Abstract
Prostaglandin E2 receptor subtype 4 agonists (EP4A) and basic fibroblast growth factor (FGF2) stimulate bone formation, but their effects on bone resorption are controversial. To provide additional insight into the skeletal effects of EP4A and FGF2, their regulation of expression of genes associated with bone formation and resorption in aged ovariectomized (OVX) rats and in cultured mouse bone marrow cells was determined. RNA was isolated from lumbar vertebrae of OVX rats (16 months of age) treated daily for 3 weeks with FGF2 or EP4A and processed for quantitative real time-PCR analyses. mRNA expression for the receptor activator of NF-κB ligand (RANKL) and cathepsin K (CTSK), but not osteoprotegerin (OPG), were upregulated by both FGF2 and EP4A. Addition of FGF2 and EP4A to the medium of cultured mouse bone marrow cells increased the formation of tartrate resistant acid phosphatase (TRAP) positive cells, upregulated the expression of RANKL and CTSK, and downregulated expression for OPG. EP4A also increased the formation of actin rings, an indicator of osteoclast activation, in a dose dependent manner in osteoclasts cultured on bone slices and triggered the formation of pits as revealed by a pitting assay. Gene expression for osterix (OSX) and IGF-2, genes associated with bone formation, was significantly greater in FGF2-treated OVX rats compared with EP4A-treated OVX rats. These findings at the molecular level are consistent with previous tissue-level histomorphometric findings, and at the doses tested, support the contention that FGF2 has a stronger bone anabolic effect than EP4A. The results of these in vivo and in vitro analyses clarify the effects of FGF2 and EP4A on bone formation and resorption, and provide insight into differences in the efficacy of two potential bone anabolic agents for restoration of lost bone mass in the osteopenic, estrogen-deplete skeleton.
Keywords: ovariectomy, bone anabolic agents, bone resorption, gene expression, osteoclastogenesis
INTRODUCTION
Osteoporosis is a disorder characterized by low bone mass and structural deterioration of bone tissue, leading to bone fragility and an increased susceptibility to fractures (1). This bone disorder affects approximately 44 million Americans, the great majority of whom are postmenopausal women (2). Most current osteoporosis therapies are anti-resorptive agents with a limited ability to restore lost bone mass to premenopausal levels. This limitation has focused attention on the development of bone anabolic agents for augmenting bone mass in osteoporotic patients. Human parathyroid hormone (PTH 1–34) is currently the only anabolic agent approved by the FDA for the treatment of osteoporosis (3). PTH has a strong stimulatory effect on bone formation, but does not restore lost cancellous bone mass completely in severely osteopenic rats, probably due to lack of adequate numbers of bone spicules to serve as a foundation for new bone formation (4). This phenomenon has not been described in osteoporotic patients treated with PTH, but has been observed in severely osteoporotic patients treated with another bone anabolic agent, sodium fluoride (5).
Basic fibroblast growth factor (FGF2) is being evaluated as an alternative bone anabolic agent with osteogenic capabilities beyond that of PTH. More specifically, FGF2 has been shown to induce formation of new bone spicules within bone marrow devoid of bone (6). It is a pluripotent and pleiotropic member of a family of polypeptides that controls the proliferation and differentiation of various cell types, and plays a major role in tissue development, repair, and regeneration (7). In vitro, FGF2 has been shown to induce proliferation of osteoblasts and bone marrow stromal cells and to stimulate osteogenesis in bone marrow cells obtained from both young and adult intact rats and from early adolescent ovariectomized (OVX) rats (8). However, the in vitro findings have sometimes been contradictory in that the biological effects of FGF2 may depend on the stage of differentiation of osteoblasts, interactions with other cytokines, and the length and mode of exposure to the growth factor in culture (9).
In vivo, FGF2 haplo-insufficiency in mice results in decreased bone mass, which indicates that FGF2 plays an important role in bone formation and is critical for the attainment of peak bone mass (10). Short-term systemic administration of FGF2 has strong bone anabolic effects in young intact rats (11) and markedly increases bone formation and augments cancellous bone mass in OVX rats (12–17).
The effects of FGF2 on bone resorption are less certain. In vitro, FGF2 is reported to either inhibit (18–20) or stimulate (21) osteoclast formation. In vivo, systemic FGF2 administration has led to either unchanged (6,22) or decreased bone resorption (13,23). We have found that osteoclast surface, an index of bone resorption, was markedly decreased in the lumbar vertebrae and proximal tibiae of FGF2-treated OVX rats (14,15,23). Also, treatment of OVX rats with FGF2 appeared to have no effect on gene expression for some cytokines associated with bone resorption such as IL-6, IFN-y and TGF-β (16). However, additional molecular analyses of osteoclast specific markers may provide insight into the effects of FGF2 on bone resorption.
Prostaglandin E2 (PGE2) is another strong stimulator of bone formation, but gastrointestinal side effects have slowed its development as an osteoporosis therapy (24,25). However, an EP4 receptor selective agonist (EP4) has been identified that appears to have the desirable stimulatory effects on bone formation with minimal gastrointestinal side effects (26). The finding of decreased cancellous bone mass and formation in EP4 receptor knockout mice is consistent with a role for this receptor in the regulation of bone formation (27). Furthermore, an EP4 agonist has been shown to prevent cancellous bone loss in OVX rats (26) and to stimulate bone formation and restore bone mass and strength in older OVX rats with established cancellous osteopenia (28). However, similar to FGF2, the effects of the EP4 agonist on bone resorption are controversial. Ke et al. (28) reported that histomorphometric indices of bone resorption were decreased by EP4 treatment in OVX rats whereas other investigators found that bone resorption was increased in aged OVX rats treated with the agonist (23). Therefore, the purpose of this study was to provide additional insight into the skeletal effects of FGF2 and EP4 by comparing their regulation of expression of genes associated with bone formation and resorption in aged OVX rats and in cultured mouse bone marrow cells.
MATERIALS AND METHODS
Animals and treatment groups
A total of 24 virgin female Sprague Dawley rats (Charles River, Wilmington, MA) that were approximately 90 days of age and weighed an average of 240g were used in this study. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Florida (Gainesville, FL). Within 2 weeks of their arrival, rats were anesthetized with an IP injection of ketamine hydrochloride and xylazine at doses of 50 mg/kg and 10 mg/kg body weight (BW), respectively. All rats were ovariectomized bilaterally from a dorsal approach. Animals were kept in pairs under standard laboratory conditions with a 13-h light, 11-h dark cycle and a constant temperature of 25°C and humidity of 48%. The food consumption of OVX rats was restricted to minimize the increase in body weight associated with ovariectomy (29). All rats were maintained for one year after surgery to allow for the development of cancellous osteopenia in the OVX animals. A group of baseline (BSL) OVX rats was sacrificed at 1 year postovariectomy when the animals were 15 months of age. Within 2 days of sacrifice of the BSL OVX group, rats from the OVX + FGF2 group (N = 8) were injected SC daily for 3 weeks with FGF2 (Chiron Corp., Emeryville, CA). The growth factor was dissolved in a vehicle of phosphate-buffered saline and administered at a dose of 1 mg/kg BW. Rats from the OVX + EP4 group (N = 8) group were injected SC daily for 3 weeks with the EP4 agonist CP-734432, which was obtained from Pfizer Global Research and Development, Groton Laboratories (Groton, CT), dissolved in a vehicle of 5% ethanol, and administered at a dose of 3 mg/kg BW.
The OVX + FGF2 and the OVX + EP4 groups were sacrificed when the animals were nearly 16 months old. Euthanasia was achieved by exsanguination from the abdominal aorta under ketamine/xylazine anesthesia. Lumbar vertebrae 1 and 2 were subjected to histological processing as previously described (30). Lumbar vertebrae 5 and 6 were excised, cleaned of soft tissue, and their vertebral processes and intervertebral disks were removed. The vertebral bodies were then immediately snap-frozen in liquid nitrogen and stored at −80°C for RT-PCR analysis.
