Abstract
Background
Peritoneal carcinomatosis (PC) originating in the appendix is a rare disease for which the long-term prognosis is poor. The aim of our study was to evaluate the results of an aggressive treatment approach used in our institution in the last decade.
Methods
We prospectively collected and analyzed data from all patients with PC. Treatment consisted of complete surgical cytoreduction of the tumour followed by intraperitoneal chemotherapy. Chemotherapy was either early postoperative intraperitoneal chemotherapy (EPIC) or hyperthermic intraperitoneal chemotherapy (HIPEC). We used Ronnett's classification for tumour grading (disseminated peritoneal adenomucinosis = grade 0, peritoneal mucinous carcinomatosis with intermediate features = grade 1 and peritoneal mucinous carcinomatosis = grade 2).
Results
From September 1997 to June 2005, 37 patients underwent laparotomy with curative intent; 13 received EPIC and 11 HIPEC. Thirteen patients could not have complete cytoreductive surgery and received no intraperitoneal chemotherapy. The estimated 5-year overall survival was 56% (95% confidence interval [CI] 34%–77%) for all patients, 58% (95% CI 30%–86%) for patients who underwent EPIC and 60% (95% CI 10%–100%) for patients who underwent HIPEC (p = 0.97). Histologic grade was an important prognostic indicator as all patients with grade 0 tumours survived whereas no patients with grade 2 tumours survived (p < 0.001). Patients with grade 1 tumours had an estimated 87% (95% CI 64%–100%) 5-year overall survival. There was no mortality attributed to surgery. The overall complication rate was 36%, including fistulas (16%), intra-abdominal abscesses (12%) and hemorrhage (9%).
Conclusion
This therapeutic approach seems both feasible and safe in select patients. Patients with high-grade tumours are poor candidates for this treatment.
Abstract
Contexte
La carcinomatose péritonéale émanant de l'appendice est une maladie rare dont le pronostic à long terme est sombre. Le but de notre étude était d'évaluer les résultats d'une approche thérapeutique radicale utilisée dans notre établissement depuis une dizaine d'années.
Méthodes
Nous avons recueilli et analysé de manière rétrospective les données concernant tous les patients atteints de carcinomatose péritonéale appendiculaire. Le traitement reposait sur une cytoréduction chirurgicale complète de la tumeur, suivie d'une chimiothérapie intrapéritonéale. La chimiothérapie était administrée sous forme de chimiothérapie intrapéritonéale postopératoire immédiate (CIPPI) ou de chimiohyperthermie intrapéritonéale (CHIP). Nous avons stadifié les tumeurs selon la classification de Ronnett (adénomucinose péritonéale diffuse = grade 0, carcinomatose péritonéale mucineuse associée à des caractéristiques intermédiaires = grade 1 et carcinomatose péritonéale mucineuse = grade 2).
Résultats
Entre septembre 1997 et juin 2005, 37 patients ont subi une laparotomie à visée curative; 13 ont reçu la CIPPI et 11, la CHIP. Treize patients n'ont pas pu subir de chirurgie cytoréductrice complète et n'ont pas reçu de chimiothérapie intrapéritonéale. La survie globale estimée à 5 ans a été de 56 % (intervalle de confiance [IC] à 95 %, 34% à 77 %) pour tous les patients, de 58 % (IC à 95 %, 30% à 86 %) pour les patients qui ont reçu la CIPPI et de 60 % (IC à 95 %, 10% à 100 %) pour les patients qui ont reçu la CHIP (p = 0,97). Le stade histologique a servi d'important indicateur pronostique puisque tous les patients présentant des tumeurs de grade 0 ont survécu, tandis qu'aucun des patients qui présentaient des tumeurs de grade 2 n'a survécu (p < 0,001). Les patients qui avaient des tumeurs de grade 1 ont présenté une survie globale estimée à 5 ans de 87 % (IC à 95 %, 64% à 100 %). Aucun décès n'a été attribué à la chirurgie. Le taux global de complications a été de 36 %, incluant des fistules (16 %), des abcès intra-abdominaux (12 %) et des hémorragies (9 %).
Conclusion
Cette approche thérapeutique semble faisable et sécuritaire chez certains patients. Les patients porteurs de tumeurs de haut grade sont avancées sont de mauvais candidats pour ce type de traitement.
