Abstract
OBJECTIVE
To examine the differential effects of intensive and conventional diabetes therapy on weight gain and body composition in adults with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Between 1982 and 1989, 1,246 adults (aged 18–39 years) in the Diabetes Control and Complications Trial were randomly assigned to either conventional therapy (1–2 injections of insulin per day) or intensive therapy (multiple daily injections or continuous subcutaneous infusion with frequent blood-glucose testing). Height and weight were measured at baseline and at annual visits for an average of 6 years (range 3–9). Body composition was assessed cross-sectionally with bioelectrical impedance analysis during 1992, at which time waist and hip circumferences were measured.
RESULTS
Intensively treated patients gained an average of 4.75 kg more than their conventionally treated counterparts (P < 0.0001). This represented excess increases in BMI of 1.5 kg/m2 among men and 1.8 kg/m2 among women. Growth-curve analysis showed that weight gain was most rapid during the first year of therapy. Intensive therapy patients were also more likely to become overweight (BMI ≥ 27.8 kg/m2 for men, ≥27.3 kg/m2 for women) or experience major weight gain (BMI increased ≥5 kg/m2). Waist-to-hip ratios, however, did not differ between treatment groups. Major weight gain was associated with higher percentages of body fat and greater fat-free mass, but among patients without major weight gain, those receiving intensive therapy had greater fat-free mass with no difference in adiposity.
CONCLUSIONS
Intensive therapy for type 1 diabetes produces substantial excess weight gain compared with conventional therapy. However, the additional weight appears to include lean tissue as well as fat.
Greater weight gain was associated with intensive compared with conventional diabetes treatment during the first year of the Diabetes Control and Complications Trial (DCCT) (1). That observation was made during the DCCT’s feasibility phase in the early 1980s, when 278 subjects were followed for 1 year. Additional patients (n = 1,163) were subsequently entered into the DCCT; a total of 1,441 patients were studied for an average of 6.5 years (range 3.5–9).
As previously reported, intensive therapy significantly reduced the risk of development and progression of retinopathy, nephropathy, and neuropathy compared with conventional therapy (2). The major adverse effect of intensive therapy was a threefold increase in severe hypoglycemia (2). After the increased rate of hypoglycemia, weight gain and heightened risk of obesity represent the most conspicuous side effects of intensive treatment observed in the DCCT. In the general population, obesity is associated with increased risks of hypertension, dyslipidemia, cardiovascular disease, gallstones and cholecystitis, respiratory dysfunction, certain forms of cancer, and type 2 diabetes. In individuals with type 1 diabetes, however, it is not clear what role excessive weight gain has in the development and/or progression of these and other disease outcomes.
RESEARCH DESIGN AND METHODS
Experimental design
The DCCT design and methods have been described in detail (3). Randomization of the first patient occurred in August 1983, and the last person began assigned therapy in June 1989. Follow-up ranged from 3.5 to 9 years, with the study ending in May 1993. A total of 29 clinical centers and 1,441 patients participated. A coordinating center, central biochemistry laboratory, central nutrition coding unit, and four other central units analyzed and managed data.
Subjects
DCCT eligibility criteria included insulin dependence for up to 15 years, as evidenced by deficient C-peptide secretion; diabetes duration for 1–15 years; age 13–39 years; and HbA1c >6.55% (>3 SD above the mean of a sample of nondiabetic people aged 13–40 years [4]). Other eligibility requirements included good general health and no more than moderate nonproliferative retinopathy. Candidates were excluded if they were obese, defined as body weight >130% of the ideal (5). Subjects were also excluded if they required >2 units of insulin per kilogram of body weight per day. Detailed eligibility criteria and baseline characteristics of the entire cohort have been published (2,3). The present report is restricted to the 1,246 subjects who were adults (age 18 years or older) when randomized.
Selected baseline characteristics are shown by treatment group in Table 1. Males randomized to intensive treatment weighed significantly less and had lower BMIs than those in the conventional treatment group. They also had a 9-month longer average duration of type 1 diabetes. There were no other significant differences between groups in these variables at baseline.
Table 1.
Selected baseline characteristics and changes in BMI and weight after 1 year of therapy for intensive and conventional treatment groups
| Adult women |
Adult men |
|||
|---|---|---|---|---|
| Intensive | Conventional | Intensive | Conventional | |
| n | 302 | 278 | 317 | 349 |
| Age (years) | 29.0 ± 6.0 | 28.1 ± 5.9 | 29.5 ± 5.4 | 29.3 ± 5.3 |
| Body weight (kg) | 63.4 ± 8.6 | 63.5 ± 8.9 | 75.6 ± 9.7 | 78.1 ± 10.5* |
| Change at 1 year | 3.0 ± 4.1† | 1.1 ± 3.8†‡ | 3.6 ± 4.8† | 1.3 ± 3.7†‡ |
| Height (cm) | 164.5 ± 6.2 | 165.2 ± 5.8 | 178.6 ± 6.7 | 178.9 ± 7.2 |
| BMI (kg/m2) | 23.4 ± 2.8 | 23.2 ± 2.9 | 23.7 ± 2.5 | 24.3 ± 2.6§ |
| Change at 1 year | 1.1 ± 1.6† | 0.4 ± 1.4†‡ | 1.1 ± 1.5† | 0.4 ± 1.2†‡ |
| HbA1c (%) | 9.0 ± 1.5 | 8.9 ± 1.7 | 8.6 ± 1.4 | 8.6 ± 1.5 |
| Duration of type 1 diabetes (month) | 72.3 ± 52.3 | 74.3 ± 52.7 | 74.8 ± 51.2 | 65.6 ± 47.5∥ |
| Insulin dose (units · kg−1 · day−1) | 0.63 ± 0.20 | 0.63 ± 0.21 | 0.63 ± 0.23 | 0.60 ± 0.20 |
Values are means ± SD. P values for group differences are from the Wilcoxon’s rank-sum test and were not significant at the 0.05 level except as noted.
P = 0.001
P < 0.0001
P < 0.0001 for changes from baseline by the Wilcoxon’s signed-rank test
P = 0.0003
P = 0.026.
Treatment groups
The details of the two treatment regimens have been published (6). Only those aspects relevant to this report will be reviewed here.
Conventional treatment
The clinical goals of conventional therapy included 1) absence of symptoms attributable to glucosuria or hyperglycemia, 2) absence of ketonuria, 3) maintenance of normal growth and development and ideal body weight, and 4) freedom from frequent or serious hypoglycemia. Insulin was administered by one or two injections per day and included mixtures of short-, intermediate-, or long-acting insulin. Self-monitoring was with urine or blood glucose testing, with the majority of patients performing daily blood glucose monitoring. Subjects were given individualized meal plans that specified amounts of food and meal times. There were no specific exercise protocols, but exercise was encouraged. Subjects in the conventional group were seen by the health care team every 3 months.
