Abstract
Infection with schistosomes results in a CD4 T cell-mediated inflammatory reaction against parasite eggs that varies greatly in magnitude both in humans as well as in mice. In the murine disease, the severe form of immunopathology correlates with high levels of IL-17. We now report that live schistosome eggs stimulate dendritic cells from high pathology-prone CBA mice to produce IL-12p40, IL-6, and TGF-β, whereas those from low pathology-prone BL/6 mice only make TGF-β. Moreover, egg-stimulated dendritic cells plus naive CD4 T cells from CBA mice resulted in increased levels of IL-6, IL-23, IL-1β, as well as IL-17 and the chemokines CXCL1, CXCL2, and CCL2, whereas similarly treated BL/6 cell cocultures instead expressed higher IL-4, IL-5, IL-10, and the transcription factor Foxp3. Neutralization of IL-23 and IL-1, but not of IL-6 or IL-21, profoundly inhibited egg-induced IL-17 production in the CBA cocultures. Conversely, stimulation with schistosome eggs in the presence of exogenous IL-23 and IL-1β induced BL/6 cells to make IL-17. These findings identify IL-23 and IL-1 as critical host factors that drive IL-17 production, and suggest that parasite recognition followed by a genetically determined innate proinflammatory response induces the development of Th17 cells and thus controls the outcome of immunopathology in schistosomiasis.
Schistosomes are trematode helminths that infect and cause widespread disease in vertebrates, including humans. The infected host mounts a circumscribed granulomatous inflammatory and fibrosing reaction against individual tissue-lodged parasite eggs. The granulomas are a manifestation of adaptive immunopathology mediated by CD4 T cells specific for egg Ags (1, 2). Though confronted with ostensibly similar parasitic loads, infected hosts display a marked variation in the intensity of disease. In the case of infection with the species Schistosoma mansoni, most humans develop mild “intestinal” schistosomiasis, which contrasts with the less frequent, but severe and lethal “hepatosplenic” form of the disease (3). Likewise, in the experimental murine model of schistosomiasis, CBA mice naturally develop pronounced hepatic granulomatous inflammation, whereas in C57BL/6 (BL/6) mice the lesions are significantly smaller (4, 5). Nevertheless, BL/6 mice can readily shift to a high pathology phenotype following immunization with a soluble preparation of schistosome egg Ags (SEA)3 emulsified in CFA (5). In the natural and induced models of severe disease, pronounced immunopathology correlates with an increase in IL-17 production by granuloma cells in the lesions of the infected animals (6, 7); both forms of high pathology can be reduced by in vivo neutralization of IL-17 (6).
IL-17-producing cells are potent proinflammatory CD4 T cells first shown to mediate the immunopathology in autoimmune diseases such as experimental allergic encephalomyelitis, collagen-induced arthritis, and inflammatory bowel disease (8-11). IL-17 production was initially described to be under the control of IL-23, a heterodimeric cytokine related to IL-12, which uses a common p40 subunit together with a unique p19 subunit, instead of IL-12’s p35 (12). In subsequent studies, IL-17 producing (Th17) cells were identified as a distinct lineage of CD4 T cells (13, 14) characterized by the expression of the transcription factor RORγt (15), and their differentiation was shown to be stimulated by a combination of IL-6, TGF-β and IL-23, with the latter serving to stabilize and expand the phenotype (16-18). IL-21 and IL-1 have also been identified as additional factors in Th17 cell development (19-22).
The demonstration of Th17 cells in the context of severe egg-induced immunopathology presupposes the activation of APCs capable of inducing a proinflammatory T cell phenotype following interaction with the parasite. However, the generation of APC acquiring such properties as a consequence of exposure to schistosomes, or helminths in general, has so far not been documented. Previous studies conducted in BL/6 mice have detected little if any departure from the resting state in dendritic cells (DC) exposed to SEA (23, 24). Moreover, such interactions were shown to be conducive to the differentiation of Th2 (25) or T regulatory cell responses (26, 27).
In this study, we report that DC can indeed initiate an innate proinflammatory cascade leading to the differentiation of pathogenic Th17 cells in response to schistosome eggs. This genetically restricted function can only be performed by DC derived from high pathology-prone CBA mice, and depends on their ability to produce IL-23 and IL-1.
Materials and Methods
Mice, parasites, and infection
Female CBA and C57BL/6 (BL/6) mice, 5–6 wk old, were purchased from The Jackson Laboratory. Swiss Webster mice were obtained from Charles River Laboratories. All mice were maintained at the Tufts University School of Medicine Animal Facility in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines. For some experiments, CBA and BL/6 mice were infected by i.p. injection with 80 cercariae of Schistosoma mansoni (Puerto Rico strain), which were shed from infected Biomphalaria glabrata snails provided to us by Dr. F. Lewis (Biomedical Research Institute, Rockville, MD), under National Institutes of Health/ National Institute of Allergy and Infectious Diseases Contract N01-AI-55270. All Swiss Webster mice were infected in an identical fashion for the purpose of isolating eggs and worms.
Cell preparations
Bone marrow-derived DC (BMDC)
Bone marrow cells were flushed from the femurs and tibias of normal CBA and BL/6 mice, RBC were lysed with Tris ammonium chloride buffer, and the remaining cells were cultured at a concentration of 2 × 105 cell/ml in 10 ml of complete-RPMI 1640 medium containing 10% FCS (Aleken Biologicals) and GM-CSF. GM-CSF-containing supernatants from the transfectant B cell hybridoma J558L (provided to us by Dr. N. Hacohen, Broad Institute, Cambridge, MA) were added to the bone marrow cells at an optimized concentration of 1/30. Three days after seeding, an additional 10 ml of GM-CSF-containing medium was added to the cultures. At day 10, nonadherent cells, which were >85% CD11c+, were harvested and used for experiments.
CD4 T cells
Single-cell suspensions were prepared from the spleens of normal CBA and BL/6 mice, RBC were lysed, and CD4 T cells were purified by negative selection using CD4 MACS columns (Miltenyi Bio-tec) in accordance with the manufacturer’s instructions. CD4 T cell purity was >95% by FACS analysis.
Parasite preparations
Schistosome eggs and worms were isolated under sterile conditions from 7-to 8-wk infected mice. For eggs, livers were blended and eggs were isolated from the tissues using a series of sieves and washes with saline solution. For worms, the circulatory system was perfused via the aorta with 20 ml of sterile PBS supplemented with 25 mM sodium citrate (Fisher Scientific). Worms were collected in a sieve and transferred to a sterile petri dish containing medium.
