Abstract
BACKGROUND:
Statin medication exhibits pleiotropic properties, such as improvement of endothelial function.
AIM:
To determine whether a high loading dose of atorvastatin prescribed before and after coronary artery bypass graft (CABG) surgery will attenuate the inflammatory response reflected in kinetic concentrations of C-reactive protein (CRP).
METHODS:
The individual area under the concentration-time curve (AUC) of CRP concentration was calculated for the first five days after CABG surgery and compared among three groups of patients: group A patients (n=16), who were on chronic statin therapy, were switched to an equivalent therapy of 20 mg atorvastatin daily for 120 h; group B patients (n=15), who were on chronic statin therapy, were switched to 80 mg atorvastatin daily (one dose 24 h before CABG surgery, one on the day of surgery and two further doses after surgery) followed by 40 mg/day up to 120 h after surgery; and group C patients (n=10), who were naive to statin therapy, underwent elective CABG surgery.
RESULTS:
The three groups were comparable according to measurements of their intra- and postoperative variables, except for their mean weight. The mean (± SEM) AUC-CRP for group B was 13,545±959.9 mg/L·h, significantly smaller (P=0.01) than that for group A (17,085±858.4 mg/L·h). In group C (statin-naïve patients), the AUC-CRP was 16,191±1447 mg/L·h, which was not significantly different from groups A and B, respectively.
CONCLUSIONS:
High loading doses of atorvastatin before CABG surgery reduced CRP concentration, expressed as AUC-CRP. This effect supports the idea that a high dose of atorvastatin is needed to attenuate the ‘negative’ inflammatory response. The present study also lends support to the possibility that high-dose atorvastatin positively improves post-open-heart surgery results.
Keywords: Coronary surgery, C-reactive protein, Statin
Elevated levels of C-reactive protein (CRP) has emerged as one of the most powerful independent predictors of myocardial infarction, development of heart failure after myocardial infarction, stroke and vascular death, with prognostic value exceeding that of low-density lipoprotein (LDL) cholesterol. In addition to being a powerful risk marker, recent evidence suggests that CRP may directly participate in lesion formation through leukocyte activation and endothelial dysfunction (1,2).
CRP facilitates endothelial cell apoptosis and inhibits angiogenesis, while promoting activation of endothelial nuclear factor-kappa B (3). Data suggest that the local concentration of CRP in atherosclerotic plaques may be much higher than the circulating levels of CRP in humans. Furthermore, the expressed CRP could act locally on endothelial monocytes/macrophages or smooth muscle cells, in an autocrine or paracrine manner, promoting atherosclerosis (4). High concentrations of CRP messenger RNA have been demonstrated to be present in situ in the atherosclerotic plaques bound to oxidized LDL, thus enhancing the ability of macrophages to phagocytize LDL and form foam cells through the CRP receptor CD32 (5–7). Other studies (8) were unable to provide evidence for or against this statement.
The half-life of CRP was established to be 19 h and is constant under all conditions of health and disease; therefore, the sole determinant of circulating CRP level represents the synthesis rate (9).
The 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, simply known as ‘statins’, are postulated to exhibit pleiotropic, nonlipid properties such as improvement of endothelial function, plaque stabilization and reduction of oxidative stress in vascular inflammation (10,11). These effects start approximately 24 h after the initiation of therapy, which suggests that an active pleiotropic effect and not a lipid-lowering effect is the underlying mechanism of action (12,13).
Several retrospective studies (14–17) have demonstrated that patients undergoing open heart surgery while on statin therapy had reduced postoperative morbidity and mortality. High CRP serum concentration measured before coronary artery bypass graft (CABG) surgery can predict early recurrence of an acute ischemic event (18) and late graft restenosis after surgery (19).
Atorvastatin, a well-known statin, is given in the acid form and is completely absorbed after oral administration. However, the drug has an extensive CYP3A-dependent first-pass metabolism with an oral bioavailability of 14%. It has an apparent volume of distribution of 5.4 L/kg with 98% protein binding, and its elimination half-life is approximately 7 h (20).