Intensive treatment
Intensive treatment had the same clinical goals as the conventional treatment group, with the additional goal of maintaining blood glucose control as close to the nondiabetic range as possible while minimizing hypoglycemia. Target ranges for glycemic control were: fasting and preprandial blood glucose 70–120 mg/dl; postprandial blood glucose <180 mg/dl; 3:00 a.m. blood glucose, tested weekly, >65 mg/dl; and monthly HbA1c ≤6.05% (mean + 2 SD of a sample of nondiabetic people aged 13–40 years). With the advice of the health care team, intensive therapy subjects could choose either multiple daily injections or continuous subcutaneous insulin infusion using an external pump. Insulin doses were guided by the results of self-monitoring of blood glucose performed at least four times per day, and they were further adjusted based on meal content and composition and anticipated exercise. Intensive treatment group subjects were hospitalized, usually for 3–4 days, to initiate therapy, and they were seen at least monthly thereafter. Although the intensive group followed a dietary protocol similar to that of the conventional group, greater attention was given to adjusting insulin and diet to achieve intensive therapy’s glycemic targets.
Diet
Dietary guidelines for the DCCT
Meal plans and educational strategies were tailored to each individual, with a caloric allowance to achieve and maintain ideal body weight and with goals of 15–20% of the energy from protein, 30–35% from fat, and 50–55% from carbohydrates (7). In response to the National Cholesterol Education Program (NCEP), the study protocol was amended in 1988 to provide all participants with counseling in the NCEP Step 1 diet (8). Instruction in the Step 2 diet was given to subjects who did not attain target levels of LDL-cholesterol, despite adherence to the Step 1 diet (7).
Weight management for intensive therapy
Once the potential for weight gain with intensive therapy was recognized, dietitians increased their emphasis and counseling on weight management. To achieve target glycemic levels without hypoglycemia or weight gain, even greater attention was given to the relation between nutrient intake and insulin. Meal plans for patients assigned to intensive therapy often subtracted 250–300 kcal from estimated daily energy needs before the DCCT to maintain weight targets. Dietary advice also focused on a healthy eating approach, emphasizing low-fat choices and recognition of hunger needs versus appetite wants, and addressing life factors that influence food choices (9). Other preventive strategies included teaching portion control and appropriate treatment of hypoglycemia, using food records, weight graphs, exercise programs, behavior modification, contracting, and goal-setting. Focus groups and weight management sessions were held to identify eating problems and discuss strategies to resolve them. Social and family pressures, eating away from home, food preparation methods, and preventing relapse were discussed (10).
Measurements
The measurements described below were obtained at the eligibility, baseline, and quarterly examinations, unless otherwise noted. Height (in centimeters) was measured with a stadiometer. Weight (in kilograms) was measured with the subject in light clothing and stocking feet on the same balance-beam scale for the duration of the trial (7). BMI was calculated by dividing weight (kilograms) by height (meters) squared.
The ratio of the natural waist-to-hip measurements (WHR) provides insight into the distribution of body mass (11). This procedure was added to the study protocol in March 1992 as part of the assessment of body composition and was performed once during the last year of the trial. Circumference measurements of hip and waist were obtained in duplicate by study-certified dietitians using inelastic tapes. If they differed by >0.5 cm, they were repeated. The waist measurement was taken at the narrowest part of the torso in a horizontal plane when viewed from behind; the hip measurement was taken at the maximum extension of the buttocks, with the subject in a relaxed standing posture (7). Fat-free body mass was estimated by tetrapolar bioelectrical body impedance analysis (BIA) using proximal electrode placement. To validate the use of this technique in type 1 diabetes, lean body mass was measured in a subset of 46 DCCT patients via dual-energy X-ray absorptiometry; a regression model specific to type 1 diabetes was developed from these data and applied studywide (12). Percent body fat was calculated as the difference between total body weight and fat-free mass, expressed as a percentage of total weight.
Subjects’ daily diets were assessed using a standardized dietary history at baseline, at years 2 and 5, and upon exit from the study (13). Dietitians were trained and certified in the collection of these data (7). Dietary data were analyzed by the DCCT Central Nutrition Coding Unit (14). Activity levels at school, work, and during leisure time were estimated using a standardized questionnaire at baseline and annually thereafter. HbA1c measurements were determined at the Central Biochemistry Laboratory with high-performance liquid chromatography (4).
Statistical methods
Major weight gain was defined as an increase in BMI of at least 5 kg/m2 from baseline, which is equivalent to ~20% weight gain, or 14 kg for most subjects (15). Overweight was defined using the National Center for Health Statistics definition for adults: BMI ≥27.8 kg/m2 for men and ≥27.3 kg/m2 for women (16). Finally, WHRs >0.85 for women or >0.9 for men were considered “increased” (17).
All data analyses were performed according to the original randomized treatment groups. Adherence to assigned therapy was 97 and 98% of study time for conventional and intensive therapy, respectively (2). Data from all women who became pregnant during the trial were censored from the time of their first conception through study end, unless otherwise noted. All P values are two-sided and are reported at their nominal levels, i.e., without formal adjustment for multiple comparisons.
The Wilcoxon’s rank-sum test (18) was used to test whether the distributions of continuous variables (including changes from baseline in continuous measures) differed between groups. A linear covariance adjustment was made to those outcome variables proving to be highly correlated with some baseline characteristics. The significance of within-group changes from baseline was assessed using Wilcoxon’s signed-rank test for paired differences (18). Fisher’s exact test (19) was used to test binary variables for association with treatment group at a single point in time.
Consistent differences in the distributions of continuous variables over time were evaluated with the nonparametric test of stochastic ordering proposed by Wei and Lachin (20), weighting each of the univariate Mann-Whitney U test differences in proportion to the corresponding sample sizes (21), after adjusting for covariance with the baseline value of the outcome. Consistent group differences in repeated dichotomous measures, such as major weight gain, were examined using the test of stochastic ordering in the multiple nonindependent 2 × 2 tables of Lachin and Wei (22).
Two-stage random-effects models (23,24) were fit via restricted maximum-likelihood estimation (25) to the consecutive measurements of BMI to obtain concise summaries of its typical rates of change. Separate models were initially fit to each sex within each treatment group, including baseline BMI and time as covariates. To minimize the cohort effects associated with staggered entry, the corresponding empirical rates of change were computed as unweighted averages of the average annual changes during the period in question.
The growth-curve fittings were carried out by Program 5V of the BMDP statistical software package, version 1990 (26). All other analyses and all data management were carried out using SAS software, Version 6 (27,28).
RESULTS
Change in weight during the first year of follow-up
Mean weight and BMI at baseline and changes after 1 year among the adult subjects in each treatment group are shown in Table 1. Overall, the intensive treatment group gained an average of 3.3 kg compared with 1.2 kg in the conventional group (P < 0.0001). The mean increase in BMI was also significantly higher in the intensive treatment group, both among all subjects (1.2 vs. 0.4 kg/m2, P < 0.0001) and within each sex.
