Abstract
Although bone mineral deficits have been identified in Rett syndrome (RTT), the prevalence of low bone mineral density and its association with skeletal fractures and scoliosis has not been characterized fully in girls and women with RTT. Accordingly, we measured total body bone mineral content (BMC) and bone mineral density (BMD) using dual energy x-ray absorptiometry in a cross-sectional group of 50 females, ages 2-38 y, with RTT. Methyl-CpG-binding 2 (MECP2) mutations, skeletal fractures, and scoliosis were documented. The prevalence of BMC and BMD z-scores <-2 SD was 59% and 45%, respectively. Although absolute BMC and BMD increased significantly with increasing age, BMC and BMD z-scores were significantly lower in older than in younger females. The prevalence of fractures and scoliosis was 28% and 64%, respectively. Low BMD z-scores were positively associated with fractures and scoliosis. Deficits in BMD were identified across a broad range of MECP2 mutations. This study identified associations among low bone mineral density, fractures, and scoliosis, and underscored the need for better understanding of the molecular mechanisms of MECP2 in the regulation of bone mineral metabolism.
Keywords: bone mineral density, skeletal fractures, scoliosis, genotype-phenotype correlations, body composition, anticonvulsants
Bone mineral deficits complicate the clinical course of Rett syndrome (RTT) (1-3), an X-linked neurodevelopmental disorder caused by mutations in the methyl-CpG binding 2 (MECP2) gene (4). Consequently, girls with RTT are at increased risk for skeletal fractures (1,3). The etiology of bone mineral deficits is unknown, although impaired ambulation and anticonvulsant therapy have been implicated as causally related factors (3). We have shown that bone mineral deficits are present, despite adequate dietary calcium (Ca) intake, increased intestinal Ca absorption, and the absence of vitamin D deficiency or hyperparathyroidism (5). Our studies (5,6) and that of Haas et al. (1) documented early, profound deficits of total body bone mineral content (BMC) and bone mineral density (BMD) in girls with RTT compared with unaffected girls and children with other neurological disorders. Haas et al. (1) showed that total body BMC did not improve with advancing age, while our study suggested that the accretion of total body BMC throughout childhood was possible (5). All of these studies were limited because of the small number and narrow age range of the participants (1,5,6). They also captured total body BMC and BMD when less emphasis was placed on the nutritional and physical rehabilitation of females with RTT.
In the present study, we expanded the number and age range of the participants and used body composition and ambulation as proxies for nutritional status and physical activity to better understand the prevalence of bone mineral deficits and the relation between bone mineral deficits, MECP2 mutations, and occurrence of bone-related disorders in RTT. We hypothesized that: 1) the early decrease in BMC and BMD persists over time in a cross-sectional cohort of females with RTT, 2) bone mineral deficits occur across a range of MECP2 mutations, and 3) the reduction in BMC and BMD correlates with the occurrence of fractures and scoliosis. We anticipated that this study would facilitate the development of strategies to promote bone mineral health in RTT.
SUBJECTS AND METHODS
Subjects
Fifty females who met the clinical criteria for RTT were enrolled (7). MECP2 mutations, if known, were recorded. All participants were female because males with MECP2 mutations have a different phenotype and rarely meet the diagnostic clinical criteria (8). The eligible age range was 0-40 y, with individuals divided among groups of 5-y intervals. Individuals were excluded if they received oral Ca supplements 6 months prior to study, had previous therapy with bis-phosphonates, had hypocalcemia, hyperparathyroidism, vitamin D deficiency, or had scoliosis requiring spinal rod placement, the latter because metal interferes with the ability of DXA to provide an accurate assessment of spine BMD.
Parents gave permission for the participation of their daughters in the research study. Assent was waived for the participants because of their cognitive impairment. The Institutional Review Board for Baylor College of Medicine approved the study protocol.
Procedures
All females with RTT were admitted overnight to the General Clinical Research Center, Texas Children's Hospital, for 1) review of their medical history, 2) physical examination, and 3) determination of their stature and body habitus, bone age, and total body and regional bone mineral status. Individual MECP2 mutations were classified as missense, early truncation, late truncation, and large deletion mutations (10). A history of fractures, seizures and anticonvulsant use, the ability to ambulate, and the presence of scoliosis were documented.
Height was measured using a fixed stadiometer or horizontal length board with a movable foot piece (Harpenden, Crymych, Great Britain). Weight was measured using an electronic balance (Scale-Tronix, Inc., Wheaton, IL). BMI was calculated as weight divided by height squared. Height, weight, and BMI measurements were converted to z-scores based on the National Center for Health Statistics normative data (11). Body fat, as a proportion of body weight, was estimated from triceps, biceps, subscapular, and suprailiac skinfold thickness (12). The bone age of females, age 20 y or less, was calculated from an x-ray of the right hand using reference standards (13).