Peritoneal carcinomatosis (PC) originating in the appendix is an uncommon disease, with poor long-term prognosis.1–6 In recent years, a number of studies have been published proposing a novel aggressive treatment approach with curative intent.2,5–12 This approach consists of treating macroscopic PC with complete cytoreductive surgery and treating residual microscopic PC with immediate intraperitoneal chemotherapy. Complete cytoreductive surgery is necessary because experimental studies show that drug penetration is limited to a few cell layers under the surface of the tumour.13,14 Intraperitoneal chemotherapy must be immediate to avoid trapping residual tumour cells in the postoperative fibrin adhesions.15 Immediate intraperitoneal chemotherapy can be delivered in 2 ways: as early postoperative intraperitoneal chemotherapy (EPIC), which is administered in normothermia, or as hyperthermic intraperitoneal chemotherapy (HIPEC), which is delivered intraoperatively along with a heating system. The latter method leads to high local concentration of antineoplastic agents;16–19 with increased cytotoxicity due to the hyperthermia,18,20 the cytotoxicity of oxaliplatin is increased by 180%.21
Results of this treatment approach for PC originating in the appendix have been encouraging, with overall 5-year survival ranging from 40% to 87% and 10-year survival ranging from 21% to 85%.1,2,8,22–29 The aim of our study was to evaluate an aggressive treatment approach combining cytoreductive surgery and intraperitoneal chemotherapy used in our institution over the last decade.
METHODS
Patients
From a prospective database we included all patients with peritoneal surface spread of appendiceal cancer treated in our tertiary care centre between January 1998 and December 2005. Both our institution's clinical trial review board and an independent ethics committee approved our study.
All patients were offered treatment consisting of maximal surgical cytoreduction with immediate intraperitoneal chemotherapy if they fulfilled the following criteria: diagnosis proven by histological examination, no evidence of visceral metastasis on computed tomography (CT) scans of the chest and abdomen, technically resectable disease and general health status good enough to tolerate major surgery. We collected and analyzed the following factors: demographic data, surgical procedures performed, pathologic diagnoses, complications and length of stay in hospital.
Surgery for peritoneal carcinomatosis
For this series, we defined maximal cytoreductive surgery as resection of all tumour deposits greater than 2 mm in diameter. This condition was mandatory before administering immediate intraperitoneal chemotherapy because this treatment is efficient only for nodules smaller than 2 mm.18,19
We approached the abdomen through a xyphopubic midline incision. After complete adhesiolysis, we confirmed the diagnosis of PC by frozen section and scored the extent of PC according to Sugarbaker's peritoneal index.30,31 This index takes into account the number of invaded areas among a total of 13, and the maximal size of tumour nodules within 3 possible groups (< 5 mm, 5 mm to 5 cm, > 5 cm). When PC seemed resectable, we removed the primary tumour and all visceral or peritoneal surface tumour deposits as completely as possible by using peritonectomy procedures, as described by Sugarbaker.32 Macroscopically detectable disease had to be completely resected before administering intraperitoneal chemotherapy. However, the presence of remaining tumour seeding smaller than 2 mm in diameter was allowed if it was located on the small bowel or stomach. Depending on the extent of disease, complete cytoreductive surgery may have required several hours to perform. If, after thorough exploration, we considered the PC resection to be incomplete, no immediate intraperitoneal chemotherapy was administered and patients received palliative surgery.
Immediate intraperitoneal chemotherapy
We performed immediate intraperitoneal chemotherapy to allow for optimal exposure to the chemotherapy solution before any adhesions formed that might impair the distribution of the drug. Patients underwent either EPIC (1998–2002) or HIPEC (2002–2005). The EPIC procedure consisted of administering normothermic intraperitoneal chemotherapy for 5 days in the immediate postoperative period. We installed 3 drains (Tenkhoff catheters) for intraperitoneal drug instillation. We administered mitomycin C (10 mg/m2) on day 1 and 5-fluorouracil (15 mg/kg) on days 2–5, given in a 2-L solution 23h/d, according to the procedure described by Sugarbaker.30,31 The intra-abdominal contents were thus soaked in the chemotherapy solution for 5 days, and we unclamped the drains thereafter to allow for complete evacuation of the drugs.
