Abstract
Activation of the descending noradrenergic system inhibits nociceptive transmission in the spinal cord. Although both α1- and α2-adrenoceptors in the spinal cord are involved in the modulation of nociceptive transmission, it is not clear how α1-adrenoceptors regulate excitatory and inhibitory synaptic transmission at the spinal level. In this study, inhibitory and excitatory postsynaptic currents (IPSCs and EPSCs, respectively) were recorded from lamina Π neurons in rat spinal cord slices. The specific α1-adrenoceptor agonist phenylephrine significantly increased the frequency of GABAergic spontaneous IPSCs in a concentration dependent manner, and this effect was abolished by the α1-adrenoceptor antagonist WB4101. Phenylephrine also significantly reduced the amplitude of monosynaptic and polysynaptic EPSCs evoked from primary afferents. The inhibitory effect of phenylephrine on evoked monosynaptic glutamatergic EPSCs was largely blocked by the GABAA receptor antagonist picrotoxin and, to a lesser extent, by the GABAB receptor antagonist CGP55845. Furthermore, blocking T-type Ca2+ channels with amiloride or mibefradil diminished the inhibitory effect produced by phenylephrine or the GABAA receptor agonist muscimol on monosynaptic EPSCs evoked from primary afferents. Collectively, these findings suggest that activation of α1-adrenoceptors in the spinal cord increases synaptic GABA release, which attenuates glutamatergic input from primary afferents mainly through GABAA receptors and T-type Ca2+ channels. This mechanism of presynaptic inhibition in the spinal cord may be involved in the regulation of nociception by the descending noradrenergic system.
Keywords: α1-adrenoceptors, descending noradrenergic modulation, presynaptic inhibition, spinal cord, GABAA receptors, GABAB receptors
Introduction
The superficial dorsal horn of the spinal cord is critically involved in the transmission and modulation of nociceptive information from the periphery. The noradrenergic nucleus locus coeruleus (the A6 cell group) also provides the major noradrenergic input to the spinal dorsal horn and contributes significantly to the analgesic effect produced by activation of the descending inhibitory pathway (Jones and Gebhart, 1987, Aston-Jones et al., 1991, West et al., 1993). Both α1- and α2-adrenoceptors are members of G protein-coupled receptors and are extensively distributed in the spinal dorsal horn (Summers and McMartin, 1993, Stone et al., 1998). Activation of α2-adrenoceptors inhibits pain transmission through pre- and postsynaptic mechanisms (Yaksh et al., 1995, Pan et al., 1999). Furthermore, spinally administered α1-adrenoceptor agonists increase nociceptive withdrawal thresholds in rats (Reddy et al., 1980, Aran and Proudfit, 1990). However, the cellular and synaptic mechanisms involved in the attenuation of nociceptive transmission at the spinal level by activations of α1-adrenoceptors are still unclear.
Glutamate released from primary afferents is a major excitatory neurotransmitter that conveys nociceptive information from primary afferents to spinal dorsal horn neurons (Yoshimura and Jessell, 1990, Randic et al., 1993, Chen et al., 2000, Pan et al., 2002). On the other hand, γ-aminobutyric acid (GABA) is a predominant inhibitory neurotransmitter that can activates both GABAA and GABAB receptors in the spinal cord (Price et al., 1984, Labrakakis et al., 2003, Wang et al., 2007). Because GABAergic interneurons and glutamatergic primary afferent terminals are intermingled in the superficial dorsal horn (Barber et al., 1978, Li et al., 2002), GABAergic inhibitory neurons in the spinal cord function as a gate to control the transmission of nociceptive impulses from primary afferents (Melzack and Wall, 1965, Cervero and Iggo, 1980, Torsney and MacDermott, 2006, Yasaka et al., 2007). Norepinephrine can stimulate GABAergic interneurons in the spinal dorsal horn (Baba et al., 2000). However, it is not clear how increased GABAergic tone in the spinal cord by α1-adrenoceptors modulates glutamatergic input from primary afferents. In the present study, using whole-cell recordings in the spinal cord slices, we determined the role of α1-adrenoceptors in the regulation of GABAergic and glutamatergic inputs to spinal dorsal horn neurons. Our study provides new information that activation of α1-adrenoceptors increases synaptic GABA release in the spinal cord, which attenuates glutamatergic input from primary afferent terminals mainly through GABAA receptors and T-type Ca2+ channels.
Methods and Materials
Animals
Male Sprague-Dawley rats (5–6 wks old; Harlan, Indianapolis, IN) were used in this study. All the surgical preparation and experimental protocols were approved by the Animal Care and Use Committee of the University of Texas M. D. Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical use of animals. All efforts were made to minimize both the suffering and number of animals used.
Spinal cord slice preparation
Rats were anesthetized using 2–3% isoflurane, and the lumbar segment of the spinal cord was removed through laminectomy at the L2-L5 level. The segment of the spinal cord was immediately placed in an ice-cold sucrose artificial cerebrospinal fluid (aCSF) presaturated with 95% O2 - 5% CO2. The sucrose aCSF contained (in mM) 206 sucrose, 2.8 KCl, 1.0 MgCl2, 1.0 CaCl2, 1.2 NaH2PO4, 25.0 glucose, and 26.0 NaHCO3. The tissue was then placed in a shallow groove formed in a gelatin block and glued onto the stage of a vibratome (Technical Product International, St. Louis, MO). Transverse spinal cord slices (400 μm) were cut in the ice-cold sucrose aCSF. Before recording, the slices were incubated in the Krebs solution gassed with 95% O2 and 5% CO2 at 34°C for at least 1 hr. The Krebs solution contained (in mM) 117.0 NaCl, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 11.0 Glucose, and 25.0 NaHCO3.
Electrophysiological recordings
Whole-cell voltage-clamp techniques were used to record the postsynaptic currents of the lamina Π neurons, as we described previously (Li et al., 2002, Zhang et al., 2005). The slice was placed in a glass-bottomed chamber and continuously perfused with Krebs solution at 5.0 ml/min at 34°C maintained by an inline solution heater and a temperature controller. The recording pipettes were made from borosilicate capillaries (1.2 mm o.d., 0.68 mm i.d) with a puller (P-97; Sutter Instruments, Novato, CA). Neurons in the lamina II in the spinal slices were identified under a fixed-stage microscope (BX50WI; Olympus, Tokyo, Japan) with differential interference contrast/infrared illumination. After forming a tight gigaohm seal, a brief negative pressure was used to obtain the whole-cell configuration.
