Abstract
AIM:
To investigate the plasma levels of atrial and brain natriuretic peptides (ANP and BNP), cardiac troponin I (cTnI) and C-reactive protein (CRP) as prognostic factors for survival in patients with sepsis.
METHOD:
Evaluation of serum levels of ANP, BNP, cTnI and CRP of patients on admission to an intensive care unit, two days later, and on the day of discharge from the intensive care unit or on the day of death.
RESULTS:
ANP levels were significantly higher in the nonsurviving patients (day 1: 70.00±49.54 pg/mL; day 2: 138.85±143.15 pg/mL; and died/discharged day: 375.70±262.66 pg/mL) than surviving patients (day 1: 23.96±29.93 pg/mL; day 2: 10.06±6.03 pg/mL; died/discharged day: 6.68±100.98 pg/mL, P<0.001). The BNP levels were significantly higher in the nonsurvivors (day 1: 254.78±308.62 pg/mL; day 2: 383.22±307.19 pg/mL; and died/discharged day: 696.47±340.33 pg/mL), than survivors (day 1: 13.72±12.95 pg/mL; day 2: 7.20±5.85 pg/mL; died/discharged day: 4.51±4.64 pg/mL, P<0.001). The cTnI levels were significantly higher in the nonsurviving patients (day 2: 0.16±0.38 μg/L; died/discharged day: 0.78±2.48 μg/L) than surviving patients (day 2: 0.04±0.07 μg/L; died/discharged day: 0.02±0.01 μg/L, P<0.05). The CRP levels were significantly higher in the nonsurvivors (day 2: 119.3±71.5 mg/L; and died/discharged day: 145.7±74.7 mg/L) than survivors (day 2: 57.0±29.7 mg/L; died/discharged day: 26.8±24.0 mg/L, P<0.05). There were no significant differences between nonsurvivors and survivors for cTnI and CRP on day 1.
CONCLUSION:
With the exception of cTnI and CRP on day 1, all of the parameters were significantly powerful to determine nonsurvivors on all days. Among these variables, BNP was the most powerful diagnostic parameter for the prediction of nonsurvivors on all days.
Keywords: C-reactive protein, Intensive care unit, Natriuretic peptides, Prognosis, Sepsis, Troponin I
Natriuretic peptides play an important role in the regulation of cardiovascular homeostasis and fluid volume. They promote natriuresis and diuresis, act as vasodilators and exert antimitogenic effects on cardiovascular tissues (1,2). Increased plasma levels of natriuretic peptide hormones have been identified as predictors of cardiac dysfunction and death in many critical care settings, including congestive heart failure, myocardial infarction and septic shock (3–7). Two members of the natriuretic family, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), are secreted by the heart mainly in response to myocardial stretch induced by volume load. Patients with septic shock show reversible left ventricular systolic dysfunction, commonly masked by a concomitant elevation in the cardiac index (8).
The cardiac-specific contractile protein troponin I (cTnI) has been suggested to be a sensitive marker of minor myocardial cell injury in systemic inflammation, sepsis or septic shock (9,10). cTnI levels have been shown to be superior to measurements of creatine kinase-MB activity for the detection of minor myocardial injury (11). Recent studies (12) have shown that troponin measurements in patients with chest pain in the emergency room allow risk stratification for poor prognosis, such as acute myocardial infarction and death. However, several studies have raised the question of the unexpectedly high percentage of elevated troponin levels in intensive care unit (ICU) patients without underlying coronary syndromes (9,13).
A number of inflammatory cells and mediators involved in the inflammatory response have been assessed for their role as potential markers of the presence and severity of the inflammatory response and organ failure (14,15). Serum levels of C-reactive protein (CRP), an acute-phase protein synthesized by the liver following stimulus by various cytokines such as tumour necrosis factor-alpha and interleukin-6, markedly increase within hours after the onset of infection or inflammation (16). Numerous studies (17–19) have demonstrated increased CRP levels in patients with sepsis, but the relation to multiple organ dysfunction and failure has not been well evaluated.
The prognostic utility of ANP, BNP, cTnI and CRP in a heterogeneous population of critically ill patients admitted to the ICU, including those admitted with sepsis, has not previously been investigated. The aim of the present study was to test whether ANP, BNP, cTnI and CRP measured around the time of ICU admission would predict mortality, and be particularly useful in predicting outcome in patients with sepsis because of the high incidence of myocardial dysfunction in these patients.
