Synopsis
Corticosteroids are used to improve lung function in infants who are progressing toward BPD. Corticosteroids facilitate extubation, but there is conflicting information about adverse effects on the developing brain. An approach to minimizing risk is to use low dose, short duration treatments in the highest risk ventilator dependent patients. Questions remain about which corticosteroid is the safest and how to dose that corticosteroid.
Keywords: Lung Injury, Alveoli, Premature, Neurodevelopment, Mechanical Ventilation
All who drink of this treatment recover in a short time, except those whom it does not help, who all die. Therefore, it is obvious that it fails only in incurable cases.
Galen, 200 AD
A Perspective
Are we using corticosteroids in the uninformed way that Galen was using his potion to treat patients almost 2000 years ago, and are treatment failures the result of the disease or the drug? Galen seemed to discount drug toxicity for those unfortunate incurable cases. There are really just two core questions that need to be asked about the use of postnatal corticosteroids for BPD - do they work? And do they have clinically concerning adverse effects? The challenge is to define what work means, what adverse effects are of concern, and what they are - as there are multiple corticosteroids used in different doses at different postnatal ages for different treatment durations. The subject of postnatal corticosteroids for BPD is blessed as being one of the subjects most evaluated by randomized-controlled trials, but the curses are the multiple treatment schedules and the limited and inconsistent outcome information. Ultimately, the clinician must weigh the statistical and population based evidence and decide if corticosteroids might help a particular infant with acceptable risks.
How Frequently are Corticosteroids Used?
The use of postnatal corticosteroids has been a bit like women's skirts, which go up and down, based on fashion or perceived benefits and risks. In 1990, about 17% of very low birth weight (VLBW) infants cared for in the Vermont Oxford Network (VON) received postnatal corticosteroids, and the use was 7% for VLBW infants in the NICHD Neonatal Research Network (NRN)1. Peak use was 28% for VON and 23% for the NRN in 1997. As follow-up information about adverse effects of corticosteroids on neurodevelopment began to appear, the American Academy of Pediatrics and the Canadian Pediatric Association strongly recommended against the use of corticosteroids to prevent or treat BPD in 20022. Use then decreased, and 8% of VLBW infants in the VON registry received postnatal corticosteroids in 2006, with a 23% use for the highest risk group (birth weights of 501-750g) (R. Soll, personal communication). Therefore, postnatal corticosteroids continue to be used selectively in infants. However, infants also receive corticosteroids for indications other than BPD. For 62 units in California in 2003, 19% of VLBW infants received postnatal steroids, but just 3.6% received steroids for BPD only; the other indications were for hypotension, airway management after extubation, or a combination of indications3. Some units also use aerosolized corticosteroids. Thus the risks and benefits of their use remain a contemporary concern. Corticosteroids are used preferentially in the smaller and earlier gestational age infants because those infants are at highest risk for developing severe BPD.
Do Corticosteroids Work for BPD?
This question means to me a corticosteroid effect on ventilator dependent infants at risk for BPD that improves lung function sufficiently to permit extubation. The use of corticosteroids soon after birth to “prevent” BPD is now less frequent because of concerns about the risk and interactions with other drugs such as indomethacin4,5. The summary conclusions of the meta-analyses by Halliday, Ehrenkranz and Doyle indicate that postnatal corticosteroids do not decrease death, although there is a trend for a death benefit for treatments started at 7-14d of age6-8 (Table 1). Corticosteroids decrease BPD and decrease extubation in failures independently of the age at which treatments were started. The trials included in these meta-analyses were mostly from the pre-surfactant treatment era and some of the trials were done before the frequent use of antenatal corticosteroids. The infants were larger than many of the infants who receive postnatal corticosteroids today. Also, there have been changes in the techniques used for mechanical ventilation and in ventilatory goals. Higher Pco2 values may be accepted for infants requiring mechanical ventilation for severe respiratory failure. A therapy that was effective in a different era on a different patient population may not work now. If postnatal corticosteroids do not work in current practice, a further discussion of their use is not relevant.
