Abstract
Objectives
The purpose of this study was to compare the effects of 3 months of estrogen replacement therapy, estrogen plus progesterone replacement therapy and a placebo, on the resting cortisol and interleukin-6(IL-6) levels in post-menopausal women.
Methods
Forty three women were randomised to one of three treatment arms: estradiol 2 mg/day (ERT), estradiol 2 mg/day plus medroxyprogesterone acetate 5 mg/day (HRT), or a placebo that was administered orally for 3 months.
Results
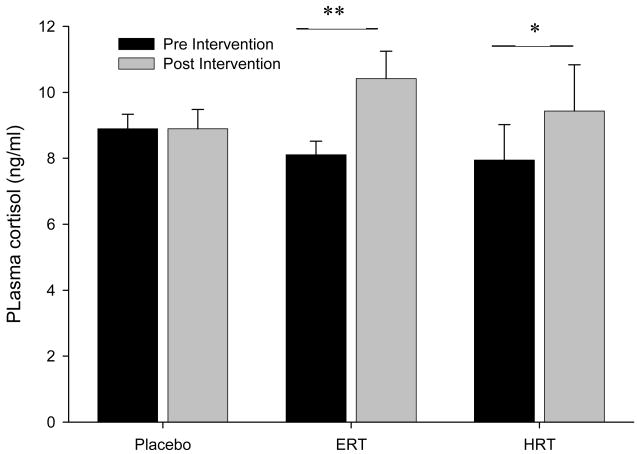
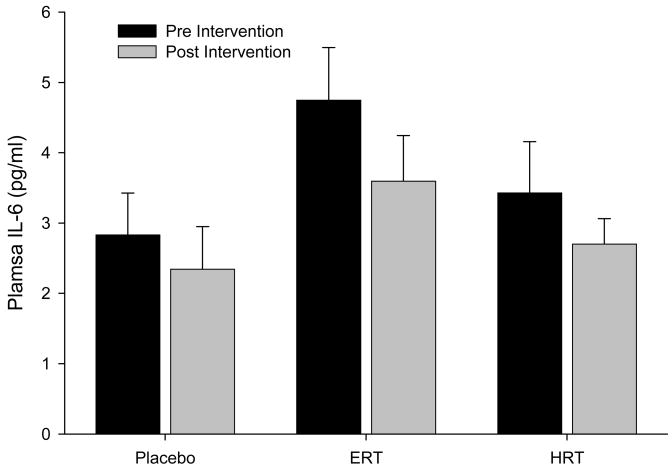
Cortisol levels showed a significant condition by intervention interaction. Post-hoc tests showed that ERT significantly increased cortisol levels after treatment compared to baseline, while in the HRT group a trend toward increased cortisol was found. No changes were observed in IL-6 levels.
Conclusions
Estrogen administration elevated cortisol levels, but this effect may be moderated by progestins. IL-6 was not altered by ERT or HRT, future studies should consider the interaction of cortisol increases on change in IL-6 expression.
Keywords: estrogen, cortisol, interleukin-6, hormone replacement therapy, menopause
Introduction
Sex dimorphisms are readily apparent in the immune system, and gonadal hormones have been shown to have important actions for immune consequences. Immune cells express receptors for gonadal hormones, revealing the possibility for direct action, but this is a complex, bidirectional relationship; for example, the conversion of androgens into estrogen is favoured by a proinflammatory environment. The occurrence of menopause allows a window into the effects of gonadal hormones, and hormone replacement therapy provides a well controlled opportunity for study. The natural changes in hormone secretion pre- and post-menopause have been cited as root cause for a myriad of health changes, but the mechanisms for these effects in many cases remain unclear. Immune parameter changes have been investigated as one potential pathway; the effects of gonadal hormones on cytokine and glucocorticoid activity.