PATIENTS AND METHODS
Patients
The study was approved by the Committee for Ethics in Human Research. Informed consent was obtained from the next of kin of each patient. All patients admitted to the medical/surgical ICU from December 2003 to November 2005 for a diagnosis of sepsis were prospectively screened for inclusion. To be included in the study, patients had to meet at least two of the following criteria for sepsis, defined by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee (20): temperature greater than 38°C or lower than 36°C; heart rate greater than 90 beats/min; respiratory rate greater than 20 breaths/min or PaC02 less than 32 mmHg; and leukocyte count greater than 12×109/L or less than 4×109/L. Although all patients who were admitted to the ICU and met the above criteria were eligible for enrollment, those with burns, under coronary care or with trauma were excluded. Other exclusions were patients who developed sepsis during their stay in the ICU, and thus did not have sepsis on admission; an immunosuppressed state (treatment with steroids, bone marrow or organ transplant recipients); leukopenia (white blood cell count less than 1×109/L) or neutropenia (polymorphonuclear granulocyte count less than 0.5×109/L); hematological malignancy; AIDS; a medical condition considered to be irreversible or lethal within 24 h after admission; and pre-existing conditions known to increase ANP and BNP plasma levels (chronic heart disease, chronic systemic hypertension, myocardial hypertrophy, chronic supraventricular arrhythmia, acute cor pulmonale, chronic obstructive lung disease associated with chronic cor pulmonale; and patients with chronic renal failure [serum creatinine greater than 180 μmol/L]).
All patients had arterial catheters and central venous catheters via the subclavian artery or vein in place, and, if required, were mechanically ventilated in volume- or pressure-controlled modes during continuous sedation with midazolam and fentanyl. All patients received routine resuscitation therapy for sepsis, including fluid administration with crystalloids and colloids. After blood and various biological specimens were collected for microbiological analysis, all patients initially received broad-spectrum antibiotics consisting of a combination of an aminoglycoside with either a fourth-generation cephalosporin or ciprofloxacin. Antibiotic treatment was adjusted based on culture results.
Adequate initial resuscitation was given to all patients with sepsis-induced tissue hypoperfusion in accordance with the protocol of Rivers et al (21). Patients were considered vasopressor-dependent if vasopressors were required to maintain the mean arterial pressure at levels greater than 65 mmHg to 70 mmHg. The vasopressor agents and corresponding dose ranges were noradrenaline (3 μg/min to 40 μg/min), adrenaline (2 μg/min to 100 μg/min), or dopamine (6 μg/kg/min to 30 μg/kg/min). The need for red blood cell (RBC) transfusion was determined by the patient’s physician. The following transfusion guideline was established for the study: no RBC transfusion if the hematocrit concentration was 24% or greater, unless there was a specific clinical indication (active bleeding, ischemia, or other); RBC transfusion for a hematocrit concentration below 24% was at the physician’s discretion.
Clinical and functional investigations
The present prospective study was designed to evaluate the predictive value of ANP, BNP, cTnI and CRP levels on the mortality and morbidity of sepsis. ANP (normal values 35 pg/mL to 50 pg/mL), BNP (normal values less than 100 pg/mL), cTnI (normal values less than 0.01 μg/L) and CRP (normal values 0 mg/L to 10 mg/L) levels were collected on admission (ie, during the first 24 h), on day 2, and on the day of discharge from the ICU or on the day of death. Patients were followed until day 28 or death. Vital signs, clinical status and severity of disease parameters (Acute Physiology and Chronic Health Evaluation [APACHE] II score) (22) were assessed daily. The APACHE II score was calculated by means of maximal daily deviations of 12 physiological variables from normal, with corrections for age and various chronic illnesses. Duration of mechanical ventilation was recorded. Survival was defined as being alive at ICU discharge.
Results of the routine blood analyses (ie, complete blood count, and serum chemistry including ANP, BNP, cTnI and CRP) were known and recorded. ANP, BNP and cTnI levels were measured at the same times. Venous blood was collected into a 10 mL sterile plain tube (without anticoagulant) before administration of any medications and stored at −20°C. Before assay, all samples were thawed to room temperature and mixed by gentle swirling or inversion. ANP and BNP plasma levels were assayed using an immunoradiometric assay (Shionora-BNP, Cisbio International, France). Two direct specific monoclonal antibodies against human ANP and BNP were used. One recognizes the carboxy-terminal sequence, and the other detects the ring structure of human ANP and BNP. Analytical performances of the ANP and BNP using Shionoria-BNP have been evaluated in the authors’ laboratory. The intra-assay coefficient of variation was between 15% and 9% for low and high concentrations, respectively. The lowest detectable limits of ANP and BNP were 1.02 pg/mL and 0.51 pg/mL, respectively.
Determination of plasma levels of cTnI was performed using the IMMUNO-1 immuno-enzymatic assay (Diasporin SPA, Italy). The lowest detectable limit of cTnI was 0.009 μg/L. Determination of plasma levels of CRP was performed using nephelometry (IMMAGE Systems, Beckman Coulter Inc, USA). The lowest detectable limit of CRP was 6.9 mg/L.
Statistical analysis
Normality distribution of the variables was tested using the one-sample Kolmogorov-Smirnov test. Demographic characteristics were compared using Student’s t test or the Mann-Whitney U test between groups, depending on distribution. Categorical variables were analyzed using the χ2 test.