The aim of the present study was to investigate whether a high loading dose of atorvastatin has a lowering effect on the kinetic concentrations of the proinflammatory marker CRP, represented by the area under the concentration-time curve (AUC) of the individual CRP serum concentration depicted in patients undergoing elective CABG surgery for coronary artery disease.
MATERIAL AND METHODS
Study design
The protocol was approved by the Rambam Health Care Campus (Haifa, Israel) Institutional Review Board (approval number 1878). The study enrolled 41 patients with stable angina pectoris scheduled for nonemergency CABG on-pump surgery. The last patient was enrolled during the last week of July 2004. Patients with any type of inflammatory response including seropositive rheumatoid arthritis, active infection, uncontrolled arterial hypertension and uncontrolled hyperglycemia were excluded (8).
Surgical procedure
Antegrade and retrograde blood cardioplegia was used for myocardial preservation. The operation was carried out under mild hypothermia (32°C to 34°C). The initial pump flow rate was aimed toward 2 L/m2/min to 2.5 L/m2/min, and pump flow was adjusted gradually to maintain a mean arterial pressure between 50 mmHg and 70 mmHg.
Study groups
The eligible patients were divided randomly into two groups:
Group A:
Sixteen patients who were on ‘standard’ statin treatment, usually simvastatin or pravastatin (20 mg/day to 40 mg/day) for at least four weeks before surgery were included; their dose was switched to atorvastatin 20 mg, 24 h before and on the day of surgery, continued up to 120 h after CABG surgery.
Group B:
Fifteen patients identical to group A patients but who received four high-loading doses of 80 mg/day of atorvastatin – one dose 24 h before CABG surgery, one on the morning of surgery, and two further doses on the next day and 48 h after CABG surgery – followed by 40 mg/day up to 120 h after surgery.
Group C:
Ten patients naive to statin therapy underwent elective CABG surgery, as in groups A and B. These subjects were considered the second ‘control’ group for the CRP kinetics.
Blood sampling for CRP determinations
Venous blood samples (2.5 mL) were collected for CRP determination in groups A and B, with the starting dose of atorvastatin and then at 24 h, 48 h, 72 h and 120 h after surgery. For group C, blood for CRP determinations was collected before starting the surgical procedure (baseline) and at the same intervals as for groups A and B. Blood samples were centrifuged at 2000 g for 7 min within 45 min of collection. Serum was stored at −40°C until analysis. Serum CRP levels were measured using high-sensitive latex-enhanced immunonephelometry on a Behring BN-ProSec Nephelometer (Dade Behring, Germany). In this assay, polystyrene beads coated with mouse monoclonal antibodies bind CRP contained in a serum sample to form aggregates. The lower limit of detection for this technique was 0.175 mg/L. The upper limit of CRP ‘normal’ value was considered to be less than 5 mg/L. Its intra-assay variation is 3.1%, 3.6%, 3.4% and 4.2% for CRP concentrations of 0.5 mg/L, 1.1 mg/L, 2.1 mg/L and 62 mg/L, respectively. The interassay variability has been established to be 2.5%, 3.2%, 2.1% and 5.2% for the same concentrations, respectively. The laboratory and the CRP kinetic and statistical analysis teams were blinded to patient therapy.
CRP kinetic analysis
The AUC-CRP (mg/L·h) after surgery was calculated from the individual CRP blood concentrations by noncompartmental kinetic analysis using the Kinetica 2000 software (Micropharm International, USA). This method uses model-independent equations, making the assumption that the terminal elimination process can be approximated by an exponential equation. This is equivalent to assuming that a straight line can approximate the logarithmic transformation of the data belonging to the terminal elimination phase of the drug. This pharmacokinetic program also allowed the determination of the time to maximum concentration (h), CRP maximum concentration (mg/L) and the CRP minimum concentration (mg/L).