The HIPEC procedure consisted of the delivery of intraperitoneal chemotherapy intraoperatively along with a heating system. We used oxaliplatin in all but 1 patient (this patient received mitomycin C) because of its efficacy against colorectal cancer, and because it is much shorter to administer than mitomycin C, according to a phase I study.18 We performed HIPEC with a continuous closed circuit using 4 36-French drains (2 inlets and 2 outlets) connected to 2 pumps. We used 1 heating unit and 2 heat exchangers to eliminate a Y connector that could reduce flow rates and heat homogeneity.19 The procedure took place with the abdomen open and the skin sutured to a retractor ring placed above the anterior surface of the abdomen. The flow rate was 1 L/min for each pump. Four thermal probes inside the peritoneal cavity provided continuous temperature feedback, and the intra-abdominal temperature was maintained everywhere between 42°C and 43°C. The duration of perfusion was 30 minutes from the time when optimal temperature (42–44°C) was reached. Usually, less than 5 minutes were necessary to reach a high homogeneous temperature, leading to a total peritoneal infusion duration of about 35 minutes. Afterwards, the infusion liquid was completely evacuated. The total oxaliplatin dose was delivered as a bolus mixed with 5% dextrose at the beginning of the procedure. The total amount of peritoneal fluid used was based, as for oxaliplatin dosage, on body surface area: 2 L/m2. Dosage of oxaliplatin was 460 mg/m2, as recommended in a previous study in humans.18 Instillation volume and oxaliplatin dosage both resulted in a similar intraperitoneal concentration of the drug in each patient.
Pathology
An experienced pathologist (A.M.) performed the pathologic classification. When surgery of the primary tumour was performed in an institution other than ours, the pathology material was sent to us for revision. We performed the tumour grading of both primary (when available) and peritoneal deposits according to Ronnett's histologic classification.33 We considered disseminated peritoneal adenomucinosis to be a grade 0 tumour; the tumour was characterized histologically by the presence of scant low-grade adenomatous mucinous epithelium within abundant extracellular mucin and associated fibrosis. We classified peritoneal mucinous carcinomatosis as a grade 2 tumour; this tumour had the cytologic and architectural features of higher-grade mucinous carcinoma associated with extracellular mucin, often with invasive components and sometimes demonstrated signet ring cell differentiation. A grade 1 tumour consisted of peritoneal mucinous carcinomatosis with intermediate features combining grade 0 and grade 2 characteristics; such tumours were derived from well-differentiated mucinous adenocarcinomas of the appendix.
Systemic chemotherapy
We administered systemic chemotherapy preoperatively for 3–6 months among patients with extensive disease. The aim of this systemic treatment was to diminish the tumour burden and maximize the chances of complete surgical cytoreduction thereafter. We administered adjuvant systemic chemotherapy only among patients who had grade 2 disease for 6 months. Chemotherapy consisted of 5-fluorouracil in addition to irinotecan or oxaliplatin once these drugs became available. In our institution, systemic chemotherapy for digestive tumours is administered by surgical oncologists.
Follow-up
We saw patients at the outpatient clinic in 3- to 4-month intervals, and we performed a physical examination. We obtained a CT scan of the abdomen and pelvis every 6 months for 5 years, and yearly thereafter.
Statistical analysis
We prospectively collected all data. No patient was lost during follow-up. We established survival curves using the Kaplan–Meier method, and we compared the results using the log-rank test. We considered the differences to be significant at p ≤ 0.05.
RESULTS
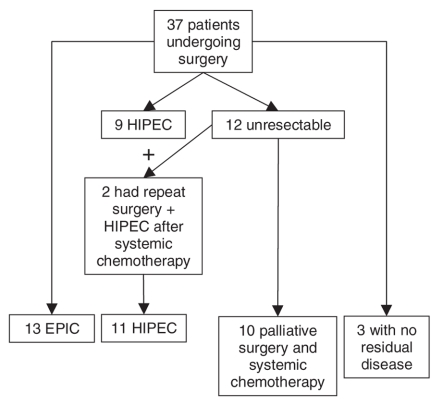
From September 1997 to June 2005, 37 patients with a PC originating in the appendix underwent laparotomy with curative intent. There were 17 men and 20 women, with a mean age of 51 (33–73) years. The primary tumour had been removed in 20 patients. At laparotomy, 12 patients had nonresectable disease, either because the PC was too extensive or because of intraoperative discovery of visceral metastasis (Fig. 1). Two of these patients underwent repeat surgery with successful cytoreduction and intraperitoneal chemotherapy after 6 months of systemic chemotherapy. Three other patients who received initial diagnoses of a mucinous tumour of the appendix with limited peritoneal disease had no evidence of PC when we performed a second-look laparotomy in our centre 6 months later. These patients had undergone appendectomy along with the complete removal of peritoneal tumours at another centre before being referred to us. Hence, these patients did not receive intraperitoneal chemotherapy.
