Abstract
Agonist replacement therapies are effective for managing substance abuse disorders including nicotine and opioid dependence. The results of preclinical laboratory studies and clinical trials indicate that agonist replacements like d-amphetamine may be a viable option for managing cocaine dependence. This experiment determined the physiological and behavioral effects of cocaine during d-amphetamine maintenance in seven cocaine-dependent participants. We predicted cocaine would be well tolerated during d-amphetamine maintenance. We also predicted d-amphetamine would attenuate the behavioral effects of cocaine. After 3–5 days of d-amphetamine maintenance (0, 15, and 30 mg/day), volunteers were administered ascending doses of cocaine (4, 30, 60 mg, IN) within a single session. Cocaine doses were separated by 90 minutes. Cocaine produced prototypical physiological (e.g., increased heart rate, blood pressure, and body temperature) and subject-rated (e.g., increased ratings of Good Effects) effects. During maintenance on the highest d-amphetamine dose, the heart-rate increasing effects of cocaine were larger than observed during placebo maintenance. These effects were not clinically significant and no unexpected or serious adverse events were observed. d-Amphetamine attenuated some of the subject-rated effects of cocaine. These results are concordant with those of previous preclinical studies, human laboratory experiments and clinical trials, further suggesting that agonist replacement therapy may be a viable strategy for managing cocaine abuse. Additional research in humans is needed to determine whether d-amphetamine attenuates the effects of cocaine under different experimental conditions (e.g., higher cocaine doses) and behavioral arrangements (e.g., drug self-administration or discrimination).
Keywords: Humans, Pharmacotherapy, Agonist Replacement Therapy, d-Amphetamine, Cocaine, Drug Reinforcement, Subjective Effects, Physiological Effects
1. Introduction
Cocaine abuse continues to be a public health concern. Data from the National Survey on Drug Use and Health (NSDUH), for example, indicate that the number of Americans that had used cocaine in the past month has remained relatively stable since 1994 (Substance Abuse and Mental Health Services Administration, [SAMHSA], 2008a). Cocaine is still the most frequently mentioned drug in emergency-room admissions (SAMHSA, Drug Abuse Warning Network [DAWN], 2008b). In addition, the percentage of 12th, 10th and 8th graders that had used cocaine in the past twelve months has remained stable since 2001 (Johnston et al., 2007). Data from the Treatment Episode Data Set (TEDS) indicate that the percentage of treatment admissions for cocaine dependence remained stable between 1995 and 2005 (SAMHSA, 2008c).
Because of persistent public-health concerns, identifying an effective pharmacotherapy for the treatment of cocaine dependence has been a priority with the National Institute on Drug Abuse (N.I.D.A.) for nearly three decades (Schuster and Snyder, 1990). Despite intense research efforts, an effective pharmacotherapy for cocaine dependence has not yet been identified (e.g., de Lima et al., 2002; Vocci and Elkashef, 2005).
Agonist replacement therapies are effective for nicotine and opioid dependence (e.g., Henningfield, 1995; Ling et al., 1994; Silagy et al., 2004; Sofuoglu and Kosten, 2004). The results of recent clinical trials suggest that agonist therapies (e.g., d-amphetamine) may also be effective for cocaine dependence (for a review, see Castells et al., 2007). The premise of this pharmacological approach is that treating patients with an agonist presumably will suppress withdrawal and produce tolerance to the abuse-related (i.e., reinforcing or positive subjective) effects of cocaine, thereby leading to the extinction of drug-taking and drug-seeking behavior (Gorelick et al., 2004). In a seminal clinical trial, cocaine dependent patients (N = 128) were randomly assigned to receive d-amphetamine (15 or 30 mg/day; N = 26 and 28, respectively) or placebo (N = 40) for 25 weeks (Grabowski et al., 2001). During the fifth week, the d-amphetamine dose was doubled. Patients maintained on higher d-amphetamine doses used significantly less cocaine during the trial than patients maintained on either the low d-amphetamine doses or placebo as determined by benzoylecgonine-free urines. These investigators as well as others have replicated these results (Grabowski et al., 2004a, 2004b; Shearer et al., 2003).
Consistent with these clinical findings, chronically treating animals with d-amphetamine attenuates the behavioral effects of cocaine (Negus, 2003; Negus and Mello, 2003a, 2003b; Peltier et al., 1996). In one study, for example, the effects of d-amphetamine maintenance therapy on cocaine self-administration were assessed in rhesus monkeys using a progressive-ratio schedule (Negus and Mello, 2003a). As expected, cocaine dose dependently increased break points when the monkeys were maintained on saline. d-Amphetamine dose dependently decreased responding for cocaine. Worth noting is that the highest dose of d-amphetamine tested, 0.1 mg/kg/hour intravenously for 10 days (i.e., approximately 168 mg/day in a 70 kg human), almost completely eliminated responding for cocaine. In another study, rats were trained to discriminate cocaine (10 mg/kg) from saline (Peltier et al., 1996). Cocaine (1–17.8 mg/kg) dose-dependently increased drug-appropriate responding. Chronic administration of subcutaneous d-amphetamine (0.32–3.2 mg/kg/12 hours) for seven days (i.e., approximately 45–90 mg/day in a 70 kg human) resulted in a significant rightward shift in the cocaine dose-response curve (i.e., attenuation of the discriminative-stimulus effects of cocaine).
We are unaware of any published reports in which the physiological and behavioral effects of cocaine were assessed under controlled laboratory conditions in humans maintained on d-amphetamine. A recent translational review of medication development for opioid and stimulant dependence noted that human laboratory studies testing the effects of sustained-release d-amphetamine on responses to cocaine were needed (Haney and Spealman, 2008). The purpose of the present experiment was to assess the physiological and behavioral effects of acute administrations of intranasal cocaine (4, 30 and 60 mg) in participants maintained on varying doses of sustained-release d-amphetamine (0, 15 and 30 mg/day for 3–5 days). The behavioral effects of cocaine were assessed using the Multiple-Choice Procedure, a putative measure of drug reinforcement, as well as a battery of subject-rated drug-effect questionnaires. We hypothesized that these cocaine doses would be well tolerated during d-amphetamine maintenance. We also hypothesized that d-amphetamine maintenance would attenuate the behavioral effects of cocaine.
2. Method
2.1. Subjects
Seven non-treatment seeking adult volunteers with recent histories of cocaine use (i.e., cocaine positive urine at the time of initial screening) and meeting criteria for cocaine dependence as determined by a computerized version of the Structured Clinical Interview for the DSM-IV completed this within-subjects, placebo-controlled study. One additional subject was enrolled but left the protocol for reasons unrelated to study procedures. Data from that individual were not included in the analyses. The Institutional Review Board of the University of Kentucky Medical Center approved this study, and volunteers gave their written informed consent prior to participating. Volunteers were paid for their participation. Previous human laboratory studies that determined the safety, tolerability and behavioral effects of cocaine during maintenance on a putative agonist replacement therapy enrolled a similar number of volunteers (Collins et al., 2006; Winhusen et al., 2006).
Prior to participation, all potential volunteers underwent a comprehensive physical- and mental-health screening. The screening measures that were used included a medical-history questionnaire, a general-health questionnaire, a mini-mental status examination, a drug-use questionnaire, an over-the-counter drug-use questionnaire, the Drug Abuse Screening Test (DAST) (Skinner, 1982), and the Michigan Alcohol Screening Test (MAST) (Selzer, 1971). The maximum score on the DAST is 28. Scores of at least 5 suggest problematic drug use. The maximum score on the MAST is 53. Scores of at least 6 suggest problematic alcohol use.