Statistical analysis when indicated was performed with the Prism3.0 software (GraphPad Software Inc, USA). Unpaired t tests and Mann-Whitney U tests were used for comparison between variables; significance was established at P<0.05. CRP kinetic analysis data are presented as mean (± SEM), while demographic and hemodynamic parameters are depicted as mean (± SD).
RESULTS
Pre- and intraoperative parameters
Table 1 shows the demographic and preoperative clinical data. Overall, the three patient groups are comparable, except for a significant difference in patients’ weight (P=0.03). Table 2 shows the intraoperative parameters that affect postoperative course, eg, cross-clamping time and cardiopulmonary bypass time. No significant differences were found between the measured parameters. This table also includes the comparison between time under anesthesia and total surgery time in the study groups. Time under mechanical ventilation (measured from anesthesia induction up to spontaneous breathing), total admission days and total ‘stay’ days in the intensive care unit are depicted in Table 3.
TABLE 1.
Demographic characteristics and presurgery parameters
| Parameters | Chronic statin, group A | Atorvastatin therapy, group B | Statin naive, group C | P |
|---|---|---|---|---|
| Number | 16 | 15 | 10 | |
| Age, years | 59.5±9.6 | 69.5±8.5 | 65.5±11.8 | NS |
| Weight, kg | 85.9±8.8 | 76.5±13.8 | 81.7±11.7 | 0.03A,B,C |
| Height, cm | 164.5±10.9 | 166.5±10.6 | 168.4±10.3 | NS |
| BUN, mmol/L | 6.2±2.1 | 6.4±2.1 | 7.7±2.8 | NS |
| Creatinine, μmol/L | 89.28±24.7 | 86.6±21.2 | 91.9±23.8 | NS |
| EuroSCORE | 2.5±1.6 | 3.2±1.7 | 3.1±2.3 | NS |
| Ejection fraction, % | 53.6±12.5 | 51.3±15.1 | 54±14.9 | NS |
Data are presented as the mean ± SD. BUN Blood urea nitrogen; EuroSCORE European System for Cardiac Operative Risk Evaluation; NS Not significant
TABLE 2.
Surgery parameters in the study groups
| Parameters | Chronic statin, group A | Atorvastatin therapy, group B | Statin naive, group C | P |
|---|---|---|---|---|
| Cross-clamp time, min | 66.5±14.6 | 68.9±21.4 | 62.8±8.2 | NS |
| CPB time, min | 112.7±19.1 | 122.2±34.0 | 105.3±10.3 | NS |
| Bypass numbers | 3.2±1 | 3.3±0.7 | 3.5±0.5 | NS |
| Anesthesia time, h | 5.2 (5.2) | 5.4 (5.5) | 5.2 (5.2) | NS |
| Surgery time, h | 4.3 (4.4) | 4.5 (4.5) | 4.3 (4.5) | NS |
Data are presented as the mean ± SD or mean (median). CBP Cardiopulmonary bypass; NS Not significant
TABLE 3.
Outcome parameters
| Parameters | Chronic statin, group A* | Atorvastatin, therapy group B | Statin naïve, group C | P |
|---|---|---|---|---|
| Days on mechanical ventilation | 5.31±14.2 | 0.7±0.4 | 1.37±0.7 | 0.001B,C 0.006A,B |
| Total days in ICU | 6.7±16.1 | 2.6±1.8 | 3±1.7 | NS |
| Total admission days | 15±19.8 | 8±3.1 | 12±7.5 | NS |
Data are presented as mean ± SD.
