Abstract
The duration of postoperative ileus following abdominal surgery is quite variable, and prolonged postoperative ileus is an iatrogenic phenomenon with important influence on patient morbidity, hospital costs and length of stay in hospital. Adequate treatment for prolonged postoperative ileus is important to improve patient morbidity and clinical efficiency. Both clinical and pharmacological management strategies have improved rapidly over the last decade, and appropriate and timely management using multimodal techniques should be used for optimal care. In this review, we define postoperative ileus, describe the pathogenesis and briefly discuss clinical management before detailing potential pharmacologic management options.
Abstract
La durée de l'iléus postopératoire consécutif à une chirurgie abdominale est assez variable, et l'iléus postopératoire prolongé est un phénomène iatrogène susceptible d'exercer un impact important sur la morbidité, de même que sur les coûts et la durée du séjour hospitalier. Il est important de traiter adéquatement l'iléus postopératoire prolongé afin de réduire la morbidité et d'améliorer l'efficacité clinique. Les stratégies de prise en charge, tant cliniques que pharmacologiques, se sont rapidement améliorées depuis une dizaine d'années; une prise en charge appropriée et précoce faisant appel à des techniques multimodales s'impose pour la prestation de soins optimaux. Dans la présente synthèse, nous définissons l'iléus postopératoire et nous en décrivons brièvement la pathogenèse et le traitement, avant d'aborder plus en détail les options pharmacothérapeutiques possibles.
In systems that try to minimize hospital stay after abdominal surgery, one of the principal limiting factors is the recovery of adequate bowel function, which can delay discharge or lead to readmission. Postoperative ileus (POI) is the term given to the cessation of intestinal function following surgery. Although all surgical procedures put the patient at risk for POI, gastrointestinal tract surgeries in particular are associated with a temporary cessation of intestinal function.
The duration of POI varies, lasting from a few hours to several weeks. Prolonged postoperative ileus, also known as pathologic postoperative ileus, can be caused by a myriad of pathologic processes that are treated with limited success by clinical and pharmacologic management. Studies of large administrative databases show that, on average, patients with a diagnosis of POI stay 5 days longer in hospital after abdominal surgery than patients without POI.1 Over the last decade, substantial efforts have been made to minimize the duration of POI, as there appears to be no associated physiologic benefit, and it is currently the primary factor delaying recovery for most patients. In this review, we define POI, describe the pathogenesis and briefly discuss clinical management before detailing current pharmacologic management options.
DEFINITIONS
Postoperative ileus has been variably defined, but involves a temporary cessation of bowel function with a variable reduction in activity sufficient to prevent effective transit of intestinal contents. Its pathogenesis is related to a complex series of inter-relations among inhibitory neural reflexes, release of neurotransmitters and inflammatory mediators, and endogenous and exogenous opioids.2 Until recently, there were no standard descriptions of the appropriate duration of POI, and studies have been inconsistent in their selection of variables defining cessation of an ileus. A reasonable working definition of POI is “the time from surgery until the passage of flatus or stool while tolerating oral diet, if appropriate.” The qualifier “if appropriate” is necessary because some patients may not be offered an oral diet owing to the type of surgery they undergo (e.g., duodenal surgery, high jejunostomy) or because of complications such as an enterocutaneous fistula. We have described toleration of diet elsewhere3 as “tolerance of part or all of 3 successive meals without nausea or vomiting suggestive of POI.”
Recently, a standardized definition of POI was proposed at a consensus conference; the condition was defined as “a transient cessation of co-ordinated bowel motility after surgical intervention, which prevents effective transit of intestinal contents and/or tolerance of oral intake.”1
A unique and important form of POI that becomes an important clinical problem occurs when the symptoms are absent or appear to resolve, but become evident 1 or more days later. When this series of events transpires after the patient has been discharged from hospital, it often presents as cessation of flatus or stool, with bloating and/or nausea and vomiting requiring readmission to rule out mechanical small bowel obstruction and to assist with rehabilitation of bowel function and symptomatic relief.