In vivo RNA Isolation and Quantitative Real Time-PCR
Lumbar vertebral bodies were individually homogenized with a Certiprep freezer mill (Spex, Edison, NJ) followed by extraction of their total RNA using a modified guanidine thiocyanate-phenol-chloroform extraction method (31). RNA pellets were then dissolved in nuclease-free water (not DEPC-treated) (Ambion, Austin, TX). RNA concentration was determined using NanoDrop ND1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE), followed by evaluation of RNA integrity by capillary electrophoresis with an Agilent 2100 Bioanalyzer (Agilent Technologies, Foster City, CA). This RNA quantification and quality evaluation was performed at the Interdisciplinary Center for Biotechnology Research Microarray Core, University of Florida (Gainesville, Fl). RNA was treated with Turbo DNA-free (Ambion), to remove contaminating DNA. RNA was further cleaned with the RNeasy Min Elute Clean Up kit 50 from Qiagen (Valencia, CA). RNA concentration and evaluation of the quality were determined again as described above. A complementary cDNA template was constructed from total RNA (1 μg) by reverse transcription using 50 μl of RNA solution mixed with 50 μl of High Capacity cDNA Archive Kit (ABI, Foster City, CA). The corresponding cDNA generated in the reverse transcription step was diluted 1:10 in nuclease- free water. Eleven ng were used as a template for Real Time PCR analysis in a total volume of 25 μl, which contained the respective TaqMan Gene Expression Assay probes and primers (ABI) and TaqMan Universal PCR Master Mix (ABI). The following genes were evaluated: COX-2 (Rn00568225_m1), VEGF (Rn00582935_m1), TGF-β1 (Rn00572010_m1), OSX (Rn01761789_m1), IGF-2 (Rn00580426_m1), OPG (Rn00563499_m1), RANKL (Rn00589289_m1) and CTSK (Rn00580723_m1). The Real Time PCR analysis was performed with an ABI 7500 Real Time PCR system. Each sample was run in triplicate wells on the sample plate to permit statistical evaluation between groups. Relative gene expression levels were normalized to Human Euk 18S rRNA (20x) from ABI (Foster City, CA) to perform relative quantification of genes involved with osteogenesis and osteoclastogenesis by using the (ddCt) comparative threshold cycle (CT) method. The data for the treatment groups are expressed as fold change relative to the BSL OVX group.
In vitro mouse marrow culture and TRAP assay
Generation of osteoclasts
Swiss-Webster mice (8–20 gram) were killed by cervical dislocation. Femora and tibiae were dissected from adherent tissue, and marrow was removed by cutting both the proximal and distal ends of the bones, inserting a syringe with a 25 gauge needle, and flushing the marrow using αMEM medium plus 10% fetal bovine serum (αMEM D10). The marrow was washed twice with αMEM D10 and 1×106 cells/cm2 were plated in 24 well culture dishes in 1 ml of αMEM D10. The cells were treated with 60 nM of the EP4 agonist (EP4A), 100 ng/ml of FGF2 (PreproTech Inc, Rocky Hill, NJ), or 10−8M 1,25-dihydroxivitamin D3 (1,25D3, Invitrogen, Ca) for 7 days or remained untreated as negative controls. Eighty percent of the medium was replaced on day 3 and fresh EP4A, FGF2, or 1,25D3 was added at this time. All cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 in air. After 7 days in culture, osteoclasts appeared and these cells were fixed with 2% paraformaldehyde, permeabilized with 0.5% Triton X-100, washed with PBS 1X, and stained for tartrate-resistant acid phosphatase activity (TRAP, Sigma Chemical Co. St. Louis, MO). TRAP+ cells were counted with an inverted microscope (x200). The cells that stained positive for TRAP in the cytoplasm and had one nuclei were counted as TRAP+ mononuclear cells, cells with 2–10 nuclei were counted as TRAP+ multinuclear cells, and cells with more that 10 nuclei were counted as TRAP+ giant cells.
We also compared the osteoclastogenic effects of PGE2 with those of EP4A since this agonist activates one of the receptors implicated in bone resorption that is also activated by PGE2. The experiment was performed with mouse bone marrow cells following the procedures mentioned above. The cells were treated with 60 nM of EP4A, 10−8M 1,25D3, or 10−6M PGE2 (Sigma) for 7 days. As a control to inhibit endogenous PGE2, we added 10−6M COX-2 inhibitor NS-398 (Cayman Chemical, Ann Arbor, MI).
Assays for osteoclast activity
Bone resorptive activity was measured by determining the number of actin rings formed by osteoclasts and by directly measuring the number and size of pits formed by osteoclasts on devitalized mouse cortical bone slices (1 cm2 × 0.1 mm). To measure actin rings, marrow cells that had been cultured for 5 days with 10−8M 1,25D3 on tissue culture plates were scraped and plated onto bone slices in 24-well dishes. Cells were cultured for 5 days in α-MEM plus 10% HIFCS, 1,25D3, and 60nM or 600nM of EP4 agonist. After incubation, cells were fixed with 2% formaldehyde in PBS for 20 min, permeabilized with 1% Triton X-100 for 10 min, and stained with Texas Red-tagged phalloidin (Sigma) to detect the actin rings (32). The total number of actin rings were counted per 1 mm2 bone slice. Pits were determined by examining bone slices using differential interference contrast optics. Three random 180,000 μm2 photos of each slice were taken, the images were transferred to Adobe photoshop, and a grid was superimposed over each picture. Area resorbed was determined by counting the number of grid intersections over pits divided by total grid intersections. Pits were defined as contiguous excavations regardless of the number of facets.
RNA isolation from mouse bone marrow cultures and quantitative Real Time– PCR
To obtain RNA from mouse bone marrow cultures, the cells were plated in sterile dishes with 10 ml αMEM D10 medium and treated under the same conditions described above. After 7 days, the cells were harvested in 1 ml of Trizol reagent (Invitrogen, Carlsbad, CA) followed by detailed protocol steps for RNA isolation from the supplier. DNases treatment and RNA clean up and RT-PCR conditions were the same as mentioned for lumbar vertebral samples. The following genes were evaluated: RANK (Mm00437135_m1), RANKL (Mm00441908_m1), OPG (Mm00435452_m1), CTSK (Mm0048036_m1), and COX-2 (Mm00478372). Each sample was run in triplicate wells on the sample plate to permit statistical evaluation between different groups of treatment. Relative gene expression levels were normalized to Human Euk 18S rRNA (20x) from ABI (Foster City, CA) to perform relative quantification of genes by using the (ddCt) comparative threshold cycle (CT) method. The data for the treatment groups are expressed as fold change relative to the untreated group as calibrator.
Statistical Analysis
Data are expressed as the mean ± SD for each group. SAS statistical software (StatView) was used for data analysis. Statistical differences in relative gene expression between treated (OVX+FGF2 and OVX+EP4A) and the untreated control group (BSL OVX) were determined by one-way ANOVA followed by the post hoc Fisher PLSD test. Probabilities (p) less than 0.05 were considered statistically significant. Values for TRAP+ mononuclear, multinuclear, and giant osteoclastic cells in the 3 groups and the statistical differences in relative gene expression from in vitro treated and untreated cultures were compared by one-way analysis of variance (ANOVA) followed by the Newman-Keuls test for multiple comparisons of pairs of means.
RESULTS
Daily treatment of aged OVX rats for 3 weeks with FGF2 and EP4A did not induce weight loss compared to BSL OVX rats. As previously described (23), FGF2-treated OVX rats were anemic with blood hematocrit levels below 20%. OVX rats treated with EP4A did not exhibit signs of gastrointestinal side effects such as diarrhea.
Histomorphometric findings
Bone histomorphometric data from the lumbar vertebral bodies of the 3 groups of rats have been previously published (30). Briefly, FGF2 treatment of OVX rats markedly increased osteoblast and osteoid surfaces by 5- to 8-fold compared with the BSL OVX group. These indices of bone formation were increased to a greater extent in FGF2-treated OVX rats compared with EP4A-treated OVX rats, which exhibited 2- to 3-fold increases in osteoblast and osteoid surfaces. Osteoclast surface (Figure 1) was significantly increased by at least of factor of 3 in EP4A-treated OVX rats compared with BSL OVX rats, but FGF2 treatment did not increase this index of bone resorption. In view of these effects on bone formation and resorption, FGF2, but not EP4A, increased cancellous bone matrix (bone + osteoid) in aged OVX rats to the level of vehicle-treated control rats (30).