Whole body, hip, and spine BMC and BMD were measured by dual-energy x-ray absorptiometry (DXA) (Delphi-A System, Hologic, Inc., Madison, WI) using standardized scan protocols. Midazolam (0.2 mg/kg/dose) was administered intravenously immediately before the scan to prevent repetitive involuntary movements that could invalidate the analysis. The data from 10 body regions were summed to provide values for total body BMC and BMD; lean body mass; and body fat using body composition software (Delphi-A System, Version 11.2, Hologic, Inc., Waltham, MA). The in vivo precision for BMC and BMD measurements is 2-3% for children and young adults (9).
Bone mineral status was expressed as BMC, which reflects bone mass, and BMD, which reflects a calculated measure of areal density, not a true volumetric density (14). Because areal density is dependent on bone size, thinning of the bone cortex and a smaller outer diameter of bone may result in diminished areal BMD, whether or not true volumetric density is diminished. To correct for changes in bone size as a function of age and body size, total body BMC and BMD were converted to z-scores based on values measured in a reference population (9). The reference database includes DXA scans for more than 2200 healthy children for whom ethnic, racial, and gender distributions are approximately equal. These data were used to develop a predictive algorithm for normative total body BMC and BMD based on age, gender, ethnicity, race, and height (6). For individuals whose ages exceeded 20 years, adult references provided with the DXA instrument were used. A significant bone mineral deficit was defined as total body BMD z-score <-2 SD (15).
Statistical Analysis
Sample size was determined from an analysis of females, age 5-12 y, with RTT (6) whose BMC averaged 458 ± 111 g and unaffected girls, ages 6-10 y, 11-14 y, and 15-18 y whose BMC averaged 775 ± 233, 1346 ± 312, and 1979 ± 289 g, respectively (9). Seven individuals within each 5-y age category allowed us to detect differences in BMC between females with RTT and the reference population of 1 SD at a significance of 0.05 and power of 0.8.
Descriptive statistics were calculated using Minitab software (Version 11.0, Minitab Statistical Software, Inc., State College, PA). One-sample t-tests were used to detect differences in height, weight, BMI, BMC, and BMD z-scores between the RTT cohort and reference population (9,11). Linear regression was applied to characterize relations: 1) between methods used to estimate the proportion of body fat in the RTT cohort; 2) among the variables: lean body mass, body fat, bone age, total body BMC, total body BMD, and age, and 3) among the variables spine, hip, and total body BMC and BMD. Two-sample t-tests were used to detect differences in lean body mass, body fat, and BMC between girls with RTT and the reference population at age-specific intervals (9). Two-sample t-tests were used to detect differences in BMC and BMD z-scores within the RTT cohort, based on gene mutations, occurrence of fractures and scoliosis, seizure and medication history, and ambulatory status. Chi-squared analysis was used to detect differences in the occurrence of fractures and scoliosis between individuals with and without bone mineral deficits. Significance was determined at P<0.05.
RESULTS
Characteristics of the RTT Cohort
The age range of the RTT cohort was 2-38 y (Table 1). The racial and ethnic distribution was predominantly Caucasian. MECP2 mutations were identified in 90% of the cohort, the most common being missense and early truncation mutations. Height, weight, and BMI z=scores were <2SD in 50%, 48%, and 28%, respectively of the RTT cohort. Mean z-scores for height, weight, and BMI, were significantly lower than those of the reference population (11). Fractures and scoliosis occurred in 28% and 64% of the cohort, respectively. Fifty percent of the cohort used anticonvulsants and 74% were ambulatory at the time of study.
Table 1.