A psychiatrist (L.R.H. or his designee) interviewed and examined each potential subject and deemed him or her to be appropriate for the study. Routine clinical laboratory blood chemistry tests, vital signs assessment and an electrocardiogram were also conducted. Potential volunteers with histories of serious physical disease or current physical disease, impaired cardiovascular functioning, chronic obstructive pulmonary disease, seizure, head trauma or CNS tumors, or current or past histories of serious psychiatric disorder (i.e., Axis I, DSM IV), other than substance abuse or dependence, were excluded from participation. Volunteers had to meet the following inclusion criteria: (1) self-reported recent cocaine use, (2) confirmation of recent cocaine use by a positive urine screen for cocaine or benzoylecgonine during the initial screening interview, and (3) fulfill diagnostic criteria for cocaine dependence on a computerized version of the SCID. All volunteers were in good health with no contraindications to stimulants.
Volunteers ranged in age from 33 to 49 years (mean: 42 years) and in weight from 63 to 93 kg (mean: 74 kg). Five of these volunteers were male and two were females. Four volunteers reported their race as African American, one reported his race as Caucasian, and two reported being of mixed race. Volunteers scored between 5 and 8 (mean: 6) on the DAST. All volunteers were current crack cocaine users, reported using cocaine 5–21 days (mean: 13) in the month prior to screening, and tested positive for cocaine at admission. Six volunteers reported using marijuana 1–31 days in the month prior to screening (mean: 8.5), but none met diagnostic criteria for cannabis dependence.
Six volunteers reported consuming alcohol (range of 0.25–30 beverages per week; mean: 8), and scored between 0 and 8 (mean: 3) on the MAST. In addition, volunteers reported that they consumed between 0 and 408 mg caffeine/day (mean: 66 mg) and 5 of the 7 volunteers reported smoking tobacco cigarettes daily (range: 7–10 cigarettes per day; mean: 9 cigarettes). One subject reported using a benzodiazepine once in the month prior to screening, while another reported using an opioid once in the month prior to screening
2.2. General Procedures
Volunteers were enrolled as inpatients at the University of Kentucky Chandler Medical Center General Clinical Research Center (GCRC) for up to 25 days and participated in one practice and up to four experimental sessions. Volunteers were informed that during their participation they would receive various drugs, administered orally or intranasally, that could include placebo, d-amphetamine or cocaine. Volunteers were instructed that these medications could be administered in combination. Other than receiving this general information, volunteers were blind to the type of drug administered. Volunteers were told that the purpose of the study was to determine how different drugs affect physiology, mood, and behavior. Other than this general explanation of purpose, volunteers were given no instruction of what they were “supposed” to do or of what outcomes might be expected.
On the day of admission to the GCRC, volunteers provided a urine sample that had to be negative for all drugs other than cocaine or THC. Volunteers were then allowed to acclimate to the GCRC for one day, during which they were observed for signs of drug or alcohol withdrawal. No volunteer showed signs of withdrawal.
2.2.1. Practice Session
Following this acclimation period, volunteers completed one practice session to familiarize them with experimental measures. Experimental medications were not administered during this session.
2.2.2. Drug Maintenance Days
Drug maintenance began on the day immediately following the practice session and continued throughout the protocol. Placebo or sustained release d-amphetamine was administered orally at 0600, 1400, and 2200 h. After three to five days of maintenance on placebo, volunteers completed the first experimental session as described below and began maintenance on the subsequent condition the following day. Every three to five days thereafter, volunteers completed an experimental session and then proceeded to the next maintenance condition until study completion. The order of drug maintenance conditions was constant across volunteers: placebo (lead in), 5 mg sustained release d-amphetamine, 10 mg sustained release d-amphetamine, and placebo (washout) administered three times per day. The total daily dose for each condition was 0, 15, 30 and 0 mg sustained release d-amphetamine. The three to five day maintenance window was used to avoid conducting experimental sessions on weekends, when medical coverage at the GCRC was limited.
2.2.3. Experimental Sessions
Volunteers received the appropriate maintenance dose at 0600 h on the morning of all experimental sessions. Experimental sessions started at 0800 h and lasted 6.5 h. Three intranasal cocaine doses were given each session in ascending order 1.5 h apart (4 [placebo], 30, and 60 mg). Volunteers completed all experimental measures prior to drug administration and every 15 min for 1 h after each cocaine dose. In addition, 1 h after each dose administration, volunteers completed the Multiple-Choice Questionnaire (described below). Immediately after completing the Multiple-Choice form for the 60 mg cocaine dose, the lottery fulfillment procedure for the Multiple Choice Procedure was completed and the condition selected by the subject (i.e., cocaine dose or money) was presented. Experimental measures were completed every 15 minutes for 1 h following fulfillment of the Multiple Choice Procedure regardless of what condition was selected.
Urine and expired breath samples were collected prior to each session to confirm drug and alcohol abstinence, respectively. Volunteers occasionally tested positive for cocaine and amphetamine, which was likely attributable to the administration of the experimental medications. Volunteers tested negative for all other drug and alcohol use. Females received urine pregnancy tests daily, which had to be negative for participation to continue.
2.2.4 Testing Room
The testing room for all sessions consisted of a table and chair for the research assistant and nurse, a hospital bed for the subject, an Apple iBook laptop computer (Apple Computer Inc., Cupertino, CA), and an automated ECG and blood pressure monitor (Dinamap Pro 1000 Vital Signs monitor, Critikon Company L.L.C., Tampa, FL). A crash cart was available in case of a medical emergency.
2.3. Physiological Measures
Heart rate, blood pressure, and oral temperature were recorded immediately prior to each cocaine dose administration and at 15 min intervals thereafter for 1 h. Cardiac rhythmicity was recorded throughout experimental sessions. If heart rate exceeded 130 beats per minute, systolic blood pressure exceeded 180 mmHg, diastolic blood pressure exceeded 120 mmHg or clinically significant ECG changes occurred following administration of cocaine at any point during the experiment, participation was terminated. No subject was excluded from participation for exceeding these parameters.
2.4 Subject-Rated Measures
Subject-rated questionnaires were administered on a microcomputer or using paper-and-pencil forms in a fixed order. Volunteers completed all experimental measures prior to the initial cocaine administration, immediately after cocaine administration, and every 15 min for 1 h after each cocaine dose.
2.4.1. Drug-Effect Questionnaire
The Drug-Effect Questionnaire consisted of 20 items, and has been shown to be sensitive to the acute effects of stimulants (Rush et al., 2003). Twenty items are presented on the video screen, one at a time. Volunteers rated each item using the computer mouse to point to and select among one of the five response options: Not at All, A Little Bit, Moderately, Quite a Bit, and Extremely (scored numerically from 0 to 4, respectively).
2.4.2. Stimulant-Sensitive Adjective-Rating Scale
The Stimulant-Sensitive Adjective-Rating Scale consists of 21 items, and has been shown to be sensitive to the acute effects of stimulants (Di Marino et al., 1998). Volunteers rated each item using a 5-point scale identical to the one described above. Responses to individual items are summed to create a composite score, with a maximum total score of 84.