BMDC-parasite cocultures
BMDC (1 × 106 cells/ml) were cultured in the presence of 100 or 500 live eggs, 2 live worms (1 male, 1 female), or LPS (10 ng/ml, Sigma-Aldrich). After 24 h, culture supernatants were collected, filtered, and assayed by ELISA for IL-12p40 and TGF-β using Ab, standards, and protocols from BD Pharmingen, and for IL-6, using Ab, standards, and protocols from R&D Systems. Potential contamination of schistosome-containing cultures with LPS was carefully ruled out using the limulus amoebocyte lysate assay (Cambrex) and by testing for possible TNF-α production by thioglycollate-elicited indicator peritoneal macrophages (28, 29). Furthermore, similar results were obtained in selected experiments in which culture medium were supplemented with 50 μg/ml polymyxin B (Sigma-Aldrich, data not shown).
BMDC-T cell-parasite cocultures
Purified CD4 T cells (1 × 106) from normal spleens plus syngeneic BMDC (2.5 × 105) were cultured in 1 ml of medium together with 100 eggs and anti-CD3/anti-CD28 coated beads (3 × 105; Dynal). After 4 days, the culture supernatants were removed and assayed by ELISA for IL-17, IL-6, and IFN-γ using Ab, standards, and protocols from R&D Systems; for IL-23, using Ab, standards, and protocols from eBioscience; and for IL-5, using Ab, standards, and protocols from BD Pharmingen. The number of cells, eggs, and anti-CD3/anti-CD28 coated beads, as well as the length of incubations were optimized in preliminary experiments.
In some experiments, neutralizing Ab against IL-12p40, IL-6 (both 2 μg/ml; BD Pharmingen), IL-21 (2 μg/ml; R&D Systems), and IL-23p19 (10 μg/ml; eBioscience), IL-1 receptor antagonist (IL-1Ra, at indicated concentrations, a gift from Dr. C. Dinarello, University of Colorado, Denver, CO) or recombinant cytokines IL-23 (20 ng/ml), IL-6 (10 ng/ml), IL-21 (80 ng/ml), IL-1β (2 ng/ml; all R&D Systems), TGF-β (8 ng/ml; Sigma-Aldrich), IL-12p40 (10 ng/ml), and IL-12p70 (20 ng/ml; both BD Pharmingen), were added either individually or in combinations to the DC-T cell-parasite cocultures, as indicated.
Real-time quantitative RT-PCR
Total RNA was isolated from individual livers of schistosome-infected CBA and BL/6 mice, as well as from DC and T cells in cocultures stimulated with eggs and anti-CD3/anti-CD28 coated beads, using the TRIzol method per the manufacturer’s instructions (Invitrogen). RNA (1–5 μg) was subjected to DNASE I treatment (Roche) and reverse-transcribed using RT2 first strand kit (C-03). Real-time quantitative RT-PCR on 10 ng of cDNA from each sample was performed by SYBR green analysis using a custom PCR Array or Taqman analysis. All reactions were performed using an ABI 7300 instrument. GAPDH levels were measured in a separate reaction and used to normalize the data. Reagents and protocols for SYBR Green and Taqman real-time quantitative PCR were obtained from Super-Array Bioscience and Applied Biosystems, respectively. Using the average mean cycle threshold (Ct) value for GAPDH and the gene of interest for each sample, the equation 1.8 e (Ct GAPDH – Ct gene of interest) × 104 was used to obtain normalized values (10).
Flow cytometry analysis
Cells from DC-T cell-egg cocultures were fluorescently labeled with allophycocyanin-conjugated anti-CD11c (clone HL3), FITC-conjugated anti-CD40 (clone 3/23), PE-conjugated anti-CD80 (clone 16–10A1), FITC-conjugated anti-CD86 (clone GL1), and PE-conjugated anti-MHC class II (clone M5/114,15.2, all from BD Pharmingen), as previously described (30). Cells were acquired using a FACSCalibur flow cytometer using CellQuest software version 3.2.1 (Becton Dickinson) and the data were analyzed using FlowJo Flow Cytometry Analysis Software.
Statistical analysis
ANOVA and Student’s t tests were used to determine the statistical analysis of the differences between groups. p values of <0.05 were considered significant and were calculated with GraphPad Prism.
Results
CBA, but not BL/6 DC produce proinflammatory cytokines in response to live schistosomes
In view of the strong association of severe egg-induced immunopathology with high levels of IL-17 (6, 7), we examined the ability of BMDC to produce the mediators of Th17 cell differentiation in response to schistosomes. For this, we assessed cytokine production by DC following a 24-h incubation with live eggs or worms, which are the stages of the schistosome life cycle present in the mammalian host. DC derived from CBA mice secreted significant amounts of IL-12p40 and IL-6 upon stimulation with eggs, while the worms elicited a considerably lower response (Fig. 1, A and B). In striking contrast, the parasites induced only marginal amounts of either cytokine in DC derived from BL/6 mice (Fig. 1, A and B) although both strains produced similar amounts of TGF-β (Fig. 1C), and of all three cytokines in response to control LPS (Fig. 1, A–C). There were no detectable amounts of IL-12p70, IL-23, or IL-10 by ELISA in either strain (data not shown). These results suggest that DC from mice that develop high pathology have the ability to react to schistosomes with a proinflammatory cytokine response.
FIGURE 1.
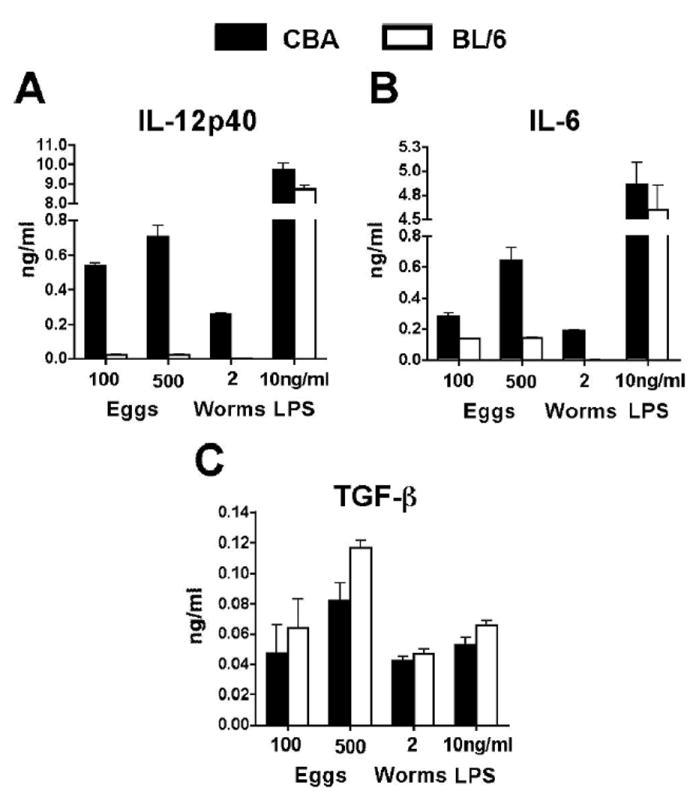
DC cytokine production induced by live schistosome eggs and worms. A–C, BMDC were cocultured with the indicated number of live eggs or worms as detailed in Materials and Methods. Cytokine levels in 24-h supernatants were measured by ELISA. DC from high pathology-prone CBA mice produce significantly more IL-12p40 (A) in comparison to BL/6 DC after exposure to live eggs or worms (p < 0.001). Similarly, significantly higher levels of IL-6 (B) were produced by CBA DC after exposure to 100 (p < 0.05) and 500 (p < 0.001) eggs or worms (p < 0.05) in comparison to their BL/6 counterparts. There were no statistically significant differences in TGF-β production (C). LPS induced similar IL-12p40, IL-6, and TGF-β production in CBA and BL/6 DC. Cytokine levels are expressed as means of triplicate ELISA determinations ± SD; background cytokine production from unstimulated DC was subtracted. Results shown are from one of eight independent experiments with similar results.