Fig. 1. Patient distribution. EPIC = early postoperative intraperitoneal chemotherapy; HIPEC = hyperthermic intraperitoneal chemotherapy.
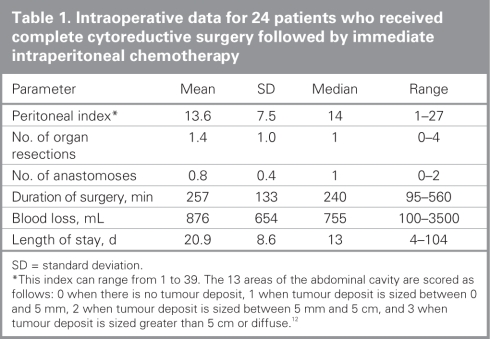
We performed complete surgical cytoreduction followed by immediate intraperitoneal chemotherapy in 24 patients: 13 EPIC and 11 HIPEC. Twelve of these patients with extensive peritoneal disease underwent preoperative systemic chemotherapy (including the 2 patients who had repeat surgery). Details of the surgical parameters are outlined in Table 1. There was no mortality attributed to surgery. The overall complication rate was 36%, including fistulas (16%), intra-abdominal abscesses (12%) and hemorrhage (9%). One patient in the HIPEC group experienced grade 2 neuropathy that lasted for 1 week after surgery. That same patient also experienced grade 3 thrombocytopenia 1 week postoperatively. There was no statistically significant difference in the complication rates between the EPIC and HIPEC groups.
Table 1
Final pathology reports showed diffuse peritoneal adenomucinosis (grade 0) in 5 patients (21%; EPIC n = 2, HIPEC n = 3), an intermediate type (grade 1) in 12 patients (50%; EPIC n = 7, HIPEC n = 5) and peritoneal mucinous carcinomatosis (grade 2) in 7 patients (29%; EPIC n = 4, HIPEC n = 3).
We administered systemic adjuvant chemotherapy in the 7 patients with grade 2 tumours. Treatments started within 6–10 weeks after surgery, depending on the patients' conditions.
Survival rates
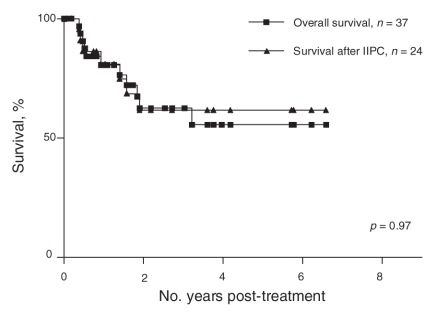
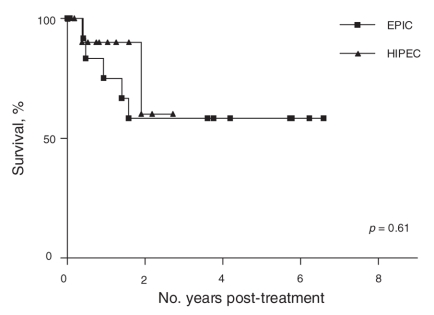
Median follow-up was 23 (range 7–81) months for the entire series. The estimated 5-year overall survival rate for the entire series was 56% (95% confidence interval [CI] 34%–77%) and 59% (95% CI 29%–88%) for patients who underwent immediate intraperitoneal chemotherapy (Fig. 2). The estimated 5-year overall survival for patients who underwent EPIC was 58% (95% CI 30%–86%), and 60% (95% CI 10%–100%) for patients who underwent HIPEC (Fig. 3). There was no statistically significant difference between these groups with regards to survival. At the time of data analysis, 5 patients had isolated peritoneal recurrence, 4 patients had visceral recurrence only and 1 patient had both.
Fig. 2. Overall survival for the entire series (intent-to-treat) and for all patients who underwent complete surgical cytoreduction followed by immediate intraperitoneal chemotherapy. IIPC = immediate intraperitoneal chemotherapy.
Fig. 3. Overall survival results: early postoperative intraperitoneal chemotherapy (EPIC) versus intraperitoneal chemohyperthermia (HIPEC).