Table 1. Postnatal Corticosteroids - Death/CLD.
| Time of Treatment | # Studies | # Pts | RR | Significance |
|---|---|---|---|---|
| Death | ||||
| <96h | 21 | 3068 | 1.01 (.89-1.15) | |
| 7-14d | 6 | 288 | 0.66(.40-1.09) | |
| >3wks | 8 | 542 | 0.99(.71-1.39) | |
| CLD | ||||
| <96h | 15 | 2415 | 0.69(.60-.80) | * |
| 7-14d | 5 | 247 | 0.62(.47-.82) | * |
| >3wks (home O2) | 5 | 481 | 0.66(.47-.92) | * |
| Failure to Extubate-7d | ||||
| <96 | 6 | 936 | 0.76(.66-.88) | * |
| 7-14d | 2 | 84 | 0.62(.46-.84) | * |
| >3wk | 5 | 288 | 0.69(.58-.82) | * |
The American Academy of Pediatrics and Canadian Pediatric Association joint condemnation of postnatal corticosteroid use for BPD curtailed the enthusiasm for further studies2. Nevertheless, two studies were attempted and both were closed before full enrollment. Watterberg, et al.5 randomized ventilated infants with birth weights 500-999g to a 15d tapered course of hydrocortisone or placebo within 2d of birth. The hypothesis was that hydrocortisone would treat the adrenal insufficiency that commonly occurs soon after birth, would improve survival and decrease BPD. The trial was stopped because of increased intestinal perforations in hydrocortisone treated infants who also received indomethacin as prophylaxis for PDA. Although there was no overall death or BPD benefit, a prospectively planned analysis of those infants exposed to histologic chorioamnionitis did demonstrate decreased BPD and death in infants randomized to hydrocortisone. This trial demonstrated benefit in a subpopulation of infants managed by current practices.
The DART study randomized 70 ventilator dependent infants with average birth weights less than 700g to a 10d tapered dose of dexamethasone or placebo at a mean postnatal age of 23d9. The initial dexamethasone dose of 0.15 mg/kg was lower than used in previous trials. Enrollment was stopped because infants could not be recruited, in part because of concerns about risks. This study was underpowered and did not demonstrate differences in BPD or death. However, the dexamethasone treatment had large effects on the lungs and successful extubation was more frequent (Table 2). This small trial demonstrates that postnatal corticosteroids do improve lung function in surfactant treated infants exposed to antenatal corticosteroids and managed by contemporary techniques.
Table 2. Responses of Ventilator-dependent Preterm Infants Randomized to a 10d Tapered Course of Dexamethasone Begun at a Median Age of 23d.
| Values at 10d | Placebo | Dexamethasone | p |
|---|---|---|---|
| N | 35 | 35 | |
| Failure to Extubate | 88% | 40% | <0.01 |
| Mean Airway Pressure (cmH2O) | 10.2 | 7.6 | <0.01 |
| Peak Airway Pressure (cmH2O) | 19.6 | 16.7 | <0.01 |
| Inspired Oxygen | 43.5% | 34.7% | <0.04 |
| Mean Blood Pressure (mmHg) | 45.7 | 52.5 | - |
Data abstracted from Doyle, et al. (9)
A recent retrospective report compared oral prednisolone (2 mg/kg/d for 5d and tapered for 9d) in 131 infants with BPD who were oxygen dependent at 38wk gestation to a matched group of 254 infants10. The prednisolone facilitated weaning of infants with Pco2 values <49 mmHg from oxygen relative to the comparison group. This use of corticosteroids differs from other trials in that infants were off ventilators and simply on oxygen. This retrospective report does suggest physiologic responses of BPD lungs late in the clinical course. Despite concerns about outcomes, neonatologists continue to use corticosteroids in some infants because the short-term improvements in lung function can be remarkable.
How do Postnatal Corticosteroids Work?
This is not a simple question. Corticosteroids are potent anti-inflammatory agents and there is a clear rationale for the use of an anti-inflammatory to treat BPD. Perhaps more than 50% of infants at risk for BPD have been exposed to the chronic indolent chorioamnionitis associated with very preterm birth, and those infants have inflamed lungs at birth11,12. Oxygen exposure, and mechanical ventilation also induce inflammatory responses13. Multiple pro-inflammatory mediators and inflammatory cells are in airway samples of infants progressing toward BPD14. Postnatal corticosteroids will decrease these indicators of inflammation15. But, indomethacin also is an anti-inflammatory and it does not decrease BPD16. Corticosteroids decrease edema as part of the anti-inflammatory effect, which may contribute to improved gas exchange and lung mechanics. Particularly in the VLBW infants, corticosteroid treatments will increase blood pressure and treat adrenal insufficiency17. The type of corticosteroid - hydrocortisone or dexamethasone - also may have different actions that contribute to clinical responses. Without a better understanding of the mediators that promote BPD at each stage of disease progression, therapies that are more targeted than corticosteroids will not be developed.