Recordings of postsynaptic currents began 5–7 min after the current reached a steady state. The input resistance was monitored, and the recording was abandoned if it changed more than 15%. Signals were processed with an amplifier (MultiClamp 700B; Axon Instruments, Union City, CA), filtered at 1–2 kHz, digitized at 10 kHz using DigiData 1322, and saved to a hard drive of a computer with pCLAMP 9.0 (Axon Instruments). The impedance of the pipette was 5–8 MΩ when filled with internal solution containing (in mM) 110.0 Cs2SO4, 5.0 TEA, 2.4 MgCl2, 0.5 CaCl2, 10.0 HEPES, 5.0 Na2ATP, 5.0 BAPTA, 0.33 GTP-Tris, and 5.0 TEA-Cl. GABAergic spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded at the holding potential of 10 mV in the presence of 0.5 μM strychnine, a glycine receptor antagonist. Excitatory postsynaptic currents (EPSCs) were recorded at the holding potential of -60 mV using the following pipette internal solution (in mM): 135.0 potassium gluconate, 5.0 KCl, 2.0 MgCl2, 0.5 CaCl2, 5.0 HEPES, 5.0 EGTA, 5.0 ATP-Mg, 0.5 Na-GTP, and 1.0 QX314 (lidocaine N-ethyl bromide). Evoked EPSCs were elicited by electrical stimulation (0.2 ms, 0.3–0.6 mA, and 0.2 Hz) through a bipolar tungsten electrode placed on the dorsal root zone (Li et al., 2002, Zhang et al., 2007). To minimize the possibility of current spread and direct activation of lamina II neurons, the neurons we selected for recording were located in the lateral dorsal horn and the distance between the stimulating electrode and recorded neuron was more than 150 μm. To block the possible postsynaptic action mediated by G protein-coupled receptors, GDP-β-S (1 mM), a general G protein inhibitor, was added into the pipette solution during the whole-cell recording.
Phenylephrine, GDP-β-S, muscimol, WB4101, and picrotoxin were purchased from Sigma-Aldrich (St. Louis, MO). Strychnine and CGP55845 were obtained from Tocris Cookson Inc. (Ellisville, MO). All the drugs and solutions were freshly prepared before the experiment and delivered using syringe pumps.
Data analysis
Data are presented as means ± S.E.M. The amplitude and frequency of sIPSCs were analyzed off-line using a peak detection program (MiniAnalysis; Synaptosoft, Decatur, GA). Detection of events was accomplished by setting a threshold above the noise level. The sIPSCs were detected by the fast rise time of the signal over an amplitude threshold (typically 6 -10 pA) above the background noise. We manually excluded the event when the noise was erroneously identified as the sIPSCs by the software program. The effect of drugs on the amplitude of eEPSCs was analyzed using Clampfit (Axon Instruments). The peak effect of drugs on sIPSCs or eEPSCs was analyzed over a period of 1.5–2 min after drug application. The cumulative probability of the amplitude and inter-event interval of sIPSCs was compared using the Kolmogorov-Smirnov test, which estimates the probability that two distributions are similar. Neurons were considered to be responsive to drugs if the frequency of sIPSCs or the amplitude of eEPSCs was altered > 15%. The effect of drugs on the frequency of sIPSCs and the amplitude of eEPSCs was determined by repeated measures ANOVA with Dunnett’ post hoc test. P < 0.05 was considered to be statistically significant.
Results
Activation of α1-adrenoceptors increases GABAergic sIPSCs
To determine the role of α1-adrenoceptors in the regulation of GABAergic synaptic inputs to spinal dorsal horn neurons, we first tested the effect of the α1-adrenoceptor agonist phenylephrine (Bylund et al., 1994, Chen et al., 2006) on GABAergic sIPSCs of lamina II neurons. Bath application of 10 μM of phenylephrine for 3 min did not significantly change the frequency of sIPSCs. At 25–100 μM, phenylephrine concentration dependently increased the frequency of sIPSCs in 23 of 30 (76.7%) neurons tested (Fig. 1). However, phenylephrine did not significant change the amplitude of sIPSCs. The phenylephrine’s effect reached maximal at 50 μM, and the frequency of sIPSCs returned to the baseline control level 15 - 20 min after washout of the drug. In the remaining 7 neurons, phenylephrine had no significant effect on the frequency and amplitude of sIPSCs. Similar to what we showed previously (Zhang et al., 2005), bath application of 20 μM bicuculline, a selective GABAA receptor antagonist, for 3 min abolished sIPSCs in all 8 neurons tested (data not shown).
Fig. 1.
Effect of phenylephrine on GABAergic sIPSCs of lamina II neurons. A, original traces of sIPSCs during control, application of 10, 25, 50, and 100 μM phenylephrine and washout in one lamina II neuron. B, cumulative probability plots of the same neuron in A show the distribution of inter-event interval and amplitude of sIPSCs during control and perfusion of 25 and 50 μM phenylephrine. C, summary data show the effect of phenylephrine on the frequency and amplitude of sIPSCs (n = 23 cells). Data are presented as means ± S.E.M. *, P < 0.05 compared with the baseline control. Phl, phenylephrine.
To assess whether the potentiating effect of phenylephrine on GABAergic sIPSCs was mediated by α1-adrenoceptors, we used a highly specific α1-adrenoceptor antagonist, WB4101 (Bylund et al., 1994). After testing the initial effect of phenylephrine on GABAergic sIPSCs, 0.5 μM WB4101 was applied for 3–4 min before bath perfusion of 50 μM phenylephrine again. WB4101 alone did not significantly change the frequency of sIPSCs, but it abolished phenylephrine-induced increases in the frequency of sIPSCs in all 9 neurons tested (Fig. 2, A-C). To ensure that the effect of phenylephrine on the frequency of sIPSCs was reproducible, we applied 50 μM phenylephrine into the tissue bath twice, at an interval of 25–30 min. In another 8 neurons, repeated application of 50 μM phenylephrine caused a similar increase in the frequency of sIPSCs (Fig. 2D). These results suggest that activation of α1-adrenoceptors increases GABAergic input to the majority of lamina Π neurons.