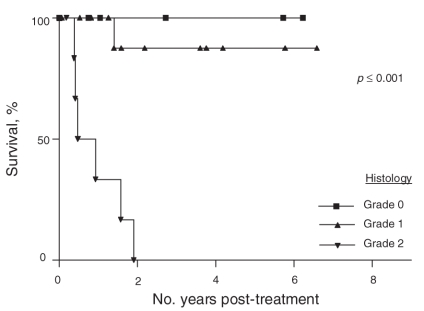
Histologic grade was an important prognostic indicator since all patients with grade 0 tumours survived, whereas no patients with grade 2 tumours survived (p < 0.001). Patients with grade 1 tumours had an estimated 87% (95% CI 64%–100%) 5-year overall survival (Fig. 4). Other parameters such as age, sex, peritoneal index, duration of surgery, blood loss and systemic chemotherapy (neoadjuvant or adjuvant) had no statistically significant influence on survival.
Fig. 4. Overall survival according to histologic grade.
Finally, we also followed the 3 patients who underwent second-look laparotomy and who we found had no disease. The first patient had a grade 0 tumour and was disease-free after 20.6 months. The second had a grade 1 tumour and was disease-free after 13.2 months. The third patient had a grade 2 tumour and received 6 months of adjuvant irinotecan-based systemic chemotherapy after her primary surgery. She was disease-free after 8.6 months.
DISCUSSION
We started treating PC patients with intraperitoneal chemotherapy more than 7 years ago. Nearly 60% of these patients are still alive today. This is comparable with most large series.2,4–6,9,10,12,34–38 This treatment approach is complex, and there is a high risk of complications in the postoperative period. Hence, a multidisciplinary approach by an experienced team is mandatory.
Our study confirmed the correlation between low histologic grade and better prognosis demonstrated in previous studies.1,39–41 Similarly, high peritoneal index has been shown to be an adverse prognostic factor in a series studying colorectal PC.26 In the presence of high-grade tumours, the procedure is not curative, especially when the peritoneal index is high. All patients in our series with high-grade tumours had a poor outcome (0% survival after 2 years). Conversely, an aggressive approach is warranted when the histopathologic grade is low or very low, even with a high peritoneal index (87% survival at 5 years). Very low-grade disease may represent truly benign disease.5,42
As the surgery is often long, with large fluid shifts and a high risk of postoperative complications, patients in our series had to be relatively young and fit to be considered for the procedure. This is an important factor reflected in the absence of procedure-related deaths. Regarding morbidity, 1 of 3 patients had significant complications after the procedure. Most were infectious (fistulas and abscesses). Intraperitoneal chemotherapy is probably largely responsible for these complications.27,38,43,44 In spite of its potential deleterious effect on tissue, we did not observe any increase in the complication rate when heat was combined with intraperitoneal chemotherapy. The shorter exposition of viscera to the chemotherapy solution (30 min v. 5 d) may explain this phenomenon.
In our series, HIPEC did not seem more effective than EPIC when used in the treatment of appendiceal PC, but it is impossible to draw any conclusion since the number of patients in each group was too small and there was no randomization. At the beginning of our study, we used EPIC, but when HIPEC became available in 2002 in our institution, we favoured this approach because there was evidence in the literature of increased chemotherapy cytotoxicity when using hyperthermia.18,20 Furthermore, HIPEC is more comfortable for the patient and is simpler for the nursing staff. Despite no demonstrable survival advantage, these reasons were deemed reasonable to justify the use of HIPEC.
Patients with a high peritoneal index can be helped by preoperative (neoadjuvant) systemic chemotherapy, even if the histologic grade is low. In our study, 10 patients who received the complete treatment of surgery and immediate intraperitoneal chemotherapy had been treated with neoadjuvant chemotherapy before surgery. Two other patients considered to be initially nonresectable at first laparotomy became potentially curable after neoadjuvant systemic treatment. The increasing availability of irinotecan and oxaliplatin has come to offer some hope for these classically refractory tumours. Furthermore, neoadjuvant treatment may allow for improved patient selection.
During HIPEC, we chose oxaliplatin for its high efficacy against colorectal cancer.45–50 Before using this drug in humans, we performed pharmacokinetics studies of oxaliplatin administered in pigs by intraperitoneal route at different temperatures. We found it possible to achieve very high intracellular concentrations of oxaliplatin (potentialized by heat) without substantial systemic absorption, making this technique relatively safe. Based on Elias' studies16,18,26 and ours, we considered it to be the drug of choice for this disease.