Two patients who were 62 and 69 days in hospital, 14 and 66 days in the intensive care unit (ICU) and 15 and 57 days on mechanical ventilation, respectively, were included in this group. NS Not significant
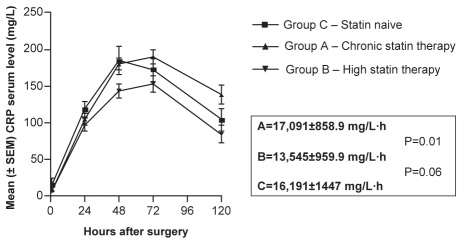
CRP kinetics
Table 4 and Figure 1 show the CRP kinetic parameters calculated from the CRP individual values. The mean (± SEM) AUC-CRP for group B was 13,545±959.9 mg/L·h (95% CI 11,486 mg/L·h to 15,604 mg/L·h), which was significantly smaller (P=0.01) than the chronic statin therapy (group A) AUC-CRP (17,085±858.4 mg/L·h; 95% CI 15,256 mg/L·h to 18,915 mg/L·h). For group C, the mean AUC-CRP was 16,191±1447 mg/L·h (95% CI 12,918 mg/L·h to 19,464 mg/L·h). Group C was not significantly different from groups A and B.
TABLE 4.
C-reactive protein (CRP) kinetic parameters in the study groups
| CRP kinetic parameters | Chronic statin, group A | Atorvastatin therapy, group B | Statin naïve, group C | P |
|---|---|---|---|---|
| Cmax, mg/L | 198.4±39.7 | 158.7±41.5 | 195.2±58.5 | 0.06A,C; 0.01B,C |
| Cmin, mg/L | 138.0±51.7 | 84.3±47 | 104.3±45.7 | 0.005A,B |
| tmax, days | 72.0±21.4 | 60.8±12.4 | 57.6±16.7 | NS |
Cmax Maximum concentration; Cmin Minimum concentration; NS Not significant; tmax Time to maximum concentration
Figure 1).
Mean (± SEM) C-reactive protein (CRP) area under the concentration-time curve
DISCUSSION
The present study confirmed that a high loading dose of atorvastatin before CABG surgery reduced CRP concentration expressed as AUC-CRP. These results support the idea that a high dose of this statin is necessary to attenuate the ‘negative’ or ‘toxic’ systemic and local inflammatory responses represented by the elevation of CRP after cardiac surgery, and their subsequent deleterious effects. Skrak et al (21) measured CRP before surgery, and on days 1 and 2 after surgery in children undergoing cardiac surgery under cardiopulmonary bypass machine conditions; this study differed from ours because the CRP kinetics was measured only for 48 h after surgery.
The theoretical basis for the prescription of atorvastatin, which differs from other statins in its potency, lies in its ability to inhibit the activation of nuclear factor-kappa B signalling in human monocytes. By significantly reducing the release of cytokines, atorvastatin also reduces the procoagulant activity of monocytes in whole human blood, supporting the hypothesis that some statin medications inhibit the inflammatory response more than others (17,22,23).
Recently, evidence has emerged that higher doses of statins than previously evaluated are needed to obtain maximum pleiotropic benefits, especially in acute coronary syndromes (24). CRP activates the complement and the coagulation cascades (22,25), and is codeposited with activated complement in all acute myocardial injuries. Considering all those actions, CRP may therefore be a therapeutic target (9) because of its ‘toxic’ effect; for this purpose, Pepys et al (26) developed an experimental substance, 1,6-bis(phosphocholine)-hexane, which is able to selectively inhibit the CRP molecule.
Individual CRP levels measured in all 41 subjects included in the present study increased markedly in the postoperative period, and did not return to baseline values for up to 120 h after surgery. These findings are similar to, and confirm, results already published by Aouifi et al (27), who compared patients who underwent on-pump and off-pump CABG, without showing differences between the two surgical techniques.
The results of the present study suggest that prescribing four doses of atorvastatin 80 mg/day (one before CABG, one the day of surgery, and two further doses after the surgery) and then 40 mg/day up to 120 h after surgery have a significant anti-inflammatory effect. This recommendation stands by the overall reduction in the measured AUC based on the individual CRP concentrations in group B compared with group A. When group B is compared with a statin-naïve group (group C), the difference between AUC-CRPs is not significant. Moreover, according to these results, it is also suggested that the traditional ‘standard’ dose of statin, which is most currently used to treat patients undergoing CABG, is not effective as an anti-inflammatory treatment. Patients included in the high-dose statin protocol (group B) were under mechanical ventilation for less time than the naïve group (group C) and group A. Those results confirm Lazar et al’s (14,28) recommendations, who prescribed statin therapy for all patients undergoing CABG based on their anti-inflammatory effects.