Drug, Dose, and Duration of Corticosteroid Treatments
An analysis of corticosteroid treatments in infants is bedeviled by the different steroids used and variable dosing schedules6-8. The early trials designed to prevent BPD used a “standard” initial dose of 0.5 mg/kg dexamethasone that was then slowly tapered over 42d - referred to as the 42d treatment. More recent trials have used lower initial doses of 0.2, 0.15, or 0.1 mg/kg dexamethasone with weaning schedules over 7 to 10d and with apparently good acute effects on lung function4,9,18. In the United States, betamethasone phosphate is not available, but it has been used and dosed similarly to dexamethasone elsewhere in the world. These two synthetic corticosteroids are 25 times more potent than hydrocortisone, but they are not equivalent. While the genomic effects are similar, non-genomic effects such as acute changes in membrane function and ion transport are very different19. Betamethasone may be more effective and safer than dexamethasone for antenatal treatment of women at risk of preterm delivery20. The betamethasone may have fewer effects on the fetal brain. Hydrocortisone is being used more frequently for treatment of hypotension in VLBW infants, and hydrocortisone has been used outside randomized controlled trials to treat BPD in Europe. Hydrocortisone is the natural hormone, which may be safer to use than the synthetic corticosteroids21. However, randomized trials of lung responses or complications are not available. Prednisolone also has been used, but not evaluated in randomized trials10. Without good trial data, clinicians are using lower doses of dexamethasone or hydrocortisone for shorter treatment periods. Treatment schedules of 0.2 mg/kg of dexamethasone or less tapered over 7 to 10d seem to avoid the hyperglycemia and hypertension frequently encountered with the higher doses and longer treatment schedules9. As toxicity from corticosteroids is related to dose and treatment duration in patients of all ages, the use of lower dose, shorter duration treatments just makes sense. Aerosolized corticosteroids are used frequently in some neonatal units and not in others for treatment of the progression of BPD. There is very little evidence of much efficacy for aerosolized steroids22,23. Aerosolization of steroids may be ineffective because very little drug gets into the lungs of preterm infants by aerosolization24.
Corticosteroids and Neurodevelopmental Outcomes
A Perspective
Cortisol is a potent agent that regulates development and normally increases prior to term birth. Cortisol also increases with preterm birth, but to lower blood levels than found after term birth (Fig. 1). The fetus is protected from maternal cortisol by placental 11β hydroxysteroid dehydrogenase, which converts cortisol to the inactive cortisone as it passes from maternal to the fetal circulation. Fetal cortisol synthesis is tightly regulated such that the fetus makes minimal cortisol until late in gestation. Stressed preterm fetuses and fetuses exposed to chorioamnionitis can “induce” cortisol synthesis and secretion25. The unstressed preterm fetus will have low blood cortisol levels and be unable to rapidly increase adrenal synthesis and secretion. Therefore, many very low birth weight infants will have low blood cortisol values and may be functionally adrenal insufficient17. The preterm newborn has an endogenous cortisol exposure much greater than the fetus for the 12wk from 26wk to term (Fig. 1). Therefore, brain growth in the preterm occurs in a high cortisol environment relative to brain growth in the fetus, independent of any antenatal or postnatal corticosteroid treatments.
Figure 1.
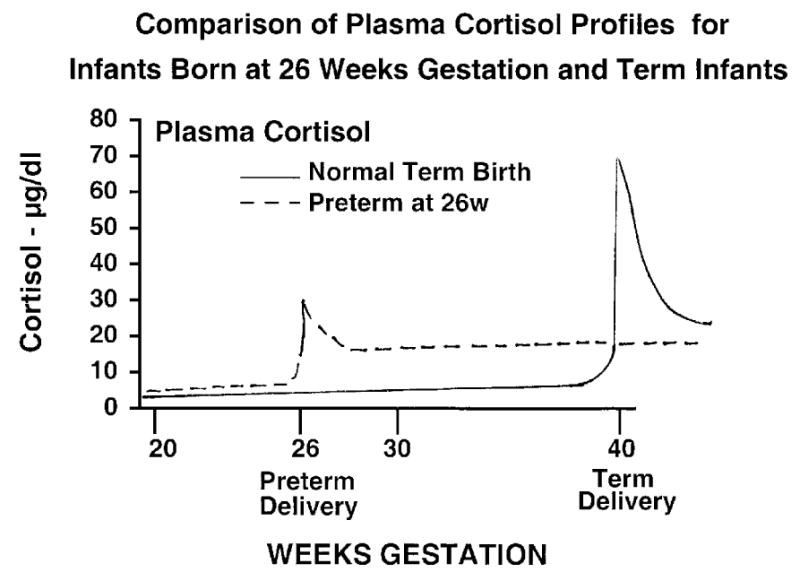
Idealized plasma cortisol profile for a normal fetus that delivers at 40 wks and for a preterm that delivers at 26 wks gestation. The preterm infant has lower cortisol levels at delivery than the term infant. However, following delivery, the preterm has higher cortisol levels than the fetus from 26 wks to 40 wks.