We cannot measure precisely the effect of selecting a particular treatment on the prognosis of patients with diffuse peritoneal adenomucinosis. This would require a randomized trial that would offer complete surgical cytoreduction followed or not by immediate intraperitoneal chemotherapy. Of note, Miner and colleagues51 recently reported on the long-term survival of patients who received treatment for pseudomyxoma peritonei by repeat surgical cytoreduction without intraperitoneal chemotherapy, with a median survival of 9.8 years; 21% of patients with low-grade disease survived 10 years.
Finally, for patients with no residual disease at second-look laparotomy 6 months after complete surgical excision of the appendix and PC, our experience showed that they were all disease-free after several months. However, longer follow-up is needed to determine whether prophylactic intraperitoneal chemotherapy is needed when no disease is found at second-look laparotomy, especially for patients with higher-grade tumours.
CONCLUSION
Although the results of this study are preliminary, the treatment of peritoneal carcinomatosis arising from the appendix by complete surgical cytoreduction followed by immediate intraperitoneal chemotherapy seems both feasible and safe in select patients. However, patients with high-grade tumours are poor candidates for this treatment.
Contributors: Drs. Sideris, Leblanc, Leclerc and Dubé designed the study. Drs. Sideris and Mitchell acquired the data, which Drs. Sideris and Drolet analyzed. Drs. Sideris and Mitchell wrote the article, which Drs. Mitchell, Drolet, Leblanc, Leclerc and Dubé reviewed. All authors approved the final version for publication.
Competing interests: None declared.
Accepted for publication Nov. 20, 2007
Correspondence to: Dr. L. Sideris Division of General Surgical Oncology Department of Surgery Hôpital Maisonneuve-Rosemont 5415 boul. de l'Assomption Montréal QC H1T 2M4 fax 514 252-0896 sideris8@sympatico.ca
References
- 1.Bradley RF, Stewart JH IV, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol 2006;30:551-9. [DOI] [PubMed]
- 2.van Ruth S, Acherman YI, van de Vijver MJ, et al. Pseudomyxoma peritonei: a review of 62 cases. Eur J Surg Oncol 2003;29:682-8. [DOI] [PubMed]
- 3.Sherer DM, Abulafia O, Eliakim R. Pseudomyxoma peritonei: a review of current literature. Gynecol Obstet Invest 2001;51:73-80. [DOI] [PubMed]
- 4.Sugarbaker PH, Shmookler B, Ronnett BM, et al. Pseudomyxoma peritonei. Br J Surg 1999;86:842. [DOI] [PubMed]
- 5.Elias D, Sabourin JC. [Pseudomyxoma peritonei. a review] [article in French]. J Chir (Paris) 1999;136:341-7. [PubMed]
- 6.Sugarbaker PH, Kern K, Lack E. Malignant pseudomyxoma peritonei of colonic origin. Natural history and presentation of a curative approach to treatment. Dis Colon Rectum 1987;30:772-9. [DOI] [PubMed]
- 7.Elias D, Dubé P, Blot F, et al. Peritoneal carcinomatosis treatment with curative intent: the Institut Gustave-Roussy experience. Eur J Surg Oncol 1997;23:317-21. [DOI] [PubMed]
- 8.Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol 2006;7:69-76. [DOI] [PubMed]
- 9.Elias D, Laurent S, Antoun S, et al. [Pseudomyxoma peritonei treated with complete resection and immediate intraperitoneal chemotherapy] [article in French]. Gastroenterol Clin Biol 2003;27: 407-12. [PubMed]
- 10.Deraco M, Gronchi A, Mazzaferro V, et al. Feasibility of peritonectomy associated with intraperitoneal hyperthermic perfusion in patients with pseudomyxoma peritonei. Tumori 2002;88:370-5. [DOI] [PubMed]
- 11.Ronnett BM, Shmookler BM, Sugarbaker PH, et al. Pseudomyxoma peritonei: new concepts in diagnosis, origin, nomenclature, and relationship to mucinous borderline (low malignant potential) tumors of the ovary. Anat Pathol 1997;2:197-226. [PubMed]