Weight over the entire study period
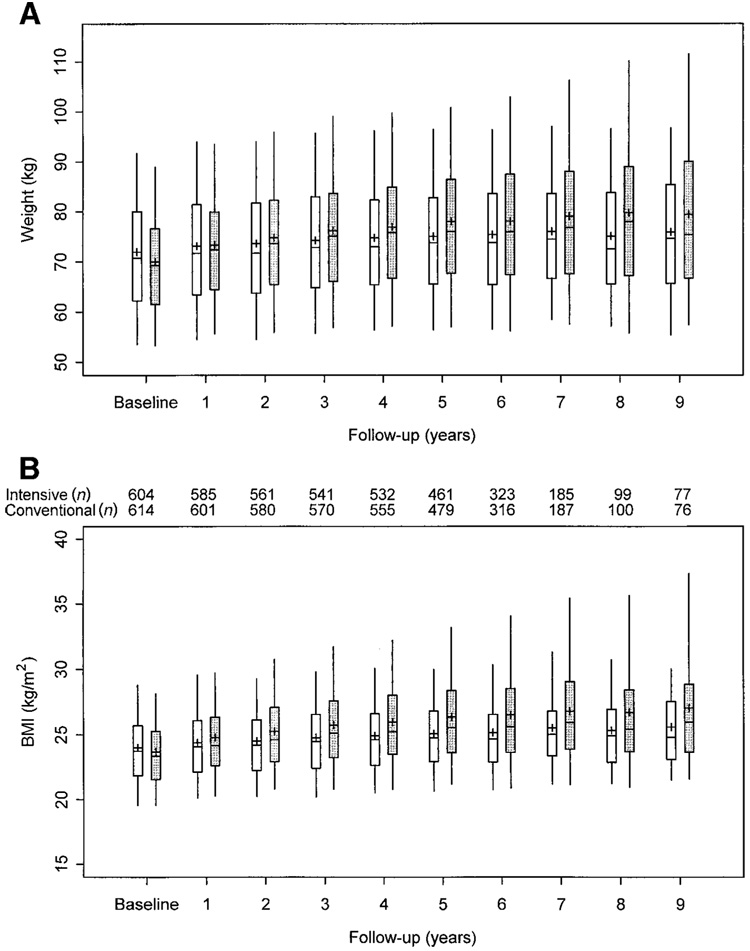
The annual distributions of weight and BMI in each treatment group are shown in Fig. 1. From year 1 onward, the medians, the upper and lower quartiles, and even the 5th and 95th percentiles were generally higher in the intensive group. The test of stochastic ordering indicates that these differences attain very high levels of statistical significance over time (P < 0.00001).
Figure 1.
Boxplots of weight (A) and BMI (B) at each annual visit for intensive ( ) and conventional treatment patients (
) and conventional treatment patients ( ). The central line is the median, the upper and lower boxes represent the upper and lower quartiles, respectively, and the upper and lower vertical lines represent the 95th and 5th percentiles, respectively. The differences between the treatment groups in both measures are significant beginning at year 1 (P < 0.0001). The number of adult subjects in the intensive and conventional treatment groups at each year is noted between A and B.
). The central line is the median, the upper and lower boxes represent the upper and lower quartiles, respectively, and the upper and lower vertical lines represent the 95th and 5th percentiles, respectively. The differences between the treatment groups in both measures are significant beginning at year 1 (P < 0.0001). The number of adult subjects in the intensive and conventional treatment groups at each year is noted between A and B.
BMI values in both treatment groups appeared to increase most sharply during the first year and then more gradually thereafter. This raises the question of whether the ongoing increases in BMI eventually level off, or even begin to reverse, in either treatment group. To address this, two-stage random-effects models were used to estimate growth curves characterizing typical changes in BMI over time within each subgroup. Details are given in the APPENDIX. Table 2 summarizes the estimated expected annual rates of change during successive 3-year intervals and contrasts them with the observed average rates over the same periods. Both the expected and observed increases in BMI were uniformly greatest during the first year following randomization, slowing distinctly thereafter. In the conventional treatment group, subsequent rates of increase were only one-half to one-third of the rate during the first year.
Table 2.
Average annual rates of change in BMI: predicted and observed*
| Sex | Baseline–Year 1 | Years 1, 2, and 3 | Years 3, 4, 5, and 6 | Years 6, 7, 8, and 9 | P value† |
|---|---|---|---|---|---|
| Female | |||||
| Conventional | |||||
| Predicted | 0.2974 | 0.1512 | 0.1589 | 0.1681 | |
| Observed | 0.3685 | 0.2228 | 0.1579 | 0.2495 | |
| n | 278 | 276 | 226 | 67 | |
| Intensive | |||||
| Predicted | 0.9925 | 0.4267 | 0.3999 | 0.3677 | 0.0005 |
| Observed | 1.1103 | 0.4892 | 0.3080 | 0.3325 | |
| n | 301 | 298 | 246 | 73 | |
| P value for difference between groups | 0.0001 | 0.0002 | 0.0058 | 0.3870 | |
| Male | |||||
| Conventional | |||||
| Predicted | 0.3903 | 0.1316 | 0.1148 | 0.0946 | |
| Observed | 0.4026 | 0.1511 | 0.1402 | 0.1324 | |
| n | 347 | 347 | 280 | 76 | |
| Intensive | |||||
| Predicted | 1.1335 | 0.3797 | 0.2940 | 0.1913 | <0.0001 |
| Observed | 1.0926 | 0.4575 | 0.3172 | 0.2323 | |
| n | 316 | 314 | 256 | 73 | |
| P value for difference between groups | <0.0001 | <0.0001 | 0.0082 | 0.3573 |
The upper figure of each pair is the average annual change in BMI predicted by the growth-curve model during the interval in question. The lower figure is the observed average rate of change for the same period. P values for the treatment-group difference in observed rates of change are from the Wilcoxon’s rank-sum test (baseline–year 1) or the Wei-Lachin test of stochastic ordering, applied separately for all other periods.
P value for test of trends over time, based on observed rates, between treatment groups within sex.
Although intensive therapy subjects gained weight less rapidly after the first year, even after 9 years, neither sex demonstrated any tendency to lose the accumulated weight. Among female subjects in particular, any slowing of weight gain was very gradual. If these patterns continued, adult women on intensive therapy would, on average, continue to gain substantial amounts of weight.