2.4.3. Adjective-Rating Scale
This scale consists of 32 items that load into two subscales: sedative and stimulant (Oliveto et al., 1992). Volunteers rated each adjective with a 5-point scale similar to the one described above. The maximum total for each subscale is 64.
2.4.4. Visual Analog Scale
Ten items from the Drug-Effect Questionnaire were completed using visual-analog scales. Volunteers rated each item by placing a mark along a 100 mm line that is anchored with “Not at All” on the left side and “Extremely” on the right side. The items rated were Active, Alert, Energetic; Euphoric; Talkative or Friendly; Good Effects; High; Rush; Like Drug; Stimulated; Willing to Take Again; and Willing to Pay For.
2.4.5 Cocaine Craving Questionnaire
Volunteers completed a modified version of the cocaine-craving questionnaire (Dudish-Poulsen and Hatsukami, 1997). Briefly, volunteers rated how much they Wanted, Needed and Craved cocaine using a 5-point rating scale identical to that described above. Responses to these three questions were summed to create a composite craving score.
2.4.6. UKU Side Effects Scale
GCRC nursing staff also completed the Udvalg for Kliniske UndersØgelser (UKU) Side Effects Rating Scale daily (Lingjaerde et al., 1987). Although not analyzed statistically, side effects reported on these scales monitored regularly by unit physicians did not lead to the discontinuation of any subject.
2.5. Multiple-Choice Procedure
The Multiple-Choice Procedure provides a contingency-based assessment of the monetary value of each dose condition (Griffiths et al., 1993; Griffiths et al., 1996). In this procedure, volunteers make a series of discrete choices between each intranasal drug dose they sampled and ascending amounts of money. For each dose, there were eight choices, resulting in a total of 24 choices per experimental session. Volunteers selected between a chance to receive the dose that they had sampled 1 h previously (labeled as Drug 1 [4 mg cocaine], Drug 2 [30 mg cocaine], or Drug 3 [60 mg cocaine]) from that day or a monetary value that would then be applied to a purchase at a local grocery store. The monetary values were: US $0.10, 0.25, 0.50, 1.00, 2.00, 4.00, 8.00, and 16.00.
After receiving the final scheduled dose of intranasal cocaine (60 mg), volunteers randomly selected one of their previous choices during that session in a lottery. This choice (drug or money) was then presented to the subject. Regardless of the outcome, volunteers completed experimental measures every 15 min for 1 h. This version of the Multiple-Choice Procedure is different from that used previously because choices made were reinforced during each session, as opposed to at the end of the entire experiment. Data from the Multiple-Choice Procedure were analyzed as “crossover point” (i.e., the maximum dollar value at which volunteers chose drug over money).
2.6. Drug Administration
All drugs were administered in a double-blind fashion. Sustained release d-amphetamine (5 and 10 mg t.i.d.; GlaxoSmithKline, Research Triangle, NC) doses were prepared by over-encapsulating commercially available drug in a size-0 capsule. Cornstarch was then used to fill the remainder of the capsule. Placebo capsules contained only cornstarch.
Cocaine doses (4 mg [placebo], 30, and 60 mg) were prepared by combining the appropriate amount of cocaine HCl (Mallinckrodt, St. Louis, MO) with lactose to equal a total of 80 mg powder. An active placebo (i.e., 4 mg cocaine) was used in an attempt to increase volunteer “blindness”. Cocaine HCL (4 mg) produces nasal numbing, but no discernible blood levels and is routinely used as the placebo dose in human laboratory studies involving intranasal drug administration (e.g., Foltin and Fischman, 1988; Higgins et al., 1990, 1993; Javaid et al., 1978).
During each administration, a nurse presented the subject with the powder, a mirror and a standard razor blade. The subject was instructed to divide the powder into two even “lines” and insufflate one line of powder through each nostril using a 65-mm plastic straw within 2 minutes.
References below to cocaine alone pertain to those instances in which an active dose, 30 or 60 mg, was administered during maintenance on 0 mg d-amphetamine. References to placebo pertain to sessions in which 4 mg cocaine (i.e., placebo) was administered during maintenance on 0 mg d-amphetamine.
2.7. Data Analysis
Two volunteers completed only 3 experimental sessions (i.e., after maintenance on 0 [lead in], 15 and 30 mg d-amphetamine per day). The remaining volunteers completed all experimental sessions. Data from experimental sessions following 0 (lead in) and 0 (wash out) mg d-amphetamine maintenance for these 5 volunteers were compared to determine differences in the cocaine dose-response curves as a result of d-amphetamine maintenance (e.g. sensitization or tolerance). Because there were no systematic differences between the phases, data for these 5 volunteers from the two 0 mg d-amphetamine maintenance conditions were averaged for subsequent analyses.
Data from the experimental sessions were analyzed as peak effect (i.e., the maximum score observed following each cocaine administration) using a two factor repeated-measures ANOVA with d-Amphetamine maintenance condition (0, 15, and 30 mg) and Cocaine dose (4 [placebo], 30 and 60 mg) as the factors (StatView, Cary, NC). If a significant effect of cocaine was detected, post-hoc tests were conducted to compare each of the eight active drug conditions with placebo (i.e., 4 mg cocaine during maintenance on 0 mg d-amphetamine). If a dose of cocaine alone differed significantly from placebo, additional post-hoc tests were conducted to compare the effects of these doses of cocaine alone and during maintenance on d-amphetamine. Effects were considered significant for p ≤ 0.05.
3. Results
3.1. Physiological Measures
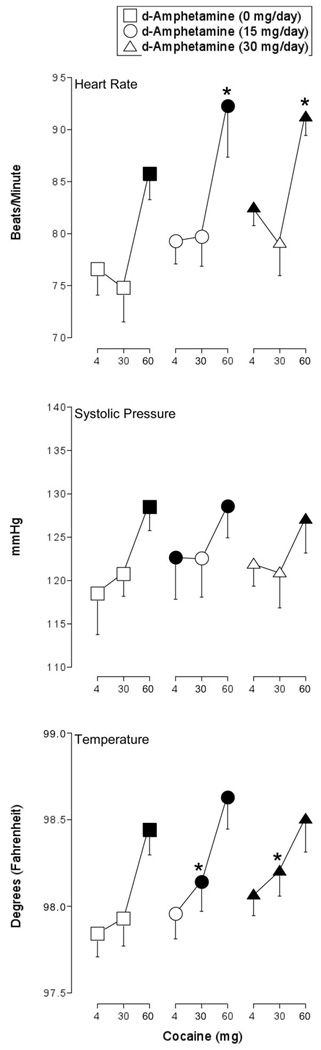
ANOVA revealed a significant effect of cocaine on heart rate, systolic pressure, and body temperature (F2,12 values > 6.3, p < 0.02). Post-hoc comparisons revealed that 60 mg cocaine alone increased heart rate, systolic pressure, and body temperature significantly above placebo levels (Figure 1). Maintenance on 15-mg/day d-amphetamine increased baseline systolic pressure (i.e., following the administration of 4 mg intranasal cocaine), while maintenance on 30-mg/day d-amphetamine increased baseline heart rate and temperature. The heart rate increasing effects of 60 mg cocaine were significantly greater during maintenance on both active doses of d-amphetamine relative to this dose of cocaine alone. d-Amphetamine maintenance did not significantly alter the systolic pressure increasing effects of cocaine. d-Amphetamine maintenance did not significantly alter the temperature-increasing effects of 60 mg cocaine. Worth noting is that while 30 mg cocaine alone failed to significantly increase body temperature during maintenance on 0 mg d-amphetamine, it did so during maintenance on both doses of d-amphetamine.