Fetal rodents are exquisitely sensitive to fluorinated corticosteroids such as betamethasone or dexamethasone that cross the placenta from mother to fetus. Fetal or neonatal exposures cause fetal growth restriction and lung maturation, as well as programming effects that result in behavioral abnormalities and hypertension as adults26. Two recent reports demonstrate that neonatal dexamethasone treatments reduce life expectancy and cause sustained atherogenic plasma lipid profiles in rats27,28. In sheep, a brief period of exposure to corticosteroids at early gestation alters kidney anatomy, function, and blood pressure regulation in adulthood29. Are these quite striking (and frightening) effects relevant to postnatal corticosteroid treatments for preterm infants? A reasonable way to think about possible relevance is to ask how corticosteroids might impact fetal or newborn weights in different species. At 80% gestation, the rat fetus increases weight by 65% in 1d, and the mouse fetus increases weight by almost 100% over 1d. A single dose of betamethasone will arrest growth in the fetal sheep for several days30. The human fetus gains only 1.6% weight over 1d at 80% gestation. Thus, corticosteroid effects on growth will be much less for the human than for shorter gestation mammals. The experimental research can be used to frame questions to be asked in humans. However, we cannot assume that corticosteroid effects will map from rodent models to the human.
Another perspective about possible effects of postnatal corticosteroids relates to the outcomes of the infants at risk. Postnatal corticosteroids are given to the sickest VLBW infants who are on ventilators and are progressing toward BPD. VLBW infants have multiple complications and neurodevelopmental problems independent of corticosteroid use. Infants with gestational ages of <26wk have MDI and PDI scores about 20 points lower than term infants at school age in population based studies from the United Kingdom31. Infants with BPD have more neurodevelopmental problems than matched infants without BPD32,33. The inflammation associated with BPD or the recurrent respiratory instabilities may contribute to neurodamage. Also, “normal” preterm infants have abnormal regional brain volumes and white matter injury at term34. These brain structural abnormalities and processing abnormalities persist to school age35. As the BPD infants tend to have more other complications of prematurity, one has to be careful about attributing outcomes to a treatment rather than to the multiple adverse occurrences experienced by this population of infants.
Neurodevelopmental Outcomes
Neonatologists have a long history of using drugs for unverified indications and without follow-up. Follow-up information became available for the use of postnatal corticosteroids in the 1990's and meta-analyses of studies with small numbers of patients in 2000 and 2001 demonstrated that postnatal corticosteroids may increase cerebral palsy (CP) and degrade neuroperformance in survivors6-8,36 (Table III). These reports resulted in the recommendation to not use postnatal corticosteroids by the American Academy of Pediatrics and the Canadian Pediatrics Association in 20022. A subsequent report by Yeh, et al. in 2004 reported more CP in infants treated with the 42d course of dexamethasone37. Another very concerning finding was that postnatal dexamethasone given for a mean of 22d decreased grey matter volume as measured by MRI38. The report by Le Flore, et al. 39 noted lower MDI and PDI scores for infants with BPD treated with dexamethasone than comparison infants using a multivariant analysis. Recently, Parikh, et al. 40 measured smaller total and regional brain tissue volumes in preterms at term who had received dexamethasone after 28d of age for a mean duration of only 6.8d (cumulative dose - 2.8 mg/kg). These last two studies were not based on randomization to postnatal corticosteroid use.
Table 3. Postnatal Corticosteroids - Follow-Up.
| Time of Treatment | # Studies | # Patients | RR | Significant |
|---|---|---|---|---|
| <96h - | ||||
| MDI<2SD | 2 | 482 | 1.24(.90-1.71) | |
| PDI<2SD | 2 | 482 | 1.18(.81-1.72) | |
| CP | 9 | 991 | 1.69(1.2-2.38) | * |
| 7-14d | ||||
| CP | 4 | 204 | 1.03(.47-2.24) | |
| >3wks | * | |||
| Abn. Neuro | 3 | 164 | 1.90(1.08-3.33) | |
However, a number of recent reports find either no adverse outcomes or benefits in follow-up of infants exposed to postnatal corticosteroids. Although O'Shea et al.41 reported an increased risk for CP in infants randomized to the 42d course of dexamethasone at 1yr of age (with no cross-over dexamethasone treatments in the control group), their composite outcomes of death or major neurodevelopmental impairment were not different from controls at school age42. Furthermore, this same cohort of infants had higher expiratory flows than controls and no adverse effects on lung function at school age43. These infants also had no differences from controls in growth, fat mass, or blood pressure at school age44. Another experience with the outcomes of a small number of infants randomized to the 42d course of dexamethasone was improved neurodevelopmental outcomes relative to controls45. A comparison of outcomes of infants randomized to dexamethasone or inhaled budesonide demonstrated no differences in neuroperformance, CP, or growth46. I interpret the budesonide treatment group to be equivalent to a no treatment group23. Lower dose dexamethasone given after the first week of life also did not adversely effect 2yr outcomes in another small randomized trial47. These reports are in contrast to mostly older reports of adverse effects of dexamethasone on neurodevelopment.