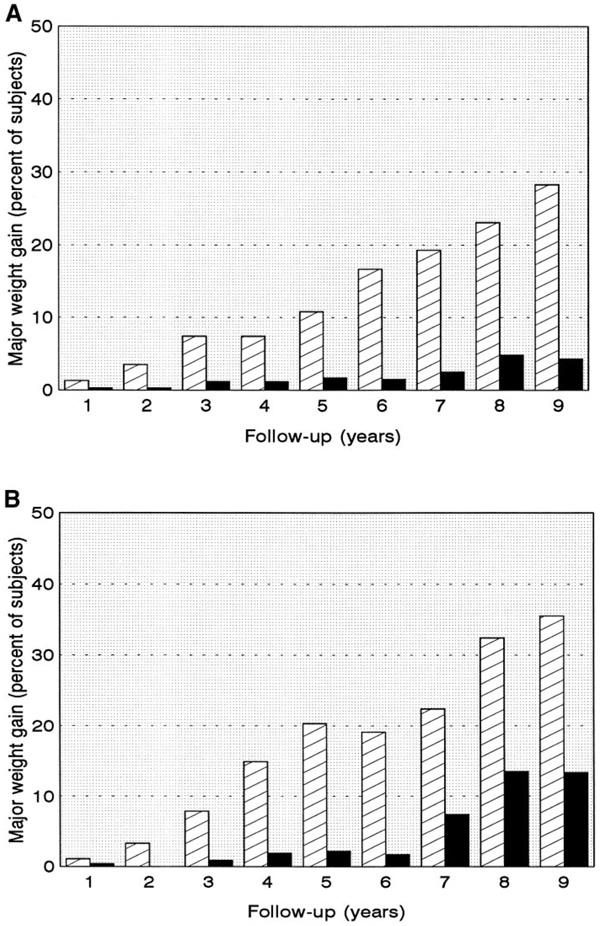
Major weight gain
The annual prevalence of major weight gain is shown in Fig. 2. The proportion of patients who gained >5 kg/m2 BMI was consistently greater in the intensive treatment group. These differences persisted throughout follow-up, and all were statistically significant at P ≤ 0.01 for both sexes.
Figure 2.
The percentage of adult men (A) and adult women (B) with major weight gain (see RESEARCH DESIGN AND METHODS for definition) in the intensive ( ) and conventional (
) and conventional ( ) treatment groups. Differences between treatment groups were significant (P < 0.01) among men at all years after year 1, and at years 2–7 they were inclusive among women. The overall pattern of differences over time was significant at P < 0.01 in both sexes.
) treatment groups. Differences between treatment groups were significant (P < 0.01) among men at all years after year 1, and at years 2–7 they were inclusive among women. The overall pattern of differences over time was significant at P < 0.01 in both sexes.
Weight change and body composition
BIA was performed once after an average of 70 months of follow-up (Table 3). Among adult women, both mean estimated percent body fat and mean estimated fat-free mass were significantly higher in the intensive treatment group after adjusting for baseline BMI (P < 0.001). Neither parameter differed significantly between treatment groups among men. Overall, 19.5% of the intensive treatment group and 17.0% of the conventional treatment group had increased WHRs (P = 0.284), and this prevalence did not differ significantly between treatment groups within either sex (data not shown). However, among women, waist circumferences were significantly greater in the intensive group (P = 0.0004).
Table 3.
Distribution of body composition variables and relevant covariates after an average of 70 months by sex and treatment group
| Quartile |
||||||
|---|---|---|---|---|---|---|
| Variable | Group | 25% | 50% | 75% | Mean ± SD | P value for treatment group differences |
| Women | ||||||
| Age when assessed (years) | INT | 30.00 | 35.00 | 40.00 | 35.11 ±6.11 | 0.1148 |
| CON | 30.00 | 34.00 | 39.00 | 34.29 ± 5.99 | ||
| Length of follow-up (months) | INT | 60.00 | 69.00 | 83.00 | 72.09 ± 19.95 | 0.8220 |
| CON | 57.00 | 69.00 | 84.00 | 72.15 ± 20.11 | ||
| Weight (kg) | INT | 62.05 | 68.90 | 78.90 | 71.79 ± 13.34 | 0.0001 |
| CON | 60.40 | 66.10 | 72.70 | 67.16 ± 9.82 | ||
| Height (cm) | INT | 161.00 | 164.90 | 169.00 | 164.80 ± 6.20 | 0.2504 |
| CON | 161.80 | 165.43 | 169.25 | 165.49 ± 5.63 | ||
| BMI (kg/m2) | INT | 22.98 | 25.46 | 28.75 | 26.42 ± 4.60 | <0.0001 |
| CON | 22.27 | 24.30 | 26.00 | 24.50 ± 3.18 | ||
| HbA1c (% of hemoglobin) | INT | 6.6 | 7.1 | 7.7 | 7.2 ± 1.0 | <0.0001 |
| CON | 7.9 | 9.1 | 10.2 | 9.1 ± 1.6 | ||
| Estimated percent body fat | INT | 28.02 | 32.70 | 36.91 | 32.51 ± 6.08 | 0.0002 |
| CON | 26.77 | 30.67 | 34.35 | 30.68 ± 5.27 | ||
| Estimated fat-free mass (kg) | INT | 43.61 | 47.22 | 51.47 | 47.84 ± 5.95 | 0.0007 |
| CON | 42.46 | 45.54 | 48.96 | 46.21 ± 4.92 | ||
| Natural WHR | INT | 0.72 | 0.76 | 0.80 | 0.76 ± 0.08 | 0.7292 |
| CON | 0.72 | 0.75 | 0.79 | 0.76 ± 0.05 | ||
| Waist circumference (cm) | INT | 71.95 | 77.05 | 84.65 | 79.03 ± 9.97 | 0.0004 |
| CON | 70.85 | 75.05 | 79.70 | 75.75 ± 7.25 | ||
| Men | ||||||
| Age when assessed (years) | INT | 31.00 | 35.00 | 40.00 | 35.23 ± 5.56 | 0.8618 |
| CON | 31.00 | 35.00 | 39.00 | 35.17 ± 5.44 | ||
| Length of follow-up (months) | INT | 58.00 | 69.00 | 83.00 | 70.95 ± 19.69 | 0.6647 |
| CON | 57.00 | 66.00 | 83.00 | 70.31 ± 18.94 | ||
| Weight (kg) | INT | 74.55 | 82.03 | 90.40 | 83.52 ± 12.84 | <0.0001 |
| CON | 73.05 | 81.10 | 88.20 | 81.60 ± 11.29 | ||
| Height (cm) | INT | 173.80 | 178.00 | 182.40 | 178.41 ± 6.60 | 0.1693 |
| CON | 174.40 | 178.70 | 184.00 | 179.02 ± 7.23 | ||
| BMI (kg/m2) | INT | 23.86 | 25.72 | 28.14 | 26.20 ± 3.46 | <0.0001 |
| CON | 23.65 | 25.16 | 27.31 | 25.43 ± 2.92 | ||
| HbA1c (% of hemoglobin) | INT | 6.6 | 7.1 | 7.6 | 7.2 ± 1.0 | <0.0001 |
| CON | 8.4 | 9.2 | 10.0 | 9.2 ± 1.3 | ||
| Estimated percent body fat | INT | 16.07 | 19.79 | 23.89 | 19.79 ± 6.13 | 0.0557 |
| CON | 14.86 | 18.46 | 23.26 | 18.92 ± 5.83 | ||
| Estimated fat-free mass (kg) | INT | 60.74 | 65.94 | 71.30 | 66.53 ± 8.10 | 0.3397 |
| CON | 60.28 | 64.90 | 70.90 | 65.84 ± 7.86 | ||
| Natural WHR | INT | 0.83 | 0.87 | 0.91 | 0.87 ± 0.07 | 0.8103 |
| CON | 0.84 | 0.87 | 0.90 | 0.87 ± 0.06 | ||
| Waist circumference (cm) | INT | 81.65 | 86.60 | 94.25 | 88.51 ± 9.82 | 0.1028 |
| CON | 81.00 | 86.00 | 91.70 | 86.99 ± 8.13 | ||
P values are from the Wilcoxon’s rank-sum test. BMI was tested after adjustment for covariance with baseline BMI. Weight, percent body fat, and fat-free mass were all adjusted for covariance with weight at baseline. INT, intensive therapy (n = 298); CON, conventional therapy (n = 337).