Figure 1.
Peak effect dose-response functions for cocaine during maintenance on placebo (squares), 15 mg/day d-amphetamine (circles), and 30 mg/day d-amphetamine (triangles) for heart rate, systolic blood pressure, and body temperature. X-axis: Intranasal cocaine dose. Data points represent means for seven volunteers. Filled symbols indicate the data point differs significantly from the placebo condition (i.e., 4 mg cocaine during maintenance on 0 mg d-amphetamine. An asterisk indicates that the data point is significantly different from the corresponding dose of cocaine during maintenance on 0 mg d-amphetamine). Unidirectional error bars (S.E.M.) are shown for clarity.
3.2. Drug-Effect Questionnaire
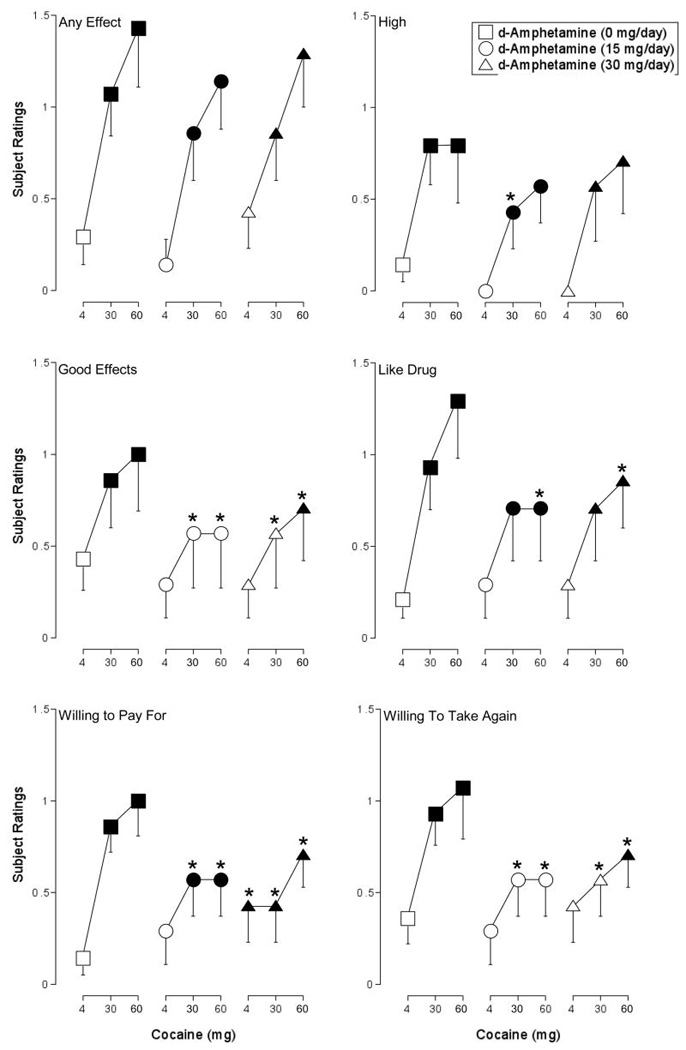
ANOVA revealed a significant effect of cocaine on six items on the Drug-Effect Questionnaire: Any Effect; Good Effects; High; Like Drug; Willing to Pay For; and Willing to Take Again (F2,12 values > 5.5, p < 0.05). For each of these items, both doses of cocaine alone increased ratings significantly above levels observed with placebo (Figure 2). Maintenance on 30-mg/day d-amphetamine increased baseline ratings of Willing to Pay For (i.e., following the administration of 4 mg intranasal cocaine). Subject ratings of Good Effects, Willing to Pay For, and Willing to Take Again following the administration of 30 and 60 mg cocaine were significantly lower during maintenance on both doses of d-amphetamine relative to when these cocaine doses were tested alone. Subject ratings of Like Drug following the administration of 60 mg cocaine were significantly lower during maintenance on both doses of d-amphetamine relative to when this cocaine dose was tested alone. Subject ratings of High following the administration of 30 mg cocaine were significantly lower during maintenance on 15 mg/day d-amphetamine relative to when this cocaine dose was tested alone. The dose-dependent effects of cocaine on ratings of Any Effect were not altered to a significant degree by either d-amphetamine maintenance condition.
Figure 2.
Peak effect dose-response functions for cocaine during maintenance on placebo (squares), 15 mg/day d-amphetamine (circles), and 30 mg/day d-amphetamine (triangles) for subject ratings of Any Effect, High, Good Effects, Like Drug, Willing to Pay For, and Willing to Take Again from the Drug-Effect Questionnaire. X-axis: Intranasal cocaine dose. The range of possible scores on these items is 0–4. Data points represent means for seven volunteers. Filled symbols indicate the data point differs significantly from the placebo condition (i.e., 4 mg cocaine during maintenance on 0 mg d-amphetamine). An asterisk indicates that the data point is significantly different from the corresponding dose of cocaine during maintenance on 0 mg d-amphetamine. Unidirectional error bars (S.E.M.) are shown for clarity.
3.3. Stimulant-Sensitive Adjective-Rating Scale
ANOVA revealed a significant effect of cocaine (F2,12 = 5.6, p < 0.02) on the Stimulant-Sensitive Adjective-Rating Scale. Both doses of cocaine alone increased ratings significantly above placebo levels (Table 1). Subject ratings following the administration of 30 and 60 mg cocaine were significantly lower during maintenance on both doses of d-amphetamine relative to when these cocaine doses were tested alone.
Table 1.