Area under the curve (AUC) of the receiver operating characteristic (ROC) curve was used to assess the predictive power of ANP, BNP, cTnI, CRP and APACHE II levels for non-survivors. A plot of true-positive rate against false-positive rate was made, and the AUC was measured. The sensitivity and specificity rates of the variables for death were estimated by cutoff points. The AUC is a measure of the overall discriminatory power of the prognostic variable. A value of 1.0 indicates perfect discrimination, a value of 0.5 equals random prediction and a value of lower than 0.5 indicates no discriminative power.
Statistica 7.0 statistical software (StatSoft Inc, USA) was used for statistical analysis. P<0.05 was considered to be statistically significant.
RESULTS
Among the 77 patients admitted for sepsis during the study period, 40 were included in the present study. Thirty-seven patients were excluded because of pre-existing cardiac disease (n=18), cor pulmonale (n=4), renal failure (n=10) and pulmonary embolism (n=5). Patient characteristics are presented in Table 1. All 40 patients included in the study were mechanically ventilated. Patients were divided into two groups: those who were alive at time of ICU discharge (survivors; n=20) and those who died (nonsurvivors; n=20); the two groups did not differ in terms of underlying etiology of severe sepsis. The primary sites of infection were the lungs (85%) and the urinary tract (15%). The majority (65%) of patients who died suffered from multiple organ failure, defined as failure of two or more vital organs.
TABLE 1.
Comparisons of ANP, BNP, cTnI and CRP levels, and APACHE II scores between survivors and nonsurvivors by days in ICU
| Characteristic | Survivors | Nonsurvivors | P |
|---|---|---|---|
| Age, years | 60.9±16.8 | 65.4±18.0 | >0.05 |
| ICU days | 18.7±3.7 | 19.4±18.0 | >0.05 |
| Ventilation, days | 14.9±3.2 | 18.7±17.1 | >0.05 |
| ANP, pg/mL | |||
| Day 1 | 23.96±29.93 | 70.00±49.54 | <0.001 |
| Day 2 | 10.06±6.03 | 138.85±143.15 | <0.001 |
| Last day | 6.68±100.98 | 375.70±262.66 | <0.001 |
| BNP, pg/mL | |||
| Day 1 | 13.72±12.95 | 254.78±308.62 | <0.001 |
| Day 2 | 7.20±5.85 | 383.22±307.19 | <0.001 |
| Last day | 4.51±4.64 | 696.47±340.33 | <0.001 |
| cTnI, μg/L | |||
| Day 1 | 0.24±0.56 | 0.19±0.48 | >0.05 |
| Day 2 | 0.04±0.07 | 0.16±0.38 | <0.05 |
| Last day | 0.02±0.01 | 0.78±2.48 | <0.05 |
| CRP, mg/L | |||
| Day 1 | 103±105 | 102.4±71.1 | >0.05 |
| Day 2 | 57.0±29.7 | 119.3±71.5 | <0.05 |
| Last day | 26.8±24.0 | 145.7±74.7 | <0.05 |
| APACHE II | |||
| Day 1 | 19.4±3.57 | 23.3±4.67 | >0.05 |
| Day 2 | 16.35±3.36 | 25.8±5.18 | <0.001 |
| Last day | 12.90±2.75 | 35.7±6.23 | <0.001 |
All data reported as mean ± SD. ANP Atrial natriuretic peptide; APACHE II Acute Physiology and Chronic Health Evaluation II; BNP Brain natriuretic peptide; cTnI Cardiac troponin I; CRP C-reactive protein; ICU Intensive care unit
There was no significant difference between the groups in terms of arterial blood analysis, hemodynamic parameters and biochemical parameters at admission into the ICU (P>0.05). Eight patients in the survivors group and nine patients in the nonsurvivors group received RBC transfusion. Ten patients in the survivors group and 16 patients in the nonsurvivors group received noradrenaline or dopamine infusion.
In the survivors and nonsurvivors groups, ventilation duration was 14.9±3.2 days and 18.7±17.1 days, respectively (P>0.05). The ICU stay of the survivors was not significantly different to that of the nonsurvivors (18.7±3.7 days versus 19.4±18.0 days, respectively; P>0.05).
Comparisons of ANP, BNP, cTnI and CRP levels, and APACHE II scores between survivors and nonsurvivors by days are shown in Table 1. ANP, BNP and APACHE II values of nonsurvivors were significantly higher than those of survivors on all days (P<0.001 for all comparisons). On the second day and last day, the cTnI and CRP values were significantly higher in nonsurvivors than survivors; however, there were no significant differences on the first day.