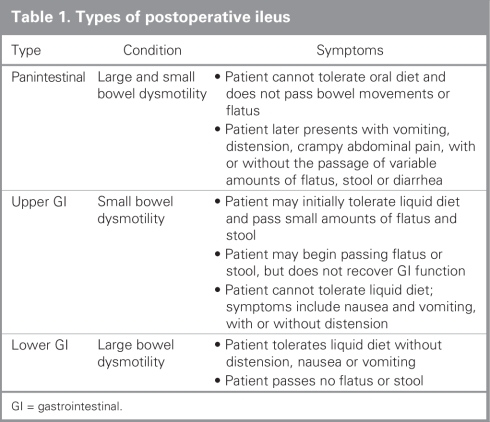
It is also important to be able to identify patients who have POI that lasts long enough to be considered clinically unacceptable or problematic. Although no standard definition exists, an example might be POI lasting longer than 5 days after laparotomy — although this would account for about 40% of patients undergoing laparotomy in the United States — or 3 days after laparoscopic surgery.1 Many surgeons consider that patients who have had no bowel function after these time points are entering the phase of clinically relevant prolonged POI. Three clinical manifestations of POI have been described in the literature, and these are outlined in Table 1.
Table 1
PATHOGENESIS
Normal bowel function requires the coordination of gastrointestinal motility, mucosal transport and defecation reflexes, and these actions are influenced by neurogenic, inflammatory and pharmacologic mechanisms.1 Both anesthesia and surgery alter the activity of these modifiers and therefore may have profound effects on bowel motility. Limiting the effect of these altered pathways forms the basis of many therapeutic options for decreasing the severity and duration of POI, and it is therefore necessary to understand the pathogenesis before understanding treatment options.1
Neurogenic mechanisms
Neurogenic mechanisms appear to play the most important role in early POI. Sympathetic stimulation inhibits gastrointestinal motility, whereas parasympathetic activity primarily stimulates it. After surgery, the sympathetic system tends to be substantially more active than the parasympathetic system, leading to decreased motility and ileus.4 Nevertheless, it is clear that other mechanisms contribute to prolonged POI.
Inflammatory mechanisms
Inflammatory mechanisms likely play an important role, but the exact nature and extent of their involvement is poorly understood.4 Investigators working with animal models have proposed a causative link between migration of leukocytes into the intestinal mucosa and gut paralysis. This may be precipitated by direct intestinal manipulation, particularly during open surgery. There are also studies that suggest tissue trauma, which leads to the release of prostaglandins, nitric oxide and several cytokines, including tumour necrosis factor-α, interleukin-1b and interleukin-6, acts directly on the enteric nervous system (ENS) and disrupts normal gastrointestinal motility.1
Pharmacologic mechanisms
Pharmacologic mechanisms have also been shown to play an important role in prolonged POI through endogenous and exogenous opioids to decrease gastrointestinal motor activity.1,5 There are 3 distinct types of opioid receptors found throughout the body and gastrointestinal system, termed Δ, μ and κ, and the effect of an opioid on the gastrointestinal tract is receptor-specific. It is thought that μ-opioid receptors play a central role in the regulation of bowel motility and have therefore become the target of new therapeutic treatments.5
CLINICAL MANAGEMENT
Until recently, the treatment options available for prolonged POI primarily consisted of nasogastric tube insertion, correction of electrolyte abnormalities and administration of intravenous fluids. Several new studies have called into question the efficacy of routine nasogastric tube placement for prevention of suspected POI, and they suggest that this common practice may actually prolong the duration of POI.1 Early postoperative ambulation and feeding have both been suggested as methods of decreasing the duration of POI, but results from clinical trials have been conflicting. More rigorously designed trials to investigate the true effects are needed to draw stronger conclusions, but at this time both techniques appear to be safe, and although early ambulation does not appear to decrease the duration of POI, starting a clear-liquid diet early after surgery probably does.1
The prevention of POI may be the most cost-effective, time-effective and patient-centred approach to management; however, no technique is currently available to prevent ileus. Several strategies may be used to reduce the clinical consequences of ileus, including minimizing intestinal trauma during surgery, using midthoracic epidural anesthesia and minimizing the need for opioids in pain management — strategies that have all been shown to reduce the risk of prolonged POI.1 A detailed discussion of the data behind these techniques is outside the scope of this paper; however, it should be noted that the pharmacologic techniques discussed herein are most effective when used as part of a multimodal strategy that combines clinical and pharmacologic management techniques as part of a concerted effort to decrease the duration of POI. The rest of our review will focus on the pharmacologic management of POI, which can be used as part of a full multimodal strategy for its prevention and treatment.6
PHARMACOLOGIC MANAGEMENT
Many of the pharmacologic management techniques available to the surgeon are still under active investigation for effectiveness and are based broadly on the known pathophysiologic influences highlighted previously. Minimizing the sympathetic inhibition of gastrointestinal motility, decreasing inflammation and stimulation of gastrointestinal μ-opioid receptors are the ultimate goals of pharmacologic management, and these can be achieved with varying success by a handful of agents.
