine level for at least 7 days prior to GBCA exposure. We compared the 1-year cumulative GBCA dose in patients with chronic renal failure with that in patients with acute renal failure at the time of index MR imaging. A 1-year cumulative GBCA dose of more than 40 mL was strongly associated with acute renal failure rather than chronic renal failure (odds ratio, 53; P = .02). Thus, patients who experienced acute renal failure tolerated relatively high levels of GBCA exposure (eg, >40 mL) for at least 1 year before acute renal failure occurred. The onset of NSF occurred a median of 24 days after GBCA administration during the acute renal failure episode.
Twelve (36%) of 33 patients received a renal transplant: The transplanted kidney was removed in five of these patients because of severe allograft dysfunction within 10 weeks of transplantation. The other seven patients had episodes of acute rejection at least 1 month prior to the onset of disease. Four of these patients continued dialysis; the remaining three recovered renal function. Five patients (15%) developed NSF within 3 months of liver transplantation, with transient renal failure caused by hepatorenal syndrome or acute tubular necrosis, as will be discussed. Nine (27%) of 33 patients had underlying diabetes mellitus or systemic lupus erythematosus.
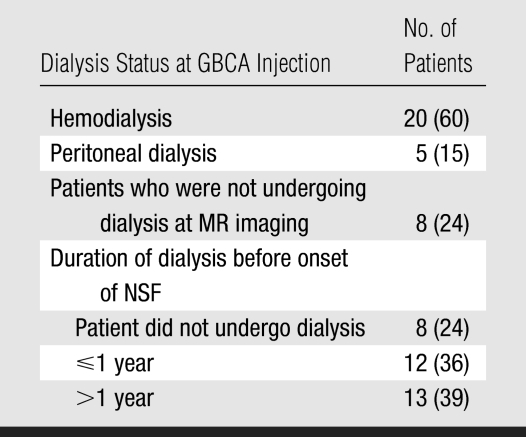
Twenty-five (76%) of the 33 patients were undergoing dialysis before GBCA exposure (Table 4). Of these patients, 20 (80%) had a schedule that called for hemodialysis three times per week, while five (20%) were undergoing peritoneal dialysis. Eight (24%) of 33 patients were not undergoing dialysis at the time of GBCA exposure or had undergone only one hemodialysis session related to acute renal failure. The period of time that patients had been undergoing dialysis was highly variable. The proportion of patients who had been undergoing regular dialysis for less than 1 year was similar to the proportion of patients who had been undergoing dialysis for more than 1 year (range, 15 days to 9 years).
Table 4.
Association between Dialysis, Injection of GBCA, and NSF Onset
Note.—Data in parentheses are percentages.
Seven patients underwent hemodialysis within 24 hours after exposure to a GBCA. (The exact number of hours between injection of a GBCA and dialysis could not be determined.) The period of time between GBCA exposure and dialysis was unavailable for 13 other patients undergoing hemodialysis. Five other patients with NSF continued to undergo peritoneal dialysis.
As indicated previously, five patients who received a liver transplant (age range, 50–64 years) developed NSF within 3 months after transplantation in the form of transient renal failure that remitted within 6 weeks. Index MR imaging was performed in all patients within 2 days of the surgical procedure. In one patient, the creatinine and blood urea nitrogen values completely normalized 3 days before the clinical onset of disease. In these five patients, normalization of kidney function was not associated with regression of NSF symptoms. Gadodiamide was used in all five patients. The average dose was 38.8 mL, the mean cumulative dose was 91.2 mL, and the mean time between injection of the GBCA and disease onset was 45 days.