Schistosome eggs induce Th17 cell differentiation in CBA, but not in BL/6, DC-CD4 T cell cocultures
To examine the ability of schistosome-stimulated DC to instruct differential T cell development, CBA and BL/6 BMDC were incubated together with syngeneic naive CD4 T cells in the presence of live schistosome eggs and anti-CD3/anti-CD28 coated beads, and cytokine production was assessed after 4 days of culture. CBA DC-CD4 T cell cocultures produced abundant IL-6 and IL-23 in response to the eggs, while identically stimulated BL/6 cocultures secreted significantly lower amounts of these cytokines (Fig. 2, A and B). Furthermore, consistent with the notion that IL-6 and IL-23 are involved in the development and expansion of Th17 cells (16-18), there was robust IL-17 production in the egg-stimulated CBA DC-CD4 T cell cocultures, while production in their BL/6 counterparts was negligible (Fig. 2C). In contrast, IL-5 production was higher in the egg-stimulated BL/6 DC-CD4 T cell cocultures in comparison to cultures comprised of CBA cells (Fig. 2D). In each case, both DC and anti-CD3/anti-CD28 stimulation were necessary for egg-induced cytokine production. Schistosome eggs did not stimulate IFN-γ or IL-10 production above that induced by anti-CD3/anti-CD28 alone, and adult schistosome worms elicited little to no cytokine production (data not shown).
FIGURE 2.
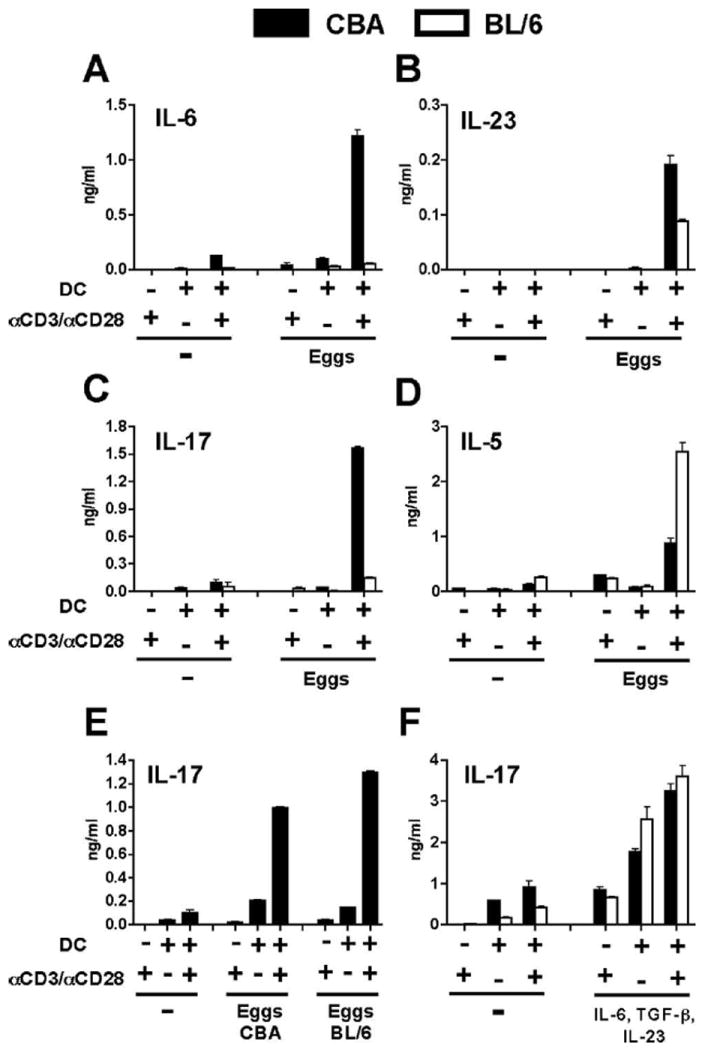
IL-17 production in DC-T cell cocultures stimulated with schistosome eggs or rIL-6, TGF-β, and IL-23. A–F, Naive CD4 T cells from CBA and BL/6 mice were cocultured with syngeneic BMDC in the presence or absence of anti-CD3/anti-CD28 coated beads, eggs or rIL-6, TGF-β, and IL-23 as described in Materials and Methods. Cytokine levels in 4-day supernatants were measured by ELISA. Stimulation with 100 eggs induced markedly higher levels of IL-6 (p < 0.001) (A), IL-23 (p < 0.001) (B), and IL-17 (p < 0.001) (C) in CBA cocultures than in BL/6 cocultures. D, IL-5 production elicited by 100 eggs was significantly higher in BL/6 cocultures than in CBA cocultures (p < 0.001). p values compare egg-stimulated CBA vs BL/6 DC-T cell cocultures in the presence of anti-CD3/ anti-CD28 coated beads. E, Significant levels of IL-17 were produced in CBA cocultures after stimulation with 100 eggs isolated from infected CBA or BL/6 mice (both p < 0.001, in comparison to unstimulated cultures). F, Both CBA and BL/6 cocultures produced significantly higher levels of IL-17 following stimulation with recombinant cytokines in comparison to unstimulated cultures (all p < 0.001). All egg preparations were obtained from infected Swiss Webster mice unless indicated otherwise. Cytokine levels are expressed as means of triplicate ELISA determinations ± SD. Results shown are from one experiment representative of seven.
Because the schistosome eggs for these experiments were isolated from the livers of infected outbred Swiss Webster mice, there existed the possibility that egg-induced cytokine production in the DC-CD4 T cell cocultures was afforded by residual, contaminating allogeneic liver tissue. However, this was not the case as similar levels of IL-17 were produced in CBA DC-CD4 T cell cocultures stimulated with eggs obtained from either infected Swiss Webster, CBA or BL/6 mice (Fig. 2, C and E).