Glucocorticoids released by the hypothalamic-pituitary-adrenal (HPA) axis are intricately linked to immune function, their actions limit and resolve inflammatory processes [1]. Glucocorticoid receptors are present in almost all cells and bind free cortisol, the biologically active form of the hormone, which is secreted by the adrenal cortex. It is generally found that levels of cortisol increase with age, with premenopausal women showing lower mean levels than men of the same age [2], but during the menopausal transition, levels increase [3]. Surprisingly few studies have investigated the effects of hormone replacement therapy (HRT) or estrogen replacement therapy (ERT) on cortisol levels, and there appear to be somewhat inconsistent findings. Several prospective and cross-sectional studies have shown that oral ERT increases total cortisol levels [4–7], although not all reports have found differences [8] and free cortisol has not been shown to increase significantly. Transdermal ERT has been shown to either decrease [9] or have no effect on cortisol levels [6, 10]. HRT has been reported to increase, decrease or not change cortisol levels. For example, Shifren et al. [11] reported that HRT increased total cortisol after 12 weeks, but did not alter free cortisol in a well controlled study, and cross-sectional data from Burleson et al. [7] found increased cortisol in HRT users. While in contrast, Patacchioli et al [12] reported that long term HRT decreased morning free cortisol levels compared to post-menopausal women not using HRT, and Pluchino et al [9] found HRT significantly reduced total cortisol after 12 months of treatment. A recent study by Kalleinen et al [13] found no differences after six months of HRT using detailed 24-hour assessments of cortisol. They suggest that the divergent effects of HRT may reflect different combinations of hormone therapy used. These and other mixed findings regarding the effects of menopause and HRT and ERT [14] indicate that further investigation is warranted.
Interleukin-6 (IL-6) is an inflammatory marker, which plays a key role in the Th1/Th2 balance, induces acute phase proteins, and affects glucose and adipose metabolism. Importantly, elevated IL-6 is a risk marker for cardiac events and atherosclerosis, which are more evident in post-menopausal women. Circulating levels of IL-6 are found to increase post-menopause, and show a continuing increase with age [15]. In line with this, it has recently been found that levels of IL-6 are negatively correlated with serum estrodiol [16]. The effects of ERT and HRT have been investigated, but data are somewhat mixed. Cross sectional studies have suggested that IL-6 is increased in post-menopausal women not on HRT, compared to women receiving HRT [17, 18]. Similarly, Cantatore at al [19] reported that IL-6 increased in control women (no treatment) 6 months after hysterectomy and bilateral oophorectomy, while women treated with ERT or HRT did not show any change in IL-6 levels. In the same direction, Rachon et al. [20] have reported that IL-6 is lowered by ERT in oral, transdermal and intranasal forms over 6 to 12 months of treatment. However, most randomised controlled trials found no change in IL-6 levels after up to 12 months of HRT treatment [21–25]. In contrast, it has also been found that HRT use is associated with increased IL-6 levels [26]. These mixed findings do not clarify the effects of HRT and ERT on IL-6 levels, and importantly have not compared the effects of each treatment with the other.
In light of the current literature of mixed findings, we analysed the concentrations of cortisol and IL-6 in post menopausal women before and after 3 months of HRT, ERT or placebo.
Methods
Participants
Forty six postmenopausal women (mean = 55 years, SD = 6.6 years) were recruited through advertisement and referral. The final sample size was 43 because 3 subjects were dropped from the analysis due to non-compliance or dropped out before the final visit. Subjects all gave written informed consent that was approved by the University of California, San Diego Institutional Review Board.
All women were normotensive (BP < 140/90 mmHG). Blood pressure was measured on two separate occasions at least 2 weeks apart, when the subject was seated after a 15-min rest. On each occasion three blood pressure readings were taken and the average of all six readings was used to assess blood pressure status. All subjects were non-smokers. Women were eligible to participate if they met the following criteria: [1] absence of a menstrual period for the previous 12 months; [2] no HRT for the previous 6 months; and [3] FSH levels in the postmenopausal range (>40 mIU/mL).