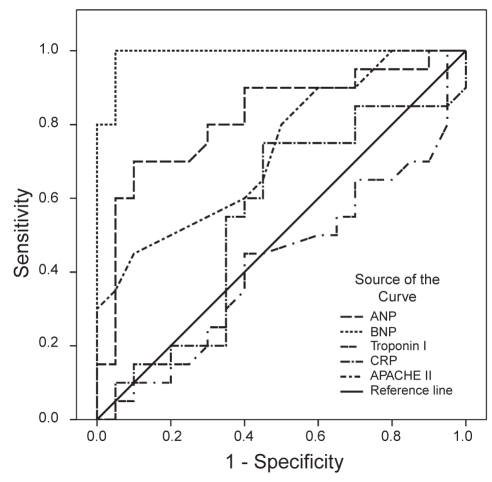
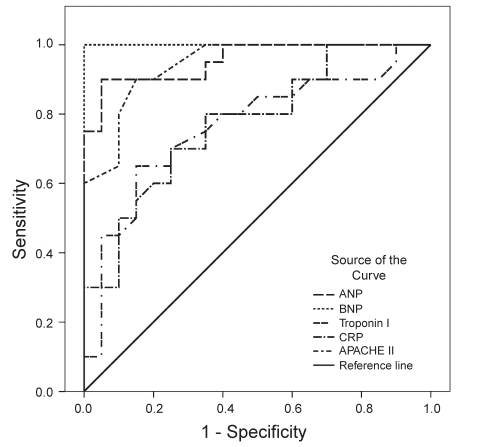
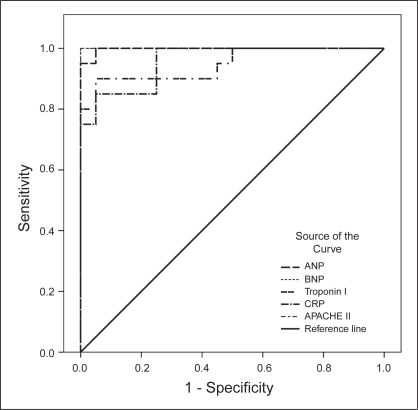
To define an optimal decision threshold for ANP, BNP, cTnI and CRP levels, and APACHE II scores, ROC curve analysis was performed. ROC curves are demonstrated in Figure 1 for day 1, in Figure 2 for day 2 and in Figure 3 for the last day.
Figure 1).
Receiver operating characteristic curves showing the association between parameters (atrial natriuretic peptides [ANP], brain natriuretic peptides [BNP], troponin I, C-reactive protein [CRP], and Acute Physiology and Chronic Health Evaluation II scores [APACHE II]) and survival outcome on day 1 in the intensive care unit
Figure 2).
Receiver operating characteristic curves showing the association between parameters (atrial natriuretic peptides [ANP], brain natriuretic peptides [BNP], troponin I, C-reactive protein [CRP], and Acute Physiology and Chronic Health Evaluation II scores [APACHE II]) and survival outcome on day 2 in the intensive care unit
Figure 3).
Receiver operating characteristic curves showing the association between parameters (atrial natriuretic peptides [ANP], brain natriuretic peptides [BNP], troponin I, C-reactive protein [CRP], and Acute Physiology and Chronic Health Evaluation II scores [APACHE II]) and survival outcome on the last day
Cut-off values, predictive accuracies and AUCs are shown in Table 2. Except for cTnI and CRP on day 1, all of the parameters were significantly powerful to discriminate non-survivors on all days; their AUCs ranged from 0.731 to 1. Among the five variables, BNP was the most powerful diagnostic parameter for predicting nonsurvivors on all days (AUC=1 on all days). APACHE II scores showed the same effect (AUC=1) on the last day.
TABLE 2.