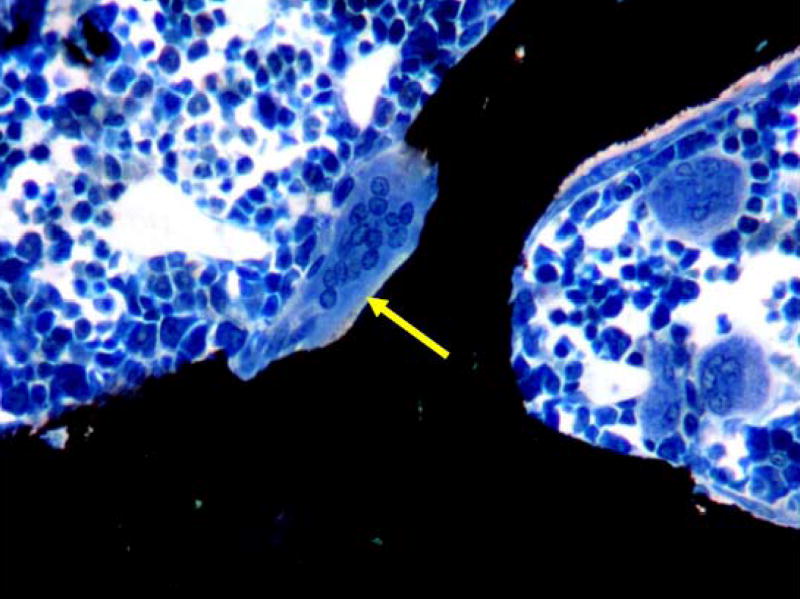
Figure 1.
Cancellous bone tissue in the lumbar vertebral body of an aged OVX rat treated for 3 weeks with the EP4 agonist, which induced formation of large, multinucleated osteoclasts (arrow) within resorptive lacunae. EP4 treatment increased osteoclast surface, an index of bone resorption, by nearly three-fold compared with baseline OVX rats. (Von Kossa/tetrachrome stain; original magnification, X400).
In vivo quantitative Real Time - PCR
We previously determined (30) that treatment of OVX rats with FGF2 and EP4A significantly upregulated expression of the following genes associated with bone formation: type 1 collagen (11.9 and 3.6 fold, respectively), osteocalcin (6.8 and 4.3 fold), and Runx2 (5.5 and 2.3 fold). FGF2 and EP4A also upregulated IGF-1 (1.8 and 1.5 fold), but neither treatment affected gene expression for BMP-2.
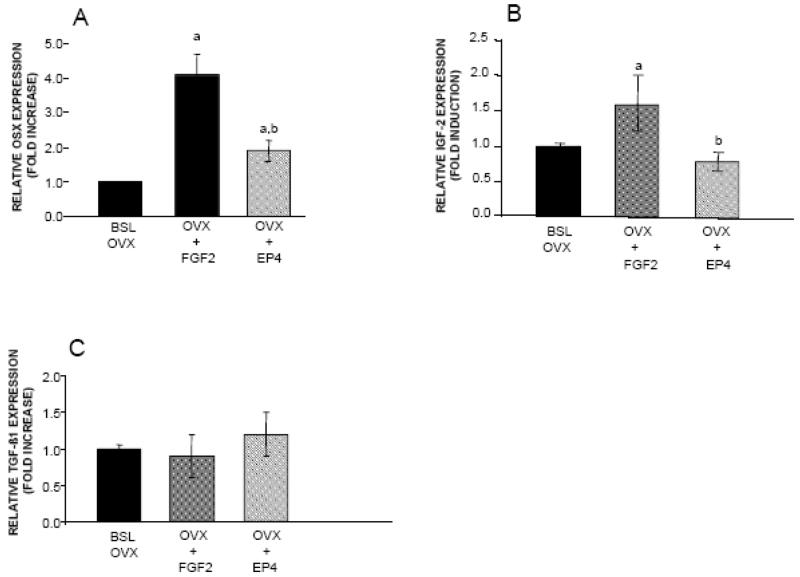
In view of these findings, we investigated the effects of both treatments on the expression of additional genes known to influence bone turnover. FGF2 and EP4A induced a significant increase in gene expression for Osx (4.4 fold and 1.9 fold respectively) relative to the BSL OVX group (Figure 2A). mRNA levels for IGF-2, a growth factor reported to be equally potent in stimulating cell proliferation as IGF-I, was also significantly increased relative to the BSL OVX group in aged OVX rats treated with FGF2 (1.6 fold change), but not with EP4A (Figure 2B). On the other hand, neither FGF2 nor EP4A affected gene expression for TGF-β1 (Figure 2C).
Figure 2.
Effects of FGF2 and EP4 on the expression of A. OSX, B. IGF-2, C. TGF-β1, in lumbar vertebral bodies from of the following groups: Baseline OVX (BSL OVX), and OVX rats treated for 3 weeks with FGF2 (OVX+FGF2) or EP4 (OVX+EP4). Bars indicate mean ± SD of 5 animals. aSignificantly different from the BSL OVX group (P<0.05). bSignificantly different from the OVX+FGF2 group (P<0.05).
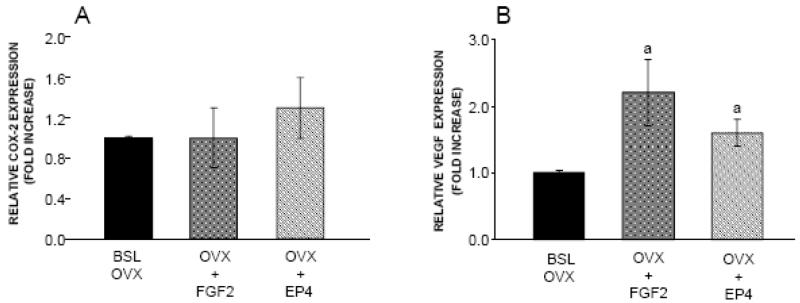
We also evaluated the effect of both treatments on gene expression for COX-2 and VEGF (Figure 3). Neither FGF2 nor EP4A affected COX-2 expression at the doses used in this in vivo experiment (Figure 3A). In comparison to the BSL OVX group, there was a significant 2.2 fold increase in gene expression for VEGF in response to FGF2 treatment. VEGF gene expression was also significantly increased by 1.6 fold in OVX rats treated with EP4A (Figure 3B).
Figure 3.
Effects of FGF2 and EP4 on the expression of A. COX-2 and B. VEGF, in lumbar vertebral bodies from of the following groups: Baseline OVX (BSL OVX), and OVX rats treated for 3 weeks with FGF2 (OVX+FGF2) or EP4 (OVX+EP4). Bars indicate mean ± SD of 5 animals. aSignificantly different from the BSL OVX group (P<0.05).
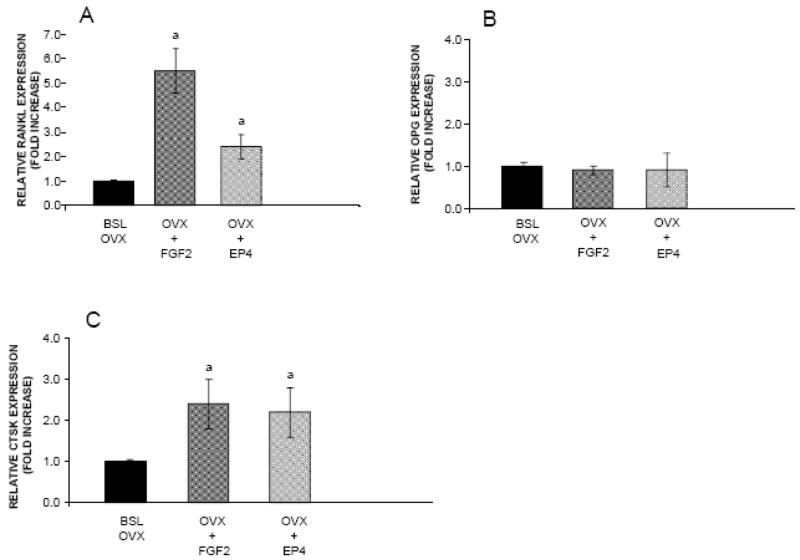
We previously found that mRNA levels for RANKL were increased significantly by 5.5 fold in FGF2-treated OVX rats compared with the BSL OVX group and by 2.4 fold in EP4A-treated OVX rats (30). In the current study (Figure 4), gene expression for cathepsin K was increased in aged OVX rats by both FGF2 (2.4 fold) and EP4A (2.2 fold) relative to the BSL OVX group. In addition, gene expression for osteoprotegerin, the decoy receptor for RANKL and a physiological inhibitor of cathepsin K, was not significantly affected by treatment with either FGF2 or EP4A. These results indicate that FGF2 and EP4A induce osteoclastogenesis in a process compatible with the RANK/RANKL/OPG signaling pathway and stimulate osteoclast activity by increasing gene expression for cathepsin K.