Participants underwent a history and physical examination by a physician and an electrocardiogram (ECG) was performed to ensure there were no cardiac abnormalities, subjects were excluded if they had history of high blood pressure, liver or renal disease, diabetes, psychosis, severe asthma, cerebrovascular disease or cancer. Subjects were not taking any prescribed medications at the time of the study. At the conclusion of the study, participants were unblinded to their assigned group and were given clinic referrals if they wanted to continue with (or begin in the case of the control group) appropriate ERT or HRT.
Procedure
Blood was drawn from participants in the early afternoon after a 30 min rest period. In a double-blind, randomised, placebo-controlled fashion, the subjects were assigned to one of three treatment arms: estradiol 2 mg/day (ERT) (N = 17), estradiol 2 mg/day plus medroxyprogesterone acetate 5 mg/day (HRT) (N = 12), or a placebo (N = 14) that was administered orally for 3 months. The randomisation process was computer generated with an SAS program (SAS Institute, Cary, NC). A random number table was used to assign treatment groups randomly with blocks. After the 3 month intervention a final blood sample was taken again in the early afternoon after a 30 min rest period.
Assays
Whole blood was drawn into tubes containing ethylenediaminetetraacetic acid (EDTA) and placed immediately on ice. EDTA tubes were centrifuged at 2500 rpm for 10 minutes at 4°C, and plasma was harvested and stored at −70°C. Levels of IL-6 and total cortisol were assessed using commercially available kits, (IL-6: HS Quantikine ELISA; R&D Systems, Minneapolis, MN, USA; Cortsiol: RIA; DSL a Becman Coulter company, Webster, TX, USA). To reduce within subject variability, all samples for each subject were assayed together.
Statistical analysis
Date were analysed using a series of 3 group (placebo, HRT, ERT) × 2 intervention period (before treatment, after treatment) multivariate analysis of variance (MANOVA) to determine the effect of intervention. Significant effects were followed by post-hoc comparisons. Eta-squared (η2), a measure of effect size, was determined. A 5% significance level was adopted throughout. Occasional missing data are reflected in the reported degrees of freedom.
RESULTS
Demographics
There were no significant differences among the three groups in terms of cause of menopause (i.e., natural vs surgical), duration of menopausal status, and body mass index. Screening and pre-intervention baseline measurements of systolic blood pressure, diastolic blood pressure and heart rate were not different among the groups.
Cortisol
In the initial analysis to determine the effect of hormone intervention, a 3 group × 2 intervention period revealed a significant group by intervention effect (F(2,34) = 3.94, p = .03, η2 = .188) showing that the intervention groups showed different patterns of cortisol responses to treatment. This was followed with a similar analysis to compare HRT and ERT (placebo excluded), no significant group by intervention interaction was found (F(1,23) = 0.89, p = .35, η2 = .037) showing that the two hormone groups did not show different patterns of response. However, post hoc tests revealed that the ERT group showed a significant increase in cortisol levels when comparing baseline and post treatment (p = .002), while the HRT group showed only a trend toward increased cortisol levels (p = .094). In the placebo group there was no difference in cortisol levels at baseline pre and post treatment.
IL-6
There were no significant effects of group or intervention, and no significant interactions.
Discussion
The current study investigated the effects of 3 months HRT or ERT in post-menopausal women on baseline IL-6 and cortisol. A significant intervention by group interaction emerged for cortisol levels which increased in a similar manner in both ERT and HRT groups from pre to post intervention. Importantly, post hoc tests showed that the ERT group showed a greater, significant, increase (+2.3 ng/ml); while the HRT group showed a trend towards increased cortisol levels pre to post intervention (+1.5 ng/ml). No effect of intervention was seen in IL-6 levels.
The current findings that cortisol was elevated by ERT and HRT treatment add to, and may help clarify, the literature. Previous reports of cross-sectional as well as prospective studies of treatment with estrogen alone, have largely found elevations in cortisol levels, in line with the current data [4–7]. The effects of estrogen when combined with progestins (HRT) is less clear, and is of course complicated by the many different doses and combinations in sequential or cyclical programs that can be used. The current data found that HRT did not cause the same degree of elevation in cortisol as ERT. We employed a regime of continuous medroxyprogesterone acetate 5 mg/day, with estradiol 2 mg/day, a fairly low estrogen: progesterone ratio. It is likely that the dosage and ratio of estrogens and progestin administered alters the effects on cortisol levels, and mediates the effects of treatment with estrogen alone. Further investigations should compare the effects of different dose combinations in HRT before this hypothesis advances beyond speculation.