Fig. 2.
Effect of phenylephrine on GABAergic sIPSCs in lamina II neurons before and after WB4101 application. A, original traces of sIPSCs during control and application of 50 μM phenylephrine with and without 0.5 μM WB4101 in one lamina II neuron. B, cumulative probability plots of the same neuron in A show the distribution of inter-event interval and amplitude of sIPSCs during control and application of phenylephrine and phenylephrine plus WB4101. C, summary data show that 0.5 μM WB4101 abolished the effect of 50 μM phenylephrine on the frequency of sIPSCs (n = 9). D, group data show the reproducible effect of 50 μM phenylephrine on the frequency of sIPSCs (n = 8). Data presented as means ± S.E.M. *, P < 0.05 compared with the baseline control. Phl, phenylephrine; WB, WB4101.
Phenylephrine inhibits glutamatergic input from primary afferents
To determine how activation of α1-adrenoceptors affects glutamatergic input from primary afferents to dorsal horn neurons, we tested the effect of phenylephrine on identified monosynaptic and polysynaptic glutamatergic EPSCs of lamina II neurons elicited from primary afferents. The evoked EPSCs (eEPSCs) were considered to be monosynaptic if the latency was constant at 20 Hz of electrical stimulation (Fig. 3A). In the case of monosynaptic eEPSCs, although the amplitude of eEPSCs was markedly attenuated, neither conduction failure nor an increase in latency occurred when stimulation frequency was increased to 20 Hz (Fig. 3A). In contrast, the latency of polysynaptic eEPSCs was increased and conduction failure was present when the stimulation frequency was increased to 20 Hz (Li et al., 2002, Zhang et al., 2007). Bath application of 50 μM phenylephrine for 3 min significantly decreased the amplitude of monosynaptic eEPSCs in 11 neurons (Fig. 3, B and C). Furthermore, 50 μM phenylephrine significantly reduced the amplitude of polysynaptic eEPSCs in another 14 lamina II neurons tested (Fig. 3C). Similar to what we reported previously (Li et al., 2002, Zhang et al., 2007), bath application of 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), a non-NMDA receptor antagonist, eliminated eEPSCs in all 7 neurons examined (data not shown). These data suggest that stimulation of spinal α1-adrenoceptors inhibits glutamatergic input from primary afferents to dorsal horn neurons.
Fig. 3.
Effect of phenylephrine on glutamatergic eEPSCs of lamina II neurons elicited from primary afferents. A, original traces of eEPSCs show identification of monosynaptic (left) and polysynaptic (right) eEPSCs in two separate lamina II neurons evoked by electrical stimulation of the dorsal root entry zone (20 Hz). B, original traces showing that 50 μM phenylephrine decreased the amplitude of polysynaptic eEPSCs of one neuron in a reproducible manner. Note that conduction failure was indicated by the arrow. C, Summary data show the reproducible effect of phenylephrine on the amplitude of both monosynaptic and polysynaptic eEPSCs of lamina II neurons. Data are presented as means ± S.E.M. *, P < 0.05 compared with the baseline control. Phl, phenylephrine.
Role of presynaptic GABAB receptors in phenylephrine-induced inhibition of glutamatergic input from primary afferents
Endogenously released GABA can activate GABAB receptors on primary afferents or dorsal horn neurons (Price et al., 1984, Li et al., 2002), which can reduce synaptic glutamate release to spinal dorsal horn neurons. Because stimulation of spinal α1-adrenoceptors causes a profound increase in synaptic GABA release, we next determined if GABAB receptors are involved in the inhibitory effect of phenylephrine on glutamatergic input to dorsal horn neurons. After testing the initial effect of phenylephrine on eEPSCs, 2 μM CGP55845, a specific GABAB receptor antagonist (Brugger et al., 1993, Bischoff et al., 1999), was applied followed by in 3 min bath application of 50 μM phenylephrine. Application of CGP55845 alone slightly but not significantly increased the baseline amplitude of eEPSCs. CGP55845 largely blocked the inhibitory effect of phenylephrine on the amplitude of polysynaptic eEPSCs in 14 lamina II neurons (Fig. 4, A and B).
Fig. 4.
Effect of CGP55845 on phenylephrine-induced inhibition of glutamatergic eEPSCs of lamina II neurons elicited from primary afferents. A, original traces of polysynaptic eEPSCs show the effect of phenylephrine on the amplitude of polysynaptic eEPSCs of one neuron before and during application of 2 μM CGP55845. B, Summary data show the effect of CGP55845 on phenylephrine-produced inhibition of the amplitude of both monosynaptic and polysynaptic eEPSCs of lamina II neurons. Data are presented as mean ± S.E.M. *, P < 0.05 compared with the baseline control. #, P < 0.05 compared with the value obtained with CGP55845 alone. Phl, phenylephrine; CGP, CGP55845.
In 12 of 16 neurons tested, phenylephrine still significantly decreased the amplitude of monosynaptic eEPSCs in the presence of 2 μM CGP55845 (Fig. 4B). In another 4 neurons, CGP55845 completely blocked the inhibitory effect of phenylephrine on the amplitude of monosynaptic eEPSCs (from 186.9 ± 13.6 to 187.3 ± 10.7 pA, P > 0.05). Thus, these findings suggest that GABAB receptors expressed on glutamatergic neurons, and, to a lesser extent, those on primary afferent terminals, contribute to reduction in glutamatergic input to dorsal horn neurons by activation of spinal α1-adrenoceptors.