Naive CD4 T cells from CBA and BL/6 mice are equally capable of developing into Th17 cells in the presence of IL-6, TGF-β and IL-23
In view of the consistent inability of egg-stimulated BL/6 DC-CD4 T cell cocultures to activate a proinflammatory response resulting in the production of IL-17, we asked whether the BL/6 T cells were inherently capable of making IL-17 under the present culture conditions. For this, CBA and BL/6 DC-CD4 T cell cocultures were stimulated with anti-CD3/anti-CD28 coated beads in the presence of rIL-6, TGF-β, and IL-23; a combination of cytokines known to promote optimal Th17 cell differentiation and expansion (16-18). As shown in Fig. 2F, and in contrast to egg-induced IL-17 secretion, the cytokines equally stimulated IL-17 production in both the CBA and BL/6 cocultures, with maximal IL-17 output dependent on DC and anti-CD3/anti-CD28 stimulation. These findings clearly indicate that BL/6 T cells are intrinsically capable of differentiating into Th17 cells, but unlike the CBA T cells, fail to do so when in the presence of syngeneic DC and schistosome eggs.
Schistosome eggs induce contrasting immune profiles in CBA vs BL/6 DC-T cell cocultures
To more broadly explore and clearly define the immune profiles that develop in the CBA vs BL/6 DC-T cell cocultures following egg stimulation, mRNA transcripts for additional mediators were measured by real-time quantitative RT-PCR analysis. Expression of IL-6, IL-12p40, IL-23p19, IL-12p35, and IL-1β was moderately to markedly enhanced in egg-stimulated CBA cocultures, whereas in the BL/6 cocultures, these cytokines were detected at much lower levels and were not significantly modulated by the eggs (Fig. 3A). Importantly, expression of IL-17 and of the chemokines CXCL1 (KC, gro-α), CXCL2 (MIP-2, gro-β), and CCL2 (MCP-1) was strongly stimulated by eggs in the CBA cocultures (Fig. 3B), while in the BL/6 cocultures they elicited no response. By comparison, eggs had no stimulatory effect on IFN-γ expression in either strain, but IL-4, as well as IL-10 and the transcription factor Foxp3, which are markers of Th2 (25, 31) and regulatory T cells, respectively (26, 27, 32, 33), were significantly enhanced in egg-stimulated BL/6 cocultures (Fig. 3C).
FIGURE 3.
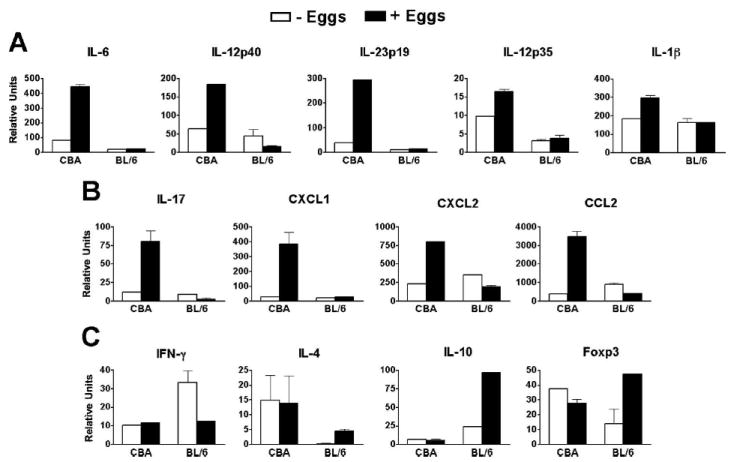
Immune profile of egg-stimulated CBA vs BL/6 DC-T cell cocultures. A–C, mRNA expression was measured by real-time quantitative RT-PCR as detailed in Materials and Methods. A, Eggs induced a significant increase in the expression of IL-6 (p < 0.001), IL-12p40 (p < 0.05), IL-23p19 (p < 0.001), and IL-1β (p < 0.05), but not of IL-12p35 in CBA cocultures. B, Transcript levels for IL-17 (p < 0.01), CXCL1 (p < 0.001), CXCL2 (p < 0.001), and CCL2 (p < 0.001)were markedly enhanced by egg stimulation in CBA cocultures; there were no increases in the BL/6 cocultures. C, IL-4 (p < 0.05), IL-10 (p < 0.001), and Foxp3 (p < 0.05) were significantly increased in egg-stimulated BL/6 cocultures, and IFN-γ was not significantly different between egg-stimulated CBA and BL/6 cocultures. p values compare egg-stimulated vs unstimulated cocultures within the CBA or BL/6 groups. Data from real-time quantitative RT-PCR analyses were normalized to GAPDH. Each bar represents the mean mRNA level from duplicate determinations ± SD from one of three independent experiments with similar results.
CD86 expression is up-regulated on CBA DC following stimulation with schistosome eggs
Because T cell lineage commitment is influenced by the phenotypic configuration of APC (34, 35), we examined the expression of costimulatory and MHC class II molecules on DC in the egg-stimulated DC-T cell cocultures. Schistosome eggs induced a 2-fold increase in CD86 expression in CD11c+ DC present in CBA, but not BL/6 cocultures, while there were no significant changes in CD40, CD80, or MHC class II expression in either case (Fig. 4A). By comparison, a low dose of LPS variably but significantly enhanced CD40, CD80, CD86, and MHC class II expression in both CBA and BL/6 DC-CD4 T cell cocultures (Fig. 4B). Taken together with the differential cytokine production, the CBA DC phenotype is consistent with an enhanced state of activation.
FIGURE 4.
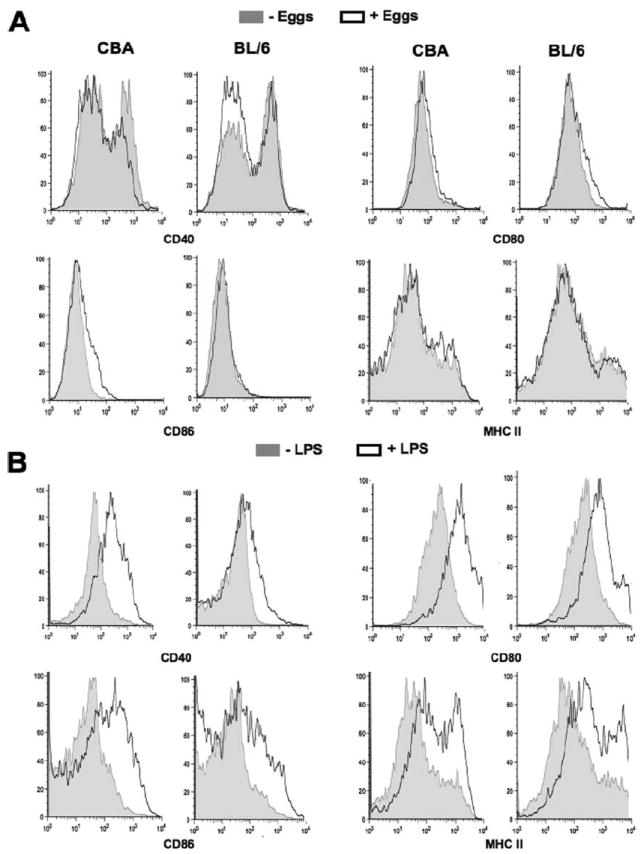
Costimulatory and MHC class II expression by CD11c+ DC. A and B, CBA and BL/6 DC-T cell cocultures were established in the presence or absence of eggs or control LPS and maintained for 4 days, after which DC were fluorescently labeled for FACS analysis as described in Materials and Methods. A, CD11c+ CBA DC exhibited a 2-fold increase in CD86 expression following egg stimulation, whereas there were no significant changes from basal expression in CD11c+ DC from BL/6 cocultures. There were no significant changes in the expression of CD40, CD80, or MHC class II induced by schistosome eggs. B, Stimulation with LPS (10 ng/ml) increased the expression of CD40, CD80, CD86, and MHC class II on both CBA and BL/6 DC. Results shown are from two experiments representative of four with similar results.