Characteristic features of girls and women (n=50) with Rett syndrome*
| Age interval | Age | Height | Height | Weight | Weight | BMI | BMI |
|---|---|---|---|---|---|---|---|
| (y) | (y) | (cm) | z-score** | (kg) | z-score** | (kg/m2) | z-score** |
| 0-5 (n = 9) | 3.4 ± 1.2 | 95.0 ± 9.7 | -0.4 ± 1.2 | 13.5 ± 3.2 | -0.9 ± 1.0 | 14.9 ± 1.5 | -0.9 ± 1.4 |
| 5-10 (n = 8) | 6.6 ± 1.1 | 113.5 ± 7.1 | -1.0 ± 0.7 | 20.6 ± 4.7 | -0.6 ± 0.9 | 15.8 ± 1.8 | 0.0 ± 1.0 |
| 10-14 (n = 7) | 11.5 ± 1.2 | 132.7 ± 9.8 | -2.2 ± 1.0 | 27.4 ± 7.1 | -2.5 ± 1.4 | 15.4 ± 2.5 | -1.5 ± 1.3 |
| 14-18 (n = 6) | 16.0 ± 1.0 | 139.5 ± 8.7 | -3.5 ± 1.3 | 37.4 ± 14.3 | -4.6 ± 4.8 | 18.9 ± 5.8 | -1.7 ± 3.3 |
| 18-24 (n = 7) | 21.7 ± 2.1 | 139.3 ± 9.4 | -3.7 ± 1.4 | 38.9 ± 7.1 | -3.7 ± 1.8 | 18.9 ± 4.1 | -2.0 ± 3.4 |
| 24-29 (n = 10) | 26.1 ± 1.5 | 146.9 ± 8.4 | -2.5 ± 1.3 | 45.9 ± 16.0 | -2.5 ± 2.2 | 21.2 ± 6.8 | -0.9 ± 1.7 |
| 29-37 (n = 3) | 33.7 ± 4.0 | 142.8 ± 2.5 | -3.1 ± 0.4 | 39.4 ± 8.4 | -3.6 ± 2.1 | 19.3 ± 4.2 | -1.3 ± 1.7 |
| Race/Ethnicity (%) | |
|---|---|
| Caucasian | 64 |
| African-American | 16 |
| Hispanic | 14 |
| Asian | 6 |
| MECP2 mutation(%)† | |
| Missense | 40 |
| Early truncation | 33 |
| Late truncation | 20 |
| Deletion | 7 |
| Fractures (%) | 28 |
| Scoliosis (%) | 64 |
| Anticonvulsant use (%) | |
| Used any time throughout lifespan | 70 |
| Used at the time of study | 50 |
| Ambulation (%) | |
| Ever walked during lifespan | 84 |
| Walked independently at study | 74 |
Expressed as mean ± SD where appropriate
P<0.05, different from the National Center for Health Statistics data (12)
Individuals with positive MECP2 mutation (n = 45)
Bone Mineral Content and Density Measures
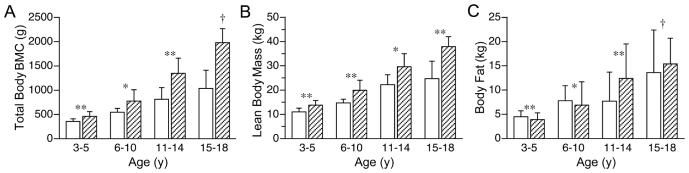
Total body BMC and BMD z scores were <-1 SD in at least three-fourths of the RTT cohort and <-2 SD in approximately one-half of the RTT cohort (Table 2). Mean z-scores for total body BMC and BMD were significantly lower in the RTT cohort than in the reference population (9). After adjusting for age, BMD, but not BMC, of African-American females with RTT tended to be greater than their Caucasian, Hispanic, and Asian counterparts (0.857 ± 0.081 vs. 0.786 ± 0.090, 0.789 ± 0.079, and 0.758 ± 0.082 g/cm 2, respectively; p<0.07). Total body BMC [BMC(g) = 32 ± 87 age(y) - 1.4 age(y) 2; p<0.001, r = 0.87] and BMD [BMD(g/cm2) = 0.54 + 0.025 age(y) - 0.0004 age(y) 2; p<0.001, r = 0.85] increased with advancing age. However, within each comparative age group, total body BMC was significantly lower in the RTT cohort than in the reference population (Figure 1a) (9). Total body BMC (Figure 2a) and BMD (Figure 2b) z-scores decreased significantly over the age range of the RTT cohort. Hip BMC (Figure 3a) and BMD (Figure 3b) were associated significantly with total body BMC and BMD, respectively. Spine BMC (p<0.001, r = 0.94) and BMD (p<0.001, r = 0.96) were associated with total body BMC and BMD, respectively. Bone age was similar to chronological age in individuals younger than age 20 y (p< 0.001, r = 0.97).
Table 2.
Characteristic features of body composition of girls and women (n = 50) with Rett syndrome
| Variable | Number of subjects* | Value** |
|---|---|---|
| Bone mineral content | ||
| Total body (g) | 49 | 850 ± 466 |
| (z-score) | 49 | -2.2 ± 1.0† |
| (% <-1 SD) | 49 | 84 |
| (% <-2 SD) | 49 | 59 |
| Spine (g) | 46 | 22 ± 13 |
| Hip (g) | 47 | 9.8 ± 5.8 |
| Bone mineral density | ||
| Total body (g/cm2) | 49 | 0.792 ± 0.140 |
| (z-score) | 49 | -1.7 ± 1.2† |
| (% <-1 SD) | 49 | 76 |
| (% <-2 SD) | 49 | 45 |
| Spine (g) | 46 | 0.597 ± 0.213 |
| Hip (g) | 47 | 0.471 ± 0.145 |
| Lean body mass (kg) | 46 | 19.6 ± 8.4 |
| (% body weight) | 46 | 66 ± 7 |
| Body fat (kg) by DXA | 46 | 10.1 ± 7.2 |
| (% body weight) | 46 | 31 + 7 |
| Body fat (kg) by anthropometry‡ | 50 | 10.1 ± 6.5 |
| (%body weight) | 50 | 30.9 ± 4.5 |
One individual unknowingly had metal devices in spine and hip; movement artifact prevented estimates of the components of body composition in some subjects
Values expressed as mean ± SD where appropriate
P<0.05, different from the reference population (10)
Determined from the sum of triceps, biceps, subscapular, and suprailiac skinfold thicknesses (13)
Figure 1.
