Abstract
Fluid shear stress is the mechanical force generated by the blood flow which is applied over the apical surface of endothelial cells in situ. The findings of a recent study suggest that stress fibers and its associated focal adhesions play roles in mechano-signal transduction mechanism. Stress fibers are present along the apical and the basal portion of the endothelial cells. Endothelial cells respond to fluid shear stress and change their morphological characteristics in both their cell shape and cytoskeletal organization. Atherosclerosis is a common disease of the arteries and it occurs in areas around the branching site of blood vessels where the cells are exposed to low fluid shear stress. The organization of stress fibers and focal adhesions are strongly influenced by shear stress, and therefore the generation of atherosclerotic lesions seem to be associated with the cytoskeletal components of endothelial cells. This review describes the possible role of the cytoskeleton as a mechano-transducer in endothelial cells in situ.
Keywords: atherosclerosis, blood vessel, endothelial cell, cytoskeleton, stress fiber, focal adhesion
Introduction
Atherosclerosis is a common disease of the arterial blood vessels. It occurs when fatty foods, cholesterol, high blood sugar, and other substances accumulate in the walls of arteries form “plaques”. The area around the branching point of blood vessels is the specific location of atherosclerotic lesions. Many studies have addressed the development of atherosclerosis. Specifically, during the early atherosclerotic development stage, plaques tend to be organized at the branching point of the vessels where the blood flow speed is low or a disturbed flow is identified. Therefore, the hemodynamic force generated by the blood flow is thought to play an important role in the organization of atherosclerosis. A previous study demonstrated that the disturbed flow at the bifurcation point of arteries is a determinant for the development of arteriosclerosis, and the steady laminar flow of high fluid shear stress is atheroprotective (Berk 2008). However, it is still unclear how this disease develops in the regions of low blood flow low at the branching site of the blood vessels.
Endothelial cells are capable of responding to fluid shear stress by altering their morphology, the distribution of cytoskeletal components, expression of certain types of proteins, etc. In endothelial cells, actomyosin-based cytoskeletal components, called stress fibers, are observed both in vivo and in situ. In vivo experiments demonstrated that stress fibers in endothelial cells respond to fluid shear stress and increase in number and thickness. In an artificial coarctation zone, where shear stress is expected to be comparatively high, stress fibers in both the apical and basal portions dramatically increased in number and thickness (Kano et al 2000). Focal adhesion-like structures which are detected at the end of stress fibers are more enlarged in the coarctation area, especially in the apical surface of endothelial cells (Kano et al 2000). In addition, stress fibers and their intimate association sites with the plasma membrane are closely attached in both the apical and the basal portions of endothelial cells, and they seem to a play role in the transmission of fluid shear stress forces (Katoh et al 1995; Kano et al 1996). We showed that there are certain areas where the apical plasma membrane and apically located stress fibers are tightly connected at the lumina surface of endothelial cells. We call such areas “apical plaques”. They seem to play an important role for sensing signal transduction of fluid shear stress, because apical plaques located at the luminar surface of endothelial cells where the fluid shear stress directly applied (Kano et al 1996, 2000). Cytoskeletal organization and its distribution changes drastically according to the magnitude and the direction of fluid shear stress applied over the endothelial cells. A flow sensing structure might be located at the part of the lumina surface of the cell where the fluid shear stress directly applied. On the surface of endothelial cells, cytoskeletal components that directly connect between the apical plasma membrane and internal side of the cell, called “apical plaques” are observed. The apical plaque is site where the fluid shear stress is directly applied (Katoh et al 1995; Kano et al 1996, 2000).
Bundles of actin filaments are associated with the plasma membrane at sites where the cells form connections with the extracellular matrix or cell-cell association sites. Stress fibers are composed of actin filaments formed by the contractile interaction of actin, myosin, alpha-actinin, tropomyosin, to name a few forming a sarcomere-like structure (Byers et al 1984; Langanger et al 1986; Burridge et al 1988). Well-spread cells exert tension on the adhering extracellular matrix and their stress fibers were pre-extended (Dembo and Wang 1999; Lu et al 2008). Stress fibers directly terminate at focal adhesions where several structural proteins that connect the cell membrane to the underlying substrates are accumulated. Isolated stress fibers from cultured fibroblasts contracted in response to Mg2+-ATP with Ca2+ (Katoh et al 1998). The contraction of nonmuscle calls, including smooth muscle and stress fibers, is caused by the phosphorylation of a myosin light chain (MLC) by a calmodulin/myosin light chain kinase (MLCK) system in a calcium dependent manner. On the other hand, Rho (Ras homology) proteins are small GTPases that are involved in signal transduction in the cell. Rho-associated kinase (called Rho-kinase, ROCK2, Rokα) (Isenberg et al 1976; Leung et al 1996; Matsui et al 1996; Katoh et al 2007b) is effectors of Rho small GTPase. The activation of Rho-kinase, which is downstream of Rho, is known to modulate the organization of stress fibers and focal adhesions (Ridley and Hall 1992; Amano et al 1996a, 1996b, 1997; Katoh et al 2007b). Moreover, Rho-kinase either directly or indirectly phosphorylates the MLC in a Ca2+-independent manner in vitro system (Amano et al 1996a, 1996b). Our recent experiments revealed that the actomyosin contraction system could be regulated by at least two independent systems: namely, the one Ca2+-dependent calmodulin/MLCK system, while the other is the Ca2+-independent Rho-kinase system. Cell tension organized by stress fibers is essential for many kinds of cell behaviors, such as cell movement, anchoring cells to extracellular matrix, the development of embryos, mechano-signal transduction into the cell, and so on. Rho and its associated Rho-kinase seems to play important roles for the maintenance of endothelial cells in situ (van Nieuw Amerongen et al 2007, 2008; van Nieuw Amerongen and van Hinsbergh 2007; van der Heijden et al 2008).