Role of presynaptic GABAA receptors in phenylephrine-induced inhibition of glutamatergic input from primary afferents
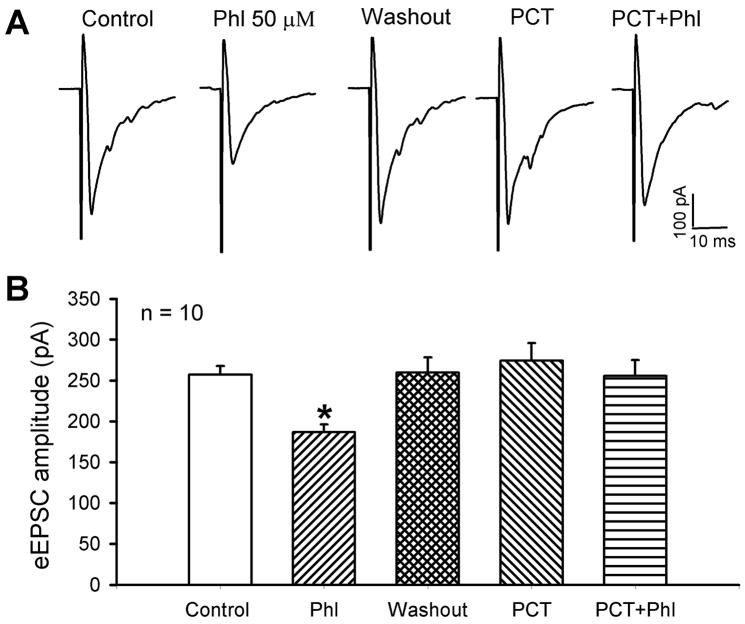
GABAA receptors are expressed on primary afferent terminals and play an important role in presynaptic inhibition (Rudomin and Schmidt, 1999). We therefore assessed the role of GABAA receptors in the reduction of glutamatergic input from primary afferents by α1-adrenoceptor activation. In 10 of 13 (84.6%) lamina II neurons tested, 50 μM picrotoxin, a GABAA receptor antagonist, completely blocked the inhibitory effect of 50 μM phenylephrine on the amplitude of monosynaptic eEPSCs (Fig. 5). In another 3 neurons, the inhibitory effect of 50 μM phenylephrine on the amplitude of monosynaptic eEPSCs was only partially attenuated by 50 μM picrotoxin (from 224.1 ± 14.3 to 173.3 ± 14.2 pA, P < 0.05). Thus, these data suggest that GABAA receptors expressed on primary afferent terminals are primarily involved in the inhibition of glutamate release by endogenously released GABA after activation of spinal α1-adrenoceptors.
Fig. 5.
Effect of picrotoxin on phenylephrine-produced inhibition of monosynaptic eEPSCs of lamina II neurons. A, original traces show the inhibitory effect of 50 μM phenylephrine on monosynaptic eEPSCs of one neuron before and during application of 50 μM picrotoxin. B, summary data show the effect of 50 μM picrotoxin on 50 μM phenylephrine-induced inhibition of the amplitude of monosynaptic eEPSCs of 10 neurons. Data are presented as means ± S.E.M. *, P < 0.05 compared with the baseline control. Phl, phenylephrine; PCT, picrotoxin.
Role of T-type Ca2+ channels in musimol- or phenylephrine-induced inhibition of glutamate release from primary afferents
In some dorsal root ganglion (DRG) neurons, T-type Ca2+ channels are involved in the excitatory effect of the GABAA receptor agonist (Aptel et al., 2007). To directly examine the role of T-type Ca2+ channels in the GABAA receptor agonist-mediated presynaptic inhibition, muscimol, a specific GABAA receptor agonist (Jang et al., 2005), and amiloride, a selective T-type Ca2+ channel blocker (Tang et al., 1988, Wu et al., 2008). Bath application of 0.5 μM muscimol for 3 min significantly decreased the amplitude of monosynaptic eEPSCs in 13 neurons (Fig. 6, A and C). After washout of the initial effect of muscimol, 500 μM amiloride was applied for 3 min before repeated application of 0.5 μM muscimol. Amiloride abolished the inhibitory effect of muscimol on the amplitude of monosynaptic eEPSCs in all 13 cells tested (Fig. 6, A and C). We also conducted experiments to ensure that muscimol-induced inhibition of monosynaptic eEPSCs was reproducible. After a 10-min washout of the initial effect of muscimol, repeated application of 0.5 μM muscimol produced a similar decrease in the amplitude of monosynaptic eEPSCs (n = 8, Fig. 6, B and D).
Fig. 6.
Effect of amiloride on muscimol-induced inhibition of monosynaptic eEPSCs of lamina II neurons. A, original traces show that 500 μM amiloride blocked the effect of 0.5 μM muscimol on the amplitude of monosynaptic eEPSCs of one lamina II neuron. B, raw traces show the reproducible effect of 0.5 μM muscimol on the amplitude of monosynaptic eEPSCs of another neuron. C, group data show the effect of 500 μM amiloride on the amplitude of monosynaptic eEPSCs of 13 lamina II neurons before and druing application of 0.5 μM muscimol. D, summary data show the reproducible effect of 0.5 μM muscimol on the amplitude of monosynaptic eEPSCs of 8 neurons. Data are presented as means ± S.E.M. *, P < 0.05 compared with the baseline control. Aml, amiloride; Mus, muscimol.
We subsequently determined whether T-type Ca2+ channels mediate the inhibitory effect of phenylephrine on glutamate release from primary afferent terminals. Bath application of 500 μM amiloride alone had no significant effect on the amplitude of monosynaptic eEPSCs. Phenylephrine (50 μM) failed to significantly decrease the amplitude of monosynaptic eEPSCs in 11 of 15 neurons in the presence of 500 μM amiloride (Fig. 7A). It has been shown that amiloride can block GABAA receptors, especially these that contain the α6-subunit (Fisher, 2002, Drafts and Fisher, 2004). However, the α6-subunit is not present in the DRG and spinal cord (Ma et al., 1993, Maddox et al., 2004). Nevertheless, we tested the effect of a structurally dissimilar T-type Ca2+ channel blocker mibefradil (Bezprozvanny and Tsien, 1995, Bao et al., 1998) on phenylephrine-induced inhibition of monosynaptic eEPSCs. In 9 of 12 lamina II neurons examined, bath application of 2.5 μM mibefradil for 3 min largely blocked the inhibitory effect of 50 μM phenylephrine on the amplitude of monosynaptic eEPSCs (Fig. 7B).