Body composition of girls (n = 50) with Rett syndrome (□) and an age-matched reference population (▨): (a) total body BMC, (b) fat-free mass, (c) body fat; *p<0.05, **p<0.01, † p<0.001.
Figure 2.
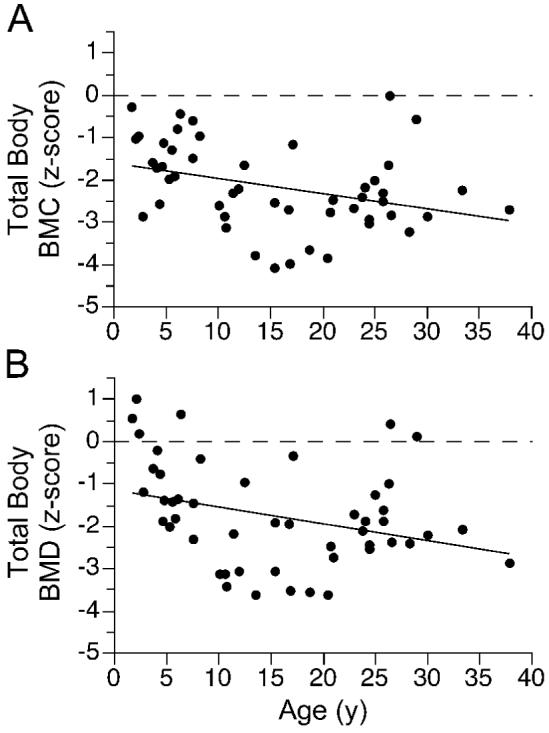
Relation between total body BMC or BMD and age in girls and women (n = 50) with Rett syndrome: (a) total body BMC(z-score) = -0.85 - 0.17 age(y) + 0.004 age2(y); p<0.01, r = -0.45; (b) total body BMD(z-score) = -0.12 - 0.22 age(y) + 0.005 age2(y); p<0.01, r = -0.46.
Figure 3.
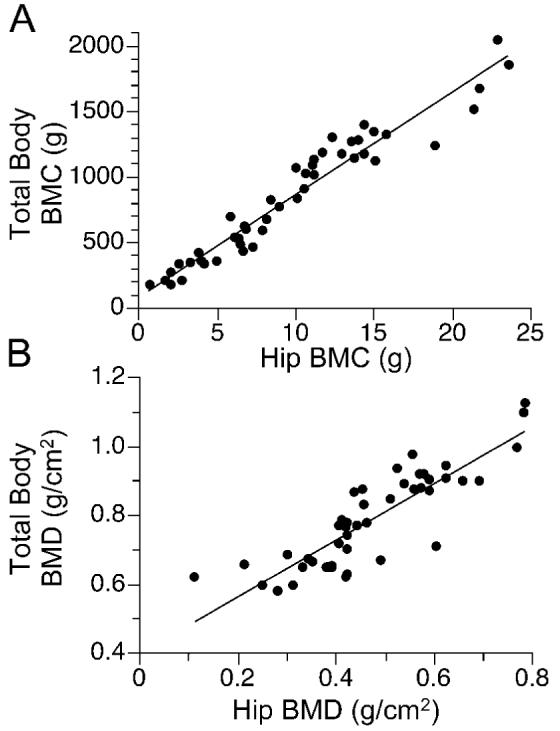
Relation between total body and hip BMC or BMD in girls and women (n = 50) with Rett syndrome: (a) total body BMC(g) = 89 + 78 hip BMC(g); p<0.001, r = 0.97]; (b) total body BMD(g/cm2) = 0.40 + 0.82 hip BMD(g/cm2); p<0.001, r = 0.86.