Contrasting immune profiles induced by schistosome eggs in in vitro CBA vs BL/6 DC-CD4 T cell cocultures parallel those induced during schistosome infection in vivo
To lend relevance to the contrasting immune responses induced by schistosome eggs in CBA vs BL/6 DC-CD4 T cell cocultures in vitro, we investigated the expression of some of these molecules in 7-wk infected mice. Real-time RT-PCR analyses of livers from infected high pathology CBA mice revealed a proinflammatory environment characterized by significantly elevated levels of IL-6, IL-23p19, IL-1β, and IL-17, whereas IL-10 expression was higher in the low pathology BL/6 strain; there were no significant differences in IL-12p40 or IL-12p35 (Fig. 5). These data clearly demonstrate that the immune profile induced by schistosome eggs in DC-CD4 T cell cocultures from these two strains in vitro is similar to that induced during the schistosome infection in vivo.
FIGURE 5.
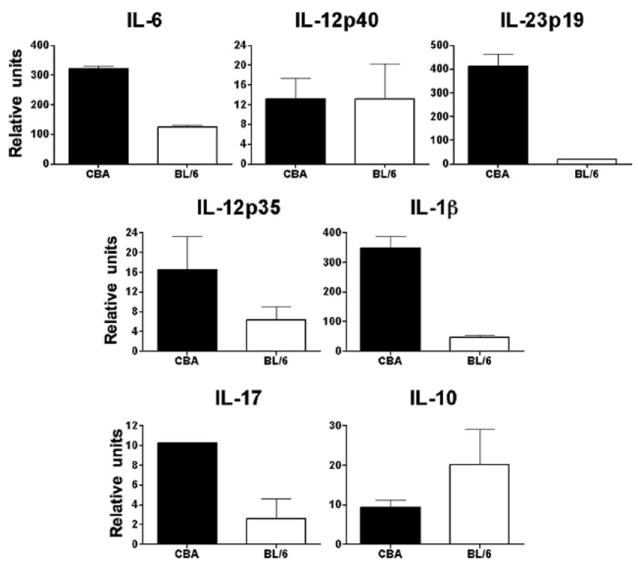
Cytokine profile of livers from schistosome-infected CBA and BL/6 mice. mRNA expression was measured by real-time quantitative RT-PCR as detailed in Materials and Methods. Livers from 7-wk infected CBA mice expressed significantly higher levels of IL-6 (p < 0.01), IL-23p19 (p < 0.01), IL-1β (p < 0.01), and IL-17 (p < 0.05); lower levels of IL-12p35, as well as higher levels of IL-10 in infected BL/6 mice were not statistically significant and there was no significant difference in IL-12p40. Data from real-time quantitative RT-PCR analyses were normalized to GAPDH. Each bar represents the mean mRNA level ± SD from two to four independent experiments with similar results.
Egg-stimulated IL-17 production in CBA DC-CD4 T cell cocultures is dependent on IL-23 and IL-1
Given that both CBA and BL/6 CD4 T cells were equally capable of developing into Th17 cells in the presence of rIL-6, TGF-β, and IL-23 (Fig. 2F), but only CBA CD4 T cells did so following stimulation with schistosome eggs (Fig. 2C), we investigated the factor(s) that facilitated Th17 cell differentiation in the CBA DC-CD4 T cell cocultures, but were absent in the BL/6. In the first approach, we neutralized candidate Th17 cell-inducing cytokines in CBA cocultures, and in the second, we supplemented these cytokines into the corresponding BL/6 co-cultures, which typically do not produce IL-17. The addition of anti-IL-12p40 or anti-IL-23p19 neutralizing Ab effectively blocked IL-17 production by egg-stimulated CBA cells, whereas, surprisingly, anti-IL-6 or anti-IL-21 Ab, individually or in combination, had no effect (Fig. 6A). IL-1 blockade with IL-1Ra, an endogenous inhibitor of IL-1 (36), also significantly inhibited IL-17 production (Fig. 6B), which is in agreement with observations from other groups demonstrating the importance of IL-1β for Th17 cell development (21, 22, 37).
FIGURE 6.
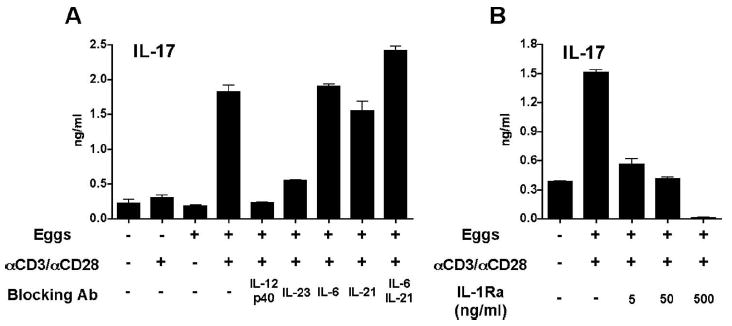
Neutralization of IL-23 and IL-1 inhibits egg-induced IL-17 production in CBA DC-T cell cocultures. A and B, DC-T cell cocultures were established in the presence or absence of eggs, anti-CD3/anti-CD28 coated beads, and the indicated blocking reagents as described in Materials and Methods. Cytokine levels in 4-day supernatants were measured by ELISA. A, Egg-induced IL-17 production in CBA cocultures was significantly blocked by anti-IL-12p40 and anti-IL-23p19 neutralizing Ab (both p < 0.001), while Ab against IL-6 or IL-21, individually or in combination, had no significant effect. B, Egg-induced IL-17 production in CBA cocultures was significantly inhibited by IL-1Ra at all tested concentrations (all p < 0.001). p values provided compare cocultures that received blocking Ab or IL-1Ra with those that did not receive either reagent. Cytokine levels are expressed as means of triplicate ELISA determinations ± SD. Results shown are from one experiment representative of two to three with similar results.