Minimizing sympathetic inhibition
Minimizing sympathetic inhibition of gastrointestinal motility has achieved greatest success through prevention of sympathetic activation with adequate pain management, minimal surgical trauma and appropriate selection of intraoperative anesthesia. When increased efferent inhibitory sympathetic activity is suspected to be influencing POI, α- and β-receptor antagonists, parasympathomimetics, midthoracic epidural blockade or local anesthetics have all been used successfully to hasten the duration of the ileus.7,8
Both propranolol, a nonspecific β-receptor antagonist, and dihydroergotamine, an α-receptor antagonist, have been investigated for treatment of POI; however, they demonstrated variable results.8 Despite some promising results from a few trials, the effects of these agents have not been adequately studied and therefore they are not used in the treatment of prolonged POI.
Neostigmine is an acetylcholinsterase inhibitor that causes an increase in cholinergic (parasympathetic) activity in the gut wall, which is believed to thereby stimulate colonic motility. Some studies have shown moderate effectiveness in alleviating acute colonic pseudo-obstruction, but the clinical usefulness of neostigmine in postoperative patients may be limited by adverse effects including abdominal cramps, excess salivation, vomiting and bradycardia.8
Use of edrophonium chloride and bethanechol chloride, which competitively inhibit acetylcholine on the binding site of acetylcholinesterase, has been reported to show improvement of POI.9 As with other acetylcholine inhibitors, however, adverse effects such as abdominal cramps, excess salivation, vomiting and bradycardia limit their clinical usefulness.
Cisapride is a serotonin (5-HT)4 receptor antagonist that promotes acetylcholine release from postganglionic nerve endings in the myenteric plexus and is thought to indirectly improve gastrointestinal motility.8 Although several studies have demonstrated its effectiveness in the management of prolonged POI, it has been withdrawn from the market owing to unacceptably high levels of cardiovascular adverse events and should no longer be considered an appropriate treatment choice.
Metocloprimide is suspected to enhance gastrointestinal motility without stimulating gastric secretion, but its use has not been substantiated for POI. Most clinical trials evaluating the effects of metoclopromide on decreasing POI have shown no difference compared with patients receiving placebo with regard to flatus, bowel sounds and bowel movements.8
Decreasing inflammation
Decreasing inflammation may be indicated in patients who are about to undergo major intestinal surgery, as this is thought to be an important contributing factor to POI.10 Inflammation may also cause secondary POI (e.g., inflammation caused by abdominal or pelvic abscess, sepsis or wound infection; surgical trauma to the intestine causing gastrointestinal paralysis). Nonsteroidal anti-inflammatory (NSAIDs) agents can be used in conjunction with opioid analgesics for their dual effects on pain control and inflammatory inhibition. Isolated clinical trials showing the effects of NSAIDs on POI are lacking, but their use as part of multimodal care pathways is well established.5 The release of inflammatory mediators as part of the stress response to surgery is thought to be an important mediator of gut motility and may support the use of anti-inflammatory agents, even in the absence of infection.