Fig. 7.
Effect of amiloride on phenylephrine-induced inhibition of monosynaptic eEPSCs of lamina II neurons. A, original traces and summary data show the effect of 50 μM phenylephrine on the amplitude of monosynaptic eEPSCs of 11 neurons before and during application of 500 μM amiloride. B, representative traces and group data show the effect of 50 μM phenylephrine on the amplitude of monosynaptic eEPSCs of 9 neurons before and during application of 2.5 μM mibefradil. Data are presented as means ± S.E.M. *, P < 0.05 compared with the baseline control. Phl, phenylephrine; Aml, amiloride; mbf, mibefradil.
In another 4 lamina Π neurons in which amiloride only partially blocked the inhibitory effect of phenylephrine on monosynaptic eEPSCs, we further examined the role of GABAB receptors in phenylephrine-produced inhibition of monosynaptic eEPSCs. After washout of the effect of phenylephrine and amiloride, 2 μM CGP55845 and 500 μM amiloride were applied together. Bath application of CGP55845 and amiloride completely blocked the inhibitory effect of phenylephrine on the amplitude of monosynaptic eEPSCs of these 4 neurons (Fig. 8). Therefore, these results strongly suggest that T-type Ca2+ channels are critically involved in the presynaptic inhibition of glutamate release from primary afferent terminals by activation of GABAA receptors and α1-adrenoceptors in the spinal cord.
Fig. 8.
Effect of amiloride plus CGP55845 on pheneylephrine-produced inhibition of the amplitude of monosynaptic eEPSC of lamina II neurons. A, original traces show the effect of 50 μM phenylephrine on the amplitude of monosynaptic eEPSCs of one neuron before and during application of 500 μM amiloride alone and 500 μM amiloride plus 2 μM CGP55845. B, summary data show the effect of phenylephrine on the amplitude of monosynaptic eEPSCs before and during application of amiloride alone and amiloride plus CGP55845 (n = 4 neurons). Data are presented as means ± S.E.M. *, P < 0.05 compared with the control. Phl, phenylephrine; Aml, amiloride; CGP, CGP55845.
Discussion
In the present study, we determined the role of α1-adrenoceptors in the regulation of glutamatergic and GABAergic synaptic inputs to spinal dorsal horn neurons. We found that the α1-adrenoceptor agonist phenylephrine significantly increased the frequency of GABAergic sIPSCs in the majority of lamina Π neurons. On the other hand, phenylephrine significantly decreased the amplitude of glutamatergic eEPSCs elicited from primary afferents. Although blocking GABAB receptors largely reduced the effect of phenylephrine on the amplitude of polysynaptic eEPSCs, it had a smaller effect on the amplitude of monosynaptic eEPSCs in the majority of neurons examined. In contrast, antagonizing the GABAA receptors nearly abolished the effect of phenylephrine on the amplitude of monosynaptic eEPSCs. In addition, blocking T-type Ca2+ channels with amiloride or mibefradil eliminated the effect of phenylephrine or the GABAA receptor agonist on the amplitude of monosynaptic eEPSCs. Collectively, our study provides new information that activation of α1-adrenoceptors increases synaptic GABA release, which reduces glutamatergic inputs from primary afferents to spinal dorsal horn neurons mainly through stimulation of GABAA receptors and T-type Ca2+ channels.
The nucleus locus coeruleus contains the major group of noradrenergic neurons and projects broadly throughout the brain and spinal cord (Aston-Jones et al., 1991). Activation of noradrenergic nucleus locus coeruleus releases norepinephrine in the spinal cord and produces analgesia (Jones and Gebhart, 1987, West et al., 1993). Stimulation of α2-adrenoceptors in the spinal dorsal horn could produce the analgesic effect through inhibition of glutamatergic synaptic transmission (Stone et al., 1998, Pan et al., 2002). Furthermore, the α1-adrenoceptor is present in the superficial dorsal horn of the spinal cord (Pieribone et al., 1994, Nalepa et al., 2005). We found that activation of α1-adrenoceptors with phenylephrine induced a dose-dependent increase in the frequency of the GABAergic sIPSCs in 76.7% of lamina Π neurons studied. Phenylephrine-induced increases in the sIPSC frequency were abolished by WB4101, a specific α1-adrenoceptor antagonist. Norepinephrine can increase spinal GABAergic transmission through α1-adrenoceptors, and this effect is blocked by tetrodotoxin (Baba et al., 2000). These electrophysiologcal data strongly suggest that α1-adrenoceptors are present on GABAergic interneurons in the spinal cord. Currently, at least three native α1-adrenoceptor subtypes (α1A, α1B, and α1D) have been identified (Hieble et al., 1995), and the α1A- and α1D-adrenoceptors have strong expression in the spinal dorsal horn (Day et al., 1997). Currently, there are no highly selective antagonists for α1-adrenoceptor subtypes. However, because WB4101 is a preferred α1A-adrenoreceptor antagonist (Bylund et al., 1994), the most likely α1-adrenoceptor mediating the phenylephrine action in the spinal dorsal horn is the α1A subtype.
The most salient finding of our study is that increased synaptic GABA release by activation of α1-adrenoceptors leads to inhibition of glutamatergic input from primary afferents. GABAergic boutons onto the primary afferent terminals provide one example of a morphological substrate for GABA-mediated presynaptic inhibition (Barber et al., 1978, Carlton and Hayes, 1991). Inhibitory neurons in the superficial dorsal horn control the relay of nociceptive signals from the periphery to the spinal cord (Melzack and Wall, 1965, Cervero and Iggo, 1980, Torsney and MacDermott, 2006). GABAB receptors are important for modulation of nociceptive transmission in the spinal dorsal horn (Iyadomi et al., 2000, Li et al., 2002, Wang et al., 2007). Similar to what has been reported previously (Cao et al., 1997), blocking GABAB receptors increased the baseline amplitude of eEPSCs, suggesting that GABAB receptors are tonically involved in the control of glutamatergic transmission in the spinal cord. Activation of GABAB receptors at the glutamatergic terminals by endogenously released GABA after stimulation of muscarinic acetylcholine receptors can limit synaptic glutamate release from primary afferents (Li et al., 2002). In the present study, we found that blocking GABAB receptors with CGP55845 primarily attenuated phenylephrine-induced inhibition of the amplitude of polysynaptic eEPSCs of lamina Π neurons. However, CGP55845 had a minor effect on phenylephrine-induced inhibition of the amplitude of monosynaptic eEPSCs in most neurons tested. This finding suggests that GABAB receptors present on glutamatergic interneurons contribute to inhibition of glutamatergic transmission in the spinal dorsal horn by α1-adrenoreceptor activation.