Although many of stress fibers run along the basal portion of the spreading cells, some of these run from the basal to the apical portion of the cell (Katoh et al 1995). Such stress fibers have been observed in both cultured and in in situ cells. On the other hand, focal adhesions are closely associated with both the apical and basal portions of endothelial cells, therefore these stress fibers seem to play a role in the transducer mechanism for the outside-in signaling of endothelial cells (Kano et al 2000; Katoh et al 2007a; Hayakawa et al 2008). This review will discuss the contractile activity generated by stress fibers and its organization in the cell. This review will also discuss the possible role of stress fibers, together with their associated focal adhesions, as a mediator for mechano-signal transduction.
Stress fibers as a model for the actomyosin contractile system
The contractile mechanism of stress fibers has been previously reported by several researchers (Hoffmann-Berling 1954; Isenberg et al 1976; Kreis and Birchmeier 1980). A procedure has been established to isolate stress fibers from a cultured cell system without any loss of contractility (Katoh et al 1998; Kano et al 2000). Using an extraction solution containing Triton X-100 detergent, stress fibers can be isolated from cultured fibroblasts, which can be used as a model system to represent the nonmuscle actomyosin contractile system. This model was used to study the regulation of stress fiber contraction in vitro. Stress fibers have a contractile structure (Katoh et al 1998, 2000). The contraction of isolated stress fiber is dependent on Ca2+ and Mg2+/ATP. The phosphorylation of the MLC occurs when stress fibers are treated with Ca2+ and Mg-ATP. This MLC phosphorylation is inhibited by protein kinase inhibitors, ML-7, one of which is specific for MLCK. The contraction of isolated stress fibers is inhibited by ML-7, thus indicating that MLC is phosphorylated by MLCK in a Ca2+-dependent manner.
The contractility of the extracted cells and actomyosin containing structures of nonmuscle cells has been previously reported. The extent of contraction of smooth muscle cell is 38% of the original length (Fay et al 1982) and the speed of contraction is 10 μm/s (Small 1977). The nonmuscle contractile apparatus of the circumferential microfilament bundles in retinal pigmented epithelial cells represent 40% of the original length (Owaribe and Masuda 1982), the contractile ring of cells represent about 30% (Cande 1980; Mabuchi et al 1988), the kidney proximal tubules of the rat represent 20% (Murakami and Ishikawa 1991), or the brush border of the intestinal epithelium represent slight contraction (Rodewald et al 1976). Several investigators have indicated that stress fibers are truly contractile and they can indeed regulate the isomeric tension within the cell (Burridge 1981). A stress fiber model with Mg2+/ATP contracts to 60% of the original length in a micro-dissected model (Isenberg et al 1976), 80% in glycerol extracted cells (Kreis and Birchmeier 1980) and 75%∼80% in digitonin-extracted cell model (Kreis and Birchmeier 1980). The essential activity for the contraction of stress fibers must be caused by the phosphorylation of MLC. Phosphorylation of the MLC occurs when isolated stress fibers are treated with Ca2+ and Mg-ATP following isolation with the Triton X-100-based extraction solution. Under those conditions, phosphorylation of MLC is inhibited by protein kinase inhibitor, which is a specific inhibitor for MLCK (ML-7). The contraction of isolated stress fibers is completely inhibited by ML-7, thus indicating that MLC is phosphorylated by MLCK in a Ca2+-dependent manner.
Many stress fiber models isolated from fibroblastic cells, that are simply extracted, represent a limited extent of the contraction activity, showing only 70%∼80% of the original length. In our model, the completely isolated stress fibers contract to 23% of the original length (Katoh et al 1998). The speed of tail recoiling of living fibroblasts is 1.5 μm/s (average: maximum 3.5 μm/s) (Chen 1981). The extent of contraction of the stress fiber is more substantial than that observed for other types of cell contractility. Stress fibers thus demonstrate the fastest actomyosin based contractile system found in nonmuscle cells.