Reports of outcomes following the use of hydrocortisone also have appeared since the recommendation to not use postnatal corticosteroids2. Hydrocortisone was randomized for infants shortly after birth to treat adrenal insufficiency and to decrease BPD5. At two years, infants randomized to hydrocortisone had improved neurodevelopmental outcomes relative to controls48. In a cohort of infants not randomized to hydrocortisone for the treatment of BPD, Rademaker, et al.49 found that relative to an older, heavier, and less sick group of comparison infants, hydrocortisone was associated with no adverse effects on multiple neurodevelopmental or MRI assessments at school age. Similar neurocognitive and MRI outcomes were reported for 8yr olds treated with hydrocortisone for BPD50. A retrospective matched cohort analysis of infants given dexamethasone or hydrocortisone suggested that the dexamethasone exposed infants had more problems with neurodevelopment21, and altered endocrine and immune function at school age51.
An Approach to Postnatal Corticosteroids
There are justified concerns about the use of postnatal corticosteroids for any indication, but especially for BPD. However, those concerns must be balanced against the clear acute physiological effects on lung function that can allow infants to come off mechanical ventilation. Shinwell, et al.52 recently reported for the Israel Neonatal Network that a decreased use of postnatal corticosteroids in VLBW infants from 23.5% in 1997-1998 to 11% in 2002-2003 was associated with an increase in BPD (oxygen use at 36wk) from 12.9% to 18.7%. This report suggests that the price one pays for decreased corticosteroid use is more BPD. The critical analysis for me is the meta-regression analysis reported by Doyle et al.53, which shows that postnatal corticosteroids decrease the risk of death or CP in populations of infants at greater than about 50% risk of BPD (Fig. 2). Treatment of low risk populations may increase risk of these adverse outcomes. The DART trial demonstrated acute effects on the lungs of ventilated infants progressing toward BPD with an initial dose of 0.15 mg/kg/d9. The general clinical experience is that some infants respond quickly to corticosteroids and others do not. I combine that information and favor corticosteroid treatment of a ventilator dependent infant who is 14-28d of age and who is progressing toward BPD. An initial dose of dexamethasone of 0.1 to 0.2 mg/kg/d for 3d may effectively achieve the goal of extubation. If extubation can be achieved or is likely to be accomplished, the dose can be tapered over 3-6d (total treatment 6-9d). If the initial 3d of treatment will not accomplish extubation, then the treatment should be stopped. This approach is not evidence based; it is an attempt to maximize benefit and minimize the risk of postnatal corticosteroids. Betamethasone or hydrocortisone might be better choices than dexamethasone, but neither have been evaluated in trials for this indication.
Figure 2.
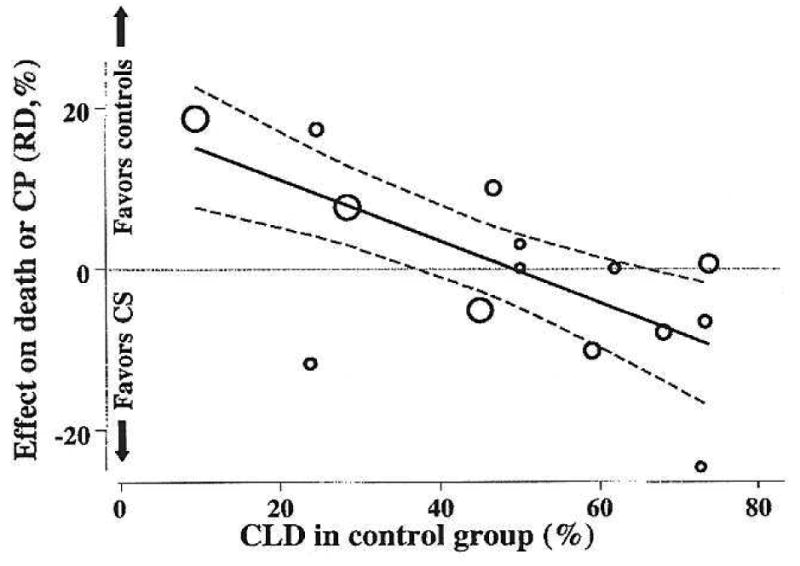
Meta-regression analysis of death or Cerebral Palsy (CP) relative to risk of developing BPD. The graph shows the outcomes as risk differences (RD) for death or CP for randomized controlled trials. The size of the circle reflects the size of the study. The outcomes are shown relative to the percent of infants in the control arm of each study who developed BPD. For infants at high risk of BPD, the risk difference for death or CP favors corticosteroid treatment. Reprinted with permission from Doyle, et al47.