- 12.Sugarbaker PH. Pseudomyxoma peritonei. Cancer Treat Res 1996;81:105-19. [DOI] [PubMed]
- 13.Los G, Ruevekamp M, Bosnie N, et al. Intraperitoneal tumor growth and chemotherapy in a rat model. Eur J Cancer Clin Oncol 1989;25:1857-66. [DOI] [PubMed]
- 14.Los G, Mutsaers PH, van der Vijgh WJ, et al. Direct diffusion of cis-diamminedichloroplatinum(II) in intraperitoneal rat tumors after intraperitoneal chemotherapy: a comparison with systemic chemotherapy. Cancer Res 1989;49:3380-4. [PubMed]
- 15.Zoetmulder FA, Sugarbaker PH. Patterns of failure following treatment of pseudomyxoma peritonei of appendiceal origin. Eur J Cancer 1996;32A:1727-33. [DOI] [PubMed]
- 16.Elias D, Raynard B, Bonnay M, et al. Heated intra-operative intraperitoneal oxaliplatin alone and in combination with intraperitoneal irinotecan: Pharmacologic studies. Eur J Surg Oncol 2006;32:607-13. [DOI] [PubMed]
- 17.Elias D, Matsuhisa T, Sideris L, et al. Heated intraoperative intraperitoneal oxaliplatin plus irinotecan after complete resection of peritoneal carcinomatosis: pharmacokinetics, tissue distribution and tolerance. Ann Oncol 2004;15:1558-65. [DOI] [PubMed]
- 18.Elias D, Bonnay M, Puizillou JM, et al. Heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol 2002;13:267-72. [DOI] [PubMed]
- 19.Elias D, Antoun S, Goharin A, et al. Research on the best chemohyperthermia technique of treatment of peritoneal carcinomatosis after complete resection. Int J Surg Investig 2000;1:431-9. [PubMed]
- 20.Ceelen WP, Hesse U, de Hemptinne B, et al. Hyperthermic intraperitoneal chemoperfusion in the treatment of locally advanced intra-abdominal cancer. Br J Surg 2000;87:1006-15. [DOI] [PubMed]
- 21.Rietbroek RC, van de Vaart PJ, Haveman J, et al. Hyperthermia enhances the cytotoxicity and platinum-DNA adduct formation of lobaplatin and oxaliplatin in cultured SW 1573 cells. J Cancer Res Clin Oncol 1997;123:6-12. [DOI] [PubMed]
- 22.Varona JF, Guerra JM, Salamanca J, et al. Pseudomyxoma peritonei: a clinicopathologic analysis and follow-up of 21 patients. Hepatogastroenterology 2005;52:812-6. [PubMed]
- 23.Sugarbaker PH, Stuart OA, Yoo D. Strategies for management of the peritoneal surface component of cancer: cytoreductive surgery plus perioperative intraperitoneal chemotherapy. J Oncol Pharm Pract 2005;11:111-9. [DOI] [PubMed]
- 24.Verwaal VJ, Zoetmulder FA. Follow-up of patients treated by cytoreduction and chemotherapy for peritoneal carcinomatosis of colorectal origin. Eur J Surg Oncol 2004;30:280-5. [DOI] [PubMed]
- 25.Iversen LH, Rasmussen PC, Wara P, et al. [Pseudomyxoma peritonei] [article in Danish]. Ugeskr Laeger 2004;166:2979-81. [PubMed]
- 26.Elias D, Pocard M, Sideris L, et al. Preliminary results of intraperitoneal chemohyperthermia with oxaliplatin in peritoneal carcinomatosis of colorectal origin. Br J Surg 2004;91:455-6. [DOI] [PubMed]
- 27.Witkamp AJ, de Bree E, Kaag MM, et al. Extensive surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in patients with pseudomyxoma peritonei. Br J Surg 2001;88:458-63. [DOI] [PubMed]
- 28.Zoetmulder FA, van der Vange N, Witkamp AJ, et al. [Hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal pseudomyxoma or peritoneal metastases of colorectal carcinoma; good preliminary results from the Netherlands Cancer Institute] [Article in Dutch]. Ned Tijdschr Geneeskd 1999;143:1863-8. [PubMed]
- 29.Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol 1999;6:727-31. [DOI] [PubMed]
- 30.Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon's role. Langenbecks Arch Surg 1999;384:576-87. [DOI] [PubMed]
- 31.Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis. Semin Surg Oncol 1998;14:254-61. [DOI] [PubMed]