Clinical Presentation and Symptoms
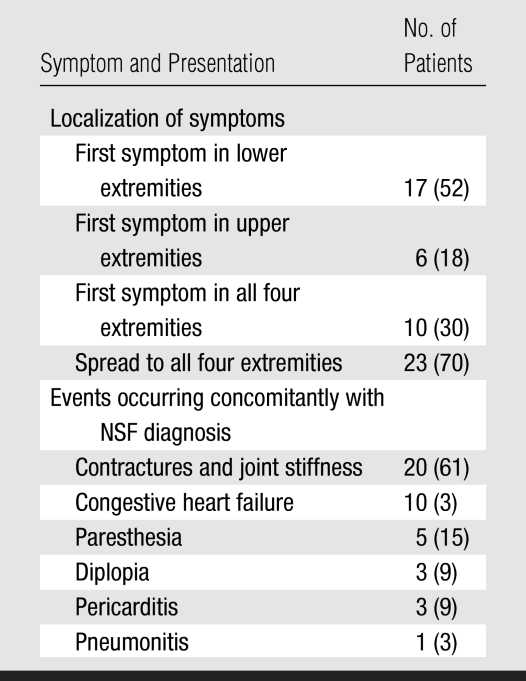
Clinical symptoms were diverse, and NSF symptoms were difficult to separate from underlying comorbidities. A summary of initial dermatologic manifestations and final clinical expression is shown in Table 5 and is briefly reviewed below.
Table 5.
Symptoms and Clinical Presentation
Note.—Data in parentheses are percentages.
In terms of dermatologic findings, all patients presented with edema. The most common pattern was edema of the lower extremities, usually extending from the ankles to just below the knees. The first manifestations were isolated to lower extremities in 17 patients (52%), to all four extremities in 10 patients (30%), and to upper limbs in six patients (18%). The edema was frequently described as having a “woody” consistency. In 23 patients (70%), symptoms progressed to affect all four extremities. Focal lesions appeared as large edematous infiltrated plaques with a peau d'orange texture. Nodules and papules were not uncommon. The lesions were frequently erythematus, pruritic, and/or painful and affected only the limbs and trunk. In some cases, there was hypopigmentation of the plaques.
Among the noncutaneous findings, the most prominent were pain (n = 23, 70%), incapacitating contractures of hands or ankles (n = 20, 61%), dyspnea (n = 10, 30%), paresthesia (n = 5, 15%), and ocular manifestations (n = 3, 9%). Two of the patients with ocular manifestations presented with diplopia of unknown origin that remitted after a few days; the third patient presented with uveitis and scleritis. Ten (30%) of 33 patients had dyspnea. Echocardiography was performed in six of these patients and revealed left atrial dilatation and pericardial effusion.
Pathologic Findings
Thirty-one (94%) of 33 patients underwent a skin biopsy performed by one of four dermatopathologists. Histologic slices revealed a dermis that was variably fibrotic and hypercellular with frequent involvement of subcutaneous fat and widening of the septae. The cellular infiltrate was composed of small, hyperchromatic spindled and ovoid fibroblasts intercalated among thick collagen bundles. As is characteristic, the process was noninflammatory, and the appearance of foci of lymphocytes was extremely rare. Although the presence of mucin was variable, more frequently Alcian blue staining did not reveal an increase in interstitial dermal mucin, so scleromyxedema was an unlikely diagnosis. The stromal spindle cells stained positive for CD34 in the 19 samples in which the staining procedure was performed.
Four (12%) patients died during the observation period. NSF was not considered the cause of the death. The death of these individuals was attributed to complications directly related to severe renal failure (n = 3) or hepatic failure (n = 1). Autopsies were performed in two of these patients and revealed findings strongly consistent with diffuse organ involvement of NSF. Fibrosis of the heart, lungs, subarachnoid space, pancreas, and colon was present to some degree in both patients. The primary cause of death was hemorrhagic and necrotizing pancreatitis in one patient and myocardial infarction and sepsis in the other.