Summary of peak means
| Measure | D-Amphetamine [0 mg/day] |
D-Amphetamine [15 mg/day] |
D-Amphetamine [30 mg/day] |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cocaine [4 mg] | Cocaine [30 mg] | Cocaine [60 mg] | Cocaine [4 mg] | Cocaine [30 mg] | Cocaine [60 mg] | Cocaine [4 mg] | Cocaine [30 mg] | Cocaine [60 mg] | |
| Physiological measures | |||||||||
| Heart rate | 76.6 | 74.8 | 85.8 | 79.3 | 79.7 | 92.3 | 82.6 | 79.1 | 91.3 |
| Systoic pressure | 118.5 | 120.8 | 128.5 | 122.7 | 122.6 | 128.5 | 122.0 | 121.0 | 127.1 |
| Diastolic pressure | 74.3 | 75.4 | 75.5 | 73.4 | 76.1 | 77.0 | 75.6 | 78.3 | 75.5 |
| Temperature | 97.8 | 97.9 | 98.4 | 98.0 | 98.1 | 98.5 | 98.1 | 98.2 | 98.5 |
| Adjective-rating scale | |||||||||
| Sedative scale | 2.1 | 1.4 | 1.8 | 2.6 | 2.1 | 2.3 | 2.0 | 3.0 | 1.9 |
| Stimulant scale | 5.8 | 7.2 | 7.8 | 5.4 | 6.6 | 5.7 | 6.3 | 8.0 | 8.3 |
| Cocaine-craving questionnaire | |||||||||
| Score | 0.0 | 0.3 | 0.4 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 |
| Drug-effect questionnaire | |||||||||
| Active, Alert, Energetic | 0.3 | 0.4 | 0.4 | 0.1 | 0.3 | 0.4 | 0.3 | 0.4 | 0.4 |
| Any Effect | 0.3 | 1.1 | 1.4 | 0.1 | 0.9 | 1.1 | 0.4 | 0.9 | 1.3 |
| Bad Effects | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.3 | 0.1 | 0.0 |
| Euphoric | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Good Effects | 0.4 | 0.9 | 1.0 | 0.3 | 0.6 | 0.6 | 0.3 | 0.6 | 0.7 |
| High | 0.1 | 0.8 | 0.8 | 0.0 | 0.4 | 0.6 | 0.0 | 0.6 | 0.7 |
| Irregular of Racing Heart | 0.1 | 0.2 | 0.3 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.3 |
| Like Drug | 0.2 | 0.9 | 1.3 | 0.3 | 0.7 | 0.7 | 0.3 | 0.7 | 0.9 |
| Nauseated | 0.1 | 0.3 | 0.3 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 |
| Nervous, Anxious | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Performance Impaired | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 |
| Performance Improved | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Restless | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.3 | 0.3 |
| Rush | 0.1 | 0.4 | 0.4 | 0.0 | 0.1 | 0.4 | 0.0 | 0.3 | 0.3 |
| Snaky, Jittery | 0.0 | 0.1 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Sluggish, Fatigued, Lazy | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.1 |
| Stimulated | 0.1 | 0.4 | 0.4 | 0.0 | 0.4 | 0.6 | 0.0 | 0.3 | 0.4 |
| Talkactive, Friendly | 0.4 | 0.5 | 0.6 | 0.6 | 0.7 | 0.6 | 0.4 | 0.7 | 0.9 |
| Willing to Pay For | 0.1 | 0.9 | 1.0 | 0.3 | 0.6 | 0.6 | 0.4 | 0.4 | 0.7 |
| Willing to Take Again | 0.4 | 0.9 | 1.1 | 0.3 | 0.8 | 0.6 | 0.4 | 0.6 | 0.7 |
| Multiple-choice procedure | |||||||||
| Crossover point | 0.3 | 2.0 | 2.0 | 0.1 | 0.7 | 1.2 | 0.1 | 1.4 | 1.3 |
| Stimulant-sensitive adjectives | |||||||||
| Score | 0.9 | 3.4 | 4.7 | 1.3 | 2.1 | 3.0 | 1.1 | 1.9 | 3.3 |
| Visual-analog scales | |||||||||
| Active, Alert, Energetic | 15.4 | 17.4 | 19.0 | 14.3 | 15.3 | 18.4 | 14.7 | 20.9 | 21.9 |
| Euphoric | 6.5 | 0.4 | 0.4 | 0.1 | 0.3 | 0.6 | 0.5 | 0.9 | 0.9 |
| Good Effects | 1.1 | 17.5 | 25.9 | 7.1 | 12.6 | 11.7 | 7.7 | 14.9 | 15.3 |
| High | 1.2 | 18.0 | 13.7 | 0.0 | 11.7 | 13.7 | 0.7 | 15.0 | 12.6 |
| Like Drug | 0.9 | 17.9 | 19.6 | 6.4 | 10.9 | 13.0 | 7.4 | 15.7 | 14.3 |
| Rush | 0.2 | 7.9 | 11.1 | 0.0 | 4.0 | 7.6 | 0.7 | 0.9 | 5.1 |
| Stimulated | 0.0 | 6.8 | 6.9 | 0.1 | 5.7 | 9.6 | 0.9 | 2.4 | 4.4 |
| Talkactive, Friendly | 9.3 | 12.4 | 20.4 | 6.9 | 9.9 | 10.3 | 9.7 | 10.1 | 12.9 |
| Willing to Pay For | 0.6 | 16.8 | 21.5 | 6.9 | 14.7 | 16.4 | 8.6 | 10.6 | 15.7 |
| Willing to Take Again | 7.6 | 18.2 | 24.1 | 6.7 | 16.5 | 16.0 | 8.3 | 16.9 | 15.0 |
3.4. Adjective-Rating Scale
There were no significant effects on the Adjective-Rating Scale (Table 1).
3.5. Visual Analog Scale
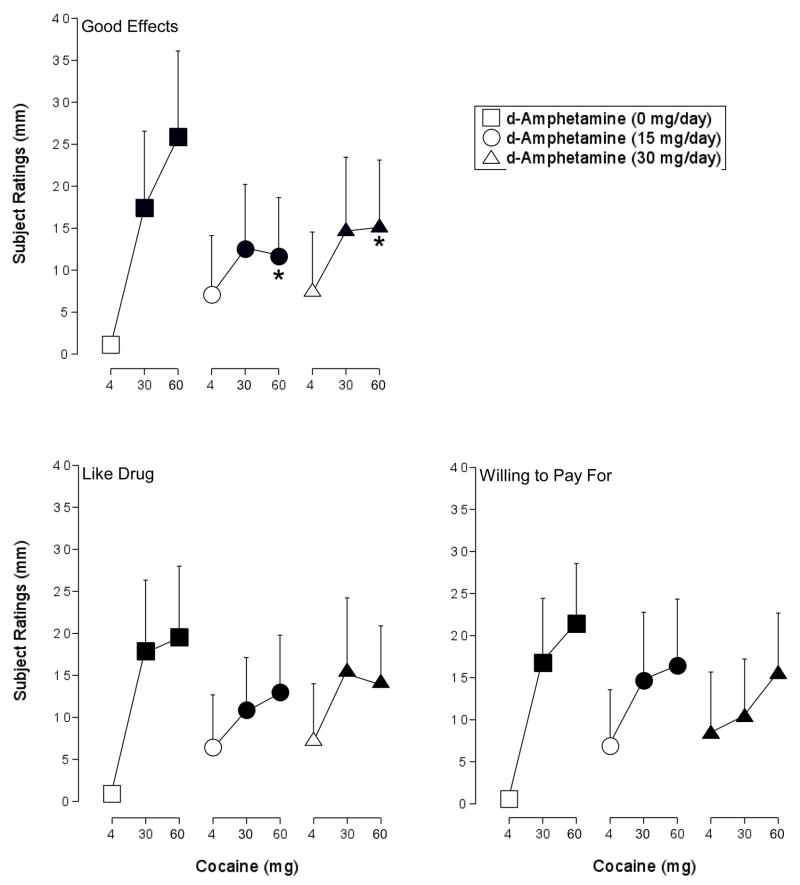
ANOVA revealed a significant effect of cocaine on six of the items on the Visual Analog Scale: Active, Alert, Energetic; Good Effects; High; Like Drug; Rush; and Willing to Pay For (F2,12 values > 3.9, p < 0.05). Both doses of cocaine alone increased ratings of Good Effects, High, Like Drug, Rush and Willing to Pay For significantly above levels observed with placebo. Maintenance on 30-mg/day d-amphetamine increased baseline ratings of Willing to Pay For (i.e., following the administration of 4 mg intranasal cocaine). Ratings of Good Effects were significantly lower following the administration of 60 mg cocaine when volunteers were maintained on either 15 or 30 mg/day d-amphetamine relative to when this cocaine dose was tested alone. Ratings of Rush were significantly lower following the administration of 30 and 60 mg cocaine when volunteers were maintained on d-amphetamine (30 mg/day) relative to when these cocaine doses were tested alone. The effects of cocaine on ratings of Like Drug, High and Willing to Pay were not altered to a significant degree by either d-amphetamine maintenance condition. Neither dose of cocaine alone increased ratings of Active, Alert, Energetic significantly above placebo levels. Figure 3 shows the cocaine dose-response function during maintenance of 0, 15, and 30 mg d-amphetamine for three measures: Good Effects, Like Drug, and Willing to Pay For.