Gaudino et al (29) demonstrated that CRP concentrations after CABG on-pump conditions did not start its decay to baseline values until after 72 h from the beginning of surgery. The mean time to maximum concentration in our study was 72 h, 60.8 h and 57.6 h for groups A, B and C, respectively. This phenomenon can be explained by the fact that roughly 10 h must elapse after the initiation of renewed liver or vascular synthesis of CRP (30).
The therapeutic intervention in the present study – targeting CRP concentration with a high-loading dose of atorvastatin before and during the first five days after CABG surgery under cardiopulmonary bypass conditions – significantly reduced AUC-CRP at the indicated time. A reduction in CRP levels will improve myocardial cell ischemia before, during and after CABG.
Limitations
The limitations of this study included the small number of participants in each study group and the fact that other inflammatory biomarkers, such as procalcitonine, interleukin-6 and tumour necrosis factor-alpha were not measured for comparison. In addition, CRP levels were only evaluated over 120 h after surgery.
Well-planned multicentre studies are needed to confirm the results of our study. Two main steps are recommended for future studies: the evaluation of the effect of a high-dose statin on other endogenous reactants of the inflammatory complex induced by open-heart surgery (not necessarily CABG surgery), and ensuring that the study is powered to detect clinical effects and significance.
Acknowledgments
This study was funded in part by the EH Friedman Cardiac Diseases Research Fund, number 182-169 and Grant number 2002268, Technion – Israel Institute of Technology VPR Fund.
REFERENCES
- 1.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: A comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol, screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 2.Suleiman M, Khatib R, Agmon Y, et al. Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction. J Am Coll Cardiol. 2006;47:962–8. doi: 10.1016/j.jacc.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 3.Verma S, Wang CH, Li SH, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–9. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 4.Venogopal SK, Devaraj S, Jialal I. Effect of C-reactive protein on vascular cells: Evidence for a proinflammatory, proatherogenic role. Curr Opinion Nephrol Hypert. 2005;14:33–7. doi: 10.1097/00041552-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Yasojima K, Schwab C, McGeer EG. Generation of C-reactive protein and complement components in atherosclerosis plaques. Am J Pathol. 2001;158:1039–51. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Q, Wright JR. Expression of C-reactive protein by alveolar macrophages. J Immunol. 1996;156:4815–20. [PubMed] [Google Scholar]
- 7.Calabro P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. 2003;108:1930–2. doi: 10.1161/01.CIR.0000096055.62724.C5. [DOI] [PubMed] [Google Scholar]
- 8.Visushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest. 1993;91:1351–7. doi: 10.1172/JCI116336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pepys MB, Hirshfield GM. C-reactive protein: A critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–9. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 11.Karaca I, Ilkay E, Akbulut M, et al. Atorvastatin effects C-reactive protein levels in patients with coronary artery disease. Curr Med Res Opin. 2003;19:187–91. doi: 10.1185/030079903125001686. [DOI] [PubMed] [Google Scholar]
- 12.Wassmann S, Nickenig G. Improvement of endothelial function by HMG-CoA reductase inhibitors. Drug News Perspect. 2002;15:85–92. doi: 10.1358/dnp.2002.15.2.840047. [DOI] [PubMed] [Google Scholar]