Incidence Rates of NSF
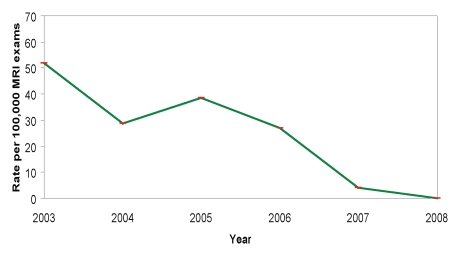
For MR examinations performed between 2003 and 2006, the overall incidence rate of NSF was 36.5 cases per 100 000 gadolinium-enhanced procedures (Fig 1). After the Food and Drug Administration issued alerts in June and December 2006 regarding the association between NSF and GBCA (21), hospital policies and procedures were developed and implemented to assess risk factors for NSF (Fig 2). Over the subsequent observation period, the corresponding NSF rate was four cases per 100 000 gadolinium-enhanced procedures (Fisher exact test, P = .001), suggesting that the new policy was associated with a significantly lower incidence of NSF.
Figure 1:
Graph shows rate of NSF cases by year.
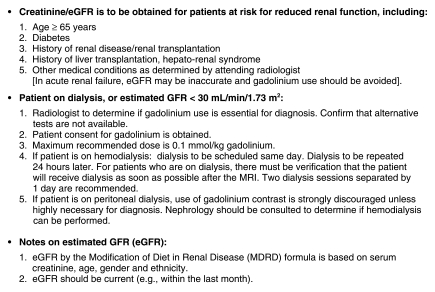
Figure 2:
Johns Hopkins policy for the prevention of NSF. eGFR = estimated glomerular filtration rate, GFR = glomerular filtration rate.
DISCUSSION
GBCA exposure in the setting of renal failure has been reported to be associated with NSF (5,7,9,11,19). Our data support these reports. All patients in our cohort had undergone an MR imaging or angiographic procedure in which a GBCA had been used prior to disease onset and had severe renal failure at the time of GBCA exposure. Several findings were frequently associated with NSF cases: Stage 5 chronic kidney disease or end-stage renal disease was seen in 79% of patients, whereas acute renal failure was seen in the remaining 21%. Also, 36% of patients were renal transplant recipients, 27% had diabetes mellitus or systemic lupus erythematosus, and 15% had undergone recent liver transplantation associated with acute renal failure.
Of note, four patients (12%) underwent catheter-based radiographic invasive arteriography with use of a GBCA. Although GBCAs were developed primarily for use in MR imaging, a number of authors have documented their use to supplement CO2 conventional angiography in patients with renal insufficiency or an allergy to iodinated contrast agents (20–22). However, this technique is associated with high doses of GBCA (up to 0.3 mmol/kg). The presumed advantage of GBCA in this setting was the reduced likelihood of iodinated contrast material–induced nephropathy.
The role of dialysis in the prevention of NSF remains uncertain. While it was once believed that dialysis was a precipitating factor in this disease, it is now generally accepted that dialysis might protect against the development of this entity. It has been proposed that making the patients undergo dialysis less than 24 hours after GBCA injection might prevent NSF. However, Saitoh et al (23) showed that one dialysis treatment was not sufficient to remove all of the gadolinium. In our study, seven patients underwent one hemodialysis session within 24 hours of GBCA exposure and still developed NSF. Five patients were undergoing peritoneal dialysis at MR imaging.
No patient in this series had not experienced a proinflammatory event (an event in which there is tissue injury, clinically relevant inflammation, or elicited immune response). All subjects in our cohort had a complicated clinical outlook at the time of the disease onset. Those who were not recuperating from a surgical procedure (including liver or kidney transplantation) were experiencing sepsis, an acute inflammatory event, or vasculitis secondary to an autoimmune disease.
Five patients had received a liver transplant shortly before NSF onset. The researchers at the University of Rochester Medical Center described their experience with two patients who received a liver transplant and subsequently developed NSF (24). A report of another such case was published recently (12). All of the patients in our study had a transient episode of renal failure, with severely deteriorated renal function at the time of GBCA exposure. The Food and Drug Administration has recently recognized this possible association and has requested that a black-box warning be placed on the package inserts of gadolinium-based products; this warning is to urge caution with use of these products in patients with liver failure and any level of renal insufficiency (25). With the exception of those in the post–liver transplantation setting, none of our patients had liver failure.