Figure 3.
Peak effect dose-response functions for cocaine during maintenance on placebo (squares), 15 mg/day d-amphetamine (circles), and 30 mg/day d-amphetamine (triangles) for subject ratings of Good Effects, Like Drug, and Willing to Pay For from the Visual Analog Scales. X-axis: Intranasal cocaine dose. The range of possible scores on these items is 0–100. Data points represent means for seven volunteers. Filled symbols indicate the data point differs significantly from the placebo condition (i.e., 4 mg cocaine during maintenance on 0 mg d-amphetamine). An asterisk indicates that the data point is significantly different from the corresponding dose of cocaine during maintenance on 0 mg d-amphetamine. Unidirectional error bars (S.E.M.) are shown for clarity.
3.6. Cocaine Craving Questionnaire
Neither intranasal cocaine nor d-amphetamine maintenance significantly altered responses on the Cocaine-Craving Questionnaire (Table 1).
3.6. Multiple-Choice Procedure
There were no significant effects on the Multiple-Choice Procedure (Table 1).
4. Discussion
The present study examined the acute physiological and behavioral effects of a range of doses of intranasal cocaine (4 [placebo], 30 and 60 mg) in patients maintained on oral d-amphetamine (0, 15 and 30 mg/day for 3–5 days). Intranasal cocaine increased heart rate, blood pressure, and body temperature as a function of dose, and produced a constellation of positive subject-rated drug effects (e.g., Good Effects). d-Amphetamine maintenance elevated baseline heart rate and body temperature, and produced modest increases in a few subject-rated drug-effect items. During maintenance on the highest d-amphetamine dose, the heart rate and temperature-increasing effects of cocaine were larger than observed during placebo maintenance. d-Amphetamine maintenance attenuated some of the subject-rated effects of cocaine.
The results of the present experiment indicate that acute administration of intranasal cocaine is safe and tolerable during oral d-amphetamine maintenance. Intranasal cocaine increased heart rate, blood pressure and body temperature. While d-amphetamine maintenance accentuated some of the physiological effects of cocaine (i.e., heart rate and body temperature), the enhancement was not clinically significant and no unexpected or serious adverse events were observed. The increased heart rate and body temperature observed with cocaine during d-amphetamine maintenance may be attributable to elevated baseline values. The present findings are concordant with those from previous human laboratory experiments that examined the physiological effects of cocaine during maintenance on putative agonist replacement therapies for cocaine dependence (e.g., Collins et al., 2006; Stoops et al., 2008; Walsh et al., 2000; Winhusen et al., 2006). Given the relative safety and tolerability of the drug combinations, future research should determine the effects of higher cocaine doses in participants maintained on higher doses of d-amphetamine.
Intranasal cocaine produced prototypical subject-rated drug effects (e.g., increased ratings of Good Effects). Worth noting is that the magnitude of the subject-rated effects of intranasal cocaine alone was small, although they were significantly different from placebo. The effects of cocaine alone on the Drug-Effect Questionnaire, for example, were less than 1.5 on a five-point scale ranging from 0–4. The doses of intranasal cocaine tested in the present experiment, 4, 30 and 60 mg, produced maximum effects of approximately 1, 18 and 26 mm, respectively, on a 100 mm visual-analog scale that assessed Good Effects.
The magnitude of the effect of cocaine alone observed in the present experiment was comparable to those observed previously with similar doses administered by various routes (e.g., oral, intranasal, intravenous and smoked) (e.g., Collins et al., 2006; Haney et al., 2005; Lile et al., 2003; Oliveto et al., 1995; Rush et al., 1999; Stoops et al., 2007, 2008; Winhusen et al., 2006). In one report, for example, the effects of intravenous cocaine (0, 16, and 48 mg/70 kg) were assessed in participants with Attention Deficit/Hyperactivity Disorder (ADHD) and histories of cocaine abuse (Collins et al., 2006). These doses produced maximum effects of approximately 21, 29 and 33 mm, respectively, on a 100 mm visual-analog scale that assessed Good Drug Effects. In another study, the effects of smoked cocaine (0, 6, 12, 25 and 50 mg) were assessed in participants with histories of cocaine abuse (Haney et al., 2005). These doses of cocaine produced maximum effects of approximately 1, 7, 15, 30 and 45 mm, respectively, on the 100 mm visual-analog scale that assessed Good Drug Effects. Nevertheless, the present findings should be interpreted cautiously because of the small magnitude effects with the doses of intranasal cocaine tested.
The reasons for the subject-rated effects of cocaine alone were small in magnitude are unknown. One possible explanation is that the cocaine was administered intranasally to participants that reported using drug primarily via inhalation (i.e., smoked crack). Future research should determine whether the effects of intranasal cocaine are more robust in participants that report insufflation as their preferred route of drug administration.
d-Amphetamine maintenance attenuated some of the subject-rated effects of cocaine. The doses of amphetamine tested in the present experiment reduced responses to intranasal cocaine by approximately 30–45 percent. The effects of d-amphetamine maintenance on responses to cocaine are quantitatively similar to those observed previously in preclinical laboratory experiments and initial clinical trials (Grabowski et al., 2004a, 2004b; Peltier et al., 1996). In the earlier study, a preclinical experiment, cocaine (1–17.8 mg/kg) dose-dependently increased drug-appropriate responding (Peltier et al., 1996). The highest dose of cocaine engendered approximately 90% drug-appropriate responding. The highest dose of cocaine engendered approximately 60% drug-appropriate responding following chronic administration of d-amphetamine (0.32 mg/kg/12 hours for seven days, s.c.). This dose of d-amphetamine is equivalent to approximately 45 mg/day in humans. In a clinical trial, cocaine dependent patients were randomly assigned to receive d-amphetamine (15 or 30 mg/day) or placebo for six months (Grabowski et al., 2004a). During the first month of treatment, the proportion of benzoylecognine positive urine screens was approximately 0.6, 0.43 and 0.48 in the groups maintained on 0, 15 and 30 mg/day d-amphetamine, respectively (i.e., 20–28 percent reduction). The d-amphetamine maintenance doses were then doubled (i.e., 0, 30 and 60 mg/day). During the second month of treatment, the proportion of benzoylecognine positive urine screens was approximately 0.56, 0.45 and 0.23 in the groups maintained on 0, 30 and 60 mg/day d-amphetamine, respectively (i.e., 20–59 percent reduction). Future human laboratory experiments should determine if maintenance on d-amphetamine (60 mg/day) might further attenuate the subject-rated effects of intranasal cocaine.