Rho A is a Rho-protein expressed in most types of the cell. Both the reorganization and contraction of stress fibers are observed when cultured fibroblasts are treated with LPA or bombesin (Ridley and Hall 1992, 1994). Because these drugs are Rho A activators, this response appears to be mediated by Rho A (Ridley and Hall 1992, 1994). Recent studies have also demonstrated the activation of Rho-kinase, which is a downstream regulator of Rho A, is observed in stress fibers (Katoh et al 2001a, 2001b). Moreover, both biochemical and immunofluorescence studies revealed Rho-kinase to have binding activity with myosin II and is thus located on the stress fibers (Kawabata et al 2004; Nakayama et al 2005). The above reports indicate that the Rho-kinase and myosin II work together on the stress fibers to control the contractile activity of the stress fiber. Biochemical studies also have shown that Rho-kinase phosphorylates the MLC of isolated smooth muscle fibers (Amano et al 1996b; Chihara et al 1997) and detergent extracted fibroblasts (Chihara et al 1997; Amano et al 1998). In vitro experiments have revealed that Rho-kinase directly phosphorylates MLC (Amano et al 1996b). Rho-kinase also known to dephosphorylate MLC indirectly with the inhibition of the myosin phosphatase activity (Amano et al 1996a). The de-phosphorylation of myosin phosphatase with active Rho-kinase causes the phosphorylation of MLC, thus resulting in the contraction of stress fibers (Katoh et al 2001a, 2001b). Stress fibers can be isolated by a glycerol-based extraction solution without Triton X-100. Stress fibers isolated with glycerol-based extraction buffer without detergent contract when Mg2+-ATP without Ca2+ is added. Since the calmodulin/MLCK contractile system essentially needs Ca2+ ion, this Ca2+ -independent contraction is caused by Rho-kinase activity (Katoh et al 2000, 2001b). The contraction of stress fibers isolated without detergent was not inhibited by MLCK inhibitors (Katoh et al 2001b). A biochemical and immunofluorescence analysis revealed that glycerol-isolated stress fibers contain both Rho A and Rho-kinase in the stress fiber. However, Triton X-100 isolated stress fiber did not contain Rho A and Rho-kinase, on the immunoblotting and immunofluorescence level. The above observations indicate that contraction of Ca2+-independent stress fibers contraction is simply caused by the Rho-kinase activity. Such contraction of stress fibers isolated without detergent is inhibited by the activity of Rho-kinase specific inhibitors, Y-27632. These results indicate the stress fiber contraction to be regulated independently in two ways: namely, a Ca2+-dependent calmodulin/MLCK system and a Ca2+-independent Rho-kinase system (Katoh et al 2001a).
Mechanism of stress fiber contraction in Ca2+-dependent and/or Ca2+-independent manner
As we mentioned above, stress fiber contraction is dually regulated: namely, by the Ca2+-dependent calmodulin/MLCK system and the Ca2+-dependent Rho-kinase system. Kinetic analyses of isolated stress fiber contraction suggest that the Ca2+-independent system causes a slower contraction (Katoh et al 2001a, 2001b). On the other hand, the Ca2+-dependent MLCK system causes a faster contraction than the Rho-kinase system. In the Rho-kinase system, both the speed and extent of contraction are less than that induced by the typical MLCK system. Two independent regulation systems exist in stress fibers together and seem to regulate different types of contraction. The calmodulin/MLCK contractile system supports transient and fast contraction. Hence, the Rho-kinase contractile system supports continuous and slow contraction. To understand the roles of MLCK and Rho-kinase for the contraction and the organization of stress fibers in living human foreskin fibroblasts, we expressed green fluorescent protein labeling actin, and treated with several MLCK or Rho-kinase inhibitors (Katoh et al 2007b). In result, the Rho-kinase inhibitors, HA1077 or Y-27632, caused a disassembly of the stress fibers that were located at the central portion of the cell within 1 hr. However, the stress fibers located in the cell periphery were not so severely affected by the Rho-kinase inhibitors. Fluorescent images revealed many thin stress fiber bundles, together with the focal adhesion at the both end of the stress fiber, at the center of the cell. These stress fibers are known as “central stress fibers” (Katoh et al 2001a). In addition, thicker stress fibers associated with focal adhesions are localized at the arched part of the lateral side of the cell. These stress fibers are known as the “peripheral stress fibers”. In both the phase contrast images and fluorescent images, the two types of stress fibers were easily distinguishable. Moreover, electron microscopy also revealed that these two types of fibers are typical stress fibers and are associated with focal adhesions, although the location and thickness are different.
The treatment of living cells with Rho-kinase inhibitor, Y-27632, specifically causes a disassembly of the stress fibers located at the central portion of the cell. The peripheral stress fibers are slightly reduced in thickness, however, they are hardly affected by the Rho-kinase inhibitor. The arched region at the leading edge of the cell gradually protrudes after the Rho-kinase inhibitor treatment, thus forming arches that are less accurate. The local movement of the plasma membrane at the cell periphery can be explained by the relaxation and loss of contractile tension at the cell. The relaxation of the cytoplasm and the cell cortex might be caused by the inhibition of Rho-kinase activity. When the activity of Rho-kinase is high, the cell tension generated by the contraction of stress fibers is respectively high. The activity of Rho-kinase seems to play a role mainly in controlling the cell tension generated by the contractile activity of stress fibers, which result in the transmission of fluid shear stress force applied over the surface of the cell to the inside of the cell.