Stimulation of gastrointestinal μ-opioid receptors
Stimulation of gastrointestinal μ-opioid receptors can theoretically influence gastrointestinal motility directly; therefore, blocking the peripheral gastrointestinal effects of centrally acting opioids used for analgesia may help prevent POI. Two novel drugs are being investigated for this reason: alvimopan and methylnaltrexone. Both drugs are μ-opioid receptor antagonists, and both appear to offer promising results for preventing prolonged POI. Opioid therapy for postoperative or chronic pain is frequently associated with adverse effects, the most common being dose-limiting and debilitating bowel dysfunction, so alvimopan and methylnaltrexone may also be useful in the treatment of chronic opioid bowel dysfunction.
Both exogenous and endogenous activation of μ-opioid receptors in the gastrointestinal tract is linked to inhibition of gut motility; therefore, inhibition of this activation becomes an optimal target for opioid-induced bowel dysfunction.11,12 Unfortunately, the currently available opioid antagonists such as naloxone are of limited use because they also act at central opioid receptors to reverse analgesia and elicit opioid withdrawal.
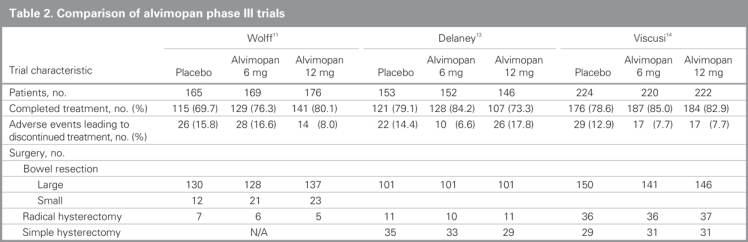
Alvimopan and methylnaltrexone are peripherally acting μ-opioid receptor antagonists that have been studied in patients undergoing abdominal and pelvic surgery and have been shown in several studies to significantly accelerate gastrointestinal recovery.12 They do not cross the blood–brain barrier and therefore do not affect the analgesic properties of centrally acting opioid receptor agonists. In a recent review of 3 phase III multicentre trials (Table 2),11,13,14 Alvimopan significantly accelerated gastrointestinal recovery after segmental bowel resection, with recovery being accelerated by about 18–22 hours in different trials. Patients who received alvimopan also had reduced postoperative morbidity, shorter stays in hospital and decreased incidence of readmission compared with those who received placebo.12 Furthermore, common adverse events such as nausea, vomiting and diarrhea all appeared to be less frequent in patients who received peripherally acting μ-opioid receptor antagonists than those who received placebo.12 Alvimopan received FDA approval for the treatment of POI on May 20, 2008, and a complete list of side effects as well as cost and safety information can be found on the Adolor website (www.adolor.com).15
Table 2
Alternative medications
Alternative medications are less well studied in the treatment of POI. For example, several laxatives are commonly used to treat constipation, but their value in the treatment of POI is unknown. In a randomized trial of bisacodyl administration versus placebo twice daily starting on postoperative day 1, patients who received bisacodyl had significantly earlier bowel movements than those who received placebo (25 h v. 56 h), but further studies are needed to assess the effect of laxatives on POI.8
CONCLUSION
Reducing the duration of POI reduces a patient's postoperative stay in hospital and thus can have profound effects on patient satisfaction, hospital efficiency and the economic burden of major abdominal surgery on the healthcare system. Although the duration of POI is variable, we now have a better understanding of the impact of perioperative opioid analgesia, the benefits of thoracic epidural analgesia with local anesthesia and the benefits of avoiding nasogastric tube placement.
Several multimodal care pathways have been effective in substantially decreasing the length of stay in hospital after major abdominal surgery, and results from these studies show the promising benefit of aggressive postoperative management for prevention and treatment of ileus. New pharmacologic agents such as μ-opioid receptor antagonists are being developed and show promise in accelerating patients' recovery from POI and return to normal diets in a shorter timeframe after surgery.
Contributors: All authors designed and wrote the article and approved the final version for publication.