The results of the present experiment are concordant with those from some previous experiments that assessed the subject-rated effects of cocaine in participants maintained on methylphenidate, another putative agonist replacement therapy (Collins et al., 2006; Winhusen et al., 2006). In the first study, participants were maintained on 0, 60 and 90 mg/day methylphenidate (Winhusen et al., 2006), while in the second study the maintenance doses were 40 and 60 mg/day (Collins et al., 2006). Participants received intravenous injections of cocaine (0, 20 and 40 mg [Winhusen et al., 2006]; 0, 16 and 48 mg [Collins et al., 2006]). Cocaine produced prototypical subject-rated effects (e.g., Good Effects) that were generally a function of dose. Methylphenidate maintenance significantly attenuated the effects of intravenous cocaine on these measures. Worth noting is that the reduction in the effects observed with methylphenidate in these previous studies was comparable to that observed with d-amphetamine in the present study when potency differences are considered (i.e., d-amphetamine is twice as potent as methylphenidate). Maintenance on 60 mg/day methylphenidate reduced peak ratings of Good Effects observed following the administration of 40–48 mg intravenous cocaine by approximately 50–57% on a visual-analog scale (Collins et al., 2006; Winhusen et al., 2006). In the present experiment, peak ratings of Good Effects on a visual-analog scale following the administration of 60 mg intranasal cocaine were reduced by approximately 40% during d-amphetamine maintenance (30-mg/day) relative to placebo maintenance.
As described above, volunteers rated a subset of items from the Drug-Effect Questionnaire using 100-mm visual analog scales. Volunteers rated these items using a five-point and 100-mm visual analog scale in an attempt to determine if drug effects might be more robust with a different scale. Rating a subset of items using both a five-point and 100-mm visual analog scale also provided a measure of internal validity. Across these two instruments, there was considerable concordance between items that were affected by the administration of intranasal cocaine (i.e., significant effects of cocaine were observed on ratings of Good Effects, High, Like Drug, and Willing to Pay For with both the five-point and 100-mm visual analog scale). However, the concordance between these two instruments was not absolute. Cocaine increased ratings of Any Effect and Willing to Take Again on the five-point Drug-Effect Questionnaire, but not on the Visual-Analog Scale. By contrast, cocaine increased ratings of Active, Alert, Energetic and Rush on the Visual-Analog Scale, but not the five-point Drug-Effect Questionnaire. Similarly, d-amphetamine maintenance attenuated cocaine-induced increases in ratings of Like Drug and Willing to Pay For on the five-point Drug-Effect Questionnaire, but not on the Visual-Analog Scale. The results must, therefore, be viewed cautiously because of uncertainty regarding the internal validity of the present experiment.
As noted above, despite intense research efforts, an effective pharmacotherapy for cocaine dependence has not yet been identified (e.g., de Lima et al., 2002; Vocci and Elkashef, 2005). The development of an effective pharmacotherapy for cocaine dependence has been limited, in part, because of uncertainty regarding the subject-rated drug-effect items that might best predict eventual clinical efficacy. In the present study the effects of intranasal cocaine alone and during d-amphetamine on ratings of Good Effects were similar on both the five-point Drug-Effect Questionnaire and 100-mm visual analog scale, suggesting good internal validity for this particular item. Moreover, as described above, the results of previous studies that maintenance on methylphenidate attenuated cocaine-induced increases in subject ratings of Good Effects (Collins et al., 2006; Winhusen et al., 2006). The attenuation of cocaine-induced increases in ratings Good Effects observed with d-amphetamine and methylphenidate is also consistent with the results of clinical trials that suggest agonist therapies reduce cocaine use in clinical trials (Grabowski et al., 2004a, 2004b). Thus, the results of human laboratory studies and clinical trials suggest this subject ratings of Good Effects may be especially useful for determining the initial efficacy of putative agonist replacement therapies for cocaine dependence.
Cocaine failed to function as a reinforcer on the Multiple-Choice Procedure, a putative measure of drug reinforcement. The most parsimonious explanation for this observation is that insufficient doses of intranasal cocaine were tested. Whether higher doses of intranasal cocaine might function as a reinforcer on the Multiple-Choice Procedure is unknown. Worth noting is that in a previous study in our laboratory, oral cocaine (0, 50, 100, 200 and 300 mg) failed to function as a reinforcer using a Multiple-Choice Procedure similar to the one used in the present experiment (Rush et al., 1999). The average “cross-over point” following the administration of placebo, 50, 100, 200 and 300 mg oral cocaine was $0.11, $0.33, $0.22, $2.11 and $2.33, respectively. Peak plasma levels following the administration of oral cocaine are at least as great as those observed following the same dose administered intranasally (Van Dyke et al., 1978). The doses of cocaine tested in our previous study were, therefore, functionally much higher than those tested in the present experiment. While the Multiple-Choice Procedure is an efficient method for assessing drug reinforcement (Griffiths et al., 1993, 1996), additional studies are needed to define the parameters under which it is useful for determining the initial efficacy of putative pharmacotherapies for cocaine dependence.
At least four caveats of the present experiment warrant discussion. First, cocaine plasma concentrations were not assayed during maintenance on placebo or d-amphetamine. Whether d-amphetamine maintenance reduced the bioavailability of cocaine is unknown. Worth noting, however, is that the cardiovascular effects of cocaine were greater during d-amphetamine maintenance relative to placebo. Similarly, the dose-related effects of cocaine on subject ratings of Any Effect, perhaps a non-specific measure of interoceptive drug effects, were not altered by d-amphetamine maintenance. These observations suggest that d-amphetamine did not significantly alter the bioavailability of cocaine. Regardless, future studies that examine the effects of d-amphetamine maintenance on the behavioral effects of cocaine should assay drug plasma concentrations. Second, a somewhat limited range of doses of intranasal cocaine was tested in the present experiment. Future studies should determine if d-amphetamine maintenance attenuates the behavioral effects of higher cocaine doses administered by different routes. Third, the doses of d-amphetamine and cocaine were tested in ascending order for safety purposes. As noted above, there were no systematic differences between the cocaine dose-response functions during a lead-in (i.e., 0 mg d-amphetamine) and wash out (0 mg d-amphetamine) phase. This observation suggests that d-amphetamine maintenance produced neither sensitization nor tolerance to the physiological and behavioral effects of cocaine. Nevertheless, future studies should administer both d-amphetamine and cocaine in a randomized fashion. Finally, the attenuation of the behavioral effects of cocaine by d-amphetamine was not an orderly function of dose. Maintenance on the low dose d-amphetamine, 15-mg/day, significantly attenuated some of the subject-rated effects of cocaine. Increasing the maintenance dose of d-amphetamine to 30 mg/day did not result in additional attenuation of the subject-rated effects of cocaine. The failure to observe a dose-related effect with d-amphetamine is unknown but could be due to testing only a two-fold range of doses. Future studies should test a wider range of doses of d-amphetamine.