Localization of stress fibers and focal adhesion in cultured and in situ cells
In cultured fibroblasts, analyses by confocal laser scanning microscopy have revealed that both stress fibers and focal adhesions are present along both the apical and the basal portion of the cell. Such stress fibers are found in cultured cell systems, including fibroblasts and endothelial cells and in in situ endothelial cells (Katoh et al 1995; Kano et al 1996, 2000). In addition to those present at the basal side of the cell, many stress fibers run from the basal side to the apical side of the cell. Such stress fibers are known as “apical stress fibers” and the macromolecular composition of these stress fibers is the same as that of general stress fibers (Katoh et al 1995; Kano et al 2000). These stress fibers attach themselves to the apical plasma membrane of the endothelial cell with plaque like structures, which contains major focal adhesion associated protein, vinculin. Detailed observations have revealed that the attachment site of the stress fibers at the apical plasma membrane thus forms focal adhesion like structures (Figure 1). When such attachment sites are stained with antibody against general focal adhesion associated proteins, such as vinculin, talin, integrin or fibronectin, they tend to show spotty plaque structures at the apical portion of the cell. These plaques are known as “apical plaques”. Several transmembrane proteins, such as integrin beta-1, fibronectin receptor and tyrosine-phosphorylated proteins are observed in apical plaque and such observations suggest that these apical plaque seems to act as a mechano-transducing structures at the apical surface of the cells. These apical stress fibers are not required by the cell to substrate adhesion sites, but rather they are needed to anchor the stress fibers to the apical plasma membrane in the cultured fibroblasts, because they are located at the dorsal side of the monolayer of endothelial cells. These apical stress fibers are attached to the plasma membrane in the apical portion of the cell principally in the same way as in basal focal adhesions. Basal focal adhesion can transmit outside-in signals from the extracellular matrix to the inside of the cell, so that the apical plaque thus seems to play a crucial role in the response to extracellular signaling, such as fluid shear stress applied over the endothelial cells.
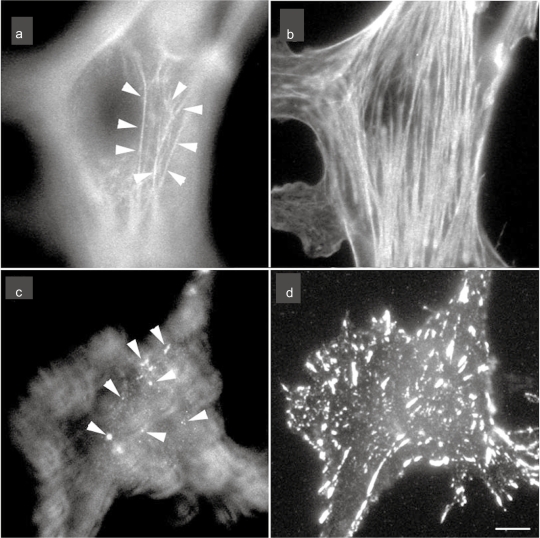
Figure 1.
The localization of stress fibers (a and d) and focal adhesion (c and d) in cultured endothelial cells. The cells were stained with rhodamine-labeled phalloidin for F-actin (stress fiber) visualization (a and b) or antivinculin for focal adhesion visualization (c and d). Micrographs are taken at two focal planes: a and c are adjusted at the apical portion of the cell; b and d are adjusted at the basal portion of the cell. Apical stress fibers are clearly visible (a; arrows) together with the typical basal stress fibers (b). Many of the anti-vinculin stained spots are observed at the apical portion of the cell (c; arrows) together with the staining of basal focal adhesions (d).
Note: Bar 20 μm.
Endothelial cells lining the inner surface of blood vessels are directly exposed to blood flow mechano-signals. Various types of endothelial cells are known to respond to the blood flow, for example, by changing their cell shape, cell migration and the re-organization of the stress fiber and focal adhesions. Many previous reports demonstrated that the mechanical forces generated by the blood flow have been shown to induce the redistribution of stress fibers and focal adhesions (Franke et al 1984; Ookawa et al 1992; Davies et al 1994; Girard and Nerem 1995). In both in situ and in vitro endothelial cells, under high fluid shear stress applied to cells, they organize thick stress fibers along the direction of the blood flow. In in situ endothelial cells, the shear stress generated by the blood flow is the frictional force that acts directly on the apical surface of the endothelial cells. Therefore, shear stress-related signal transduction is highly expected to initially start at sites where “chemical signaling events” caused by the mechanical signaling may be initiated. Therefore, one possible candidate to receive mechano-signaling by the blood flow is the “apical plaque”. Observations by confocal laser scanning microscopy revealed that thick stress fibers are present mainly along the basal portion of the cell. However, thin stress fibers are also found in the apical portion of the cell (Kano et al 1996). Such apical stress fibers observed in the endothelial cells run along the whole length of the cell in the same direction as the blood flow (Kano et al 1996, 2000) (Figure 2). Some stress fibers run vertically, connecting the basal and the apical side of the plasma membrane as in cultured fibroblasts (Katoh et al 1995). Focal adhesion associated proteins, such as vinculin, talin, paxillin or fibronectin receptors have been also located in both the basal and the apical portions of the cell-forming plaque (Kano et al 1996, 2000).
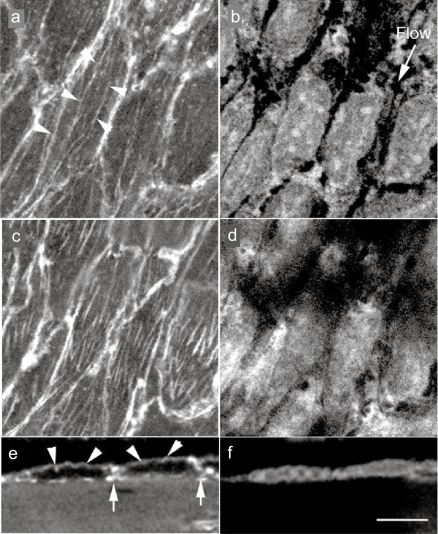
Figure 2.