Figure 4.
FGF2 and EP4 regulate the expression of A. RANKL, B. OPG, and C. CTSK mRNAs associated with bone resorption in lumbar vertebral bodies from ovariectomized (OVX) rats. Figures present fold changes relative to r18s from of the following experimental groups: Baseline OVX (BSL OVX), and OVX rats treated for 3 weeks with FGF2 (OVX+FGF2) or EP4 (OVX+EP4). Values are the mean ± SD from 5 OVX rats per group. aSignificantly different from BSL OVX (P<0.05).
Evaluation of in vitro osteoclast-like cell formation by TRAP assay and osteoclast activity by pitting assay
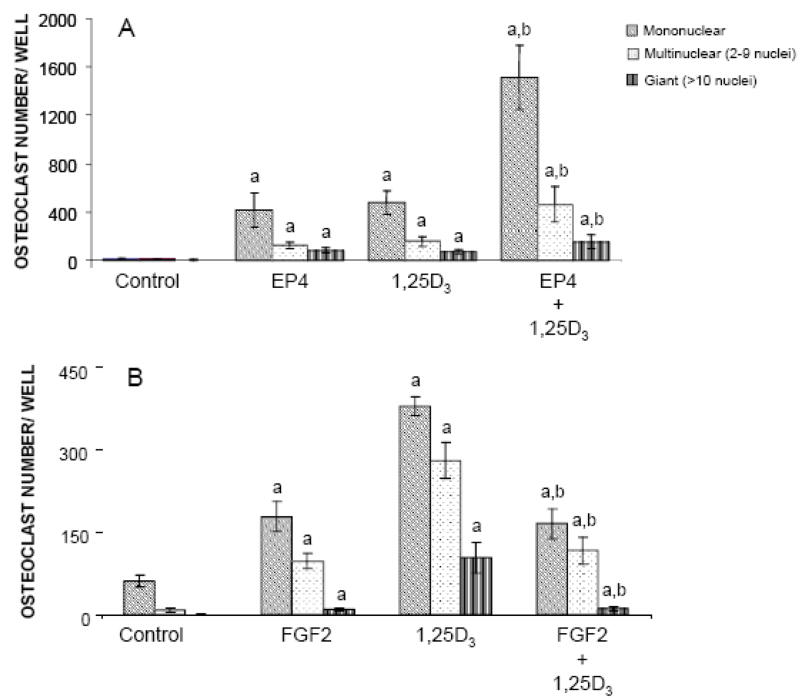
EP4A treatment caused a significant increase in the number of mononuclear, multinuclear, and giant osteoclasts in bone marrow culture compared with the number of TRAP+ osteoclasts formed in untreated control cultures (Figure 5A). EP4A stimulated formation of TRAP+ mononuclear cells more than formation of TRAP+ multinuclear and giant cells. Furthermore, there was no significant difference in the increase in the number of TRAP+ mononuclear, multinuclear, and giant osteoclasts between marrow cultures treated with EP4A and 1,25D3. Interestingly, co-treatment of mouse marrow cultures with EP4A and 1,25D3 enhanced the number of TRAP+ mononuclear (P<0.001), multinuclear (P<0.01), and giant cells (P<0.05) compared to treatment with 1,25D3 alone. These in vitro findings regarding the stimulatory effects of EP4A on osteoclast formation are consistent with our in vivo histomorphometric and gene expression findings in aged OVX rats treated with EP4A. Similar effects on osteoclastogenesis were found in mouse bone marrow cultures treated with FGF2 (Figure 5B). There were significant increases in the numbers of FGF2-stimulated TRAP+ mononuclear, multinuclear, and giant cells compared with the number of these osteoclastic cells in untreated bone marrow cultures. We also found that co-treatment of mouse marrow cultures with FGF2 and 1,25D3 significantly decreased the formation of TRAP+ cells compared to treatment with 1,25D3 alone as a positive control.
Figure 5.
Effects of FGF2, EP4, and 1,25-dihydroxivitamin D3 (1,25D3) on osteoclast formation. Mouse bone marrow was cultured with FGF2, EP4, 1,25D3, FGF2 + 1,25D3 and EP4 + 1,25D3 for 7 days. TRAP-positive cells were identified as mononuclear (one nucleus), multinuclear (2–10 nuclei), or giant (>10 nuclei) cells. Values are expressed as the mean ± SD for triplicate cultures. aSignificantly different from control (untreated mouse bone marrow culture) (P<0.05). bSignificantly different from mouse bone marrow culture treated with 1,25D3 alone (P<0.05).
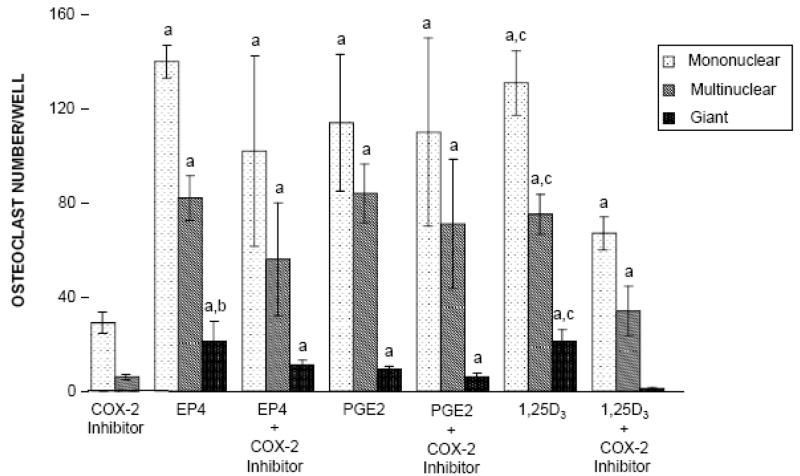
As shown in Figure 6, the stimulatory effects of EP4A, PGE2, and 1,25D3 on osteoclast differentiation were statistically significant relative to cell cultures treated with the COX-2 inhibitor alone, which suppressed endogenous PGE2. There was no significant difference in osteoclast differentiation between cultures treated with PGE2 and EP4A. Additionally, when the cell culture was treated with PGE2 in combination with the COX-2 inhibitor, there was no significant difference on osteoclast differentiation compared with treatment with PGE2 alone. When the cell culture was treated with EP4A in combination with the COX-2 inhibitor, there was a significant decrease in TRAP+ giant osteoclast formation, but not in TRAP+ mono- and multinuclear cells, when compared to treatment with EP4A alone. However, there was a significant reduction in TRAP+ mononuclear, multinuclear, and giant osteoclast formation in cultures treated with 1,25D3 in combination with the COX-2 inhibitor compared with 1,25D3 alone.
Figure 6.