Body composition of patients with and without major weight gain at the time of evaluation (further stratified by sex and treatment group) is described in Table 4. Wilcoxon tests compare the estimated percentage of body fat and fat-free body mass between treatment groups within each weight-gain stratum and between patients with and without major weight gain in each treatment group. Both characteristics were highly correlated with weight at baseline, so these tests were performed after a linear adjustment for that covariance.
Table 4.
Body composition characteristics associated with major weight gain
| Characteristic | Major weight gain | Treatment group | n | First quartile | Median | Third quartile | Adjusted mean* | P value for treatment group† | P value for weight gain‡ |
|---|---|---|---|---|---|---|---|---|---|
| Percent body fat | |||||||||
| Men | Yes | INT | 38 | 24.5 | 27.1 | 31.7 | 25.9 | <0.0001 | |
| CON | 5 | 23.0 | 24.7 | 28.7 | 24.3 | 0.3733 | |||
| No | INT | 260 | 15.1 | 19.2 | 22.7 | 17.8 | 0.1479 | ||
| CON | 332 | 14.7 | 18.4 | 23.2 | 17.2 | 0.0076 | |||
| Women§ | Yes | INT | 67 | 36.8 | 38.9 | 41.6 | 39.8 | <0.0001 | |
| CON | 9 | 36.3 | 38.1 | 40.8 | 40.1 | 0.5958 | |||
| No | INT | 220 | 27.0 | 30.7 | 34.0 | 32.3 | 0.2342 | ||
| CON | 249 | 26.8 | 30.3 | 33.9 | 31.9 | <0.0001 | |||
| Fat-free mass (kg) | |||||||||
| Men | Yes | INT | 38 | 65.8 | 69.4 | 78.5 | 68.8 | <0.0001 | |
| CON | 5 | 67.6 | 67.9 | 73.7 | 66.9 | 0.7189 | |||
| No | INT | 260 | 60.4 | 65.3 | 70.6 | 63.2 | <0.0001 | ||
| CON | 332 | 60.3 | 64.8 | 70.9 | 61.6 | 0.0131 | |||
| Women | Yes | INT | 67 | 47.8 | 53.3 | 56.5 | 54.9 | <0.0001 | |
| CON | 9 | 47.4 | 50.9 | 54.8 | 53.8 | 0.2537 | |||
| No | INT | 220 | 43.1 | 45.7 | 49.5 | 51.0 | 0.0019 | ||
| CON | 249 | 42.4 | 45.3 | 48.7 | 50.1 | 0.0005 |
Least-squares estimates of cell means after adjusting for covariance with weight at baseline
from the Wilcoxon’s test of differences between treatment groups within weight-gain categories after adjusting for covariance with weight at baseline
from the Wilcoxon’s test of differences between patients with and without major weight gain within treatment groups after adjusting for covariance with weight at baseline
includes all women randomized as adults. Excluding women who became pregnant during the trial yields substantially identical results. INT, intensive; CON, conventional.
As expected, patients with major weight gain tended to have significantly higher proportions of body fat than similarly treated patients without major weight gain. However, they also tended to have significantly greater fat-free mass. Among patients without major weight gain, intensive therapy was associated with greater fat-free mass but no concomitant difference in adiposity. No treatment-related differences in body composition were observed among patients with major weight gain, but the small number of conventionally treated subjects in these strata limits the statistical power of these comparisons.
The associations among treatment group, weight gain, and body composition were confirmed in simultaneous tests provided by linear analysis-of-covariance models for fat-free mass and percentage of body fat (not shown). In addition to their covariance with baseline weight, both variables showed significant differences associated with sex and major weight gain. Fat-free mass also differed significantly by treatment, whereas percent fat did not; and neither variable suggested any interaction between treatment group and major weight gain. However, fat-free mass did show a nominally significant interaction between treatment and sex.
Effect of other baseline and intrastudy variables
Baseline variables, including BMI, age, insulin dose, HbA1c, duration of type 1 diabetes, total calories consumed, percentage of calories from fat, and activity level (sedentary compared with not sedentary) were examined to determine their potential impact on weight gain during the study. Intrastudy variables included mean insulin dose, self-reported calorie and fat intake, cigarette smoking, and hypoglycemia. Because of differences between sexes and treatment groups, these analyses were conducted separately in each of the four sex/treatment combinations. Overall, the baseline covariates of age (stratified adjusted relative risk 1.44, CI 1.01–2.05), BMI (1.63, 1.13–2.35), and HbA1c (1.43, 1.00–2.05) were all positively and significantly associated with major weight gain. However, only age was consistent between all four sex/treatment subgroups. None of the intrastudy variables examined was significantly associated with major weight gain.
CONCLUSIONS
Weight gain and hypoglycemia have been identified as the major adverse effects of intensive diabetes therapy (1,29) or improved glucose control (30) in type 1 diabetes. The current report provides new information on the rate of weight gain for up to 9 years of intensive therapy as well as data on body composition and body fat distribution. On average, adult subjects randomly assigned to intensive therapy gained 4.8 kg more during a mean 6.0 years of follow-up than those receiving conventional treatment. In both women and men, the rate of weight gain declined markedly with longer follow-up. Whether this is a natural consequence of long-term intensive treatment or a result of greater attention to weight gain by patients and study personnel is not clear. Similarly, the mechanism(s) of the weight gain cannot be directly addressed by this study.
Results from the 1-year follow-up data from the feasibility phase (1) established a correlation between weight gain and both a lower HbA1c and the presence of severe hypoglycemia. In a study of 6 adult type 1 diabetes patients studied before and after intensive therapy, Carlson and Campbell (29) estimated that 70% of their weight gain could be accounted for by elimination of glycosuria, and the remaining 30% by a 5% decrease in daily energy expenditure, as assessed by whole-room calorimetry. An increase in energy expenditure associated with poor glycemic control has previously been reported (31,32).
To our knowledge, an increase in fat-free mass associated with intensive treatment has not previously been reported. In the Carlson and Campbell study (29), 2.4 of the 2.6 kg of increased weight was accounted for by an increase in fat mass. However, their study sample was small (n = 6) and was followed for only 2 months, and thus it lacked power to find relatively modest increases in fat-free mass (29). Goodship et al. (33) found no difference in fat-free mass in 31 subjects with type 1 diabetes compared with age- and sex-matched nondiabetic volunteers. Fat-free mass was calculated from anthropometry, and the subjects represented a wide range of glycemic control. Librenti et al. (34) found no significant difference in body composition between type 1 diabetic and nondiabetic subjects, but their subjects were not receiving intensive therapy. Body composition was assessed with BIA using standard equations. In contrast, we assessed body composition in 1,180 individuals using a BIA method specifically validated for our population.