Confocal laser scanning optical images of in situ endothelial cells in guinea pig aorta endothelial cells were doubly stained with rhodamine-labeled phalloidin for F-actin (stress fiber) visualization (a, c, and e) and with propidium iodide for nuclei (b, d, and f). Stress fibers are observed at both the apical side of the cell (arrows) and the basal side of the cell (b), along with the direction of the blood flow (b; flow). e and f; A side view of the endothelium. Apical stress fibers (e; arrows) and the F-actin accumulation of cell-cell apposition sites (e; arrows) are visible.
Note: Bar 20 μm.
Possible mechanisms of stress fibers as a mediator for mechano-signaling
Fluid shear stress is a determinant of endothelial cell morphology. It is definite that the laminar fluid flow and the strong fluid shear stress induces elongation of endothelial cells and directional stress fiber organization along with the direction of blood flow. On the other hand, endothelial cells applied to disturbed flow or low fluid shear stress showed cobblestone like morphology and exhibit loosely arranged stress fibers in the cell. Cell shape and cytoskeletal arrangement of endothelial cells is highly dependent of the magnitude of fluid shear stress. It is well known that the endothelial cells at the atherosclerosis lesion shows much reduced endothelial cell polarized morphology. In this area, atherosclerosis develops at the branching site of the aorta showing earliest fatty streaks. At the branching site, endothelial cells suffered high shear stress due to the laminar blood flow, thereby protecting them from forming fatty streak organization, and therefore this area is considered to be an atheroprotective region (Berk 2008; Vartanian et al 2008). On the other hand, in the disturbed flow area, endothelial cells showed a cobblestone like morphology without any developed stress fibers. Cell apoptosis are also observed in disturbed flow area (Tardy et al 1997; Tricot et al 2000). In many case, atherosclerosis lesions develop in the area where the fluid shear stress is low, so that the low shear stress area is atherogenic. In this region, the elongation of endothelial cells and the organization of cytoskeletal components, such as stress fibers and focal adhesions, are highly prohibited. The above observations strongly suggest that the down regulation in the organization of certain cytoskeletal components by low fluid shear stress seems to be earlier event for the development of atherosclerosis lesions.
Endothelial elongation and the distribution of cytoskeletal components are not simply determined by the magnitude fluid shear stress (Katoh et al 2007b; van Nieuw Amerongen et al 2007, 2008; Vartanian et al 2008). The cells applied a sustained and uni-directional flow in venous endothelial cells showed well elongated morphology, but organized small number of stress fibers (Katoh et al 2007a). Endothelial cells cultured on micropatterning substrates showed cell shape change independently with fluid shear stress (Vartanian et al 2008). Thrombin stimulation induces stress fiber organization and cell elongation in endothelial cells (van Nieuw Amerongen et al 2008). Mechanical cyclic stretch also causes morphological changes and induces cytoskeletal organization (Naruse et al 1998; Kakisis et al 2004). However, at least in in situ arterial endothelial cells, major determinant of endothelial cell morphology seems to be fluid shear stress.
Our recent study showed direct evidence of a disturbed flow at the bifurcation point of the common iliac artery in living animals using an ultrasound micro imaging system (Katoh et al 2007a). Even in the aorta, not all endothelial cells are exposed to the same blood flow. Observations of the flow pattern in the aorta demonstrated that there are certain areas where a disturbed blood flow is generated (Davies et al 2003; VanderLaan et al 2004; Katoh et al 2007a). As we already mentioned, the organization of stress fibers and focal adhesions changes according to the magnitude of fluid shear stress in aortic endothelial cells (Davies et al 1994, 2003) (Figure 3). A surgical coarctation made at the straight portion of abdominal aorta, where the artificially high shear stress is applied over endothelial cell in in situ, increases the number and size of both stress fibers and focal adhesions (Kano et al 2000). Even under physiological conditions, the localization and distribution of stress fibers and focal adhesions seem to be affected by the power of the local fluid shear stress force (Katoh et al 2007a).
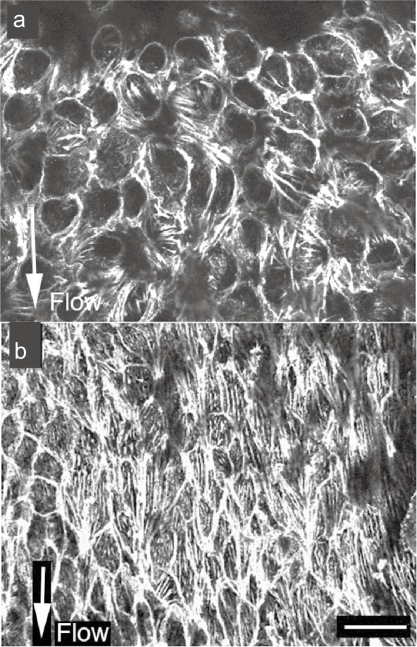
Figure 3.
Stress fibers in in situ endothelial cells in a low blood flow area (a) and a high blood flow area (b). In the low blood flow area, cell shape is circular and the expression of stress fibers is low (a). In the direction of high blood flow, the endothelial cells are elongated and the expression of stress fibers is increased along the direction of the blood flow (b).
Note: Bar 20 μm.