We have moved beyond Galen because we know a fair bit about the treatment - it can work and it has risks. The follow-up data are particularly confusing as bad or good outcomes have been reported with early or late treatment and with higher or lower exposures. There may be other aspects of our care that interact with corticosteroids and contribute to these inconsistent outcomes. Clearly, the use of corticosteroids and indomethacin together soon after birth increases gastrointestinal perforations4,5. For example, good nutrition might blunt adverse effects of postnatal corticosteroids. Following the lead of Doyle, et al.53, very selective treatment of the highest risk infants with a low dose (but what dose?) for as short a duration as possible (presently undefined?) with a corticosteroid (but which one?) is the best we can do for now. The recommendation to limit postnatal corticosteroid use should not discourage careful studies of postnatal corticosteroids in high risk VLBW infants.
Acknowledgments
No funding was received for this article. I have no financial disclosures related to this work.
Footnotes
Declaration: Corticosteroid use for BPD is not an FDA approved use for this class of drugs.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walsh MC, Yao Q, Horbar JD, et al. Changes in the use of postnatal steroids for bronchopulmonary dysplasia in 3 large neonatal networks. Pediatrics. 2006;118:e1328. doi: 10.1542/peds.2006-0359. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics - American Academy of Pediatrics Committee on Fetus and Newborn and Canadian Paediatric Society. Fetus and Newborn CommitteePostnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics. 2002;109:330. doi: 10.1542/peds.109.2.330. [DOI] [PubMed] [Google Scholar]
- 3.Finer NN, Powers RJ, Ou CH, et al. Prospective evaluation of postnatal steroid administration: a 1-year experience from the California Perinatal Quality Care Collaborative. Pediatrics. 2006;117:704. doi: 10.1542/peds.2005-0796. [DOI] [PubMed] [Google Scholar]
- 4.Stark AR, Carlo WA, Tyson JE, et al. Adverse effects of early dexamethasone in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. New England Journal of Medicine. 2001;344:95. doi: 10.1056/NEJM200101113440203. [DOI] [PubMed] [Google Scholar]
- 5.Watterberg KL, Gerdes JS, Cole CH, et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics. 2004;114:1649. doi: 10.1542/peds.2004-1159. [DOI] [PubMed] [Google Scholar]
- 6.Halliday HL, Ehrenkranz RA, Doyle LW. Moderately early (7-14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2003:CD001144. doi: 10.1002/14651858.CD001144. [DOI] [PubMed] [Google Scholar]
- 7.Halliday HL, Ehrenkranz RA, Doyle LW. Delayed (>3 weeks) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2003:CD001145. doi: 10.1002/14651858.CD001145. [DOI] [PubMed] [Google Scholar]
- 8.Halliday HL, Ehrenkranz RA, Doyle LW. Early postnatal (<96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2003:CD001146. doi: 10.1002/14651858.CD001146. [DOI] [PubMed] [Google Scholar]
- 9.Doyle LW, Davis PG, Morley CJ, et al. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics. 2006;117:75. doi: 10.1542/peds.2004-2843. [DOI] [PubMed] [Google Scholar]
- 10.Bhandari A, Schramm CM, Kimble C, et al. Effect of a short course of prednisolone in infants with oxygen-dependent bronchopulmonary dysplasia. Pediatrics. 2008;121:e344. doi: 10.1542/peds.2006-3668. [DOI] [PubMed] [Google Scholar]
- 11.Watterberg KL, Demers LM, Scott SM, et al. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210. [PubMed] [Google Scholar]
- 12.Andrews WW, Goldenberg RL, Faye-Petersen O, et al. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol. 2006;195:803. doi: 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 13.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 14.De Dooy J, Colpaert C, Schuerwegh A, et al. Relationship between histologic chorioamnionitis and early inflammatory variables in blood, tracheal aspirates, and endotracheal colonization in preterm infants. Pediatr Res. 2003;54:113. doi: 10.1203/01.PDR.0000069702.25801.D1. [DOI] [PubMed] [Google Scholar]