Exogenous IL-23 and IL-1β enhance egg-induced IL-17 production in BL/6 DC-T cell cocultures
The reciprocal experiments using exogenous cytokines demonstrated that IL-23 significantly enhanced IL-17 production in egg-stimulated BL/6 cocultures, and to a lesser extent did so by itself, whereas IL-12p40, IL-12p70, IL-6, or IL-21, either individually or in combination, had no effect (Fig. 7A). Exogenous IL-1β similarly facilitated significant IL-17 production, albeit less than IL-23, while both cytokines together had no additive effect (Fig. 7B). Moreover, only the addition of exogenous IL-12p70 was capable of inducing robust IFN-γ production, independent of the presence of schistosome eggs (Fig. 7C). However, none of the added cytokines induced significant additional IL-4, IL-5, or IL-10 production (data not shown). Taken together, these findings demonstrate that IL-23 and IL-1 produced by DC in response to schistosome eggs represents a plausible mechanism by which Th17 cell differentiation is induced in the CBA strain.
FIGURE 7.
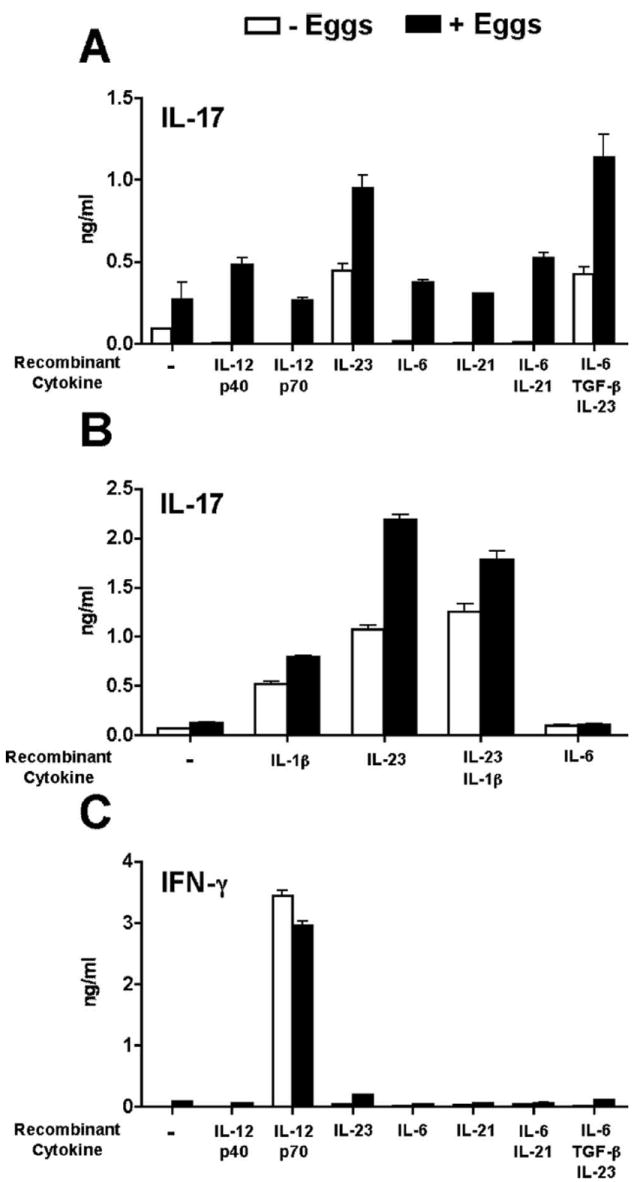
Exogenous IL-23 and IL-1β enhance egg-induced IL-17 production in BL/6 DC-T cell cocultures. A–C, DC-T cell cocultures were established in the presence or absence of eggs, anti-CD3/anti-CD28 coated beads, and recombinant cytokines as described in Materials and Methods. Cytokine levels in 4-day supernatants were measured by ELISA. A, Egg-stimulated IL-17 production in BL/6 cocultures was significantly increased in the presence of IL-23 alone or in combination with IL-6 and TGF-β (both p < 0.001) in comparison to cocultures that received no recombinant cytokines. IL-12p40, IL-12p70, IL-6, IL-21, individually or in combination, had no significant effect. B, Egg-stimulated IL-17 production was significantly increased in the presence of IL-23 (p < 0.001) or IL-1β (p < 0.05), either individually or together (p < 0.01). C, IFN-γ production was significantly increased only in the presence of IL-12p70 (p < 0.001) independent of the presence of eggs. Cytokine levels are expressed as means of triplicate ELISA determinations ± SD. Results shown are from one experiment representative of three with similar results.
Discussion
Th17 cells have been shown to mediate chronic inflammation in several autoimmune diseases including autoimmune encephalomyelitis, collagen induced arthritis, and inflammatory bowel disease (8-11). More recently, they were also shown to play a role in host defense against a variety of extracellular pathogens (38-40). Our laboratory has demonstrated that severe egg-induced immunopathology associated with the schistosome infection in CBA mice, or SEA/CFA-immunized BL/6 mice, strongly correlates with a proinflammatory cytokine environment characterized by high levels of IL-17, and that in the absence of immunization, BL/6 mice develop a significantly milder immunopathology and IL-17 levels are typically low or absent (6).
To further dissect the mechanisms leading to differential Th17 cell development in schistosomiasis, we used an in vitro model in which naive lymphoid cells were directly stimulated with live parasites. CBA DC significantly responded to this stimulation by producing IL-12p40, IL-6, and TGF-β; neither IL-12p70 nor IL-23 were demonstrable by ELISA. Although the sole detection of IL-12p40 could denote monomeric or dimeric forms of this molecule, similar egg-stimulated DC in the presence of CD4 T cells clearly produced IL-23. This was supported by the marked increase in mRNA transcripts for IL-12p40 in conjunction with IL-23p19. These findings are consistent with the observation that significant levels of IL-23 are only attained following interaction with, and feedback from T cells; a process that has been linked to CD40 ligation (41). However, the addition of soluble CD40L (CD40L, CD154) to egg-stimulated DC cultures consistently failed to substitute for T cells in the production of measurable IL-23 protein or message (data not shown).
In CBA cell cultures, stimulation with schistosomes led to the production of IL-6, TGF-β, IL-23, and IL-1, a cytokine configuration known to promote Th17 cell differentiation (16-18, 21, 22). The increase in IL-17 was accompanied by a rise in the leukocyte chemoattractants CXCL1, CXCL2, and CCL2 and by an enhanced state of APC activation reflected by the increase of CD86 costimulatory molecule expression. Of all these mediators, the BL/6 cells only produced TGF-β, an immunomodulatory cytokine linked to the development of T regulatory cells (16). Moreover, the BL/6 cells responded to egg stimulation with an increase in IL-4, IL-5, IL-10, and Foxp3, further suggesting the induction of Th2 (25, 31) and T regulatory cells (26, 27, 32, 33). The striking Th17 cell response induced by schistosome eggs in CBA cell cocultures starkly contrasts with the weaker and largely anti-inflammatory response seen in the BL/6 cells, a finding that is supported by similar cytokine profiles observed in the lesional environment of schistosome-infected CBA vs BL/6 mice in vivo (7). Of note, our observations in the BL/6 strain are in agreement with previous studies using SEA as the stimulus (23, 24), but are at odds with a report suggesting that schistosome eggs instruct proinflammatory responses in DC from this strain (42). Such dissenting findings, which may result from the use of up to a hundredfold higher egg to cell ratios, still need to be reconciled.