In January 2007, our institution implemented policies regarding the use of GBCA in patients with severe renal dysfunction, with the aim being to reduce the incidence of NSF (Fig 2). These policies were associated with a significant reduction in the incidence of NSF. Besides the previously reported epidemiologic considerations of NSF associations, the success of such a policy in the reduction of NSF due to GBCA exposure strengthens the belief that there is a causal relationship between GBCA and NSF.
A remaining controversy is the relationship between different types of GBCAs and NSF. In our series, the mean gadodiamide dose in patients with NSF was significantly higher than the mean gadopentetate dose in these patients (0.15 mmol/kg vs 0.1 mmol/kg); this may have accounted for the higher number of NSF cases associated with gadodiamide use. Since the overall mean dose of each GBCA was not available for all patients at our institution who received these agents, our data do not favor either GBCA as a more likely candidate for association with NSF.
A limitation of our study was its retrospective nature. The records for six patients contained only information obtained after the diagnosis of NSF was made. The analysis of progress notes and other records was retrospective and may have introduced bias. We took into account only unequivocal clinical descriptions that were accompanied by follow-up information on subsequent days. Additional work needs to be performed to design a case-control study that will help us better understand the epidemiology of NSF.
Our findings support the association between NSF and administration of a GBCA in patients with acute and chronic severe renal failure, with possible cofactors related to events involving a strong immune response or tissue injury. Implementation of hospital policies consistent with Food and Drug Administration recommendations regarding assessment of patient risk for NSF in relation to GBCA may reduce the incidence of disease.
ADVANCE IN KNOWLEDGE
Implementation of an institutional policy to assess risk for nephrogenic systemic fibrosis (NSF) before gadolinium-based contrast agent (GBCA) administration reduced the incidence of NSF, supporting the claim that there is an association between GBCA and NSF.
IMPLICATIONS FOR PATIENT CARE
While the overall incidence of NSF is low (36.5 cases per 100 000 gadolinium-enhanced MR images), all patients who developed NSF had acute renal failure, end-stage renal disease, or severe renal failure with an estimated glomerular filtration rate of less than 15 mL/min/1.73 m2.
Knowledge of at-risk patient populations can reduce the incidence of NSF.
Abbreviations
GBCA = gadolinium-based contrast agent
NSF = nephrogenic systemic fibrosis
Author contributions: Guarantors of integrity of entire study, J.P., D.M.F., D.A.B.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, all authors; clinical studies, J.P., D.M.F., D.A.B.; statistical analysis, J.P., S.L., D.M.F., D.A.B.; and manuscript editing, all authors
Authors stated no financial relationship to disclose.
Funding: D.A.B. is an employee of the National Institutes of Health Clinical Center.
References
- 1.Cowper SE. Nephrogenic systemic fibrosis: the nosological and conceptual evolution of nephrogenic fibrosing dermopathy. Am J Kidney Dis 2005;46:763–765. [DOI] [PubMed] [Google Scholar]
- 2.Kucher C, Steere J, Elenitsas R, Siegel DL, Xu X. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis with diaphragmatic involvement in a patient with respiratory failure. J Am Acad Dermatol 2006;54(2 suppl):S31–S34. [DOI] [PubMed] [Google Scholar]
- 3.Ting WW, Stone MS, Madison KC, Kurtz K. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol 2003;139:903–906. [DOI] [PubMed] [Google Scholar]
- 4.Daram SR, Cortese CM, Bastani B. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis 2005;46:754–759. [DOI] [PubMed] [Google Scholar]