The current study measured total cortisol in plasma, which includes the free and bound portions. In the blood approximately 95% of cortisol is bound, principally to cortisol-binding globulin (CBG). Measures of salivary cortisol are, in contrast, validated measures of free cortisol. A recent study which compared oral and transdermal estrogen administration effects on total cortisol, salivary free cortisol and CBG found that oral, but not transdermal estrogen increased serum total cortisol and CBG, but did not alter salivary free cortisol [6]. It is likely then that the finding of increased plasma cortisol after ERT in the current study reflects the effects of increased CBG. Estrogens are known to stimulate hepatic protein production including CBG, and oral administration leads to a far higher local concentration of estrogens in the portal circulation compared to transdermal administration [27]. This may help explain why oral ERT more consistently elevates total cortisol levels compared to transdermal ERT. The effects of progesterone on CBG production is less established, but it has been reported that high concentrations of progesterone can significantly suppress CBG mRNA expression in cell lines [28]. If oral progesterone leads to high local concentrations in the portal circulation similar to estrogens, the smaller increase in cortisol seen in HRT may be explained by the suppression of CBG by progesterone. However, this remains speculation until the actions of progesterone and hormone combinations on CBG levels in vivo are determined.
We did not find any evidence for ERT or HRT effects on IL-6 levels. IL-6 is a multi-functional cytokine which plays a key regulation role in inflammation, it is the primary stimulant for C-reactive protein (CRP) production by the liver. CRP is a risk factor for cardiovascular events, and has provided a link between hormone treatment and increased coronary risk in women taking hormone treatment [29]. Many studies have shown that hormone therapy, particularly ERT, increases levels of CRP, but in line with current data, there does not appear to be a concurrent increase in IL-6 [30]. Silvestri et al. [31] have suggested that the increase in CRP must be due to metabolic hepatic stimulation rather than the IL-6 acute-phase pathway. It is possible that the elevation in cortisol with hormone therapy, may also play a role. Cortisol is a predominantly anti-inflammatory hormone, which suppresses IL-6 production [32], thus any stimulation toward increased IL-6 hormone therapy may have may be offset by the inhibitory effect that elevated cortisol exerts.
The current study shows that estrogen administration leads to elevated cortisol, and that that this effect may be moderated by progestins. The importance of understanding the role of gonadal hormones in health is highlighted by changes within a lifetime, such as the menopause, which significantly alter health risks. The effect of hormone treatment, and particularly the importance of increased cortisol in the context of CRP elevation without any increase in IL-6, therefore deserves further study.
Figure 1.
Mean (±SE) baseline plasma cortisol concentrations (ng/ml) pre and post intervention. Difference from baseline (** p < .05, * p < .1).
Figure 2.
Mean (±SE) baseline plasma IL-6 concentrations (pg/ml) pre and post intervention
Acknowledgments
This work was supported by grant HL57265 and HL073355 from the National Institutes of Health and the UCSD General Clinical Research Center (MO1RR-00827).