Calculated cut-off, sensitivity, specificity, and AUC values for ANP, BNP, cTnI and CRP levels, and APACHE II scores at discharge/death outcome (last day)
| Cut-off | Sensitivity (%) | Specificity (%) | AUC | P | ||
|---|---|---|---|---|---|---|
| Day 1 | ANP | >47.6 | 70 | 90 | 0.819 | 0.001 |
| BNP | >32.1 | 100 | 95 | 0.990 | <0.001 | |
| cTnI | ≤0.03 | 50 | 65 | 0.425 | 0.417 | |
| CRP | >7.1 | 75 | 55 | 0.551 | 0.579 | |
| APACHE II | >23 | 45 | 90 | 0.731 | 0.012 | |
| Day 2 | ANP | >15.4 | 90 | 95 | 0.955 | <0.001 |
| BNP | >23.9 | 100 | 100 | 1.000 | <0.001 | |
| cTnI | >0.03 | 65 | 85 | 0.764 | 0.004 | |
| CRP | >5.6 | 80 | 65 | 0.774 | 0.003 | |
| APACHE II | >20 | 90 | 85 | 0.943 | <0.001 | |
| Last day | ANP | >9.8 | 100 | 95 | 0.998 | <0.001 |
| BNP | >20.1 | 100 | 100 | 1.000 | <0.001 | |
| cTnI | >0.03 | 85 | 95 | 0.948 | <0.001 | |
| CRP | >6.6 | 85 | 95 | 0.958 | <0.001 | |
| APACHE II | >17 | 100 | 100 | 1.000 | <0.001 | |
APACHE II Acute Physiology and Chronic Health Evaluation; AUC Area under the curve; ANP Atrial natriuretic peptides, pg/mL; BNP Brain natriuretic peptides, pg/mL; CRP C-reactive protein, mg/L; cTnI Cardiac troponin I, μg/L
On the first day, optimal cut-off points for ANP, BNP and APACHE II levels were greater than 47.6 pg/mL, 32.1 pg/mL and 23, respectively. At these cut-off points, the sensitivity rates for correct prediction of nonsurvivors were 70%, 100% and 45%, respectively, whereas the specificity rates were 90%, 95% and 90%. On the second day, optimal cut-off points for ANP, BNP, cTnI, CRP and APACHE II levels were higher than 15.4 pg/mL, 23.9 pg/mL, 0.03 μg/L, 56 mg/L and 20, respectively. At these cut-off points, the sensitivity rates for correct prediction of nonsurvivors were 90%, 100%, 65%, 80% and 90%, respectively, whereas the specificity rates were 95%, 100%, 85%, 65% and 85%. On the last day, the optimal cut-off points for ANP, BNP, cTnI, CRP and APACHE II levels were higher 9.8 pg/mL, 20.1 pg/mL, 0.03 μg/L, 66 mg/L and 17, respectively. At these cut-off points, the sensitivity rates for correct prediction of nonsurvivors were 100%, 100%, 85%, 85% and 100%, respectively, whereas the specificity rates were 95%, 100%, 95%, 95% and 100%.
DISCUSSION
We investigated the plasma levels of ANP, BNP, cTnI and CRP, as well as APACHE II scores, as a prognostic indicators for survival in patients with sepsis. ANP, BNP and APACHE II values of nonsurvivors were significantly higher than survivors in all days (P<0.001 for all comparisons). On the second day and last day, cTnI and CRP values were significantly higher in nonsurvivors than survivors; however, there were no significant differences on the first day. Except for cTnI and CRP on day 1, all of the parameters were significantly powerful to discriminate nonsurvivors on all days. Among the five variables, BNP was the most powerful diagnostic parameter for the prediction of nonsurvivors on all days. APACHE II scores on the last day had the same power of prediction.
Despite advances in therapy, sepsis causes more than 200,000 deaths per year in the United States, equalling the number of patients dying from myocardial infarction (23). Myocardial dysfunction is a common complication in patients with severe sepsis, and early recognition and aggressive supportive therapy are mandatory because mortality in patients with septic shock is still high (24). The ambiguity of clinical findings and unclear risk stratification in sepsis have been major problems in sepsis intervention trials (25). Within this context, there is a need for biomarkers to tackle the challenges of sepsis monitoring and treatment (26).
Other biological markers have been used to detect myocardial dysfunction in sepsis (27). Hartemink et al (28) measured α-ANP, the C-terminal circulating immunoreactive ANP, in 14 patients with septic shock. They found high α-ANP plasma levels in patients with high cardiac filling pressures. Mazul-Sunko et al (29) showed that the N-terminal fragment of ANP (pro-ANP) was increased in patients with sepsis and was inversely correlated to myocardial performance. However, neither α-ANP nor pro-ANP had a prognostic value in these studies. In 14 patients with septic shock, the mean plasma ANP level was fivefold higher than that in healthy subjects (30). Hartemink et al (28) reported inverse correlations between ANP and left ventricular stroke work index and right ventricular stroke work index, and a positive relationship between ANP and dopamine dose in 14 patients with septic shock on day 1 in the ICU. In the study by Witthaut et al (31), ANP values were found to be several-fold higher in patients with septic shock than in control subjects. There was a good correlation between ANP and interleukin-6, whereas ANP was not significantly correlated with any hemodynamic parameter. In our study, we found that ANP values of nonsurvivors were significantly higher than survivors on all days (P<0.001 for all comparisons).
Several studies (27,31–34) have addressed the questions whether BNP is of prognostic value in patients with sepsis. However, studies on the value of BNP testing in critically ill patients revealed conflicting results in sepsis patients. In a study (32) of patients with severe sepsis or septic shock, BNP levels were markedly higher in patients with impaired systolic left ventricular function than in those with preserved systolic left ventricular function at days 1 to 4 during their ICU stay. In addition, at days 2 and 3, BNP levels were higher in nonsurvivors than in survivors. A prognostic impact of BNP with respect to mortality was also found by Tung et al (34) in evaluating BNP levels in 49 ICU patients with shock, mainly of noncardiac origin. In contrast, a more recent study (33) analyzing 78 patients who had been admitted consecutively to a general ICU revealed a trend toward higher BNP levels in survivors compared with nonsurvivors. When the analysis was restricted to the patients with sepsis, who had higher BNP levels than those without sepsis, the same trend was observed. Other studies (35,36) did not find any prognostic information using BNP levels in critically ill patients. In addition, Berendes et al (37) showed that plasma levels of natriuretic peptides were elevated in patients admitted to the ICU after major surgery, but levels of natriuretic peptides were not predictive of outcome. In our study, we investigated ANP, BNP, cTnI, CRP and APACHE II to determine cut-off values, predictive accuracies and AUCs. We found that, among the five variables, BNP was the most powerful predictor of nonsurvival on all days (AUC=1 in all days).