Body Composition Measures
Lean body mass and body fat comprised 66 ± 7% and 31 ± 7% of body weight, respectively, in the RTT cohort (Table 2). The proportion of body fat obtained by DXA correlated positively with that obtained from the thickness of four skinfolds (p< 0.001, r = 0.80). Lean body mass [Lean body mass(kg) = 3.74 ± 1.69 age(y) - 0.03 age(y) 2, p<0.001, r = 0.85] and body fat [body fat(kg) = 0.33 ± 1.09 age(y) - 0.02 age(y) 2; p<0.01, r = 0.56] increased with advancing age. However, within each age group, the lean body mass (Figure 1b), but not body fat (Figure 1c), was significantly lower in the RTT cohort than in the reference population (9). When expressed as a proportion of body weight, body fat was higher for girls with RTT, age 3-5 y (30 ± 4% vs. 21 ± 5%, p<0.001) and 6-10 y (33 ± 7% vs. 23 ± 8%, p<0.01) than that of the respective reference groups, but not for girls with RTT, age 11-14 y (23 ± 12% vs. 27 ± 8%) and 15-18 y (32 ± 9% vs. 27 ± 6%) (9). When adjusted for age, the accretion of bone mineral content was associated positively with the lean body mass (p<0.001, r = 0.97), but not body fat.
Mutational Analysis
Total body BMC and BMD z-scores were not significantly different among the classes of gene mutations.
Anticonvulsants and Ambulation
Total body BMC and BMD z-scores were significantly lower in those who had seizures than in those who did not (Table 3). Total body BMC and BMD z-scores were significantly lower in those who received anticonvulsants previously, but not at the time of study, than in those who did not. Total body BMC and BMD z-scores did not differ between females who ambulated independently and those who walked with assistance or never walked.
Table 3.
Total body BCM and BMD z-scores versus the presence or absence of skeletal fractures, scoliosis, seizures, anticonvulsant use, and ambulatory status in girls and women (n = 50) with Rett syndrome
| Variable | Number of subjects | BMC z-score* | BMD z-score* |
|---|---|---|---|
| Presence of seizures | |||
| Yes | 32 | -2.4 ± 1.0** | -2.0 ± 1.1 |
| No | 17 | -1.8 ± 0.9 | -1.3 ± 1.2 |
| Ever used anticonvulsants | |||
| Yes | 34 | -2.4 ± 0.9† | -2.1 ± 1.0† |
| No | 15 | -1.5 ± 0.9 | -1.0 ± 1.3 |
| Currently using anticonvulsants | |||
| Yes | 25 | -2.2 ± 1.2 | -1.8 ± 1.3 |
| No | 24 | -2.1 ± 0.8 | -1.6 ± 1.1 |
| Presence of scoliosis | |||
| Yes | 31 | -2.4 ± 1.0 | -2.0 ± 1.1** |
| No | 18 | -1.8 ± 1.0 | -1.3 ± 1.3 |
| Ever walked | |||
| Yes | 42 | -2.2 ± 1.0 | -1.9 ± 1.0 |
| No | 7 | -1.9 ± 1.2 | -0.6 ± 1.5 |
| Currently walks without assistance | |||
| Yes | 37 | -2.2 ± 0.9 | -1.9 ± 1.0 |
| No | 12 | -2.2 ± 1.3 | -1.2 ± 1.7 |
| Occurrence of skeletal fractures | |||
| Yes | 13 | -3.0 ± 0.9‡ | -2.6 ± 1.0† |
| No | 36 | -1.8 ± 0.9 | -1.4 ± 1.1 |
Values expressed as mean ± SD
P<0.05
P<0.01
P<0.001
Skeletal Fractures and Scoliosis
An age effect was apparent for both fractures and scoliosis in the RTT cohort; females with fractures (20.3 ± 6.7 vs. 13.6 ± 10.1 y, p<0.01) and/or scoliosis (18.0 ± 8.9 vs. 11.0 ± 9.5 y, p<0.01) were older. Fractures were found in 50% of females with, but only in 7% of those without, bone mineral deficits (X2 = 11.3, p<0.001). Total body BMC and BMD z-scores were significantly lower in those with than without fractures (Table 3). Fractures ranged from one to five per individual and occurred at variable sites. Scoliosis was found in 77% of those with, and in 52% of those without, bone mineral deficits (X2 = 3.3, p<0.07). Total body BMD, but not BMC, z-scores were significantly lower in those with than without scoliosis (Table 3).
DISCUSSION
The natural history of bone mineral deficits and bone-related disorders has not been characterized fully in females with RTT. In this cross-sectional study, low BMD was common, occurring in 45% of the RTT cohort. However, the variability across the age range studied precluded our ability to identify individual susceptibility. Although absolute BMC and BMD increased with advancing age, BMC and BMD z-scores were lower in older than in younger females. Skeletal fractures and scoliosis also were common, occurring in 28% and 64%, respectively, of the RTT cohort, and were associated with lower total body BMC and BMD. Bone mineral deficits were identified across a broad range of MECP2 mutations. These findings highlight the importance of early diagnosis of bone mineral deficits in RTT and underscore the need to better understand the molecular mechanisms of MECP2 in the regulation of bone mineral metabolism.