In addition to GABAB receptors, GABAA receptors are also involved in presynaptic inhibition of primary afferent input in the spinal cord (Rudomin and Schmidt, 1999). GABAA receptors are expressed on various subpopulations of DRG neurons that terminate in laminae I–III (Labrakakis et al., 2003, Aptel et al., 2007). It has been shown that local application of GABAA receptor agonists to the DRG reduces neuropathic pain (Naik et al., 2008). We found that the GABAA receptor agonist muscimol mimicked the inhibitory effect of phenylephrine on the amplitude of monosynaptic eEPSCs elicited from primary afferents. Furthermore, the GABAA receptor antagonist picrotoxin largely blocked the effect of phenylephrine on the amplitude of monosynaptic eEPSCs. Our data suggest that endogenously released GABA by α1-adrenoreceptor activation in the spinal cord can activate GABAA receptors on primary afferent terminals to reduce the amount of glutamate release. It is not fully known about how GABAA receptor activation produces presynaptic inhibition in the spinal cord. Although excessive primary afferent depolarization may lead to the generation of dorsal root reflexes that are pro-nociceptive (Cervero and Laird, 1996, Willis, 1999, Cervero et al., 2003, Lin et al., 2003), primary afferent depolarization is the prevailing mechanism of GABAergic inhibition of central terminals of nociceptive afferent fibers (Rudomin and Schmidt, 1999). GABAA receptor activation causes membrane depolarization because of a high intracellular chloride concentration in primary sensory neurons (Alvarez-Leefmans et al., 1988, Sung et al., 2000). This membrane depolarization, by inactivating Na+ channels, could sufficiently decrease or block action potential invasion into the primary afferent terminals, thereby inhibiting excitatory transmitter release (Rudomin and Schmidt, 1999, Willis, 1999).
Low voltage-activated (T-type) Ca2+ channels are expressed in DRG neurons (Talley et al., 1999, Wu et al., 2004, 2008). The electrophysiological hallmarks of this channel include low voltage activation, fast (transient) inactivation kinetics, and low unitary conductance (Keja and Kits, 1994, Wu et al., 2004). Calcium entry through T-type Ca2+ channels mediates membrane depolarization and increases intracellular calcium. There are three genes forming the α1 subunit of T-type Ca2+ channels, CaV3.1 for α1G, CaV3.2 for α1H, and CaV3.3 for α1I. CaV3.2 is the major subtype expressed in small- and medium-sized DRG neurons (Talley et al., 1999). Amiloride preferentially inhibits T-type currents mediated by CaV3.2 subunits (Williams et al., 1999) and has a moderate affinity for CaV3.1 channels (Monteil et al., 2000). Activation of GABAA receptors increases intracellular Ca2+ concentration [Ca2+] and induces membrane depolarization through T-type Ca2+ channels in the mouse DRG neurons (Aptel et al., 2007). The T-type Ca2+ channels could promote GABA-induced neuronal excitability allowing an action potential to be triggered (Aptel et al., 2007). We therefore determined the role of T-type Ca2+ channels in musimol- and phenylephrine-induced inhibition of glutamate release from primary afferents. We found that the inhibitory effect of phenylephrine on the amplitude of monosynaptic eEPSCs elicited from primary afferents was largely blocked by amiloride and mibefradil, two structurally distinct T-type Ca2+ channel blockers (Tang et al., 1988, Bezprozvanny and Tsien, 1995, Bao et al., 1998, Wu et al., 2008). Amiloride also completely blocked the muscimol effect on the amplitude of monosynaptic eEPSCs. Thus, T-type Ca2+ channels are critically involved in the α1-adrenoceptor agonist-induced inhibition of glutamatergic input from primary afferent terminals. In a subpopulation of neurons in which amiloride alone did not fully block the inhibitory effect of phenylephrine, subsequent application of CGP55845 abolished the remaining effect of phenylephrine on monosynaptic eEPSCs. Therefore, our findings suggest that both GABAA (via T-type Ca2+ channels) and GABAB receptors contribute to attenuation of primary afferent inputs by activation of α1-adrenoceptors.
In summary, our study provides important new information that activation of α1-adrenoceptors in the spinal cord leads to increased synaptic release of GABA, which inhibits glutamatergic input from primary afferents to dorsal horn neurons through GABAA receptors, and to a lesser extend, GABAB receptors. T-type Ca2+ channels are critically involved in presynaptic inhibition of glutamate release from primary afferents by stimulation of α1-adrenoceptors and GABAA receptors in the spinal dorsal horn. These mechanisms probably contribute to the regulation of nociceptive transmission in the spinal cord and participate in the antinociceptive effect produced by activation of the descending noradrenergic system and by spinally administered α1-adrenoceptor agonists.
Acknowledgments
This study was supported by the National Institutes of Health Grants GM64830 and NS45602.