Recently, several studies (38,39) have raised the question of an unexpectedly high percentage of elevated troponin levels in ICU patients without underlying coronary syndromes. The mechanism and prognostic value of cTnI elevation in sepsis and systemic inflammatory response syndrome are poorly understood. Cytokines and endotoxins from Gram-negative microorganisms may lead to myocardial depression and ventricular dilation (40). In some patients, elevated cTnI levels may indicate bacterial myocarditis or ischemic damage due to extremely elevated O2 consumption, reduced perfusion pressure and decreased O2 delivery to the cardiac muscle (41). A recent report (42) has shown that tumour necrosis factor-alpha increases the permeability of endothelial monolayers to macromolecules and lower molecular weight solutes. It may be speculated that similar alteration of permeability also occurs at the level of myocyte cell membranes, thus leading to leakage of cTnI. Elevated troponin levels have been shown to be related to the severity of the disease as expressed by global scores such as APACHE II score or simplified acute physiology score II (43,44), as well as short-term prognosis (42,45); the results of these studies were very similar in concluding that elevated troponin levels in patients with sepsis indicate a higher severity of disease, the presence of myocardial dysfunction and a worse prognosis. In our study, we found that cTnI was a prognostic factor for survival in patients with sepsis, except on admission (ie, during the first 24 h) in ICU.
CRP is predominantly produced and secreted by hepatocytes, although other cells including alveolar macrophages may also synthesize CRP (46). CRP is thought to represent a measure of cytokine-induced protein synthesis. The relatively short half-life of approximately 19 h makes it a useful monitor for follow-up of inflammatory response, infection and antibiotic treatment. In addition, laboratory tests for CRP are easily available and less costly than cytokine tests. In patients with sepsis, Presterl et al (47) demonstrated a correlation among the plasma levels of CRP, interleukin-6 and tumour necrosis factor-sR, and the APACHE III and mortality probability model II scores. Both scoring systems, as well as CRP levels, were significantly higher in the nonsurvivors compared with the survivors. In our study, we found CRP to be a prognostic factor for survival in patients with sepsis, except on admission (ie, during the first 24 h) in ICU.
The APACHE II score – a complex algorithm – was not originally developed for individual outcome prediction in sepsis patients (22). Despite its limitations, outcome predictors such as the extensively evaluated APACHE II score are helpful in identifying those septic patients who are at high risk for death and who are more likely to benefit from intervention (48).
CONCLUSIONS
Increased ANP, BNP, cTnI and CRP concentrations may be a valuable addition to APACHE II scores to predict the risk of death. Serial measurements of ANP, BNP, cTnI and CRP concentrations in critically ill patients may help to identify patients who may require more aggressive diagnostic and therapeutic interventions to avoid complications. Among the five variables, BNP had the most powerful diagnostic parameter to predict nonsurvivors on all days. ANP, cTnI, CRP and especially BNP concentrations may also be helpful in clinical trials, to identify high-risk patients who would benefit from new therapeutic interventions.
Acknowledgments
This work was supported by the Trakya University research grant no TUBAP 712.