BMC in healthy, prepubertal and postpubertal children increases annually by 11 ± 2% and 4 ± 0.5%, respectively (16). In the present study, the rate of increase in total body BMC and BMD in females with RTT was substantially lower than that of healthy, unaffected girls, ages 8-16 y (9), but paralleled changes observed in children with other neurological disabilities (15,17). Others have reported deficits in cortical bone thickness, radial bone density, and tibial bone strength, measured by skeletal radiographs, densitometry, and ultrasonography in girls with RTT (3,18,19). Histomorphometric studies of trabecular bone in girls with RTT suggest that deficits in BMD may be the consequence of decreased bone formation rather than increased resorption because the number and metabolic activity of osteoblasts are decreased, while the number of osteoclasts may be normal or diminished (20). Thus, bone mineral deficits in RTT may be a manifestation of growth failure rather than a degenerative disorder (17).
Although bone mineral deficits were apparent at an early age, we found marked variability in total body BMC and BMD among the RTT cohort. Genetic factors are thought to be responsible for 70% of the variance in bone mass (21). However, the variability in total body BMC or BMD in the RTT cohort could not be explained by their mutations because the small number of subjects within each group precluded phenotype-genotype correlations. Others have suggested that R133C and T158M mutations confer a protective effect against bone mineral deficits (19), but we did not observe such an effect. In the present study, deficits in lean body mass, but not body fat, paralleled bone mineral deficits (22). Being African-American, as in unaffected individuals, tended to protect against low bone density (9). BMC and BMD z-scores were lower in individuals who had seizures and received anticonvulsants, both known risk factors (15).
Ambulation protects against bone mineral deficits in children with neurological disorders (23). In children with cerebral palsy, programs using upright stands, load-bearing physical activities, or vibrating platforms improved vertebral, femoral neck, and tibial BMD (24-26). In our study, however, total body BMC and BMD z-scores were not significantly different between those who ambulated independently and those who walked with assistance or never walked. The explanation for this finding was not readily apparent although, in contrast to other studies (23), body size did not differ between our ambulatory and non-ambulatory groups. Despite our findings, we routinely emphasize physical therapy for females with RTT to improve their functional abilities (27).
Low BMD is thought to contribute to fracture risk, although prospective measures of BMD in the lumbar spine of children with cerebral palsy did not predict subsequent fracture risk (29). In healthy girls, each 1 SD reduction of total body BMD doubles the risk for new fractures (30). The annual fracture rate for healthy females 6 years of age and older is 3 per 100 person-y (28) and for girls with spastic quadriplegia, 2.7 per 100 person-y (29). In the present study, one to five skeletal fractures occurred in 28% of the RTT cohort, resulting in a fracture rate of 3.6 per 100 person-y. Scoliosis occurred in 64% of the RTT cohort, a value in the range of other reports (31-34). However, we may have underestimated the prevalence of scoliosis and the severity of low bone mineral density in females with RTT because we excluded females with spinal rod placement. Nevertheless, for future DXA applications, the correlation among spine, hip, and total body BMC and BMD in the RTT cohort is of practical importance for those in whom repetitive movements or spinal rod placement preclude total body scans. The associations among low BMC and BMD, fractures, and scoliosis suggest that bone mineral metabolism is important for bone mineral health.
In summary, bone mineral deficits and bone-related disorders were common in RTT. Bone mineral deficits were greater in older than in younger females with RTT. Fractures and scoliosis were associated with lower BMC and BMD. Although factors commonly associated with protection from, or increased risk of, bone mineral deficits were identified, a better understanding of molecular mechanisms that regulate the interaction between MECP2 and bone mineral metabolism is crucial to the development of therapeutic strategies for RTT.
Acknowledgments
The authors thank J. Kenard Fraley and E. O'Brian Smith for assistance with data management and statistical analysis, and the families who permitted their daughters to participate in this study.
Financial Support: This work is a publication of the United States Department of Agriculture/Agricultural Research Service Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX. This project has been funded in part with federal funds from the Agricultural Research Service of the United States Department of Agriculture under Cooperative Agreement number 6250-51000; the National Institutes of Health, M01-RR00188 General Clinical Research Center; and with funds provided by the Rett Syndrome Association of Illinois and The Blue Bird Circle.
Abbreviations
- BMC
bone mineral content
- BMD
bone mineral density
- DXA
dual-energy x-ray absorptiometry
- MECP2
methyl-CpG-binding protein 2
- RTT
Rett syndrome
Footnotes
The contents of this publication do not necessarily reflect the views or policies of the United States Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States government.