Footnotes
Authors have no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol. 2002;20:125–63. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- 2.Van CE, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81:2468–73. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- 3.Woods NF, Carr MC, Tao EY, Taylor HJ, Mitchell ES. Increased urinary cortisol levels during the menopausal transition. Menopause. 2006;13:212–21. doi: 10.1097/01.gme.0000198490.57242.2e. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca E, Basurto L, Velazquez S, Zarate A. Hormone replacement therapy increases ACTH/dehydroepiandrosterone sulfate in menopause. Maturitas. 2001;39:57–62. doi: 10.1016/s0378-5122(01)00192-x. [DOI] [PubMed] [Google Scholar]
- 5.Gudmundsson A, Goodman B, Lent S, et al. Effects of estrogen replacement therapy on the circadian rhythms of serum cortisol and body temperature in postmenopausal women. Exp Gerontol. 1999;34:809–18. doi: 10.1016/s0531-5565(99)00044-3. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi AC, Bahri A, Breen LA, et al. The influence of the route of oestrogen administration on serum levels of cortisol-binding globulin and total cortisol. Clin Endocrinol. 2007;66:632–5. doi: 10.1111/j.1365-2265.2007.02784.x. [DOI] [PubMed] [Google Scholar]
- 7.Burleson MH, Malarkey WB, Cacioppo JT, et al. Postmenopausal hormone replacement: effects on autonomic, neuroendocrine, and immune reactivity to brief psychological stressors. Psychosom Med. 1998;60:17–25. doi: 10.1097/00006842-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Komesaroff PA, Esler MD, Sudhir K. Estrogen supplementation attenuates glucocorticoid and catecholamine responses to mental stress in perimenopausal women. J Clin Endocrinol Metab. 1999;84:606–10. doi: 10.1210/jcem.84.2.5447. [DOI] [PubMed] [Google Scholar]
- 9.Pluchino N, Genazzani AD, Bernardi F, et al. Tibolone, transdermal estradiol or oral estrogen-progestin therapies: effects on circulating allopregnanolone, cortisol and dehydroepiandrosterone levels. Gynecol Endocrinol. 2005;20:144–9. doi: 10.1080/09513590400021169. [DOI] [PubMed] [Google Scholar]
- 10.Cucinelli F, Soranna L, Barini A, et al. Estrogen treatment and body fat distribution are involved in corticotropin and cortisol response to corticotropin-releasing hormone in postmenopausal women. Metabolism. 2002;51:137–43. doi: 10.1053/meta.2002.29971. [DOI] [PubMed] [Google Scholar]
- 11.Shifren JL, Desindes S, McIlwain M, Doros G, Mazer NA. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause. 2007;14:985–94. doi: 10.1097/gme.0b013e31803867a. [DOI] [PubMed] [Google Scholar]
- 12.Patacchioli FR, Simeoni S, Monnazzi P, Pace M, Capri O, Perrone G. Menopause, mild psychological stress and salivary cortisol: influence of long-term hormone replacement therapy (HRT) Maturitas. 2006;55:150–5. doi: 10.1016/j.maturitas.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Kalleinen N, Polo-Kantola P, Irjala K, et al. 24-hour serum levels of growth hormone, prolactin, and cortisol in pre- and postmenopausal women: the effect of combined estrogen and progestin treatment. J Clin Endocrinol Metab. 2008;93:1655–61. doi: 10.1210/jc.2007-2677. [DOI] [PubMed] [Google Scholar]
- 14.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–78. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Cioffi M, Esposito K, Vietri MT, et al. Cytokine pattern in postmenopause. Maturitas. 2002;41:187–92. doi: 10.1016/s0378-5122(01)00286-9. [DOI] [PubMed] [Google Scholar]
- 16.Yasui T, Maegawa M, Tomita J, et al. Changes in serum cytokine concentrations during the menopausal transition. Maturitas. 2007;56:396–403. doi: 10.1016/j.maturitas.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Scheidt-Nave C, Bismar H, Leidig-Bruckner G, et al. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab. 2001;86:2032–42. doi: 10.1210/jcem.86.5.7445. [DOI] [PubMed] [Google Scholar]