- 32.Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [DOI] [PMC free article] [PubMed]
- 33.Ronnett BM, Yan H, Kurman RJ, et al. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer 2001;92:85-91. [DOI] [PubMed]
- 34.Sugarbaker PH. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg 1994;219:109-11. [DOI] [PMC free article] [PubMed]
- 35.Prayson RA, Hart WR, Petras RE. Pseudomyxoma peritonei. A clinicopathologic study of 19 cases with emphasis on site of origin and nature of associated ovarian tumors. Am J Surg Pathol 1994;18:591-603. [PubMed]
- 36.Pan H, McGary C, LaVorgna KA, et al. Pseudomyxoma peritonei. Gastroenterologist 1998;6:147-50. [PubMed]
- 37.Jivan S, Bahal V. Pseudomyxoma peritonei. Postgrad Med J 2002;78:170-2. [DOI] [PMC free article] [PubMed]
- 38.Jähne J. Extensive surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in patients with pseudomyxoma peritonei (Br J Surg 2001; 88: 458-63). Br J Surg 2001;88:1130. [DOI] [PubMed]
- 39.Pai RK, Longacre TA. Appendiceal mucinous tumors and pseudomyxoma peritonei: histologic features, diagnostic problems, and proposed classification. Adv Anat Pathol 2005;12:291-311. [DOI] [PubMed]
- 40.Jacquemin G, Laloux P. Pseudomyxoma peritonei: review on a cluster of peritoneal mucinous diseases. Acta Chir Belg 2005;105:127-33. [PubMed]
- 41.Galani E, Marx GM, Steer CB, et al. Pseudomyxoma peritonei: the ‚controversial' disease. Int J Gynecol Cancer 2003;13:413-8. [DOI] [PubMed]
- 42.Vana J, Adamicova K, Johanes R, et al. [Appendiceal mucocele causing pseudomyxoma peritonei] [Article in German]. Zentralbl Chir 2005;130:177-80. [DOI] [PubMed]
- 43.Witkamp AJ, de Bree E, Kaag MM, et al. Extensive cytoreductive surgery followed by intraoperative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur J Cancer 2001;37:979-84. [DOI] [PubMed]
- 44.El-Malt M, Ceelen W, van den Broecke C, et al. Healing of experimental colonic anastomoses: effects of combined preoperative high-dose radiotherapy and intraperitoneal 5-fluorouracil. Int J Cancer 2001;96:297-304. [DOI] [PubMed]
- 45.Reddy GK, Gibson AD, Price N. Evolution of FOLFOX regimens in the treatment of advanced colorectal cancer. Clin Colorectal Cancer 2005;4:296-9. [DOI] [PubMed]
- 46.Park SH, Sung JY, Han SH, et al. Oxaliplatin, folinic acid and 5-fluorouracil (FOLFOX-4) combination chemotherapy as second-line treatment in advanced colorectal cancer patients with irinotecan failure: a Korean single-center experience. Jpn J Clin Oncol 2005;35:531-5. [DOI] [PubMed]
- 47.Maindrault-Goebel F, de Gramont A, Louvet C, et al. Evaluation of oxaliplatin dose intensity in bimonthly leucovorin and 48-hour 5-fluorouracil continuous infusion regimens (FOLFOX) in pretreated metastatic colorectal cancer. Oncology Multidisciplinary Research Group (GERCOR). Ann Oncol 2000;11:1477-83. [DOI] [PubMed]
- 48.Mabro M, Artru P, André T, et al. A phase II study of FOLFIRI-3 (double infusion of irinotecan combined with LV5FU) after FOLFOX in advanced colorectal cancer patients. Br J Cancer 2006;94:1287-92. [DOI] [PMC free article] [PubMed]
- 49.Hebbar M, Tournigand C, Lledo G, et al. Phase II trial alternating FOLFOX-6 and FOLFIRI regimens in second-line therapy of patients with metastatic colorectal cancer (FIREFOX study). Cancer Invest 2006;24:154-9. [DOI] [PubMed]
- 50.de Gramont A, Tournigand C, Louvet C, et al. [Oxaliplatin, folinic acid and 5-fluorouracil (folfox) in pretreated patients with metastatic advanced cancer. The GERCOD] [article in French]. Rev Med Interne 1997;18:769-75. [DOI] [PubMed]
- 51.Miner TJ, Shia J, Jaques DP, et al. Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg 2005;241:300-8. [DOI] [PMC free article] [PubMed]