REFERENCES
- 1.Mair J, Hammerer-Lercher A, Puschendorf B. The impact of cardiac natriuretic peptide determination on the diagnosis and management of heart failure. Clin Chem Lab Med. 2001;39:571–88. doi: 10.1515/CCLM.2001.093. [DOI] [PubMed] [Google Scholar]
- 2.Clerico A. Pathophysiological and clinical relevance of circulating levels of cardiac natriuretic hormones: Are they merely markers of cardiac disease? Clin Chem Lab Med. 2002;40:752–60. doi: 10.1515/CCLM.2002.129. [DOI] [PubMed] [Google Scholar]
- 3.Hall C, Rouleau JL, Moyè L, et al. N-terminal proatrial natriuretic factor: An independent predictor of long-term prognosis after myocardial infarction. Circulation. 1994;89:1934–42. doi: 10.1161/01.cir.89.5.1934. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb S, Kukin ML, Ahern D, Packer M. Prognostic importance of atrial natriuretic peptide in patients with chronic heart failure. J Am Coll Cardiol. 1989;13:153–9. doi: 10.1016/0735-1097(89)90344-6. [DOI] [PubMed] [Google Scholar]
- 5.Mitaka C, Nagura T, Sakanishi N, Tsunoda Y, Toyooka H. Plasma alpha-atrial natriuretic peptide concentrations in acute respiratory failure associated with sepsis: Preliminary study. Crit Care Med. 1990;18:1201–3. doi: 10.1097/00003246-199011000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Mukoyama M, Nakao K, Saito Y, et al. Increased human brain natriuretic peptide in congestive heart failure. N Engl J Med. 1990;313:757–8. doi: 10.1056/NEJM199009133231114. [DOI] [PubMed] [Google Scholar]
- 7.Witthaut R, Busch C, Fraunberger P, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: Impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med. 2003;29:1696–702. doi: 10.1007/s00134-003-1910-0. [DOI] [PubMed] [Google Scholar]
- 8.Price S, Anning SB, Mitchell JA, Evans TW. Myocardial dysfunction in sepsis: Mechanisms and therapeutic implications. Eur Heart J. 1999;20:715–24. doi: 10.1053/euhj.1998.1358. [DOI] [PubMed] [Google Scholar]
- 9.Turner A, Tsamitros M, Bellomo R. Myocardial cell injury in septic shock. Crit Care Med. 1999;27:1775–80. doi: 10.1097/00003246-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Ammann P, Fehr T, Minder EI, Günter C, Bertel O. Elevation of troponin I in sepsis and septic shock. Intensive Care Med. 2001;27:965–9. doi: 10.1007/s001340100920. [DOI] [PubMed] [Google Scholar]
- 11.Adams JE, 3rd, Bodor GS, Davila-Roman VG, et al. Cardiac troponin-I. A marker with high specificity for cardiac injury. Circulation. 1993;88:101–6. doi: 10.1161/01.cir.88.1.101. [DOI] [PubMed] [Google Scholar]
- 12.Hamm CW, Goldmann BU, Heeschen C, Kreymann G, Berger J, Meinertz T. Emergency room triage of patients with acute chest pain by means of rapid testing for cardiac troponin T or troponin I. N Engl J Med. 1997;337:1648–53. doi: 10.1056/NEJM199712043372302. [DOI] [PubMed] [Google Scholar]
- 13.Guest TM, Ramanathan AV, Tuteur PG, Schechtman KB, Ladenson JH, Jaffe AS. Myocardial injury in critically ill patients. A frequently unrecognized complication. JAMA. 1995;273:1945–9. [PubMed] [Google Scholar]
- 14.Marty C, Misset B, Tamion F, et al. Circulating interleukin-8 concentrations in patients with multiple organ failure of septic and nonseptic origin. Crit Care Med. 1994;22:673–9. doi: 10.1097/00003246-199404000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Roumen RM, Redl H, Schlag G, et al. Inflammatory mediators in relation to the development of multiple organ failure in patients after severe blunt trauma. Crit Care Med. 1995;23:474–80. doi: 10.1097/00003246-199503000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Thijs LG, Hack CE. Time course of cytokine levels in sepsis. Intensive Care Med. 1995;21(Suppl 2):S258–S63. doi: 10.1007/BF01740764. [DOI] [PubMed] [Google Scholar]
- 17.Póvoa P, Almeida E, Moreira P, et al. C-reactive protein as an indicator of sepsis. Intensive Care Med. 1998;24:1052–6. doi: 10.1007/s001340050715. [DOI] [PubMed] [Google Scholar]
- 18.Yentis SM, Soni N, Sheldon J. C-reactive protein as an indicator of resolution of sepsis in the intensive care unit. Intensive Care Med. 1995;21:602–5. doi: 10.1007/BF01700168. [DOI] [PubMed] [Google Scholar]
- 19.Smith RP, Lipworth BJ, Cree IA, et al. C-reactive protein: A clinical marker in community-acquired pneumonia. Chest. 1995;108:1288–91. doi: 10.1378/chest.108.5.1288. [DOI] [PubMed] [Google Scholar]
- 20.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 21.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 22.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 23.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 25.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–7. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 27.Maeder M, Fehr T, Rickli H, Amman P. Sepsis-associated myocardial dysfunction: Diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest. 2006;129:1349–66. doi: 10.1378/chest.129.5.1349. [DOI] [PubMed] [Google Scholar]