Effects of COX-2 inhibitor on osteoclast differentiation. Mouse bone marrow cells were cultured with EP4, PGE2, 1,25D3, or EP4 + 1,25D3 with and without COX-2 inhibitor (NS-398) for 7 days. TRAP-positive cells were identified as mononuclear (one nucleus), multinuclear (2–10 nuclei), or giant (>10 nuclei) cells. No TRAP+ giant cells were detected in the cultures treated with the COX-2 inhibitor alone. Values are expressed as the mean ± SD for triplicate cultures. aSignificantly different from control (COX-2 inhibitor-treated mouse bone marrow culture) (P<0.05). bSignificantly different from mouse bone marrow culture treated with EP4 + COX-2 inhibitor (P<0.05). CSignificantly different from mouse bone marrow culture treated with 1,25D3 + COX-2 inhibitor (P<0.05).
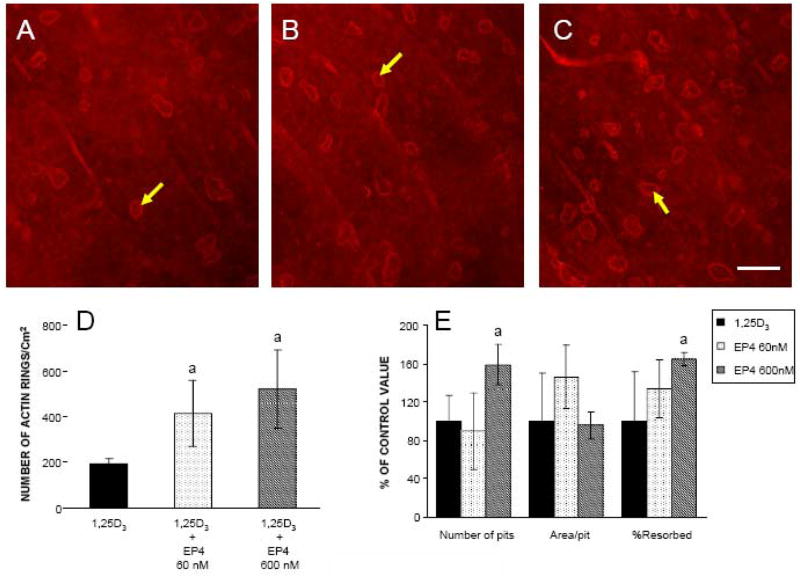
Osteoclastic bone resorption is associated with the formation of actin rings that result from the reorganization of the actin cytoskeleton of osteoclasts (32). EP4A caused a dose dependent increase in the number of actin rings compared with 1,25D3 alone (Figure 7A–D). Consistent with these results, we found that 600 nM of EP4A significantly increased the number of pits and % area resorbed compared with 1,25D3 (Figure 7E).
Figure 7.
Effects of EP4 and 1,25D3 on the formation of actin rings and resorptive pits on bone slices. Marrow cells that had been cultured for 5 days with 1,25D3 were scraped and plated on bone slices. Pits were determined by examining bone slices using differential interference contrast optics. A. Cultures treated with 1,25D3 alone, B. Cultures treated with 60 nM EP4 + 1,25D3, C. Cultures treated with 600 nM EP4 +1,25D3 with arrows showing the actin rings. Scale bar = 25 μm, D. Quantitation of the actin rings showing that their formation in response to EP4 is dose dependent, E. Quantitation of the pitting is shown in the bar graph. Values are expressed as the mean ± SD of 5 measurements. aSignificantly different from culture treated with 1,25D3 (P<0.05).
Quantitative Real Time– PCR in mouse bone marrow cultures
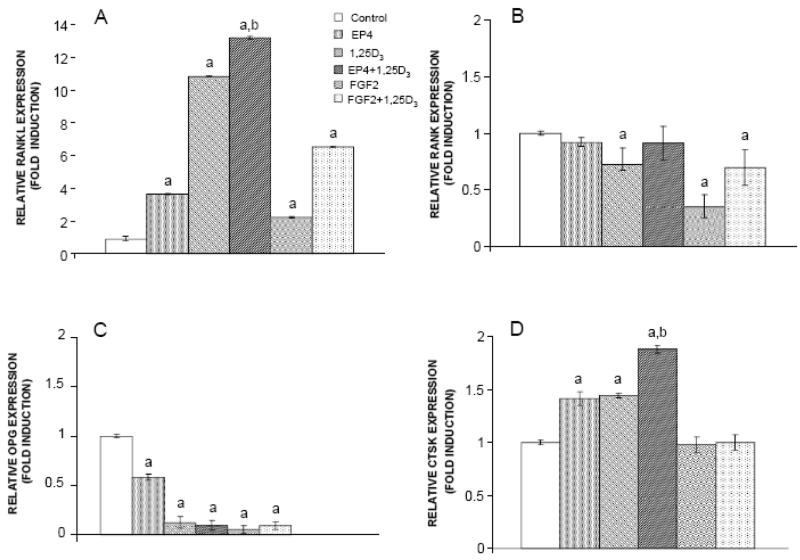
As shown in Figure 8, RANKL gene expression was upregulated significantly by 3.6 fold (P<0.001) relative to the calibrator in mouse bone marrow cultures treated with EP4A alone, by 2.2 fold in cultures treated with FGF2 alone, by 10.8 fold in cultures treated with 1,25D3 alone, by 13.1 fold in cultures co-treated with EP4A and 1,25D3, and by 6.5 fold in cultures co-treated with FGF2 and 1,25D3. The upregulation of RANKL in the co-treated cultures was significantly greater when compared to the upregulation by treatment with EP4A and FGF2 alone. Similarly, gene expression for cathepsin K was increased significantly 1.4 fold by treatment with EP4A and 1,25D3 alone, and the combination of both treatments significantly enhanced the upregulation of this gene by 1.9 fold (P<0.001) relative to control and single treatments. FGF2 alone or in combination with 1,25D3 failed to affect CTSK expression. In addition, both EP4A and FGF2 downregulated gene expression for osteoprotegerin (OPG), the decoy receptor for RANKL. Gene expression for RANK, the RANKL receptor, was slightly reduced in the presence of EP4A, FGF2, 1,25D3, or in combination.
Figure 8.
Effects of FGF2 and EP4 on the expression of A. RANKL, B. RANK, C. OPG, and D. CTSK mRNA in mouse bone marrow cells. Cultures were treated with FGF2, EP4, 1,25D3, FGF2 + 1,25D3 or EP4 + 1,25D3. Figures represent the fold induction relative to the untreated bone marrow culture. aSignificantly different from control (untreated mouse bone marrow culture) (P<0.05). bSignificantly different from mouse bone marrow culture treated with 1,25D3 alone (P<0.05).
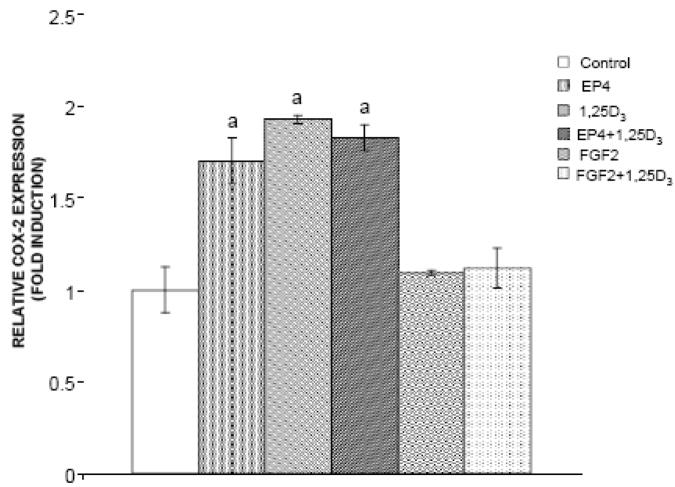
As shown in Figure 9, EP4A, 1,25D3, and both agents in combination induced a statistically significant increase in COX-2 gene expression (1.7, 1.9, and 1.8 fold changes, respectively) relative to control. In contrast, FGF2 did not affect COX-2 gene expression in the bone marrow cultures, and inhibited COX-2 expression in the presence of 1,25D3.
Figure 9.
Effects of FGF2 and EP4 on gene expression for COX-2 in mouse bone marrow cells. Cultures were treated with FGF2, EP4, 1,25D3, FGF2 + 1,25D3 or EP4 + 1,25D3. Figures represent the fold induction relative to the untreated bone marrow culture (control). aSignificantly different from control (P<0.05).