Tyrosine residues of certain proteins involved in signal transduction are phosphorylated when the proteins are functionally activated. The level of activation of tyrosine residues in in situ endothelial cells is a reflection of the signal transduction of a local portion of the blood vessel. In high shear stress areas, at both the bifurcation point of the artery or a region of surgical coarctation, the expression level of phosphotyrosine containing proteins, such as c-Src and platelet endothelial cell adhesion molecule-1 (PECAM-1) are strictly increased at the cell-cell apposition sites (Kano et al 2000; Osawa et al 2002; Katoh et al 2007a). This suggests that the area of cell-cell appositions in endothelial cells, where apical stress are fibers directly connected, is a 1 site of mechano-signal transduction (Kano et al 2000; Osawa et al 2002). PECAM-1 and c-Src are proteins that are tyrosine phosphorylated in a shear stress-dependent manner. Both proteins are observed at the cell-cell apposition sites in in situ endothelial cells, especially in the high shear stress areas at the branching point and a surgical coarctation zone of the aorta. Upregulation of PECAM-1 and c-Src seems to have a key role in the organization of stress fibers and focal adhesions in aortic endothelial cells. Further experiments are expected to solve this signal-transduction mechanisms in endothelial cells.
In the straight abdominal aorta, the blood flow pattern applied over the endothelial cells is considered to be laminar. However, a disturbed flow around the branching point is expected to generate atherosclerosis disease. Endothelial cells at the disturbed flow in the branching side of blood vessel are exposed to lower shear stress than the straight portion of the aorta. It is possible that the fluid shear stress generated by the blood flow is transmitted directly from the apical plaque (focal adhesion-like structures) to the focal adhesions at the basal side of the endothelial cells and/or from the lateral cell to cell apposition sites via the stress fibers. This strongly suggests that the apical plaques and their associated stress fibers are possible sites where signal transduction takes place (Kano et al 2000).
As we mentioned previously, the macromolecular composition of the “apical plaque” is essentially the same as that for the focal adhesions observed at the basal portion of the cell in both in vitro and in situ cell systems. The apical stress fibers in both cultured and in situ cells are firmly anchored to the plasma membrane at the areas of apical plaques with focal adhesion-associated proteins. Using cultured cell systems, other researchers have demonstrated direct evidence that the stress fibers transmit mechanical stimuli from the outside to the inside of the cell (Hayakawa et al 2008). The cellular structure to transmit mechanical force from the apical to the basal portion of the cell must be relatively stiff and should be easily controlled according to the response to the mechanical stimuli (see the schematic illustration in Figure 4 and Figure 5). The apical plaque and apical stress fibers may work together as “mechano-transducers” which thus directly transmit a mechanical force applied over the apical surface of the endothelial cell to the internal side of the cell.
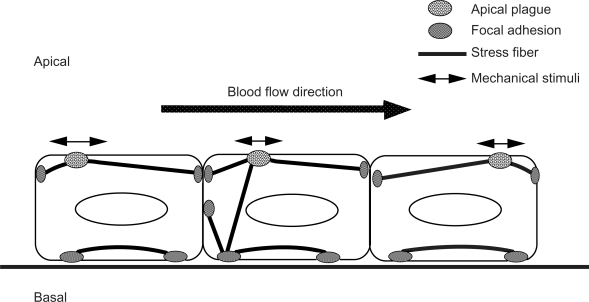
Figure 4.
Schematic illustrations of the stress fibers, focal adhesions, and apical plaques in endothelial cells. Stress fibers run not only on the basal side of the cell, but they also run from the basal side to the apical portion of the cell. Focal adhesion-like structures, called “apical plaques” are localized at the apical side of the endothelial cells in situ.
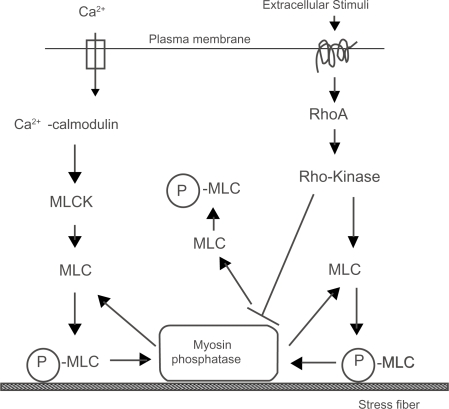
Figure 5.
A schematic illustration of two regulatory systems for actomyosin contraction in nonmuscle cells. Phosphorylated MLC (P-MLC) induces contraction and the organization of stress fibers.
Note: *Active Rho-kinase inhibit the activity of myosin phosphatase result in P-MLC.