Competing interests: None declared for Dr. Zeinali and Mr. Stulberg. Dr. Delaney is a paid consultant with Adolor Corporation and Wyeth, and has received honoraria from both companies, and travel assistance and speaker fees from Adolor.
Correspondence to: Dr. C.P. Delaney Professor and Chief, Division of Colorectal Surgery Vice-Chairman, Department of Surgery Case Western Reserve University University Hospitals of Cleveland 11100 Euclid Ave. Cleveland OH 44106-5047 fax 216 844-5957 conor.delaney@UHhospitals.org
References
- 1.Delaney C, Kehlet H, Senagore A, et al. Postoperative ileus: profiles, risk factors, and definitions—a framework for optimizing surgical outcomes in patients undergoing major abdominal colorectal surgery. In: Bosker G, editor. Clinical consensus update in general surgery. Roswell (GA): Pharmatecture, LLC); 2006. Available: www.clinicalwebcasts.com/pdfs/GenSurg_WEB.pdf (accessed 2008 May 15).
- 2.Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg 2000;87:1480-93. [DOI] [PubMed]
- 3.Delaney CP, Zutshi M, Senagore AJ, et al. Prospective randomized controlled trial between a pathway of Controlled Rehabilitation with Early Ambulation and Diet (CREAD) and traditional postoperative care after laparotomy and intestinal resection. Dis Colon Rectum 2003;46:851-9. [DOI] [PubMed]
- 4.Mattei P, Romgeau JL. Review of the pathophysiology and management of postoperative ileus. World J Surg 2006;30:1382-91. [DOI] [PubMed]
- 5.Delaney CP. Clinical perspective on postoperative ileus and the effect of opiates. Neurogastroenterol Motil 2004;16(Suppl 2):1-4. [DOI] [PubMed]
- 6.Delaney CP, Fazio VW, Senagore AJ, et al. Fast-track post-operative management protocol for patients with high comorbidity undergoing complex abdominal and pelvic colorectal surgery. Br J Surg 2001;88:1533-8. [DOI] [PubMed]
- 7.Kehlet H, Holte K. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630-41. [DOI] [PubMed]
- 8.Holte K, Kehlet H. Postoperative ileus: progress towards effective management. Drugs 2002;62:2603-15. [DOI] [PubMed]
- 9.Luckey A, Livingston E, Tache Y. Mechanisms and treatment of postoperative ileus. Arch Surg 2003;138:206-14. [DOI] [PubMed]
- 10.Bauer AJ, Boeckxstaens GE. Mechanisms of postoperative ileus. Neurogastroenterol Motil 2004;16(suppl 2):54-60. [DOI] [PubMed]
- 11.Wolff BG, Michelassi F, Gerkin TM, et al. Alvimopan, a novel, peripherally acting mu-opioid antagonist. Ann Surg 2004;240: 728-35. [DOI] [PMC free article] [PubMed]
- 12.Delaney CP, Wolff BG, Viscusi ER, et al. Alvimopan, for postoperative ileus following bowel resection: a pooled analysis of phase III studies. Ann Surg 2007;245:355-63. [DOI] [PMC free article] [PubMed]
- 13.Delaney CP, Weese JL, Hyman NH, et al. Phase III trial of alvimopan, a novel, peripherally acting, mu opioid antagonist, for postoperative ileus after major abdominal surgery. Dis Colon Rectum 2005;48:1114-25. [DOI] [PubMed]
- 14.Viscusi ER, et al. Alvimopan, a peripherally acting mu-opioid receptor antagonist, compared with placebo in postoperative ileus after major abdominal surgery: results of a randomized, double-blind, controlled study. Surg Endosc 2006;20:64-70. [DOI] [PubMed]
- 15.Adolor Corporation and GlaxoSmithKline. Adolor and GlaxoSmithKline announce FDA approval of Entereg(R) (alvimopan) for the management of postoperative ileus (POI) [press release]. Available: http://phx.corporate-ir.net/phoenix.zhtml?c=120919&p=irol-newsArticle&t=Regular&id=1148660& (accessed 2008 May 20).