In summary, the results of human laboratory studies, preclinical experiments, and initial clinical trials suggest that agonist replacement therapy may be a viable strategy for the management of cocaine dependence (see Castells et al., 2007 for a review; Grabowski et al., 2001). Clinicians may, however, be reluctant to use amphetamine derivatives to manage cocaine dependency because of their abuse potential. The viability of the agonist replacement approach for cocaine dependence may hinge on identifying novel agonist replacement therapies that have less abuse potential and are more acceptable to clinicians.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Castells X, Casas M, Vidal X, Bosch R, Roncero C, Ramos-Quiroga JA, Capella D. Efficacy of central nervous system stimulant treatment for cocaine dependence: a systematic review and meta-analysis of randomized controlled clinical trials. Addiction. 2007;102:1871–1887. doi: 10.1111/j.1360-0443.2007.01943.x. [DOI] [PubMed] [Google Scholar]
- Collins SL, Levin FR, Foltin RW, Kleber HD, Evans SM. Response to cocaine, alone and in combination with methylphenidate, in cocaine abusers with ADHD. Drug Alcohol Depend. 2006;82:158–67. doi: 10.1016/j.drugalcdep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- de Lima MS, de Oliveira Soares BG, Reisser AA, Farrell M. Pharmacological treatment of cocaine dependence: A systematic review. Addiction. 2002;97:931–949. doi: 10.1046/j.1360-0443.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- Di Marino ME, Haberny KA, Felch LJ, Walsh SL, Preston KL, Bigelow GE. Development of a subject- rated rating scale sensitive to acute cocaine administration. In: Harris L, editor. Problems of Drug Dependence, 1997: Proceedings of the 59th Annual Scientific Meeting. National Institute on Drug Abuse Research Monograph Series; Rockville, MD. 1998. p. 139. [Google Scholar]
- Dudish-Poulsen SA, Hatsukami DK. Dissociation between subjective and behavioral responses after cocaine stimuli presentation. Drug Alcohol Depend. 1997;47:1–9. doi: 10.1016/s0376-8716(97)00054-9. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW. Ethanol and cocaine interactions in humans: Cardiovascular consequences. Pharmacol Biochem Behav. 1988;31:877–883. doi: 10.1016/0091-3057(88)90399-1. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: A double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004a;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: Two double-blind randomized clinical trials. Neuropsychopharmacology. 2004b;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Rush CR, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Exp Clin Psychopharmacol. 1996;4:97–106. [Google Scholar]
- Griffiths RR, Troisi JR, II, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4:3–13. [PubMed] [Google Scholar]
- Haney M, Hart C, Collins ED, Foltin RW. Smoked cocaine discrimination in humans: effects of gabapentin. Drug Alcohol Depend. 2005;80:53–61. doi: 10.1016/j.drugalcdep.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology. 2008 doi: 10.1007/s00213-008-1079-x. Epub ahead of print Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE. Nicotine medications for smoking cessation. N Engl J Med. 1995;333:1196–1203. doi: 10.1056/NEJM199511023331807. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Bickel WK, Hughes JR, Lynn M, Capeless MA, Fenwick JW. Effects of intranasal cocaine on human learning, performance and physiology. Psychopharmacology. 1990;10:451–458. doi: 10.1007/BF02247124. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Rush CR, Hughes JR, Bickel WK, Lynn M, Capeless MA. Acute behavioral and cardiac effects of cocaine and alcohol combinations in humans. Psychopharmacology. 1993;111:285–294. doi: 10.1007/BF02244943. [DOI] [PubMed] [Google Scholar]
- Javaid JI, Fischman MW, Schuster CR, Dekirmenjian H, Davis JM. Cocaine plasma concentration: relation to physiological and subjective effects in humans. Science. 1978;202:227–228. doi: 10.1126/science.694530. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. University of Michigan News Service; Ann Arbor, MI: 2007. [accessed 02/04/07]. Overall, illicit drug use by American teens continues gradual decline in 2007. [Online]. Available: www.monitoringthefuture.org. [Google Scholar]
- Lile JA, Stoops WW, Allen TS, Glaser PEA, Hays LR, Rush CR. Baclofen does not alter the reinforcing, subject-rated or cardiovascular effects of intranasal cocaine in humans. Psychopharmacology. 2003;171:441–449. doi: 10.1007/s00213-003-1598-4. [DOI] [PubMed] [Google Scholar]
- Ling W, Rawson RA, Compton MA. Substitution pharmacotherapies for opioid addiction: from methadone to LAAM and buprenorphine. J Psychoactive Drugs. 1994;26:119–128. doi: 10.1080/02791072.1994.10472259. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl. 1987;334:1–100. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology. 2003a;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003b;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: Acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- Oliveto AH, Rosen MI, Woods SW, Kosten TR. Discriminative stimulus, self-reported and cardiovascular effects of orally administered cocaine in humans. J Pharmacol Exp Ther. 1995;272:231–241. [PubMed] [Google Scholar]
- Peltier RL, Li DH, Lytle D, Taylor CM, Emmett-Oglesby MW. Chronic d-amphetamine or methamphetamine produces cross-tolerance to the discriminative and reinforcing stimulus effects of cocaine. J Pharmacol Exp Ther. 1996;277:212–218. [PubMed] [Google Scholar]
- Rush CR, Baker RW, Wright K. Acute physiological and behavioral effects of oral cocaine in humans: a dose-response analysis. Drug Alcohol Depend. 1999;55:1–12. doi: 10.1016/s0376-8716(98)00164-1. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PEA, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Snyder M. NIDA’s Medication Development Program - 1989. In: Harris LS, editor. National Institute on Drug Abuse. Public Health Service: Alcohol, Drug Abuse, and Mental Health Administration; Problems of Drug Dependence, 1989: Proceedings of the 51st Annual Scientific Meeting. Research Monograph 95; Rockville, Maryland. DHHS (ADM): U.S. Department of Health and Human Services; 1990. pp. 90–1663.pp. 64–73. [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Shearer J, Wodak A, van Beek I, Mattick RP, Lewis J. Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction. 2003;98:1137–1141. doi: 10.1046/j.1360-0443.2003.00447.x. [DOI] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004:CD000146. doi: 10.1002/14651858.CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The Drug Abuse Screening Test. Addict Behav. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Kosten TR. Pharmacologic management of relapse prevention in addictive disorders. Psychiatr Clin North Am. 2004;27:627–648. doi: 10.1016/j.psc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Lofwall MR, Rush CR. The safety, tolerability, and subject-rated effects of acute intranasal cocaine administration during aripiprazole maintenance. Am J Drug Alcohol Abuse. 2007;33:769–776. doi: 10.1080/00952990701651556. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Blackburn JW, Hudson DA, Hays LR, Rush CR. Safety, tolerability and subject-rated effects of acute intranasal cocaine administration during atomoxetine maintenance. Drug Alcohol Depend. 2008;92:282–285. doi: 10.1016/j.drugalcdep.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies (SAMHSA) [Accessed 02/15/08];National Survey on Drug Use and Health (NSDUH), 2006. 2008a Available: http://www.samhsa.gov/
- SAMHSA. Drug Abuse Warning Network (DAWN), 2005. [Accessed 02/15/08];2008b Available: http://www.samhsa.gov/
- SAMHSA. Treatment Episode Data Set (TEDS): 1995–2005. National Admissions to Substance Abuse Treatment Services. [Accessed 02/15/08];2008c Available: http://www.samhsa.gov/
- Van Dyke C, Jatlow P, Ungerer J, Barash PG, Byck R. Oral cocaine: plasma concentrations and central effects. Science. 1978;200:211–213. doi: 10.1126/science.24895. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry. 2005;18:265–270. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Haberny KA, Bigelow GE. Modulation of intravenous cocaine effects by chronic oral cocaine in humans. Psychopharmacology (Berl) 2000;150:361–73. doi: 10.1007/s002130000439. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, Singal BM, Harrer J, Apparaju S, Mezinskis J, Desai P, Elkashef A, Chiang CN, Horn P. Methylphenidate and cocaine: a placebo-controlled drug interaction study. Pharmacol Biochem Behav. 2006;85:29–38. doi: 10.1016/j.pbb.2006.06.023. [DOI] [PubMed] [Google Scholar]