Our findings indicate that IL-23 and/or IL-1 produced by schistosome ligand-activated DC are capable of inducing the development of Th17 cells in genetically susceptible hosts. This observation contrasts with the notion that Th17 cell induction is largely a function of IL-6 and TGF-β (16-18); in fact, neutralization of IL-6 did not abolish IL-17 production induced by eggs. Additionally, neutralization of IL-21, which has been implicated as an IL-6-independent Th17-inducing cytokine (19, 20), also failed to abrogate egg-stimulated IL-17 production, individually or in combination with anti-IL-6 Ab. On the other hand, IL-23 has been widely recognized as an important factor in Th17 cell development, either by itself or together with IL-6 and TGF-β (16-18). IL-1 has also been shown to play a critical role in the induction of pathogenic Th17 cells as demonstrated in experimental models of autoimmune encephalomyelitis and arthritis (22, 43, 44). Importantly, IL-1 alone or together with, IL-23, IL-6, and TGF-β is an essential cytokine for Th17 cell development in humans (37, 45, 46). IL-23 and IL-1 appear to be equally capable of eliciting schistosome egg-induced Th17 cell development in CBA cell cocultures although the addition of exogenous cytokines to the BL/6 cocultures suggests IL-23 to be more potent than IL-1; no synergistic effect between the two cytokines was observed. Regardless, the requirement of anti-CD3/anti-CD28 stimulation for optimal IL-17 production suggests that T cell feedback through TCR engagement further enhances IL-23 and IL-1 production, thus sparking an amplification loop for maximal Th17 cell differentiation (22, 47). These scenarios are consistent with the notion that expression of the Th17-specific transcription factor RORγt can be induced by a variety of cytokine combinations involving IL-6, TGF-β, IL-23, IL-1β, and IL-21 (20, 37, 48, 49). The considerable variations of cytokine requirements observed in the different systems may ultimately depend on the nature of the pathogen-specific ligands, as well as the engaged pattern recognition receptors and downstream signaling pathways.
An unresolved issue concerns the schistosome products that elicit differential host innate immune and consequent immunopathological responses. Our current observations suggest that these are most strongly expressed by the eggs, as the worms elicited little or no responses despite their larger mass; in fact, their activity may be attributable to egg laying during the culture. Of the ligands that have received the most attention are the schistosome glycans, which are expressed on these parasites while residing in their vertebrate hosts (50, 51). For example, egg glycans terminating in Lewis (Le)x or pseudo-Lewisy have been proposed to respectively signal via TLR-4 (52) and the C-type lectin receptor (CLR) DC-specific intracellular adhesion molecule grabbing nonintegrin (DC-SIGN, CD209) (53, 54), while LacdiNAc is recognized by the S-type lectin, galectin-3 (51, 55). Additionally, a schistosome-specific lipid, lyso-phosphatidylserine, has also been identified as a putative TLR-2 agonist (56). Although these ligands have been a subject of considerable scientific scrutiny, a consensus about one or more distinct schistosome ligand-innate receptor interactions that significantly impacts the host immune response has yet to be reached. Nevertheless, because schistosome surfaces are heavily glycosylated, pattern-recognition receptors such as the CLR remain attractive host receptor candidates (51, 57), particularly because the yeast zymosan (β glucan)-binding CLR, Dectin-1, was capable of inducing Th17 cell development through a TLR-independent pathway involving the Syk-CARD9 signaling cascade (40). Not only did this study demonstrate that CLR were capable of linking the innate and adaptive immune responses, but relevant to our present report, it also highlighted the requirement of IL-23 for the differentiation of Th17 cells in a pathogen-based system (40).
Although a significant increase in Th17 cells is clearly linked with the exacerbation of inflammation in autoimmune and infectious disease including schistosomiasis, recent work indicates that IL-17 secretion per se does not automatically afford pathogenicity to these cells. In fact, McGeachy et al. have described two types of Th17 cells of markedly diverging pathogenicity based on the circumstances of their induction. Both cell types depended on IL-6 and TGF-β, but the pathogenic form was induced in the presence of IL-23, whereas the absence of IL-23 gave rise to a nonpathogenic form characterized by the coproduction of IL-10 (58). These findings are in agreement with our previous observations in schistosomiasis that IL-23 is not only required for the severe form of the disease but also profoundly inhibits IL-10 (7). They also suggest that the production of IL-23 and IL-17, but not IL-10, in egg-stimulated CBA DC-T cell cocultures reflects the generation of Th17 cells of the pathogenic type. IL-27, another member of the IL-12 family of heterodimeric cytokines, has been proposed as a likely candidate that confers to Th17 cells the ability to coproduce IL-10, thereby reducing their pathogenicity (58-60). However, in our experience with schistosomiasis, IL-27 signaling did neither significantly affect the magnitude of immunopathology nor the levels of IL-17 or IL-10 (61).
Taken together, our results demonstrate that genetically diverse hosts differentially interpret and respond to schistosome products resulting in the development of distinct innate and adaptive immune profiles leading to contrasting immunopathologies. Although the full extent of the underlying pathways remains to be elucidated, Th17 cells driven by IL-23 and IL-1-producing APC promote pathology exacerbation, whereas IL-10-, TGF-β-, and Foxp3-expressing T regulatory cells (33, 62), likely in conjunction with Th2 cytokine-driven alternatively activated macrophages (63), serve to curtail the severity of disease. Although the bases of immunopathology are likely to differ among the various pathogens and autoimmune conditions, an increased understanding of the underlying mechanisms and their regulation will make it possible to envisage strategies to prevent or treat the most severe forms of disease.
Footnotes
This work was supported by Public Health Service Grant R01-18919.
Abbreviations used in this paper: SEA, schistosome egg Ag; DC, dendritic cell; BMDC, bone marrow-derived DC; Ct, cycle threshold; CLR, C-type lectin receptor.
Disclosures The authors have no financial conflict of interest.