Conclusion
The majority of the stress fibers are located within the basal side of endothelial cells, and both their ends are tightly associated with the focal adhesion. Among the basally located stress fibers, some stress fibers are located along the apical plasma membrane of the endothelial cells via specialized attachment sites called “apical plaques”. Isometrically contractile stress fibers can form hard connections between apical plaques and focal adhesions located at the basal side of the cells. Such connections have possibility to transmit mechanical force to the focal adhesion and cell-cell adhesion sites where signaling events can be initiated. Since the apical plaques and apical stress fibers are located at the surface of endothelial cells in situ, they seems to act as transducer of mechanical stimuli generated by the blood flow. Apical plaques include the several kinds of signal transduction-associated proteins, such as phosphotyrosine containing proteins, focal adhesion kinase and c-Src, so that the apical plaque and its associated stress fibers together seem to play an important role in the mechano-signal transduction mechanism in endothelial cells in situ.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- Amano M, Chihara K, Kimura K, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–11. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Nakamura N, et al. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–88. doi: 10.1046/j.1365-2443.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996b;271:20246–9. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M, Mukai H, Ono Y, et al. Identification of a putative target for Rho as the serine-threonin kinase protein kinase N. Science. 1996a;271:648–50. doi: 10.1126/science.271.5249.648. [DOI] [PubMed] [Google Scholar]
- Berk B. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation. 2008;117:1082–9. doi: 10.1161/CIRCULATIONAHA.107.720730. [DOI] [PubMed] [Google Scholar]
- Burridge K. Are stress fibers contractile? Nature. 1981;294:691–2. doi: 10.1038/294691a0. [DOI] [PubMed] [Google Scholar]
- Burridge K, Fath K, Kelly T, et al. Focal adhesions: Transmembrane junctions between the extracellular matrix and the cytoskeleton. Ann Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Byers HR, White GE, Fujiwara K. Organization and function of stress fibers in cells in vitro and in situ. Cell Muscle Motil. 1984;5:83–137. doi: 10.1007/978-1-4684-4592-3_2. [DOI] [PubMed] [Google Scholar]
- Cande WZ. A permeabilized cell model for studying cytokinesis using mammalian tissue culture cells. J Cell Biol. 1980;88:326–35. doi: 10.1083/jcb.87.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. Mechanism of retraction of the trailing edge during fibroblast movement. J Cell Biol. 1981;90:187–200. doi: 10.1083/jcb.90.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara K, Amano M, Nakamura N, et al. Cytoskeletal rearrangements and transcriptional activation of c-fos serum response element by Rho-kinase. J Biol Chem. 1997;272:25121–7. doi: 10.1074/jbc.272.40.25121. [DOI] [PubMed] [Google Scholar]
- Davies PF, Robotewskyj A, Griem ML. Quantitative studies of endothelial cell adhesion. Directional remodeling of focal adhesion sites in response to flow forces. J Clin Invest. 1994;93:2031–8. doi: 10.1172/JCI117197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PF, Zilberberg J, Helmke BP. Spatial microstimuli in endothelial mechanosignaling. Circ Res. 2003;92:359–70. doi: 10.1161/01.RES.0000060201.41923.88. [DOI] [PubMed] [Google Scholar]
- Dembo M, Wang WJ. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–16. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay FS, Fogarty K, Fujiwara K, et al. Contractile mechanism of single isolated smooth muscle cells. In: Dewey BM, editor. Basic Biology of Muscles: A Comparative Approach. New York: Raven Press; 1982. pp. 143–57. [PubMed] [Google Scholar]
- Franke RP, Gräfe M, Schnittler H, et al. Induction of human vascular endothelial stress fibers by fluid shear stress. Nature. 1984;16:648–50. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- Girard PG, Nerem RM. Shear stress modulates endothelial cell morphology and F-actin organization through and regulation of focal adhesion-associated proteins. J Cell Physiol. 1995;163:179–93. doi: 10.1002/jcp.1041630121. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Tatsumi H, Sokabe M. Actin stress fibers transmit and focus force to activate mechanosensitive channels. J Cell Sci. 2008;121:496–503. doi: 10.1242/jcs.022053. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Berling H. Adenosintriphosphat als Betriebsstoff von Zellbewegungen. Biochim biophys Acta (Amst) 1954;14:182–94. doi: 10.1016/0006-3002(54)90157-2. [DOI] [PubMed] [Google Scholar]
- Isenberg G, Rathke PC, Hülsmann N, et al. Cytoplasmic actomyosin fibrils in tissue culture cells. Direct proof of contractility by visualization of ATP-induced contraction in fibrils isolated by laser microbeam dissection. Cell Tissue Res. 1976;166:427–43. doi: 10.1007/BF00225909. [DOI] [PubMed] [Google Scholar]
- Kakisis JD, Liapis CD, Sumpio BE. Effects of cyclic strain on vascular cells. Endothelium. 2004;11:17–28. doi: 10.1080/10623320490432452. [DOI] [PubMed] [Google Scholar]
- Kano Y, Katoh K, Fujiwara K. Lateral zone of cell-cell adhesion as the major fluid shear stress-related signal transduction site. Circ Res. 2000;86:425–33. doi: 10.1161/01.res.86.4.425. [DOI] [PubMed] [Google Scholar]