- 5.Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006;21:1104–1108. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Nephrogenic fibrosing dermopathy associated with exposure to gadolinium-containing contrast agents: St Louis, Missouri, 2002–2006. MMWR Morb Mortal Wkly Rep 2007;56:137–141. [PubMed] [Google Scholar]
- 7.Broome DR, Girguis MS, Baron PW, Cottrell AC, Kjellin I, Kirk GA. Gadodiamide-associated nephrogenic systemic fibrosis: why radiologists should be concerned. AJR Am J Roentgenol 2007;188:586–592. [DOI] [PubMed] [Google Scholar]
- 8.Khurana A, Runge VM, Narayanan M, Greene JF Jr, Nickel AE. Nephrogenic systemic fibrosis: a review of 6 cases temporally related to gadodiamide injection (omniscan). Invest Radiol 2007;42:139–145. [DOI] [PubMed] [Google Scholar]
- 9.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006;17:2359–2362. [DOI] [PubMed] [Google Scholar]
- 10.Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 2007;243:148–157. [DOI] [PubMed] [Google Scholar]
- 11.Moreno-Romero JA, Segura S, Mascaro JM Jr, et al. Nephrogenic systemic fibrosis: a case series suggesting gadolinium as a possible aetiological factor. Br J Dermatol 2007;157:783–787. [DOI] [PubMed] [Google Scholar]
- 12.Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic fibrosing dermopathy. J Am Acad Dermatol 2007;56:27–30. [DOI] [PubMed] [Google Scholar]
- 13.High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 2007;56:21–26. [DOI] [PubMed] [Google Scholar]
- 14.High WA, Ayers RA, Cowper SE. Gadolinium is quantifiable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 2007;56:710–712. [DOI] [PubMed] [Google Scholar]
- 15.Mackay-Wiggan JM, Cohen DJ, Hardy MA, Knobler EH, Grossman ME. Nephrogenic fibrosing dermopathy (scleromyxedema-like illness of renal disease). J Am Acad Dermatol 2003;48:55–60. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict GFR from S-creatinine [abstr]. J Am Soc Nephrol 2000;11(suppl):155A. [Google Scholar]
- 17.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med 2006;354:2473–2483. [DOI] [PubMed] [Google Scholar]
- 18.Mehta CR, Patel NR. Exact inference for categorical data. In: Encyclopedia of biostatistics. New York, NY: Wiley, 1998;1411:–1422.
- 19.Cowper SE. Nephrogenic fibrosing dermopathy. NFD/NSF Web site. http://www.icnfdr.org/. Accessed September 18, 2007.
- 20.Spinosa DJ, Matsumoto AH, Angle JF, et al. Safety of CO(2)- and gadodiamide-enhanced angiography for the evaluation and percutaneous treatment of renal artery stenosis in patients with chronic renal insufficiency. AJR Am J Roentgenol 2001;176:1305–1311. [DOI] [PubMed] [Google Scholar]
- 21.Spinosa DJ, Matsumoto AH, Angle JF, et al. Gadolinium-based contrast and carbon dioxide angiography to evaluate renal transplants for vascular causes of renal insufficiency and accelerated hypertension. J Vasc Interv Radiol 1998;9:909–916. [DOI] [PubMed] [Google Scholar]
- 22.Spinosa DJ, Matsumoto AH, Angle JF, Hagspiel KD, McGraw JK, Ayers C. Renal insufficiency: usefulness of gadodiamide-enhanced renal angiography to supplement CO2-enhanced renal angiography for diagnosis and percutaneous treatment. Radiology 1999;210:663–672. [DOI] [PubMed] [Google Scholar]
- 23.Saitoh T, Hayasaka K, Tanaka Y, Kuno T, Nagura Y. Dialyzability of gadodiamide in hemodialysis patients. Radiat Med 2006;24:445–451. [DOI] [PubMed] [Google Scholar]
- 24.Maloo M, Abt P, Kashyap R, et al. Nephrogenic systemic fibrosis among liver transplant recipients: a single institution experience and topic update. Am J Transplant 2006;6:2212–2217. [DOI] [PubMed] [Google Scholar]
- 25.FDA requests boxed warning for contrast agents used to improve MRI images. U.S. Food and Drug Administration Web site. http://www.fda.gov/bbs/topics/NEWS/2007/NEW01638.html. Accessed February 28, 2008.