- 28.Hartemink KJ, Groeneveld AB, de Groot MC, et al. Alpha-atrial natriuretic peptide, cyclic guanosine monophosphate, and endothelin in plasma as markers of myocardial depression in human septic shock. Crit Care Med. 2001;29:80–7. doi: 10.1097/00003246-200101000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Mazul-Sunko B, Zarkovic N, Vrkic N, et al. Pro-atrial natriuretic peptide hormone from right atria is correlated with cardiac depression in septic patients. J Endocrinol Invest. 2001;24:RC22–RC4. doi: 10.1007/BF03343878. [DOI] [PubMed] [Google Scholar]
- 30.Mitaka C, Hirata Y, Makita K, et al. Endothelin-1 and atrial natriuretic peptide in septic shock. Am Heart J. 1993;126:466–8. doi: 10.1016/0002-8703(93)91074-o. [DOI] [PubMed] [Google Scholar]
- 31.Witthaut R, Busch C, Fraunberger P, et al. Plasma atrial natriuretic peptide and brain natriuretic peptide are increased in septic shock: Impact of interleukin-6 and sepsis-associated left ventricular dysfunction. Intensive Care Med. 2003;29:1696–702. doi: 10.1007/s00134-003-1910-0. [DOI] [PubMed] [Google Scholar]
- 32.Charpentier J, Luyt CE, Fulla Y, et al. Brain natriuretic peptide: A marker of myocardial dysfunction and prognosis during severe sepsis. Crit Care Med. 2004;32:660–5. doi: 10.1097/01.ccm.0000114827.93410.d8. [DOI] [PubMed] [Google Scholar]
- 33.Cuthbertson BH, Patel RR, Croal BL, et al. B-type natriuretic peptide and the prediction of outcome in patients admitted to intensive care. Anaesthesia. 2005;60:16–21. doi: 10.1111/j.1365-2044.2004.03972.x. [DOI] [PubMed] [Google Scholar]
- 34.Tung RH, Garcia C, Morss AM, et al. Utility of B-type natriuretic peptide for the evaluation of intensive care unit shock. Crit Care Med. 2004;32:1643–7. doi: 10.1097/01.ccm.0000133694.28370.7f. [DOI] [PubMed] [Google Scholar]
- 35.Forfia PR, Watkins SP, Rame JE, et al. Relationship between B-type natriuretic peptides and pulmonary capillary wedge pressure in the intensive care unit. J Am Coll Cardiol. 2005;45:1667–71. doi: 10.1016/j.jacc.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 36.Jefic D, Lee JW, Jefic D, et al. Utility of B-type natriuretic peptide an N-terminal Pro B-type nariuretic peptide in evaluation of respiratory failure in critically ill patients. Chest. 2005;128:288–95. doi: 10.1378/chest.128.1.288. [DOI] [PubMed] [Google Scholar]
- 37.Berendes E, Van Aken H, Raufhake C, et al. Differential secretion of atrial and brain natriuretic peptide in critically ill patients. Anesth Analg. 2001;93:676–82. doi: 10.1097/00000539-200109000-00029. [DOI] [PubMed] [Google Scholar]
- 38.Heidenreich PA, Alloggiamento T, Melsop K, McDonald KM, Go AS, Hlatky MA. The prognostic value of troponin in patients with non-ST-elevation acute coronary syndromes: A meta-analysis. J Am Coll Cardiol. 2001;38:478–85. doi: 10.1016/s0735-1097(01)01388-2. [DOI] [PubMed] [Google Scholar]
- 39.Guest TM, Ramanathan AV, Tuteur PG, Schechtman KB, Ladenson JH, Jaffe AS. Myocardial injury in critically ill patients: A frequently unrecognized complication. JAMA. 1995;273:1945–9. [PubMed] [Google Scholar]
- 40.Turner A, Tsamitros M, Bellomo R. Myocardial cell injury in septic shock. Crit Care Med. 1999;27:1775–80. doi: 10.1097/00003246-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Arlati S, Brenna S, Prencipe L, et al. Myocardial necrosis in ICU patients with acute non-cardiac disease: A prospective study. Intensive Care Med. 2000;26:31–7. doi: 10.1007/s001340050008. [DOI] [PubMed] [Google Scholar]
- 42.Ammann P, Fehr T, Minder EI, Gunter C, Bertel O. Elevation of troponin I in sepsis and septic shock. Intensive Care Med. 2001;27:965–9. doi: 10.1007/s001340100920. [DOI] [PubMed] [Google Scholar]
- 43.Ver Elst KM, Spapen HD, Nguyen DN, et al. Cardiac troponin I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem. 2000;46:650–7. [PubMed] [Google Scholar]
- 44.Metha NJ, Khan IA, Gupta V, et al. Cardiac troponin predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol. 2004;95:13–7. doi: 10.1016/j.ijcard.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Ammann P, Maggiorini M, Bertel O, et al. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol. 2003;41:2004–9. doi: 10.1016/s0735-1097(03)00421-2. [DOI] [PubMed] [Google Scholar]
- 46.Dong Q, Wright JR. Expression of C-reactive protein by alveolar macrophages. J Immunol. 1996;156:4815–20. [PubMed] [Google Scholar]
- 47.Presterl E, Staudinger T, Pettermann M, et al. Cytokine profile and correlation to the APACHE III and MPM II scores in patients with sepsis. Am J Respir Crit Care Med. 1997;156:825–32. doi: 10.1164/ajrccm.156.3.9607131. [DOI] [PubMed] [Google Scholar]
- 48.Eichacker PQ, Parent C, Kalil A, et al. Risk and the efficacy of antiinflammatory agents: Retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002;166:1197–205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]