- 13.Wassmann S, Laufs U, Muller K, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22:300–5. doi: 10.1161/hq0202.104081. [DOI] [PubMed] [Google Scholar]
- 14.Lazar HL, Bao Y, Zhang Y, Bernard SA. Pretreatment with statins enhances myocardial protection during coronary revascularization. J Thorac Cardiovasc Surg. 2003;125:1037–42. doi: 10.1067/mtc.2003.177. [DOI] [PubMed] [Google Scholar]
- 15.Pan W, Pinter T, Anton J, Lee VV, Vaughn WK, Collard CD. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation. 2004;110(Suppl 2):45–9. doi: 10.1161/01.CIR.0000138316.24048.08. [DOI] [PubMed] [Google Scholar]
- 16.Clark LL, Ikonomidis JS, Crawford FA, Jr, Crumbly A, 3rd, Kratz JM, Stroud MR. Preoperative statin treatment is associated with reduced post operative mortality and morbidity in patients undergoing cardiac surgery: An 8-year retrospective cohort study. J Thorac Cardiovasc Surg. 2006;131:679–85. doi: 10.1016/j.jtcvs.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Chello M, Patti G, Candure D, et al. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Crit Care Med. 2006;34:660–7. doi: 10.1097/01.CCM.0000201407.89977.EA. [DOI] [PubMed] [Google Scholar]
- 18.Milazzo D, Biasucci LM, Luciani N, et al. Elevated levels of C-reactive protein before coronary artery bypass grafting predict recurrence of ischemic events. Am J Cardiol. 1999;84:459–61. doi: 10.1016/s0002-9149(99)00333-1. [DOI] [PubMed] [Google Scholar]
- 19.Buffon A, Liouzzo G, Biasucci LM. Pre-procedural serum levels of C-reactive protein predict early complications and late restenosis after coronary angioplasty. J Am Coll Cardiol. 1999;34:1512–21. doi: 10.1016/s0735-1097(99)00348-4. [DOI] [PubMed] [Google Scholar]
- 20.Lennernas H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42:1141–60. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]
- 21.Skrak P, Kovacikova L, Kunovsky P. Procalcitonin, neopterin and C-reactive protein after pediatric cardiac surgery with cardiopulmonary bypass. Bratisl Lek Listy. 2007;108:501–5. [PubMed] [Google Scholar]
- 22.Hilgendorf A, Muth H, Parviz B, et al. Statins differ in their ability to block NF-kappaB activation in human blood monocytes. Int J Clin Pharmacol Ther. 2003;41:397–401. doi: 10.5414/cpp41397. [DOI] [PubMed] [Google Scholar]
- 23.Wong S, Schwartz RC, Pestka JJ. Superinduction of TNF-alpha and IL-6 in macrophages by vomitoxin (deoxynivalenol) modulated by mRNA stabilization. Toxicology. 2001;161:139–49. doi: 10.1016/s0300-483x(01)00331-6. [DOI] [PubMed] [Google Scholar]
- 24.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 25.Bisoendial RJ, Kastelein JJ, Levels JH, et al. Activation of inflammation and coagulation after infusion of C-reactive protein in humans. Circ Res. 2005;96:714–6. doi: 10.1161/01.RES.0000163015.67711.AB. [DOI] [PubMed] [Google Scholar]
- 26.Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–21. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 27.Aouifi A, Piriou V, Blanc P, et al. Effect of cardiopulmonary bypass on serum procalcitonin and C-reactive protein concentrations. Br J Anaesth. 1999;83:602–7. doi: 10.1093/bja/83.4.602. [DOI] [PubMed] [Google Scholar]
- 28.Lazar H. Should all patients receive statins before cardiac surgery: Are more data necessary? J Thorac Cardiovasc Surg. 2006;131:520–2. doi: 10.1016/j.jtcvs.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Gaudino M, Nasso G, Andreotti F, et al. Preoperative C-reactive protein level and outcome following coronary surgery. Eur J Cardio Thorac Surg. 2002;22:521–6. doi: 10.1016/s1010-7940(02)00436-0. [DOI] [PubMed] [Google Scholar]
- 30.Ehl S, Gehring B, Pohlandt F. A detailed analysis of changes in serum C-reactive protein levels in neonates treated for bacterial infection. Eur J Pediatr. 1999;158:238–42. doi: 10.1007/s004310051058. [DOI] [PubMed] [Google Scholar]