List of abbreviations
- eEPSCs
evoked excitatory postsynaptic currents
- DRG
dorsal root ganglion
- GABA
γ-aminobutyric acid
- sIPSCs
spontaneous inhibitory postsynaptic currents
- WB4101
2-(2,6-dimethoxyphenoxy)ethylaminomethyl-1,4-benzodioxane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aptel H, Hilaire C, Pieraut S, Boukhaddaoui H, Mallie S, Valmier J, Scamps F. The Cav3.2/alpha1H T-type Ca2+ current is a molecular determinant of excitatory effects of GABA in adult sensory neurons. Mol Cell Neurosci. 2007;36:293–303. doi: 10.1016/j.mcn.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Aran S, Proudfit HK. Antinociceptive interactions between intrathecally administered alpha noradrenergic agonists and 5′-N-ethylcarboxamide adenosine. Brain Res. 1990;519:287–293. doi: 10.1016/0006-8993(90)90090-x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Baba H, Goldstein PA, Okamoto M, Kohno T, Ataka T, Yoshimura M, Shimoji K. Norepinephrine facilitates inhibitory transmission in substantia gelatinosa of adult rat spinal cord (part 2): effects on somatodendritic sites of GABAergic neurons. Anesthesiology. 2000;92:485–492. doi: 10.1097/00000542-200002000-00031. [DOI] [PubMed] [Google Scholar]
- Bao J, Li JJ, Perl ER. Differences in Ca2+ channels governing generation of miniature and evoked excitatory synaptic currents in spinal laminae I and II. J Neurosci. 1998;18:8740–8750. doi: 10.1523/JNEUROSCI.18-21-08740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber RP, Vaughn JE, Saito K, McLaughlin BJ, Roberts E. GABAergic terminals are presynaptic to primary afferent terminals in the substantia gelatinosa of the rat spinal cord. Brain Res. 1978;141:35–55. doi: 10.1016/0006-8993(78)90615-7. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Tsien RW. Voltage-dependent blockade of diverse types of voltage-gated Ca2+ channels expressed in Xenopus oocytes by the Ca2+ channel antagonist mibefradil (Ro 40-5967) Mol Pharmacol. 1995;48:540–549. [PubMed] [Google Scholar]
- Bischoff S, Leonhard S, Reymann N, Schuler V, Shigemoto R, Kaupmann K, Bettler B. Spatial distribution of GABA(B)R1 receptor mRNA and binding sites in the rat brain. J Comp Neurol. 1999;412:1–16. [PubMed] [Google Scholar]
- Brugger F, Wicki U, Olpe HR, Froestl W, Mickel S. The action of new potent GABAB receptor antagonists in the hemisected spinal cord preparation of the rat. Eur J Pharmacol. 1993;235:153–155. doi: 10.1016/0014-2999(93)90836-7. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Jr, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors: alpha 1-adrenergic receptor subtypes, inositol phosphates, and sources of cell Ca2+ Pharmacol Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- Cao CQ, Tse HW, Jane DE, Evans RH, Headley PM. Metabotropic glutamate receptor antagonists, like GABA(B) antagonists, potentiate dorsal root-evoked excitatory synaptic transmission at neonatal rat spinal motoneurons in vitro. Neuroscience. 1997;78:243–250. doi: 10.1016/s0306-4522(96)00579-9. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hayes ES. GABAergic vesicle-containing dendrites and spines: a critical element in processing sensory input in the monkey dorsal horn. Neurosci Lett. 1991;121:40–42. doi: 10.1016/0304-3940(91)90644-9. [DOI] [PubMed] [Google Scholar]
- Cervero F, Iggo A. The substantia gelatinosa of the spinal cord: a critical review. Brain. 1980;103:717–772. doi: 10.1093/brain/103.4.717. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JM. Mechanisms of touch-evoked pain (allodynia): a new model. Pain. 1996;68:13–23. doi: 10.1016/S0304-3959(96)03165-X. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JM, Garcia-Nicas E. Secondary hyperalgesia and presynaptic inhibition: an update. Eur J Pain. 2003;7:345–351. doi: 10.1016/s1090-3801(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Chen Q, Li DP, Pan HL. Presynaptic alpha1 adrenergic receptors differentially regulate synaptic glutamate and GABA release to hypothalamic presympathetic neurons. J Pharmacol Exp Ther. 2006;316:733–742. doi: 10.1124/jpet.105.094797. [DOI] [PubMed] [Google Scholar]
- Chen SR, Eisenach JC, McCaslin PP, Pan HL. Synergistic effect between intrathecal non-NMDA antagonist and gabapentin on allodynia induced by spinal nerve ligation in rats. Anesthesiology. 2000;92:500–506. doi: 10.1097/00000542-200002000-00033. [DOI] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Drafts BC, Fisher JL. Structural determinants of the pharmacological properties of the GABAA receptor alpha6 subunit. J Pharmacol Exp Ther. 2004;309:1108–1115. doi: 10.1124/jpet.103.064360. [DOI] [PubMed] [Google Scholar]
- Fisher JL. Amiloride inhibition of gamma-aminobutyric acid(A) receptors depends upon the alpha subunit subtype. Mol Pharmacol. 2002;61:1322–1328. doi: 10.1124/mol.61.6.1322. [DOI] [PubMed] [Google Scholar]
- Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ, Minneman KP, Ruffolo RR., Jr International Union of Pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- Iyadomi M, Iyadomi I, Kumamoto E, Tomokuni K, Yoshimura M. Presynaptic inhibition by baclofen of miniature EPSCs and IPSCs in substantia gelatinosa neurons of the adult rat spinal dorsal horn. Pain. 2000;85:385–393. doi: 10.1016/S0304-3959(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Jang IS, Ito Y, Akaike N. Feed-forward facilitation of glutamate release by presynaptic GABA(A) receptors. Neuroscience. 2005;135:737–748. doi: 10.1016/j.neuroscience.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Jones SL, Gebhart GF. Spinal pathways mediating tonic, coeruleospinal, and raphe-spinal descending inhibition in the rat. J Neurophysiol. 1987;58:138–159. doi: 10.1152/jn.1987.58.1.138. [DOI] [PubMed] [Google Scholar]
- Keja JA, Kits KS. Single-channel properties of high- and low-voltage-activated calcium channels in rat pituitary melanotropic cells. J Neurophysiol. 1994;71:840–855. doi: 10.1152/jn.1994.71.3.840. [DOI] [PubMed] [Google Scholar]