DISCUSSION
FGF2 and EP4 agonists are well known to stimulate bone formation (12–15,26,28,30), but their effects on bone resorption are controversial. Although some in vitro studies indicate that FGF2 enhances osteoclastogenesis (19,21), osteoclast surface, a histomorphometric index of bone resorption, is unchanged (30) or even decreased (13,14,23) in OVX rats treated with the growth factor. Regarding EP4 agonists, Ke et al. (28) reported, in contrast to our previous study (30), a decrease in osteoclast surface in EP4A-treated OVX rats. The results of the current study help resolve this controversy by indicating that both FGF2 and EP4A induced expression of genes associated with bone resorption in vivo as well as stimulated osteoclastogenesis in vitro. In the lumbar vertebral body of aged OVX rats, gene expression for RANKL and cathepsin K, but not OPG, were upregulated by FGF2 and EP4A treatment. These findings indicate that both agents stimulated osteoclastogenesis and the activity of these bone resorbing cells in vivo. Furthermore, addition of FGF2 and EP4A to the medium of cultured mouse bone marrow cells increased the formation of TRAP+ mono- and multinuclear cells committed to the osteoclast lineage, upregulated gene expression for RANKL and cathepsin K, and downregulated gene expression for OPG. EP4A also increased the formation of actin rings in osteoclasts cultured on bone slices and enhanced the resorptive activity of these cells as revealed by a pitting assay. These data are consistent with EP4A stimulating osteoclast formation, activation, and bone resorption, and are consistent with the findings that EP4A stimulates RANKL and inhibits OPG. These in vivo and in vitro findings indicate that both FGF2 and EP4A have a stimulatory effect on bone resorption.
The above molecular and cellular evidence for enhanced osteoclastogenesis by EP4A is consistent with the histomorphometric finding of a 2.7-fold increase in osteoclast surface in EP4A-treated OVX rats. On the other hand, despite similar evidence for a stimulatory effect on osteoclastogenesis by FGF2, histomorphometric analysis revealed that osteoclast surface was unaffected by systemic treatment with the growth factor. This inconsistency may be a consequence of the high percentage (>80%) of cancellous bone surfaces lined by osteoid in FGF2-treated OVX rats, since osteoclasts are rarely found adjacent to such unmineralized bone surfaces. FGF2 is known to impair bone mineralization (13,14), which probably results in low calcium concentrations in the bone microenvironment. In vitro studies have shown that osteoclastic survival is markedly reduced in calcium-free medium (33), and conversely, high extracellular calcium levels stimulate osteoclast-like cell formation in osteoblast-containing, mouse bone cell cultures (34). Therefore, the inconsistent results generated by the molecular and histomorphometric analyses in FGF2-treated OVX rats may be due, at least in part, to low calcium levels along osteoid-lined, cancellous bone surfaces.
In mouse bone marrow cultures, both EP4A and FGF2 enhanced the formation of TRAP+ mono- and multinuclear cells. However, these agents had a much smaller effect on the formation of TRAP+ giant cells representative of mature osteoclasts. This finding suggests that EP4A and FGF2 have their primary effect on the initial commitment of bone marrow cells to the osteoclast lineage. In addition, the observed increase in gene expression for cathepsin K indicates that both FGF2 and EP4A stimulate osteoclastic activity as well as osteoclastogenesis. This finding regarding EP4A is not surprising in view of a previous report that the stimulatory effect of PGE2 on bone resorption in vitro is mediated mainly by the EP4 receptor (35).
Since 1,25D3 is well known to stimulate osteoclastogenesis in vitro (36), it seemed appropriate to compare its effects with those of FGF2 and EP4A on this process. FGF2 increased the numbers of mono- and multinucleated TRAP+ cells, but not to the same extent as 1,25D3, and the magnitude of the increase in these osteoclastic cells was reduced in the marrow cell cultures co-treated with FGF2 and 1,25D3. QRT-PCR analyses of gene expression in these cells revealed that FGF2 and EP4A as well as 1,25D3 upregulated RANKL and downregulated OPG. Furthermore, co-treatment of marrow cell cultures with EP4A and 1,25D3 had a greater stimulatory effect on the formation of mono- and multinuclear TRAP+ cells, greater increases in gene expression for RANKL and cathepsin K, and a greater downregulation of OPG than treatment with either agent alone. These findings suggest that EP4A and 1,25D3 stimulate bone resorption via a common pathway, namely the RANK/RANKL/OPG signaling pathway. In addition, our observation of increased gene expression for COX-2 by EP4A and 1,25D3 treatment in mouse bone marrow cell cultures suggests an interactive role for the COX-2 signaling pathway in the regulation of osteoclastogenesis by these agents.
In view of the current finding that EP4A induces COX-2 in vitro and a similar, previous finding in cultured mouse osteoblastic cells (37), we evaluated the potential for endogenous prostaglandin production to contribute to the stimulatory effects of this agonist as well as 1,25D3 on osteoclastogenesis in mouse bone marrow cells. Co-treatment with a COX-2 inhibitor diminished the increase in all 3 types of TRAP+ cells induced by 1,25D3, which indicates that this hormone works, at least in part, through endogenous prostaglandin synthesis and the COX-2 pathway. In contrast, the stimulatory effect of PGE2 on osteoclastogenesis was not affected by co-treatment with a COX-2 inhibitor, and only the EP4A-induced formation of TRAP+ giant cells, but not the mono- and multinuclear TRAP+ cells, was decreased by co-treatment with this inhibitor. These findings indicate that PGE2 stimulates osteoclastogenesis downstream from COX-2. Regarding EP4A treatment, the initial increase in commitment of bone marrow cells to the osteoclast lineage (TRAP+ mono- and multinuclear cells) appears to occur downstream from COX-2, but the EP4A-induced differentiation of giant TRAP+ cells may depend, in part, on endogenous prostaglandin synthesis and the COX-2 pathway. The observed increase in gene expression for COX-2 induced by EP4A in mouse bone marrow cells is consistent with this latter finding.
Our previous study (30) demonstrated that several genes associated with bone formation such type I collagen, osteocalcin, and Runx2 were upregulated in the lumbar vertebral body of aged OVX rats treated with FGF2 and EP4A. In particular, the transcription factor Runx2 appears to be a downstream target for FGF2 and EP4A signaling that may mediate the transactivation of downstream genes characteristic of osteoblast differentiation and function such as osteocalcin and type I collagen. The current study expanded on these findings by determining that FGF2 and EP4A treatment also increased gene expression for osterix, which is a transcription factor involved in the control of Runx2 function and the regulation of bone formation. The observed upregulation of osterix was significantly greater in FGF2-treated OVX rats compared with EP4A-treated OVX rats. This was also the case for genes such type I collagen and Runx2 (30). These findings at the molecular level are consistent with the tissue-level histomorphometric findings (30), and add further support to the contention that FGF2 has a stronger bone anabolic effect than EP4A (23).
One of the limitations of the study is that only one dose of FGF2 and EP4A was tested, and the possibility that a different dose of EP4A may have induced an anabolic effect comparable to that of FGF2 cannot be ruled out. Another limitation is that the in vivo portion of the study was performed in rats, whereas the osteoclastogenesis studies were performed in mouse bone marrow cells. Therefore, potential species differences in the skeletal responses to FGF2 and EP4A may complicate comparisons between the in vivo and vitro findings. However, FGF2 has similar bone anabolic effects without stimulating bone resorption in OVX rats and mice (30,38). To our knowledge, the skeletal effects of EP4A treatment have not been reported in mice, but the observation of osteopenia and decreased bone formation in EP4 receptor knockout mice (27) is consistent with a stimulatory effect of an EP4 agonist on bone formation in this species.