- 15.Speer CP. Inflammation and bronchopulmonary dysplasia. Seminars in Neonatology. 2003;8:29. doi: 10.1016/s1084-2756(02)00190-2. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt B, Roberts RS, Fanaroff A, et al. Indomethacin prophylaxis, patent ductus arteriosus, and the risk of bronchopulmonary dysplasia: further analyses from the Trial of Indomethacin Prophylaxis in Preterms (TIPP) J Pediatr. 2006;148:730. doi: 10.1016/j.jpeds.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 17.Watterberg KL. Adrenocortical function and dysfunction in the fetus and neonate. Seminars in Neonatology. 2004;9:13. doi: 10.1016/j.siny.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Durand M, Mendoza ME, Tantivit P, et al. A randomized trial of moderately early low-dose dexamethasone therapy in very low birth weight infants: dynamic pulmonary mechanics, oxygenation, and ventilation. Pediatrics. 2002;109:262. doi: 10.1542/peds.109.2.262. [DOI] [PubMed] [Google Scholar]
- 19.Buttgereit F, Brand MD, Burmester GR. Equivalent doses and relative drug potencies for non-genomic glucocorticoid effects: a novel glucocorticoid hierarchy. Biochem Pharmacol. 1999;58:363. doi: 10.1016/s0006-2952(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 20.Lee BH, Stoll BJ, McDonald SA, et al. Neurodevelopmental outcomes of extremely low birth weight infants exposed prenatally to dexamethasone versus betamethasone. Pediatrics. 2008;121:289. doi: 10.1542/peds.2007-1103. [DOI] [PubMed] [Google Scholar]
- 21.Karemaker R, Heijnen CJ, Veen S, et al. Differences in behavioral outcome and motor development at school age after neonatal treatment for chronic lung disease with dexamethasone versus hydrocortisone. Pediatr Res. 2006;60:745. doi: 10.1203/01.pdr.0000246200.76860.de. [DOI] [PubMed] [Google Scholar]
- 22.Shah SS, Ohlsson A, Halliday H, et al. Inhaled versus systemic corticosteroids for the treatment of chronic lung disease in ventilated very low birth weight preterm infants. Cochrane Database Syst Rev. 2003:CD002057. doi: 10.1002/14651858.CD002057. [DOI] [PubMed] [Google Scholar]
- 23.Cole CH, Colton T, Shah BL, et al. Early inhaled glucocorticoid therapy to prevent bronchopulmonary dysplasia. N Engl J Med. 1999;340:1005. doi: 10.1056/NEJM199904013401304. see comments. [DOI] [PubMed] [Google Scholar]
- 24.Dubus JC, Montharu J, Vecellio L, et al. Lung deposition of HFA beclomethasone dipropionate in an animal model of bronchopulmonary dysplasia. Pediatr Res. 2007;61:21. doi: 10.1203/01.pdr.0000250055.26148.42. [DOI] [PubMed] [Google Scholar]
- 25.Watterberg KL, Scott SM, Naeye RL. Chorioamnionitis, cortisol, and acute lung disease in very low birth weight infants. Pediatrics. 1997;99:E6. doi: 10.1542/peds.99.2.e6. [DOI] [PubMed] [Google Scholar]
- 26.Jobe AH, Ikegami M. Fetal Responses to Glucocorticoids. In: Mendelson CR, editor. Endocrinology of the Lung. Totowa: Humana Press; 2000. p. 45. [Google Scholar]
- 27.Kamphuis PJ, de Vries WB, Bakker JM, et al. Reduced life expectancy in rats after neonatal dexamethasone treatment. Pediatr Res. 2007;61:72. doi: 10.1203/01.pdr.0000249980.95264.dd. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Havinga R, Bloks VW, et al. Postnatal treatment with dexamethasone perturbs hepatic and cardiac energy metabolism and is associated with a sustained atherogenic plasma lipid profile in suckling rats. Pediatr Res. 2007;61:165. doi: 10.1203/pdr.0b013e31802d89ff. [DOI] [PubMed] [Google Scholar]
- 29.Wintour EM, Moritz KM, Johnson K, et al. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol. 2003;549:929. doi: 10.1113/jphysiol.2003.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikegami M, Jobe AH, Newnham J, et al. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med. 1997;156:178. doi: 10.1164/ajrccm.156.1.9612036. [DOI] [PubMed] [Google Scholar]
- 31.Marlow N, Hennessy EM, Bracewell MA, et al. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120:793. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- 32.Short EJ, Klein NK, Lewis BA, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112:e359. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 34.Inder TE, Warfield SK, Wang H, et al. Abnormal cerebral structure is present at term in premature infants. Pediatrics. 2005;115:286. doi: 10.1542/peds.2004-0326. [DOI] [PubMed] [Google Scholar]
- 35.Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121:306. doi: 10.1542/peds.2007-0414. [DOI] [PubMed] [Google Scholar]