References
- 1.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 2.Gause WC, Urban JF, Jr, Stadecker MJ. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–277. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 3.Bica I, Hamer DH, Stadecker MJ. Hepatic schistosomiasis. Infect Dis Clin North Am. 2000;14:583–604. doi: 10.1016/s0891-5520(05)70122-7. [DOI] [PubMed] [Google Scholar]
- 4.Cheever AW, Duvall RH, Hallack TA, Jr, Minker RG, Malley JD, Malley KG. Variation of hepatic fibrosis and granuloma size among mouse strains infected with Schistosoma mansoni. Am J Trop Med Hyg. 1987;37:85–97. doi: 10.4269/ajtmh.1987.37.85. [DOI] [PubMed] [Google Scholar]
- 5.Rutitzky LI, Hernandez HJ, Stadecker MJ. Th1-polarizing immunization with egg antigens correlates with severe exacerbation of immunopathology and death in schistosome infection. Proc Natl Acad Sci USA. 2001;98:13243–13248. doi: 10.1073/pnas.231258498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutitzky LI, Lopes da Rosa JR, Stadecker MJ. Severe CD4 T cell-mediated immunopathology in murine schistosomiasis is dependent on IL-12p40 and correlates with high levels of IL-17. J Immunol. 2005;175:3920–3926. doi: 10.4049/jimmunol.175.6.3920. [DOI] [PubMed] [Google Scholar]
- 7.Rutitzky LI, Bazzone L, Shainheit MG, Joyce-Shaikh B, Cua DJ, Stadecker MJ. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J Immunol. 2008;180:2486–2495. doi: 10.4049/jimmunol.180.4.2486. [DOI] [PubMed] [Google Scholar]
- 8.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 9.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 13.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 15.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 17.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the TH17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 18.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 21.Matsuki T, Nakae S, Sudo K, Horai R, Iwakura Y. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 22.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald AS, Straw AD, Bauman B, Pearce EJ. CD8- dendritic cell activation status plays an integral role in influencing Th2 response development. J Immunol. 2001;167:1982–1988. doi: 10.4049/jimmunol.167.4.1982. [DOI] [PubMed] [Google Scholar]
- 24.Cervi L, MacDonald AS, Kane C, Dzierszinski F, Pearce EJ. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J Immunol. 2004;172:2016–2020. doi: 10.4049/jimmunol.172.4.2016. [DOI] [PubMed] [Google Scholar]
- 25.Pearce EJ, Kane M, Sun CJ, Taylor J, McKee AS, Cervi L. Th2 response polarization during infection with the helminth parasite Schistosoma mansoni. Immunol Rev. 2004;201:117–126. doi: 10.1111/j.0105-2896.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 26.Hesse M, Piccirillo CA, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever AW, Shevach EM, Wynn TA. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- 27.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 28.Rashid G, Luzon AA, Korzets Z, Klein O, Zeltzer E, Bernheim J. The effect of advanced glycation end-products and aminoguanidine on TNFα production by rat peritoneal macrophages. Peritoneal Dial Int. 2001;21:122–129. [PubMed] [Google Scholar]
- 29.Weiel JE, Hamilton TA, Adams DO. LPS induces altered phosphate labeling of proteins in murine peritoneal macrophages. J Immunol. 1986;136:3012–3018. [PubMed] [Google Scholar]
- 30.Rutitzky LI, Mirkin GA, Stadecker MJ. Apoptosis by neglect of CD4+ Th cells in granulomas: a novel effector mechanism involved in the control of egg-induced immunopathology in murine schistosomiasis. J Immunol. 2003;171:1859–1867. doi: 10.4049/jimmunol.171.4.1859. [DOI] [PubMed] [Google Scholar]
- 31.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Down-regulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–166. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Wing K, Miyara M. Regulatory T cells: a brief history and perspective. Eur J Immunol. 2007;37(Suppl 1):S116–S123. doi: 10.1002/eji.200737593. [DOI] [PubMed] [Google Scholar]
- 34.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 35.Reis ESC. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 36.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 37.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 38.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 39.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, Reis e Sousa C. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 41.Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- 42.Vanhoutte F, Breuilh L, Fontaine J, Zouain CS, Mallevaey T, Vasseur V, Capron M, Goriely S, Faveeuw C, Ryffel B, Trottein F. Toll-like receptor (TLR)2 and TLR3 sensing is required for dendritic cell activation, but dispensable to control Schistosoma mansoni infection and pathology. Microbes Infect. 2007;9:1606–1613. doi: 10.1016/j.micinf.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Di Padova FE, Boots AM, Gram H, Joosten LA, van den Berg WB. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167:141–149. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 46.Gerosa F, Baldani-Guerra B, Lyakh LA, Batoni G, Esin S, Winkler-Pickett RT, Consolaro MR, De Marchi M, Giachino D, Robbiano A, et al. Differential regulation of interleukin 12 and interleukin 23 production in human dendritic cells. J Exp Med. 2008;205:1447–1461. doi: 10.1084/jem.20071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS, et al. STAT3 and NF-κB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J Immunol. 2006;176:5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 48.Kattah MG, Wong MT, Yocum MD, Utz PJ. Cytokines secreted in response to Toll-like receptor ligand stimulation modulate differentiation of human Th17 cells. Arthritis Rheum. 2008;58:1619–1629. doi: 10.1002/art.23497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 50.Cummings RD, Nyame AK. Schistosome glysoconjugates. Bio-chim Biophys Acta 1455. 1999:363–374. doi: 10.1016/s0925-4439(99)00063-0. [DOI] [PubMed] [Google Scholar]
- 51.Hokke CH, Yazdanbakhsh M. Schistosome glycans and innate immunity. Parasite Immunol. 2005;27:257–264. doi: 10.1111/j.1365-3024.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- 52.Thomas PG, Carter MR, Atochina O, Da’Dara AA, Piskorska D, McGuire E, Harn DA. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J Immunol. 2003;171:5837–5841. doi: 10.4049/jimmunol.171.11.5837. [DOI] [PubMed] [Google Scholar]
- 53.van Die I, van Vliet SJ, Nyame AK, Cummings RD, Bank CM, Appelmelk B, Geijtenbeek TB, van Kooyk Y. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13:471–478. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- 54.Meyer S, van Liempt E, Imberty A, van Kooyk Y, Geyer H, Geyer R, van Die I. DC-SIGN mediates binding of dendritic cells to authentic pseudo-LewisY glycolipids of Schistosoma mansoni cercariae, the first parasite-specific ligand of DC-SIGN. J Biol Chem. 2005;280:37349–37359. doi: 10.1074/jbc.M507100200. [DOI] [PubMed] [Google Scholar]
- 55.van den Berg TK, Honing H, Franke N, van Remoortere A, Schiphorst WE, Liu FT, Deelder AM, Cummings RD, Hokke CH, van Die I. LacdiNAc-glycans constitute a parasite pattern for galectin-3-mediated immune recognition. J Immunol. 2004;173:1902–1907. doi: 10.4049/jimmunol.173.3.1902. [DOI] [PubMed] [Google Scholar]
- 56.van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt-Jones EA, Espevik T, de Jong EC, Kapsenberg ML, Golenbock DT, et al. A novel host-parasite lipid cross-talk: schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–48129. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 57.Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7:1258–1265. doi: 10.1038/ni1417. [DOI] [PubMed] [Google Scholar]
- 58.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 59.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 60.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 61.Shainheit MG, Saraceno R, Bazzone LE, Rutitzky LI, Stadecker MJ. Disruption of interleukin-27 signaling results in impaired γ interferon production but does not significantly affect immunopathology in murine schistosome infection. Infect Immun. 2007;75:3169–3177. doi: 10.1128/IAI.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 63.Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]