- 18.Straub RH, Hense HW, Andus T, Scholmerich J, Riegger GA, Schunkert H. Hormone replacement therapy and interrelation between serum interleukin-6 and body mass index in postmenopausal women: a population-based study. J Clin Endocrinol Metab. 2000;85:1340–4. doi: 10.1210/jcem.85.3.6355. [DOI] [PubMed] [Google Scholar]
- 19.Cantatore FP, Loverro G, Ingrosso AM, et al. Effect of oestrogen replacement on bone metabolism and cytokines in surgical menopause. Clin Rheumatol. 1995;14:157–60. doi: 10.1007/BF02214935. [DOI] [PubMed] [Google Scholar]
- 20.Rachon D, Suchecka-Rachon K, Hak L, Mysliwska J. Effects of intranasal 17beta-estradiol administration on serum bioactive interleukin-6 and C-reactive protein levels in healthy postmenopausal women. Menopause. 2006;13:840–5. doi: 10.1097/01.gme.0000227400.60816.52. [DOI] [PubMed] [Google Scholar]
- 21.Walsh BW, Paul S, Wild RA, et al. The effects of hormone replacement therapy and raloxifene on C-reactive protein and homocysteine in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2000;85:214–8. doi: 10.1210/jcem.85.1.6326. [DOI] [PubMed] [Google Scholar]
- 22.Zanger D, Yang BK, Ardans J, et al. Divergent effects of hormone therapy on serum markers of inflammation in postmenopausal women with coronary artery disease on appropriate medical management. J Am Coll Cardiol. 2000;36:1797–802. doi: 10.1016/s0735-1097(00)00952-9. [DOI] [PubMed] [Google Scholar]
- 23.Lacut K, Oger E, Le GG, et al. Differential effects of oral and transdermal postmenopausal estrogen replacement therapies on C-reactive protein. Thromb Haemost. 2003;90:124–31. [PubMed] [Google Scholar]
- 24.Cooper BC, Burger NZ, Toth MJ, Cushman M, Sites CK. Insulin resistance with hormone replacement therapy: associations with markers of inflammation and adiposity. Am J Obstet Gynecol. 2007;196:123–7. doi: 10.1016/j.ajog.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koh KK, Schenke WH, Waclawiw MA, Csako G, Cannon RO., III Statin attenuates increase in C-reactive protein during estrogen replacement therapy in postmenopausal women. Circulation. 2002;105:1531–3. doi: 10.1161/01.cir.0000013837.81710.da. [DOI] [PubMed] [Google Scholar]
- 26.Herrington DM, Brosnihan KB, Pusser BE, et al. Differential effects of E and droloxifene on C-reactive protein and other markers of inflammation in healthy postmenopausal women. J Clin Endocrinol Metab. 2001;86:4216–22. doi: 10.1210/jcem.86.9.7799. [DOI] [PubMed] [Google Scholar]
- 27.Mazer NA. Interaction of estrogen therapy and thyroid hormone replacement in postmenopausal women. Thyroid. 2004;14:S27–S34. doi: 10.1089/105072504323024561. [DOI] [PubMed] [Google Scholar]
- 28.Misao R, Nakanishi Y, Fujimoto J, Tamaya T. Effects of sex steroid hormones on corticosteroid-binding globulin gene expression in human endometrial cancer cell line Ishikawa. Ann Clin Biochem. 1998;35:637–42. doi: 10.1177/000456329803500507. [DOI] [PubMed] [Google Scholar]
- 29.Writing Group for the Women’s Health Initiative Investigators. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results From the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 30.Lakoski SG, Herrington DM. Effects of hormone therapy on C-reactive protein and IL-6 in postmenopausal women: a review article. Climacteric. 2005;8:317–26. doi: 10.1080/13697130500345109. [DOI] [PubMed] [Google Scholar]
- 31.Silvestri A, Gebara O, Vitale C, et al. Increased levels of C-reactive protein after oral hormone replacement therapy may not be related to an increased inflammatory response. Circulation. 2003;107:3165–9. doi: 10.1161/01.CIR.0000074208.02226.5E. [DOI] [PubMed] [Google Scholar]
- 32.Dovio A, Sartori ML, Masera RG, Racca S, Angeli A. Inhibitory effect of physiological concentrations of cortisol but not estradiol on interleukin (IL)-6 production by human osteoblast-like cell lines with different constitutive IL-6 expression. Cytokine. 2001;15:47–52. doi: 10.1006/cyto.2001.0892. [DOI] [PubMed] [Google Scholar]