Much attention has been focused on body fat distribution because of increasing evidence that abdominal obesity, as assessed by WHR (12,35,36) or waist circumference (37), may be a risk factor for macrovascular disease. We found no significant differences in WHR associated with intensive therapy, despite greater weight gain, although waist circumferences were significantly greater among intensively treated women. Whether waist circumference or WHR is a better predictor of cardiovascular disease risk is debatable. A previous report noted that the intensively treated DCCT patients in the highest quartile of weight gain had increased WHR and BMI associated with higher blood pressure and a relatively atherogenic serum lipid profile (38). These individuals may be predisposed to the insulin resistance syndrome associated with type 2 diabetes, and their tendency toward abdominal obesity may have been exposed by intensive insulin treatment (39).
In conclusion, intensive diabetes treatment is associated with weight gain for up to 9 years. The rate of increase appears to decrease with time, however, and includes an increase in fat-free mass. Given the marked benefits of improved glycemic control in preventing the micro-vascular complications of type 1 diabetes (2), the risk of weight gain should not deter initiation of intensive treatment in appropriate patients. Nonetheless, better understanding of the causes of weight gain and methods to control it are desirable. Besides the physical consequences of excessive weight, concern with body image could make the fear of weight gain an obstacle to successful implementation of intensive diabetes therapy.
Acknowledgments
The DCCT is sponsored by the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, through cooperative agreements and a research contract. Additional support was provided by the National Heart, Lung and Blood Institute; the National Eye Institute; and the General Clinical Research Program of the National Center for Research Resources.
Abbreviations
- BIA
bioelectrical body impedance analysis
- DCCT
Diabetes Control and Complications Trial
- NCEP
National Cholesterol Education Program
- WHR
waist-to-hip ratio
APPENDIX
Changes in BMI over time
Table A1 provides detailed descriptions of the distributions of BMI at each year of follow-up by treatment group within sexes. In both sexes, the intensive group’s tendency toward higher BMI attained a very high level of statistical significance within the first year, and this initial separation clearly persisted for the remainder of the trial. Less apparent from the raw data is whether the extent of the group difference increases, decreases, or remains essentially constant thereafter.
Table A1.
BMI during follow-up
| Year group | n | First quartile | Median | Third quartile | Mean | SD | Z | P |
|---|---|---|---|---|---|---|---|---|
| Women | ||||||||
| Baseline | ||||||||
| Intensive | 302 | 21.29 | 23.35 | 25.26 | 23.41 | 2.76 | — | — |
| Conventional | 278 | 21.22 | 22.96 | 24.97 | 23.24 | 2.87 | — | — |
| 1 | ||||||||
| Intensive | 286 | 22.19 | 24.07 | 26.62 | 24.43 | 3.08 | −5.99 | <0.001 |
| Conventional | 265 | 21.47 | 23.25 | 25.26 | 23.59 | 2.96 | ||
| 2 | ||||||||
| Intensive | 267 | 22.28 | 24.24 | 26.89 | 24.82 | 3.40 | −6.11 | <0.001 |
| Conventional | 252 | 21.59 | 23.49 | 25.61 | 23.79 | 2.94 | ||
| 3 | ||||||||
| Intensive | 243 | 22.45 | 24.59 | 27.47 | 25.21 | 3.63 | −5.70 | <0.001 |
| Conventional | 233 | 21.56 | 23.70 | 25.67 | 23.96 | 3.12 | ||
| 4 | ||||||||
| Intensive | 229 | 22.62 | 24.82 | 28.21 | 25.54 | 3.90 | −4.78 | <0.001 |
| Conventional | 223 | 21.86 | 24.07 | 26.01 | 24.29 | 3.12 | ||
| 5 | ||||||||
| Intensive | 195 | 22.97 | 25.14 | 28.49 | 26.22 | 4.29 | −5.93 | <0.001 |
| Conventional | 184 | 21.92 | 24.08 | 26.19 | 24.41 | 3.11 | ||
| 6 | ||||||||
| Intensive | 134 | 23.20 | 25.28 | 29.00 | 26.25 | 4.37 | −4.66 | <0.001 |
| Conventional | 121 | 22.02 | 24.28 | 26.61 | 24.66 | 3.39 | ||
| 7 | ||||||||
| Intensive | 78 | 23.83 | 25.69 | 29.71 | 26.90 | 4.85 | −2.71 | 0.007 |
| Conventional | 68 | 22.90 | 24.12 | 26.97 | 25.20 | 3.53 | ||
| 8 | ||||||||
| Intensive | 34 | 22.73 | 24.53 | 30.45 | 26.58 | 5.48 | −1.93 | 0.054 |
| Conventional | 38 | 22.84 | 25.19 | 27.91 | 25.84 | 3.84 | ||
| 9 | ||||||||
| Intensive | 31 | 22.96 | 24.87 | 30.04 | 26.99 | 5.64 | −1.73 | 0.083 |
| Conventional | 30 | 23.27 | 26.00 | 28.17 | 26.07 | 3.34 | ||
| Overall (test of stochastic ordering) | 6.87 | <0.0001 | ||||||
| Men | ||||||||
| Baseline | ||||||||
| Intensive | 317 | 21.71 | 23.39 | 25.28 | 23.67 | 2.54 | ||
| Conventional | 349 | 22.42 | 24.28 | 26.07 | 24.35 | 2.64 | ||
| 1 | ||||||||
| Intensive | 316 | 22.92 | 24.19 | 26.20 | 24.77 | 2.72 | −6.06 | <0.001 |
| Conventional | 347 | 22.81 | 24.60 | 26.49 | 24.74 | 2.76 | ||
| 2 | ||||||||
| Intensive | 315 | 23.22 | 24.71 | 26.92 | 25.26 | 2.97 | −7.84 | <0.001 |
| Conventional | 347 | 23.05 | 24.80 | 26.71 | 24.86 | 2.67 | ||
| 3 | ||||||||
| Intensive | 312 | 23.56 | 25.17 | 27.28 | 25.69 | 3.19 | −8.82 | <0.001 |
| Conventional | 346 | 23.10 | 25.13 | 26.92 | 25.06 | 2.73 | ||
| 4 | ||||||||
| Intensive | 311 | 23.92 | 25.28 | 27.87 | 25.92 | 3.29 | −9.18 | <0.001 |
| Conventional | 344 | 23.26 | 24.99 | 27.15 | 25.15 | 2.74 | ||
| 5 | ||||||||
| Intensive | 269 | 23.82 | 25.59 | 28.03 | 26.09 | 3.40 | −7.66 | <0.001 |
| Conventional | 299 | 23.47 | 24.96 | 27.24 | 25.34 | 2.81 | ||
| 6 | ||||||||
| Intensive | 192 | 24.04 | 25.80 | 28.38 | 26.52 | 4.44 | −7.50 | <0.001 |
| Conventional | 200 | 23.53 | 24.77 | 26.97 | 25.24 | 2.84 | ||
| 7 | ||||||||
| Intensive | 109 | 23.80 | 26.17 | 28.80 | 26.53 | 3.66 | −5.31 | <0.001 |
| Conventional | 119 | 23.69 | 25.18 | 26.85 | 25.46 | 2.97 | ||
| 8 | ||||||||
| Intensive | 65 | 24.37 | 25.78 | 28.93 | 26.88 | 4.06 | −4.61 | <0.001 |
| Conventional | 63 | 22.83 | 24.90 | 26.75 | 25.11 | 2.74 | ||
| 9 | ||||||||
| Intensive | 46 | 23.96 | 26.03 | 30.12 | 27.18 | 4.37 | −3.75 | <0.001 |
| Conventional | 46 | 23.55 | 24.79 | 27.02 | 25.44 | 2.98 | ||
| Overall (test of stochastic ordering) | −9.84 | <0.0001 |
Test statistics and P values for group differences at each individual year are from the large-sample approximation to the Wilcoxon’s rank-sum test using the Wei-Lachin variance estimate. The “overall” results are based on the Wei-Lachin test of stochastic ordering.