In view of the well known angiogenic effects of FGF2 (39), it is not surprising that OVX rats treated with the growth factor exhibited increased gene expression for VEGF. Based on reports that osteoblastic cells express VEGF and its receptors (40) and that this growth factor is associated with osteogenesis (41), VEGF may play a role in the marked increase in bone formation induced by FGF2 treatment. Interestingly, gene expression for IGF-I, which is known to induce VEGF in osteoblast-like cells (42), was also increased in FGF2-treated OVX rats (30). Furthermore, the current study showed that these animals were characterized by increased gene expression for IGF-2, which is a known stimulator of bone formation (43). Therefore, the strong bone anabolic effect of FGF2 most likely involves interactions with signaling pathways for other growth factors such as VEGF and the IGFs. On the other hand, gene expression for TGF-β1, another TGF-β family member that plays an important role in bone formation (44), was unaffected by treatment with FGF2 and EP4A. This finding regarding TGF-β1 is somewhat unexpected in that FGF2 has been reported to upregulate gene expression for TGF-β1 in osteoblast-like cells in vitro (45) and in intact rats treated with FGF2 for 7 days (22). However, this discrepancy may be a consequence of the longer duration of FGF2 treatment (3 weeks) in the current study, which does not allow for detection of early changes in gene expression in response to FGF2. This may also be the reason for the lack of an effect of FGF2 and EP4A on gene expression for COX-2 in bone tissue from aged OVX rats, despite evidence that this gene is induced by EP4A and FGF2 in vitro (37,46) and involved in the regulation of bone resorption (47).
In summary, the current study generated in vivo and in vitro evidence for a stimulatory effect of FGF2 and EP4A on osteoclastogenesis. Regarding FGF2, this effect is evident at the molecular level in vivo and in cultured mouse bone marrow cells, but is masked in vivo at the tissue level by excessive osteoid on cancellous bone surfaces, which is not conducive to the attachment of mature osteoclasts. Therefore, treatment with FGF2 is strongly anabolic in favor of bone formation and a marked accumulation of new bone matrix. EP4A treatment was also found to induce molecular events associated with osteoclastogenesis, but in contrast to FGF2, this was reflected by an increase in osteoclast numbers and bone resorption in aged OVX rats treated with this agent. Therefore, the observed increase in bone formation was in balance with the increase in bone resorption so that EP4A failed to increase cancellous bone mass in these animals. These in vivo and in vitro findings provide insight into differences in the efficacy of two potential bone anabolic agents for restoration of lost bone mass in the osteopenic, estrogen-deplete skeleton.
Acknowledgments
This research was supported by NIH grant R37 AG09241 from the National Institute on Aging. The authors are grateful to Dr. David Moraga, Mercedes Rivera, and Sally Vanegas for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Consensus Development Conference V. Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1994;90:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Foundation NO. America’s Bone Health: The state of osteoporosis and low bone mass in our nation. National Osteoporosis Foundation; 2000. [Google Scholar]
- 3.Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocrine Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- 4.Qi H, Li M, Wronski TJ. Comparison of the bone anabolic effects effects of parathyroid hormone at skeletal sites with moderate and severe osteopenia in aged ovariectomized rats. J Bone Miner Res. 1995;10:948–955. doi: 10.1002/jbmr.5650100616. [DOI] [PubMed] [Google Scholar]
- 5.Zerwekh JE, Hagler HK, Sakhaee K, Gottschalk F, Peterson RD, Pak CYC. Effects of slow release sodium fluoride on cancellous bone histology and connectivity in osteoporosis. Bone. 1994;15:691–699. doi: 10.1016/8756-3282(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 6.Iwaniec UT, Magee KA, Mitova-Caneva NG, Wronski TJ. Bone anabolic effects of subcutaneous treatment with basic fibroblast growth factor alone and in combination with estrogen in osteopenic ovariectomized rats. Bone. 2003;33:380–386. doi: 10.1016/s8756-3282(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 7.Dupree M, Pollack S, Levine L, Laurencin C. Fibroblast growth factor 2 induced proliferation in osteoblasts and bone marrow stromall cells: a whole cell model. Biophys J. 2006;91:3097–3112. doi: 10.1529/biophysj.106.087098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varkey M, Kucharski C, Haque T, Sebald W, Uludag H. In vitro osteogenic response of rat bone marrow cells to bFGF and BMP-2 treatments. Clin Orthop Relat Res. 2006;443:113–123. doi: 10.1097/01.blo.0000200236.84189.87. [DOI] [PubMed] [Google Scholar]
- 9.Fakhry A, Ratisoontorn C, Vedhachalam C, Salhab I, Koyama E, Leboy P, Paifici M, Kirschner R, Nah H. Effects of FGF2/9 in calvarial bone cell cultures: differentiation stage-dependent mitogenic effect, inverse regulation of BMP-2 and noggin, and enhancement of osteogenic potential. Bone. 2005;36:254–266. doi: 10.1016/j.bone.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Naganawa T, Xiao L, Aboundee E, Sobue T, Kalajzic I, Sabbieti M, Agas D, Hurley MM. In vivo and in vitro comparison of the effects of FGF-2 null and haplo-insufficiency on bone formation in mice. Biochem Biophys Res Commun. 2006;339:490–498. doi: 10.1016/j.bbrc.2005.10.215. [DOI] [PubMed] [Google Scholar]
- 11.Nagai H, Tsukuda R, Mayahara H. Effects on basic fibroblast growth factor (bFGF) on bone formation in growing rats. Bone. 1995;16:367–373. doi: 10.1016/8756-3282(94)00049-2. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Kurokawa T, Aoyama I, Hanada K, Tamura M, Kawaguchi H. Stimulation of bone formation by intraosseous injection of basic fibroblast growth factor in ovariectomized rats. Int Orthop. 1998;22:49–54. doi: 10.1007/s002640050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang H, Pun S, Wronski TJ. Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology. 1999;140:5780–5788. doi: 10.1210/endo.140.12.7195. [DOI] [PubMed] [Google Scholar]
- 14.Iwaniec UT, Mosekilde L, Mitova-Caneva NG, Thomsen JS, Wronski TJ. Sequential treatment with basic fibroblast growth factor and PTH is more efficacious that treatment with PTH alone for increasing vertebral bone mass and strength in osteopenic ovariectomized rats. Endocrinology. 2002;143:2515–2526. doi: 10.1210/endo.143.7.8884. [DOI] [PubMed] [Google Scholar]
- 15.Power RA, Iwaniec UT, Magee KA, Mitova-Caneva NG, Wronski TJ. Basic fibroblast growth factor has rapid bone anabolic effects in ovariectomized rats. Osteoporos Int. 2004;15:716–723. doi: 10.1007/s00198-004-1595-4. [DOI] [PubMed] [Google Scholar]
- 16.Power RA, Iwaniec UT, Wronski TJ. Changes in gene expression associated with the bone anabolic effects of basic fibroblast growth factor in aged ovariectomized rats. Bone. 2002;31:143–148. doi: 10.1016/s8756-3282(02)00799-8. [DOI] [PubMed] [Google Scholar]
- 17.Haque T, Uludag H, Zernicke R, Winn S, Sebald W. Bone marrow cells from normal and ovariectomized rats respond differently to basic fibroblast growth factor and bone morphogenetic protein 2 treatment in vitro. Tissue Engineering. 2005;11:634–644. doi: 10.1089/ten.2005.11.634. [DOI] [PubMed] [Google Scholar]