REFERENCES
- 1.Haas RH, Dixon SD, Sartoris DJ, Hennessy MJ. Osteopenia in Rett syndrome. J Pediatr. 1997;131:771–774. doi: 10.1016/s0022-3476(97)70113-6. [DOI] [PubMed] [Google Scholar]
- 2.Leonard H, Thomson M, Bower C, Fyfe S, Constantinou J. Skeletal abnormalities in Rett syndrome: increasing evidence for dysmorphogenetic defects. Am J Med Genet. 1995;58:282–285. doi: 10.1002/ajmg.1320580316. [DOI] [PubMed] [Google Scholar]
- 3.Leonard H, Thomson MR, Glasson EJ, Fyfe S, Leonard S, Bower C, Christodoulou J, Ellaway C. A population-based approach to the investigation of osteopenia in Rett syndrome. Dev Med Child Neurol. 1999;41:323–328. doi: 10.1017/s0012162299000717. [DOI] [PubMed] [Google Scholar]
- 4.Amir RE, Van den Veyer IB, Schultz R, Malicki DM, Tran CQ, Dahle EJ, Philippi A, Timar L, Percy AK, Motil KJ, Lichtarge O, Smith EO, Glaze DG, Zoghbi HY. Influence of mutation type and X chromosome inactivation on Rett syndrome phenotypes. Ann Neurol. 2000;47:670–679. [PubMed] [Google Scholar]
- 5.Motil KJ, Schultz RJ, Abrams S, Ellis KJ, Glaze DG. Fractional calcium absorption is increased in girls with Rett syndrome 2006. J Pediatr Gastroenterol Nutr. 2006;42:419–426. doi: 10.1097/01.mpg.0000189370.22288.0c. [DOI] [PubMed] [Google Scholar]
- 6.Ellis KJ, Shypailo RJ, Hardin DS, Perez MD, Motil KJ, Wong WW, Abrams SA. Z score prediction model for assessment of bone mineral content in pediatric diseases. J Bone Miner Res. 2001;16:1658–1664. doi: 10.1359/jbmr.2001.16.9.1658. [DOI] [PubMed] [Google Scholar]
- 7.Trevathan E, Moser HW. Diagnostic criteria for Rett syndrome. Ann Neurol. 1988;23:425–428. doi: 10.1002/ana.410230432. [DOI] [PubMed] [Google Scholar]
- 8.Villard L. MECP2 mutations in males. J Med Genet. 2007;44:417–423. doi: 10.1136/jmg.2007.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis KJ, Abrams SA, Wong WW. Body composition of a young, multiethnic female population. Am J Clin Nutr. 1997;65:724–731. doi: 10.1093/ajcn/65.3.724. [DOI] [PubMed] [Google Scholar]
- 10.Huppke P, Held M, Hanefeld R, Engel W, Laccone F. Influence of mutation type and location on phenotype in 123 patients with Rett syndrome. Neuropediatrics. 2002;33:63–68. doi: 10.1055/s-2002-32365. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services National Center for Health Statistics; National Health and Nutrition Examination Survey. Retrieved from http://www.cdc.gov/growthcharts on January 28, 2008.
- 12.Durnin JV, Rahaman MM. The assessment of the amount of fat in the human body from measurements of skinfold thickness. Br J Nutr. 1967;21:681–689. doi: 10.1079/bjn19670070. [DOI] [PubMed] [Google Scholar]
- 13.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Stanford University; Palo Alto: 1959. [Google Scholar]
- 14.Compston JE. Bone density: BMC, BMD, or corrected BMD? Bone. 1995;16:5–7. doi: 10.1016/8756-3282(95)80004-A. [DOI] [PubMed] [Google Scholar]
- 15.Henderson RC, Lark RK, Gurka MJ, Worley G, Fung EB, Conaway M, Stallings VA, Stevenson RD. Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy. Pediatrics. 2002;110:e5. doi: 10.1542/peds.110.1.e5. [DOI] [PubMed] [Google Scholar]
- 16.Braillon PM. Annual changes in bone mineral content and body composition during growth. Horm Res. 2003;60:284–290. doi: 10.1159/000074246. [DOI] [PubMed] [Google Scholar]
- 17.Henderson RC, Kairalla JA, Barrington JW, Abbas A, Stevenson RD. Longitudinal changes in bone density in children and adolescents with moderate to severe cerebral palsy. J Pediatr. 2005;146:769–775. doi: 10.1016/j.jpeds.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Cepollaro C, Gonnelli S, Bruni D, Pacini S, Maritni S, Franci MB, Gennari L, Rossi S, Hayek G, Zappella M, Gennari C. Dual x-ray absorptiometry and bone ultrasonography in patients with Rett syndrome. Calcif Tissue Int. 2001;69:259–262. doi: 10.1007/s002230010027. [DOI] [PubMed] [Google Scholar]