Addressing this question involves identifying typical patterns of change underlying the variation between individuals. A growth-curve model does so by estimating the equation defining a curve around which individual observations are assumed to be randomly scattered. A two-stage random-effects model essentially does this by fitting curves of the same general shape to each individual; assuming these coefficients are randomly distributed across the population, their mean values define a model representing the entire group.
To estimate growth curves for BMI in the DCCT, we considered a general class of models in which the rate of change undergoes long-term acceleration or deceleration with, at most, a single change of direction (e.g., from increase to decrease). This takes the form of an equation involving both time and its square (imparting the curvature). The strong correlation between baseline and follow-up BMIs provided considerable increases in precision by including each individual’s baseline BMI.
Initial models were fit separately within each combination of sex and treatment group. The results in some “adjacent” strata (e.g., conventionally treated men and women) were sufficiently similar to combine them into a single model using an indicator of the stratification variable and tests for interactions between that variable and the other variables used. However, to maintain the model’s simplicity and generality, no further covariates were added.
Interactions that were not significant were dropped, and the models were refit. Indicator variables were likewise dropped if neither they nor any interaction involving them contributed significantly to the fit. Finally, the resulting “minimal” models were again compared between the remaining strata, and further combination and model reduction were considered. In the end, a single model, described in Table A2, proved to fit all adult patients reasonably well.
Table A2.
Estimated growth curves
| Term | Estimate | SEM | P |
|---|---|---|---|
| Average | 1.94174 | 0.34355 | <0.0001 |
| Gender difference | ±0.08470 | 0.03967 | 0.0328 |
| Treatment difference | ±0.20489 | 0.05059 | 0.0001 |
| Men | |||
| Conventional | 1.82155 | ||
| Intensive | 2.23133 | ||
| Women | |||
| Conventional | 1.65215 | ||
| Intensive | 2.06193 | ||
| Baseline BMI | 0.93540 | 0.01434 | <0.0001 |
| Time (linear) | |||
| Average | 0.29666 | 0.02962 | <0.0001 |
| Treatment difference | ±0.15155 | 0.02962 | <0.0001 |
| Conventional | 0.14511 | ||
| Intensive | 0.44821 | ||
| Time (quadratic) | |||
| Average | −0.00440 | 0.00369 | 0.2325 |
| Gender difference | ±0.00248 | 0.00151 | 0.0993 |
| Treatment difference | ±0.00685 | 0.00369 | 0.0632 |
| Treatment-by-gender | ±0.00340 | 0.00150 | 0.0236 |
| interaction | |||
| Men | |||
| Conventional | 0.00337 | ||
| Intensive | −0.01713 | ||
| Women | |||
| Conventional | 0.00153 | ||
| Intensive | −0.00537 |
Constant terms reflect treatment and sex effects on the initial weight gain. For any fixed value of baseline BMI, men tended to gain ~0.169 kg/m2 more than women in the same treatment group, whereas intensively treated patients gained ~0.410 kg/m2 more than patients of the same sex on conventional therapy.
The rates of change thereafter also differed between genders and between treatment groups, although the most pronounced effect was a more rapid increase in the intensive group. This increase did slow over time, particularly among men, for whom the quadratic (curvature) term had about three times the magnitude estimated for women. Conventionally treated patients increased more slowly, but at a rate that remained much more constant over time; the curvature was essentially zero for women and small but positive for men.
Our model estimates that the average increase in BMI in the conventional group between years 1 and 2 is 0.155 kg/m2 for men and 0.150 kg/m2 for women. From year 4 to year 5, the estimated increases are 0.175 and 0.159 kg/m2, respectively; and between years 8 and 9, they are 0.202 and 0.171 kg/m2, respectively. In the intensive treatment group, the estimated increases during the second year of follow-up are 0.397 kg/m2 among men and 0.432 kg/m2 among women. Between the fourth and fifth annual visits, typical increases in the intensive group were 0.294 and 0.400 kg/m2, respectively, whereas in the last year of follow-up, they were 0.157 and 0.357 kg/m2, respectively. Clearly, this model suggests that substantial weight gain continues to affect intensively treated female patients in particular.
Footnotes
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.