- 18.Jimi E, Shuto T, Ikebe T, Jingushi S, Hirata M, Koga T. Basic fibroblast growth factor inhibits osteoclast-like cell formation. J Cell Physiol. 1996;168:395–402. doi: 10.1002/(SICI)1097-4652(199608)168:2<395::AID-JCP18>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Chikazu D, Katagiri M, Ogasawara T, Ogata N, Shimoaka T, Takato T, Nakamura K, Kawaguchi H. Regulation of osteoclast differentiation by fibroblast growth factor 2: Stimulation of receptor activator of nuclear factor KB ligand/osteoclast differentiation factor expression in osteoblasts and inhibition of macrophage colony-stimulating factor function in osteoclast precursors. J Bone Miner Res. 2001;16:2074–2081. doi: 10.1359/jbmr.2001.16.11.2074. [DOI] [PubMed] [Google Scholar]
- 20.Zuo J, Jiang J, Dolce C, Holliday S. Effects of basic fibroblast growth factor on osteoclasts and osteoclast-like cells. Biochem Biophys Res Comm. 2004;318:162–167. doi: 10.1016/j.bbrc.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Chikazu D, Hakeda Y, Ogata N, Nemoto K, Itabashi A, Takato T, Kumegawa M, Nakamura K, Kawaguchi H. Fibroblast growth factor (FGF)-2 directly stimulates mature osteoclast function through activation of FGF receptor 1 and p42/p44 MAP kinase. J Biol Chem. 2000;275:31444–31450. doi: 10.1074/jbc.M910132199. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Hanada K, Tamura M, Shibanushi T, Nigi H, Tagawa M, Fukumoto S, Matsumoto T. Stimulation of endosteal bone formation by systemic injections of recombinant basic fibroblast growth factor in rats. Endocrinology. 1995;136:1276–1284. doi: 10.1210/endo.136.3.7867582. [DOI] [PubMed] [Google Scholar]
- 23.Iwaniec UT, Moore K, Rivera MF, Myers SE, Vanegas SM, Wronski TJ. A comparative study of the bone-restorative efficacy of anabolic agents in aged ovariectomized rats. Osteoporosis Int. 2007;18:351–362. doi: 10.1007/s00198-006-0240-9. [DOI] [PubMed] [Google Scholar]
- 24.Jee WSS, Ma YF. The in vivo anabolic actions of prostaglandins in bone. Bone. 1997;21:297–304. doi: 10.1016/s8756-3282(97)00147-6. [DOI] [PubMed] [Google Scholar]
- 25.Mori S, Jee WSS, Li XJ. Production of new trabecular bone in osteopenic ovariectomized rats by prostaglandin E2. Calcif Tissue Int. 1992;50:80–87. doi: 10.1007/BF00297302. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida K, Oida H, Kobayashi T, Maruyama T, Tanaka M, Katayama T, Yamaguchi K, Segi E, Tsuboyama T, Matsushita M, Ito K, Ito Y, Sugimoto Y, Ushikubi F, Ohuchida S, Kondo K, Nakamura T, Narumiya S. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc Natl Acad Sci. 2002;99:4580–4585. doi: 10.1073/pnas.062053399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Healy DR, Li Y, Simmons HA, Crawford DT, Ke HZ, Pan LC, Brown TA, Thompson DD. Osteopenia and impaired fracture healing in aged EP4 receptor knockout mice. Bone. 2005;37:46–54. doi: 10.1016/j.bone.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 28.Ke HZ, Crawford DT, Qi H, Simmons HA, Owen TA, Paralkar VM, Li M, Lu B, Grasser WA, Cameron KO, Lefker BA, DaSilva-Jardine P, Scott DO, Zhang Q, Tian XY, Jee WSS, Brown TA, Thompson DD. A nonprostanoid EP4 receptor selective prostaglandin E2 agonist restores bone mass and strength in aged, ovariectomized rats. J Bone Miner Res. 2006;21:565–575. doi: 10.1359/jbmr.051110. [DOI] [PubMed] [Google Scholar]
- 29.Wronski TJ, Schenck PA, Cintron M, Walsh CC. Effect of body weight on osteopenia in ovariectomized rats. Calcif Tissue Int. 1987;40:155–159. doi: 10.1007/BF02555700. [DOI] [PubMed] [Google Scholar]
- 30.Aguirre JI, Leal ME, Rivera MF, Vanegas SM, Jorgensen M, Wronski TJ. Effects of basic fibroblast growth factor and prostaglandin E2 receptor subtype 4 agonist on osteoblastogenesis and adipogenesis in aged ovariectomized rats. J Bone Miner Res. 2007;22:877–888. doi: 10.1359/jbmr.070313. [DOI] [PubMed] [Google Scholar]
- 31.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 32.Holliday LS, Welgus HG, Hanna J, Lee BS, Lu M, Jeffrey JJ, Gluck SL. Interstitial collagenase activity stimulates the formation of actin rings and ruffled membranes in mouse marrow osteoclasts. Calcif Tissue Int. 2003;72:206–214. doi: 10.1007/s00223-002-1008-7. [DOI] [PubMed] [Google Scholar]
- 33.Mentaverri R, Kamel S, Brazier M. Involvement of capacitive calcium entry and calcium store refilling in osteoclastic survival and bone resorption process. Cell Calcium. 2003;34:169–175. doi: 10.1016/s0143-4160(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 34.Kaji H, Sugimoto T, Kanatani M, Chihara K. High extracellular calcium stimulates osteoclast-like cell formation and bone-resorbing activity in the presence of osteoblastic cells. J Bone Miner Res. 1996;11:912–920. doi: 10.1002/jbmr.5650110707. [DOI] [PubMed] [Google Scholar]
- 35.Suzawa T, Miyaura C, Inada M, Maruyama T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 36.Lee S-K, Kalinowski J, Jastrzebski S, Lorenzo JA. 1,25(OH)2 Vitamin D3-stimulated osteoclast formation in spleen-osteoblast co-cultures is mediated in part by enhanced IL-1α and receptor activator of NF-Kb ligand production in osteoblasts. J Immunology. 2002;169:2374–2380. doi: 10.4049/jimmunol.169.5.2374. [DOI] [PubMed] [Google Scholar]
- 37.Sakuma Y, Li Z, Pilbeam CC, Alander CB, Chikazu D, Kawaguchi H, Raisz LG. Stimulation of cAMP production and cyclooxygenase-2 by prostaglandin E2 and selective prostaglandin receptor agonists in murine osteoblastic cells. Bone. 2004;34:827–834. doi: 10.1016/j.bone.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Yao W, Balooch G, Balooch M, Jiang Y, Nalla RK, Kinney J, Wronski TJ, Lane NE. Sequential treatment of ovariectomized mice with bFGF and risedronate restored trabecular bone microarchitecture and mineralization. Bone. 2006;39:460–469. doi: 10.1016/j.bone.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Gospodarowicz D, Ferrara N, Schweigerer L, Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocrine Rev. 1987;8:95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- 40.Deckers MM, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Lowik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667–1674. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- 41.Zelzer E, McLean W, Ng YS, Fukai N, Reginato AM, Lovejoy S, D’More PA, Olsen BR. Skeletal defects in VEGF (120/120) mice reveal multiple roles for VEGF in skeletogenesis. Development. 2002;129:1893–1904. doi: 10.1242/dev.129.8.1893. [DOI] [PubMed] [Google Scholar]
- 42.Akeno N, Robins J, Zhang M, Czyzyk-Krzeska MF, Clemens TL. Induction of vascular endothelial growth factor by IGF-I in osteoblast-like cells is mediated by the PI3K signaling pathway through the hypoxia-inducible factor-2 alpha. Endocrinology. 2002;143:1545–1553. doi: 10.1210/endo.143.2.8639. [DOI] [PubMed] [Google Scholar]
- 43.Fisher M, Meyer C, Garber G, Dealy C. Role of IGFBP2, IGF-I and IGF-II in regulating long bone growth. Bone. 2005;37:741–750. doi: 10.1016/j.bone.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Locklin RM, Williamson MC, Beresford JN, Triffitt JT, Owen ME. In vitro effects of growth factors and dexamethasone on rat marrow stromal cells. Clin Orthop. 1995;313:27–35. [PubMed] [Google Scholar]
- 45.Dennler S, Goumans MJ, Dijke PT. Transforming growth factor β signal transduction. J Leukocyte Biol. 2002;71:731–740. [PubMed] [Google Scholar]
- 46.Kawaguchi H, Pilbeam CC, Gronowicz G, Abreu C, Fletcher BS, Herschman HR, Raisz LG, Hurley MM. Transcriptional induction of prostaglandin G/H synthase-2 by basic fibroblast growth factor. J Clin Invest. 1995;96:923–930. doi: 10.1172/JCI118140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X-H, Kirschenbaum A, Yao S, Levine AC. Interactive effect of interleukin-6 and prostaglandin E2 on osteoclastogenesis via the OPG/RANKL/RANK system. Ann NY Acad Sci. 2006;1068:225–233. doi: 10.1196/annals.1346.047. [DOI] [PubMed] [Google Scholar]