- 19.Zysman L, Lotan M, Ben-Zeev B. Osteoprosis in Rett syndrome: a study on normal values. ScientificWorldJournal. 2006;6:1619–1630. doi: 10.1100/tsw.2006.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budden SS, Gunness ME. Possible mechanisms of osteopenia in Rett syndrome: bone histomorphometric studies. J Child Neurol. 2003;18:698–702. doi: 10.1177/08830738030180100401. [DOI] [PubMed] [Google Scholar]
- 21.Slemenda CW, Christian JC, Williams CJ, Norton JA, Johnston CC., Jr Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res. 1991;6:561–567. doi: 10.1002/jbmr.5650060606. [DOI] [PubMed] [Google Scholar]
- 22.Glastre C, Braillon P, David L, Cochat P, Meunier PJ, Delmas PD. Measurement of bone mineral content of the lumbar spine by dual energy x-ray absorptionetry in normal children: correlations with growth parameters. J Clin Endocrinol Metab. 1990;70:1330–1333. doi: 10.1210/jcem-70-5-1330. [DOI] [PubMed] [Google Scholar]
- 23.Chad KE, McKay HA, Zello GA, Bailey DA, Faulkner RA, Snyder RE. Body composition in nutritionally adequate ambulatory and non-ambulatory children with cerebral palsy and a healthy reference group. Dev Med Child Neurol. 2000;42:334–339. doi: 10.1017/s001216220000058x. [DOI] [PubMed] [Google Scholar]
- 24.Caulton JM, Ward KA, Alsop CW, Dunn G, Adams JE, Mughal MZ. A randomized controlled trial of standing programme on bone mineral density in non-ambulant children with cerebral palsy. Arch Dis Child. 2004;89:131–135. doi: 10.1136/adc.2002.009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chad KE, Bailey DA, McKay HA, Zello GA, Snyder RE. The effect of a weight-bearing physical activity program in bone mineral content and estimated volumetric density in children with spastic cerebral palsy. J Pediatr. 1999;135:115–117. doi: 10.1016/s0022-3476(99)70340-9. [DOI] [PubMed] [Google Scholar]
- 26.Ward K, Alsop C, Caulton J, Rubin C, Adams J, Mughal Z. Low magnitude mechanical loading is osteogenic in children with disabling conditions. J Bone Miner Res. 2004;19:360–369. doi: 10.1359/JBMR.040129. [DOI] [PubMed] [Google Scholar]
- 27.Lotan M, Isakov E, Merrick J. Improving functional skills and physical fitness in children with Rett syndrome. J Intellect Disabil Res. 2004;48:730–735. doi: 10.1111/j.1365-2788.2003.00589.x. [DOI] [PubMed] [Google Scholar]
- 28.Grazier KL, Holbrook TL, Kelsey JL, Stauffer RN. Amercan Academy of Orthopedic Surgeons. Chicago: 1984. The frequency of occurrence, impact, and cost of musculoskeletal conditions in the United States; pp. 72–80. [Google Scholar]
- 29.Henderson RC. Bone density and other possible predictors of fracture risk in children and adolescents with spastic quadriplegia. Dev Med Child Neurol. 1997;39:224–227. doi: 10.1111/j.1469-8749.1997.tb07415.x. [DOI] [PubMed] [Google Scholar]
- 30.Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res. 2000;15:2011–2018. doi: 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]
- 31.Kerr AM, Webb P, Prescott RJ, Milne Y. Results of surgery for scoliosis in Rett syndrome. J Child Neurol. 2003;18:703–708. doi: 10.1177/08830738030180101201. [DOI] [PubMed] [Google Scholar]
- 32.Harrison DJ, Webb PJ. Scoliosis in the Rett syndrome: natural history and treatment. Brain Dev. 1990;12:154–156. doi: 10.1016/s0387-7604(12)80200-2. [DOI] [PubMed] [Google Scholar]
- 33.Bassett GS, Tolo VT. The incidence and natural history of scoliosis in Rett syndrome. Dev Med Child Neurol. 1990;32:963–966. doi: 10.1111/j.1469-8749.1990.tb08118.x. [DOI] [PubMed] [Google Scholar]
- 34.Ager S, Fyfe S, Christodoulou J, Jacoby P, Schmitt L, Leonard H. Predictors of scoliosis in Rett syndrome. J Child Neurol. 2006;21:809–813. doi: 10.1177/08830738060210091501. [DOI] [PubMed] [Google Scholar]