- Kano Y, Katoh K, Masuda M, et al. Macromolecular composition of stress fiber-plasma membrane attachment sites in endothelial cells in situ. Circ Res. 1996;79:1000–6. doi: 10.1161/01.res.79.5.1000. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, et al. Stress fiber organization regulated by MLCK and Rho-kinase in cultured human fibroblast. Am J Cell Physiol. 2001a;280:C1669–79. doi: 10.1152/ajpcell.2001.280.6.C1669. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, et al. Rho-kinase -mediated contraction of isolated stress fibers. J Cell Biol. 2001b;153:569–83. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Fujiwara K. Isolation and in vitro contraction of stress fibers. Meth Enzym. 2000;325:369–80. doi: 10.1016/s0076-6879(00)25458-x. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Masuda M, et al. Isolation and contraction of the stress fiber. Mol Biol Cell. 1998;9:1919–38. doi: 10.1091/mbc.9.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Ookawara S. Morphological differences between guinea pig aortic and venous endothelial cells in situ. Cell Biol Int. 2007a;31:554–64. doi: 10.1016/j.cellbi.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Ookawara S. Rho-kinase dependent organization of stress fibers and focal adhesions in cultured fibroblasts. Genes Cells. 2007b;12:623–38. doi: 10.1111/j.1365-2443.2007.01073.x. [DOI] [PubMed] [Google Scholar]
- Katoh K, Masuda M, Kano Y, et al. Focal adhesion proteins associated with apical stress fibers of human fibroblasts. Cell Motil Cytoskel. 1995;31:177–95. doi: 10.1002/cm.970310302. [DOI] [PubMed] [Google Scholar]
- Kawabata S, Usukura J, Moroe N, et al. Interaction of Rho-kinase with myosin II at stress fibers. Genes Cells. 2004;9:653–60. doi: 10.1111/j.1356-9597.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- Kreis TI, Birchmeier W. Stress fiber sarcomeres of fibroblasts are contractile. Cell. 1980;22:555–61. doi: 10.1016/0092-8674(80)90365-7. [DOI] [PubMed] [Google Scholar]
- Langanger G, Moreremans M, Daneels G, et al. The molecular organization of myosin in stress fibers of cultured cells. J Cell Biol. 1986;102:200–9. doi: 10.1083/jcb.102.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, et al. The p160 RhoA-binding linase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–27. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Feng Y, Hucker W, et al. Actin stress fiber pre-extension in human aortic endothelial cells. Cell Motil Cytoskeleton. 2008;65:281–94. doi: 10.1002/cm.20260. [DOI] [PubMed] [Google Scholar]
- Mabuchi I, Tsukita S, Tsukita S, et al. Cleavage furrow isolated from newt eggs: Contraction, organization of the actin filaments, and protein components of the furrow. Proc Natl Acad Sci U S A. 1988;85:5996–70. doi: 10.1073/pnas.85.16.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–16. [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Ishikawa H. Stress fibers in situ in proximal tubules of the rat kidney. Cell Struct Funct. 1991;16:231–40. doi: 10.1247/csf.16.231. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Amano M, Katsumi A, et al. Rho-kinase and myosin II activities are required for cell type and environment specific migration. Genes Cells. 2005;10:107–17. doi: 10.1111/j.1365-2443.2005.00823.x. [DOI] [PubMed] [Google Scholar]
- Naruse K, Yamada T, Sokabe M. Involvement of SA channels in orienting response of cultured endothelial cells to cyclic stretch. Am J Physiol. 1998;274:H1532–8. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- Ookawa K, Sato M, Ohshima N. Changes in the microstructure of cultured porcine aortic endothelial cells in the early stage after applying a fluid-imposed shear stress. J Biomech. 1992;25:1321–8. doi: 10.1016/0021-9290(92)90287-b. [DOI] [PubMed] [Google Scholar]
- Osawa M, Masuda M, Kusano K, et al. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773–85. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaribe K, Masuda H. Isolation and characterization of circumferential microfilament bundles from retinal pigmented epithelial cells. J Cell Biol. 1982;95:310–5. doi: 10.1083/jcb.95.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in responce to growth factors. Cell. 1992;70:389–99. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. Signal transduction pathways regulating Rho-mediated stress fiber formation: requirement for a tyrosine kinase. EMBO J. 1994;13:2600–10. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R, Newman SB, Karnovsky MJ. Contraction of isolated brush borders from the intestinal epithelium. J Cell Biol. 1976;70:541–54. doi: 10.1083/jcb.70.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV. Studies on isolated smooth muscle cells: The contractile apparatus. J Cell Sci. 1977;24:327–49. doi: 10.1242/jcs.24.1.327. [DOI] [PubMed] [Google Scholar]
- Tardy Y, Resnick N, Nagel T, et al. Shear stress gradients remodel endothelial monolayers in vitro via a cell proliferation-migration-loss cycle. Arterioscler Thromb Vasc Biol. 1997;17:3102–6. doi: 10.1161/01.atv.17.11.3102. [DOI] [PubMed] [Google Scholar]
- Tricot O, Mallat Z, Heymes C, et al. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000;101:2450–3. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- van der Heijden M, Versteilen A, Sipkema P, et al. Rho-kinase-dependent F-actin rearrangement is involved in the inhibition of PI3-kinase/Akt during ischemia-reperfusion-induced endothelial cell apoptosis. Apoptosis. 2008;13:404–12. doi: 10.1007/s10495-007-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuw Amerongen G, Beckers C, Achekar I, et al. Involvement of Rho kinase in endothelial barrier maintenance. Arterioscler Thromb Vasc Biol. 2007;27:2332–9. doi: 10.1161/ATVBAHA.107.152322. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen G, Musters R, Eringa E, et al. Thrombin-induced endothelial barrier disruption in intact microvessels: role of RhoA/Rho kinase-myosin phosphatase axis. Am J Physiol Cell Physiol. 2008;294:C1234–41. doi: 10.1152/ajpcell.00551.2007. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen G, van Hinsbergh V. Endogenous RhoA inhibitor protects endothelial barrier. Circ Res. 2007;101:7–9. doi: 10.1161/CIRCRESAHA.107.156513. [DOI] [PubMed] [Google Scholar]
- VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- Vartanian K, Kirkpatrick S, Hanson S, et al. Endothelial cell cytoskeletal alignment independent of fluid shear stress on micropatterned surfaces. Biochem Biophys Res Commun. 2008;371:787–92. doi: 10.1016/j.bbrc.2008.04.167. [DOI] [PubMed] [Google Scholar]