- 36.Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr. 2001;1:1. doi: 10.1186/1471-2431-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh TF, Lin YJ, Lin HC, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
- 38.Murphy BP, Inder TE, Huppi PS, et al. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001;107:217. doi: 10.1542/peds.107.2.217. [DOI] [PubMed] [Google Scholar]
- 39.LeFlore JL, Salhab WA, Broyles RS, et al. Association of antenatal and postnatal dexamethasone exposure with outcomes in extremely low birth weight neonates. Pediatrics. 2002;110:275. doi: 10.1542/peds.110.2.275. [DOI] [PubMed] [Google Scholar]
- 40.Parikh NA, Lasky RE, Kennedy KA, et al. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics. 2007;119:265. doi: 10.1542/peds.2006-1354. [DOI] [PubMed] [Google Scholar]
- 41.O'Shea TM, Kothadia JM, Klinepeter KL, et al. Randomized placebo-controlled trial of a 42 day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: Outcome of study participants at 1 year adjusted age. Pediatrics. 1999;104:15. doi: 10.1542/peds.104.1.15. [DOI] [PubMed] [Google Scholar]
- 42.O'Shea TM, Washburn LK, Nixon PA, et al. Follow-up of a randomized, placebo-controlled trial of dexamethasone to decrease the duration of ventilator dependency in very low birth weight infants: neurodevelopmental outcomes at 4 to 11 years of age. Pediatrics. 2007;120:594. doi: 10.1542/peds.2007-0486. [DOI] [PubMed] [Google Scholar]
- 43.Nixon PA, Washburn LK, Schechter MS, et al. Follow-up study of a randomized controlled trial of postnatal dexamethasone therapy in very low birth weight infants: effects on pulmonary outcomes at age 8 to 11 years. J Pediatr. 2007;150:345. doi: 10.1016/j.jpeds.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Washburn LK, Nixon PA, O'Shea TM. Follow-up of a randomized, placebo-controlled trial of postnatal dexamethasone: blood pressure and anthropometric measurements at school age. Pediatrics. 2006;118:1592. doi: 10.1542/peds.2006-0973. [DOI] [PubMed] [Google Scholar]
- 45.Gross SJ, Anbar RD, Mettelman BB. Follow-up at 15 years of preterm infants from a controlled trial of moderately early dexamethasone for the prevention of chronic lung disease. Pediatrics. 2005;115:681. doi: 10.1542/peds.2004-0956. [DOI] [PubMed] [Google Scholar]
- 46.Wilson TT, Waters L, Patterson CC, et al. Neurodevelopmental and respiratory follow-up results at 7 years for children from the United Kingdom and Ireland enrolled in a randomized trial of early and late postnatal corticosteroid treatment, systemic and inhaled (the Open Study of Early Corticosteroid Treatment) Pediatrics. 2006;117:2196. doi: 10.1542/peds.2005-2194. [DOI] [PubMed] [Google Scholar]
- 47.Doyle LW, Davis PG, Morley CJ, et al. Outcome at 2 years of age of infants from the DART study: a multicenter, international, randomized, controlled trial of low-dose dexamethasone. Pediatrics. 2007;119:716. doi: 10.1542/peds.2006-2806. [DOI] [PubMed] [Google Scholar]
- 48.Watterberg KL, Shaffer ML, Mishefske MJ, et al. Growth and neurodevelopmental outcomes after early low-dose hydrocortisone treatment in extremely low birth weight infants. Pediatrics. 2007;120:40. doi: 10.1542/peds.2006-3158. [DOI] [PubMed] [Google Scholar]
- 49.Rademaker KJ, Uiterwaal CS, Groenendaal F, et al. Neonatal hydrocortisone treatment: neurodevelopmental outcome and MRI at school age in preterm-born children. J Pediatr. 2007;150:351. doi: 10.1016/j.jpeds.2006.10.051. [DOI] [PubMed] [Google Scholar]
- 50.Lodygensky GA, Rademaker K, Zimine S, et al. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics. 2005;116:1. doi: 10.1542/peds.2004-1275. [DOI] [PubMed] [Google Scholar]
- 51.Karemaker R, Kavelaars A, ter Wolbeek M, et al. Neonatal dexamethasone treatment for chronic lung disease of prematurity alters the hypothalamus-pituitary-adrenal axis and immune system activity at school age. Pediatrics. 2008;121:e870. doi: 10.1542/peds.2007-2454. [DOI] [PubMed] [Google Scholar]
- 52.Shinwell ES, Lerner-Geva L, Lusky A, et al. Less postnatal steroids, more bronchopulmonary dysplasia: a population-based study in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2007;92:F30. doi: 10.1136/adc.2006.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doyle LW, Halliday HL, Ehrenkranz RA, et al. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk for chronic lung disease. Pediatrics. 2005;115:655. doi: 10.1542/peds.2004-1238. [DOI] [PubMed] [Google Scholar]