REFERENCES
- 1.The DCCT Research Group. Weight gain associated with intensive therapy in the Diabetes Control and Complications Trial. Diabetes Care. 1988;11:567–573. doi: 10.2337/diacare.11.7.567. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. NEJM. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.The DCCT Research Group. The Diabetes Control and Complications Trial (DCCT): design and methodologic considerations for the feasibility phase. Diabetes. 1986;35:530–545. [PubMed] [Google Scholar]
- 4.The DCCT Research Group. Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter center. Clin Chem. 1987;33:2267–2271. [PubMed] [Google Scholar]
- 5.Metropolitan Life Foundation. 1983 Metropolitan height and weight tables. Stat Bull Metrop Life Found. 1983;64:2–9. [PubMed] [Google Scholar]
- 6.The DCCT Research Group. Implementation of treatment protocols in the Diabetes Control and Complications Trial. Diabetes Care. 1995;18:361–375. doi: 10.2337/diacare.18.3.361. [DOI] [PubMed] [Google Scholar]
- 7.The DCCT Research Group. DCCT Manual of Operations. Springfield, VA: U.S. Department of Commerce, National Technical Information Service; 1993. (publ. no. 93-183382) [Google Scholar]
- 8.Report of the National Cholesterol Education Program Expert Panel on Detection. Evaluation and treatment of high blood cholesterol in adults. Arch Intern Med. 1988;148:36–39. [PubMed] [Google Scholar]
- 9.Simkins SW. Lessons from the DCCT: the nutritional challenges of implementing intensive therapy. Diabetes Spectrum. 1994;7:294–295. [Google Scholar]
- 10.The DCCT Research Group. Expanded role of the dietitian in the Diabetes Control and Complications Trial: implications for clinical practice. J Am Diet Assoc. 1993;93:758–767. doi: 10.1016/0002-8223(93)91748-f. [DOI] [PubMed] [Google Scholar]
- 11.Kisselbah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, Adams PW. Relation of body fat distribution to metabolic complication of obesity. J Clin End Metab. 1982;54:254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 12.Leiter LA, Lukaski HC, Kenny DJ, Barnie A, Camelon K, Ferguson RS, Maclean S, Simkins S, Zinman B, Cleary P for the DCCT Research Group. The use of bioelectrical impedance analysis (BIA) to estimate body composition in the Diabetes Control and Complications Trial. Int J Obesity. 1994;18:829–834. [PubMed] [Google Scholar]
- 13.Burke BS. The diet history as a tool in research. J Am Diet Assoc. 1947;23:1041–1046. [Google Scholar]
- 14.Schmidt LE, Cox MS, Buzzard IM, Cleary PA the DCCT Research Group. Reproducibility of a comprehensive diet history in the Diabetes Control and Complications Trial. J Am Diet Assoc. 1994;94:1392–1397. doi: 10.1016/0002-8223(94)92541-0. [DOI] [PubMed] [Google Scholar]
- 15.Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med. 1990;150:665–672. [PubMed] [Google Scholar]
- 16.Najjar MF, Rowland M. Anthropometric Reference Data and Prevalence of Overweight, United States, 1976–80. Washington, DC: U.S. Govt. Printing Office; Oct, 1987. (Vital and Health Statistics Series 11, no. 238). (Public Health Service DHHS publ. no. 87–1688) [PubMed] [Google Scholar]
- 17.Lonn EM, Yusuf S, Doris CI, Sabine MJ, Dzavik V, Hutchison K, Riley WA, Tucker J, Pogue J, Taylor W. Study design and baseline characteristics of the Study to Evaluate Carotid Ultrasound Changes in Patients Treated With Ramipril and Vitamin E: SECURE. Am J Cardiol. 1996;78:914–919. doi: 10.1016/s0002-9149(96)00467-5. [DOI] [PubMed] [Google Scholar]
- 18.Hollander M, Wolfe DAA. Nonparametric Statistical Methods. New York: John Wiley & Sons; 1973. [Google Scholar]
- 19.Agresti A. Categorical Data Analysis. New York: John Wiley & Sons; 1990. pp. 60–64. [Google Scholar]
- 20.Wei LJ, Lachin JM. Two-sample asymptotically distribution-free tests for incomplete multivariate observations. JASA. 1984;79:653–661. [Google Scholar]
- 21.Lachin JM. Some large sample distribution-free estimators and tests for multivariate partially incomplete observations for two populations. Stat Med. 1992;11:1151–1170. doi: 10.1002/sim.4780110903. [DOI] [PubMed] [Google Scholar]
- 22.Lachin JM, Wei LJ. Estimation and tests in the analysis of multiple nonindependent 2×2 tables with partially missing observations. Biometrics. 1988;44:513–528. [PubMed] [Google Scholar]
- 23.Jennrich RI, Schluchter MD. Unbalanced repeated-measure models with structured covariance matrices. Biometrics. 1986;42:805–820. [PubMed] [Google Scholar]
- 24.Schluchter MD. Analysis of incomplete multivariate data using linear models with structured covariance matrices. Stat Med. 1988;7:317–324. doi: 10.1002/sim.4780070132. [DOI] [PubMed] [Google Scholar]
- 25.Laird NM, Ware JH. Random-effect models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 26.Schluchter MD. Unbalanced Repeated Measures With Structured Covariance Matrices. In: Dixon, Brown, Engelman, Jennrich, editors. BMDP Statistical Software Manual. Vol. 2. Berkeley, CA: University of California Press; 1990. pp. 1207–1244. [Google Scholar]
- 27.SAS Institute. SAS Language. Version 6. 1st Edition. Cary, NC: SAS; 1990. [Google Scholar]
- 28.SAS Institute. SAS/STAT. Version 6, Volumes 1 and 2. 4th Edition. Cary, NC: SAS; 1990. [Google Scholar]
- 29.Carlson MG, Campbell PJ. Intensive insulin therapy and weight gain in T1DM. Diabetes. 1993;42:1700–1707. doi: 10.2337/diab.42.12.1700. [DOI] [PubMed] [Google Scholar]
- 30.Wing RR, Klein R, Moss SE. Weight gain associated with improved glycemic control in population-based sample of subjects with type I diabetes. Diabetes Care. 1990;13:1106–1109. doi: 10.2337/diacare.13.11.1106. [DOI] [PubMed] [Google Scholar]
- 31.Molnár D, Decsi T, Zoltész G. Resting energy expenditure and food induced thermogenesis in diabetic children receiving continuous subcutaneous insulin infusion. Diabetes Res. 1988;7:117–121. [PubMed] [Google Scholar]
- 32.Muller MJ, von zur Mühlen A, Lautz HU, Schmidt FW, Daiber M, Hurter P. Energy expenditure in children with type I diabetes: evidence for increased thermogenesis. BMJ. 1989;299:487–491. doi: 10.1136/bmj.299.6697.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodship THJ, Butler PC, Rodham D, Clayton B, Holm PD, Alberti KGMM. Total body potassium insulin-dependent diabetes mellitus. Clin Sci (Lond) 1990;78:377–381. doi: 10.1042/cs0780377. [DOI] [PubMed] [Google Scholar]
- 34.Librenti MC, Vedani P, Micossi P, Pozza G. Validazione di una metodica di rilevazione impedenziometrica per la valutazione dela composiziioine corporea in soggetti diabetici e controllii normali. Minerva Endocrinol. 1991;16:27–30. [PubMed] [Google Scholar]
- 35.Larsson B, Svardsudd K, Weilin L, Wilhensen L, Bjorntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13-year follow-up of participants in men born in 1913. Br Med J. 1984;288:1401–1404. doi: 10.1136/bmj.288.6428.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lapidus L, Bengtsson C, Larsson B, Pennert K, Roybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Br Med J. 1984;289:1257–1261. doi: 10.1136/bmj.289.6454.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hartz AJ, Rupley DC, Rimm AH. The association of girth measurement with disease in 32,856 women. Am J Epidemiol. 1984;119:71–80. doi: 10.1093/oxfordjournals.aje.a113727. [DOI] [PubMed] [Google Scholar]
- 38.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure. JAMA. 1998;280:140–146. doi: 10.1001/jama.280.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hokanson JE, Dev RU, Purnell JQ, Steffes MW, Cleary PA, Brunzell JD. A family history of type 2 diabetes predicts excessive weight gain in subjects with type 1 diabetes or intensive insulin therapy in the DCCT (Abstract) Diabetes. 1999;48(Suppl 1):A44. [Google Scholar]