- Labrakakis C, Tong CK, Weissman T, Torsney C, MacDermott AB. Localization and function of ATP and GABAA receptors expressed by nociceptors and other postnatal sensory neurons in rat. J Physiol. 2003;549:131–142. doi: 10.1113/jphysiol.2002.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan YZ, Levey AI, Pan HL. Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. J Physiol. 2002;543:807–818. doi: 10.1113/jphysiol.2002.020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Zou X, Fang L, Willis WD. Sympathetic modulation of acute cutaneous flare induced by intradermal injection of capsaicin in anesthetized rats. J Neurophysiol. 2003;89:853–861. doi: 10.1152/jn.00568.2002. [DOI] [PubMed] [Google Scholar]
- Ma W, Saunders PA, Somogyi R, Poulter MO, Barker JL. Ontogeny of GABAA receptor subunit mRNAs in rat spinal cord and dorsal root ganglia. J Comp Neurol. 1993;338:337–359. doi: 10.1002/cne.903380303. [DOI] [PubMed] [Google Scholar]
- Maddox FN, Valeyev AY, Poth K, Holohean AM, Wood PM, Davidoff RA, Hackman JC, Luetje CW. GABAA receptor subunit mRNA expression in cultured embryonic and adult human dorsal root ganglion neurons. Brain Res Dev Brain Res. 2004;149:143–151. doi: 10.1016/j.devbrainres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Monteil A, Chemin J, Bourinet E, Mennessier G, Lory P, Nargeot J. Molecular and functional properties of the human alpha(1G) subunit that forms T-type calcium channels. J Biol Chem. 2000;275:6090–6100. doi: 10.1074/jbc.275.9.6090. [DOI] [PubMed] [Google Scholar]
- Naik AK, Pathirathna S, Jevtovic-Todorovic V. GABA(A) receptor modulation in dorsal root ganglia in vivo affects chronic pain after nerve injury. Neuroscience. 2008;154:1539–1553. doi: 10.1016/j.neuroscience.2008.04.061. [DOI] [PubMed] [Google Scholar]
- Nalepa I, Vetulani J, Borghi V, Kowalska M, Przewlocka B, Pavone F. Formalin hindpaw injection induces changes in the [3H]prazosin binding to alpha1-adrenoceptors in specific regions of the mouse brain and spinal cord. J Neural Transm. 2005;112:1309–1319. doi: 10.1007/s00702-005-0279-3. [DOI] [PubMed] [Google Scholar]
- Pan HL, Chen SR, Eisenach JC. Intrathecal clonidine alleviates allodynia in neuropathic rats: interaction with spinal muscarinic and nicotinic receptors. Anesthesiology. 1999;90:509–514. doi: 10.1097/00000542-199902000-00027. [DOI] [PubMed] [Google Scholar]
- Pan YZ, Li DP, Pan HL. Inhibition of glutamatergic synaptic input to spinal lamina II(o) neurons by presynaptic alpha(2)-adrenergic receptors. J Neurophysiol. 2002;87:1938–1947. doi: 10.1152/jn.00575.2001. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of alpha 1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GW, Wilkin GP, Turnbull MJ, Bowery NG. Are baclofen-sensitive GABAB receptors present on primary afferent terminals of the spinal cord? Nature. 1984;307:71–74. doi: 10.1038/307071a0. [DOI] [PubMed] [Google Scholar]
- Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SV, Maderdrut JL, Yaksh TL. Spinal cord pharmacology of adrenergic agonist-mediated antinociception. J Pharmacol Exp Ther. 1980;213:525–533. [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hokfelt T, Riedl MS, Elde R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. J Neurosci. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers RJ, McMartin LR. Adrenoceptors and their second messenger systems. J Neurochem. 1993;60:10–23. doi: 10.1111/j.1471-4159.1993.tb05817.x. [DOI] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CM, Presser F, Morad M. Amiloride selectively blocks the low threshold (T) calcium channel. Science. 1988;240:213–215. doi: 10.1126/science.2451291. [DOI] [PubMed] [Google Scholar]
- Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–1843. doi: 10.1523/JNEUROSCI.4584-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Zhang HM, Chen SR, Pan HL. Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol. 2007;579:849–861. doi: 10.1113/jphysiol.2006.126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West WL, Yeomans DC, Proudfit HK. The function of noradrenergic neurons in mediating antinociception induced by electrical stimulation of the locus coeruleus in two different sources of Sprague-Dawley rats. Brain Res. 1993;626:127–135. doi: 10.1016/0006-8993(93)90571-4. [DOI] [PubMed] [Google Scholar]
- Williams ME, Washburn MS, Hans M, Urrutia A, Brust PF, Prodanovich P, Harpold MM, Stauderman KA. Structure and functional characterization of a novel human low-voltage activated calcium channel. J Neurochem. 1999;72:791–799. doi: 10.1046/j.1471-4159.1999.0720791.x. [DOI] [PubMed] [Google Scholar]
- Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. Differential sensitivity of N- and P/Q-type Ca2+ channel currents to a mu opioid in isolectin B4-positive and -negative dorsal root ganglion neurons. J Pharmacol Exp Ther. 2004;311:939–947. doi: 10.1124/jpet.104.073429. [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. Distinct inhibition of voltage-activated Ca(2+) channels by delta-opioid agonists in dorsal root ganglion neurons devoid of functional T-type Ca(2+) currents. Neuroscience. 2008;153:1256–1267. doi: 10.1016/j.neuroscience.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Pogrel JW, Lee YW, Chaplan SR. Reversal of nerve ligation-induced allodynia by spinal alpha-2 adrenoceptor agonists. J Pharmacol Exp Ther. 1995;272:207–214. [PubMed] [Google Scholar]
- Yasaka T, Kato G, Furue H, Rashid MH, Sonohata M, Tamae A, Murata Y, Masuko S, Yoshimura M. Cell-type-specific excitatory and inhibitory circuits involving primary afferents in the substantia gelatinosa of the rat spinal dorsal horn in vitro. J Physiol. 2007;581:603–618. doi: 10.1113/jphysiol.2006.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Pan HL. Regulation of glutamate release from primary afferents and interneurons in the spinal cord by muscarinic receptor subtypes. J Neurophysiol. 2007;97:102–109. doi: 10.1152/jn.00586.2006. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Li DP, Chen SR, Pan HL. M2, M3, and M4 receptor subtypes contribute to muscarinic potentiation of GABAergic inputs to spinal dorsal horn neurons. J Pharmacol Exp Ther. 2005;313:697–704. doi: 10.1124/jpet.104.079939. [DOI] [PubMed] [Google Scholar]