Abstract
Oxidative/nitrative stress caused by peroxynitrite, the reaction product of
superoxide (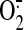 ) and nitric
oxide (NO), is the primary cause of myocardial ischemia/reperfusion injury.
The present study determined whether INO-4885
[5,10,15,20-tetra[N-(benzyl-4′-carboxylate)-2-pyridinium]-21H,23H-porphine
iron(III) chloride], a new peroxynitrite decomposition catalyst, may provide
cellular protection and protect heart from myocardial ischemia/reperfusion
injury. Adult male mice were subjected to 30 min of ischemia and 3 or 24 h of
reperfusion. Mice were randomized to receive vehicle, INO-4885 without
catalytic moiety, or INO-4885 (3-300 μg/kg i.p.) 10 min before reperfusion.
Infarct size, apoptosis, nitrotyrosine content,
NO/
) and nitric
oxide (NO), is the primary cause of myocardial ischemia/reperfusion injury.
The present study determined whether INO-4885
[5,10,15,20-tetra[N-(benzyl-4′-carboxylate)-2-pyridinium]-21H,23H-porphine
iron(III) chloride], a new peroxynitrite decomposition catalyst, may provide
cellular protection and protect heart from myocardial ischemia/reperfusion
injury. Adult male mice were subjected to 30 min of ischemia and 3 or 24 h of
reperfusion. Mice were randomized to receive vehicle, INO-4885 without
catalytic moiety, or INO-4885 (3-300 μg/kg i.p.) 10 min before reperfusion.
Infarct size, apoptosis, nitrotyrosine content,
NO/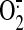 production, and
inducible nitric-oxide synthase (iNOS)/NADPH oxidase expression were
determined. INO-4885 treatment reduced ischemia/reperfusion-induced protein
nitration and caspase 3 activation in a dose-dependent fashion in the range of
3 to 100 μg/kg. However, doses exceeding 100 μg/kg produced nonspecific
effects and attenuated its protective ability. At the optimal dose (30
μg/kg), INO-4885 significantly reduced infarct size (p < 0.01),
decreased apoptosis (p < 0.01), and reduced tissue nitrotyrosine
content (p < 0.01). As expected, INO-4885 had no effect on
ischemia/reperfusion-induced iNOS expression and NO overproduction. To our
surprise, this compound significantly reduced superoxide production and
partially blocked NADPH oxidase overexpression in the ischemic/reperfused
cardiac tissue. Additional experiments demonstrated that INO-4885 provided
better cardioprotection than N-(3-(aminomethyl)benzyl)acetamidine
(1400W, a selective iNOS inhibitor), apocynin (an NADPH oxidase inhibitor), or
Tiron (a cell-permeable superoxide scavenger). Taken together, our data
demonstrated that INO-4885 is a cardioprotective molecule that attenuates
myocardial reperfusion injury by facilitating peroxynitrite decomposition and
inhibiting NADPH oxidase-derived
production, and
inducible nitric-oxide synthase (iNOS)/NADPH oxidase expression were
determined. INO-4885 treatment reduced ischemia/reperfusion-induced protein
nitration and caspase 3 activation in a dose-dependent fashion in the range of
3 to 100 μg/kg. However, doses exceeding 100 μg/kg produced nonspecific
effects and attenuated its protective ability. At the optimal dose (30
μg/kg), INO-4885 significantly reduced infarct size (p < 0.01),
decreased apoptosis (p < 0.01), and reduced tissue nitrotyrosine
content (p < 0.01). As expected, INO-4885 had no effect on
ischemia/reperfusion-induced iNOS expression and NO overproduction. To our
surprise, this compound significantly reduced superoxide production and
partially blocked NADPH oxidase overexpression in the ischemic/reperfused
cardiac tissue. Additional experiments demonstrated that INO-4885 provided
better cardioprotection than N-(3-(aminomethyl)benzyl)acetamidine
(1400W, a selective iNOS inhibitor), apocynin (an NADPH oxidase inhibitor), or
Tiron (a cell-permeable superoxide scavenger). Taken together, our data
demonstrated that INO-4885 is a cardioprotective molecule that attenuates
myocardial reperfusion injury by facilitating peroxynitrite decomposition and
inhibiting NADPH oxidase-derived
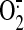 production.
production.
Since its conceptual introduction in the early 1980s, ischemic/reperfusion injury has widely been accepted as the leading cause of tissue damage occurring in pathology such as myocardial infarction, stroke, organ transplantation, cardiopulmonary bypass, and end-organ damage complicating circulatory shock. In the ischemic heart, initial cardiac damage is prompted by diminished blood supply. Swift restoration of normal blood supply is imperative to minimize cardiac injury. Unfortunately, reperfusion itself can lead to additional injury in the form of cardiac dysfunction, reperfusion arrhythmias, and exacerbated myocardial infarction.
For many years, the mechanisms of reperfusion injury have been
investigated, and reactive oxygen species such as the superoxide
(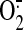 ) anion, hydroxyl
radical, and hydrogen peroxide have been identified to play prominent roles in
I/R injury. Of the variety of reactive oxygen and nitrogen species generated
during oxidative stress, peroxynitrite (ONOO-) is one of the most
reactive and toxic players (Beckman and
Koppenol, 1996). Produced by spontaneous combination of NO and
) anion, hydroxyl
radical, and hydrogen peroxide have been identified to play prominent roles in
I/R injury. Of the variety of reactive oxygen and nitrogen species generated
during oxidative stress, peroxynitrite (ONOO-) is one of the most
reactive and toxic players (Beckman and
Koppenol, 1996). Produced by spontaneous combination of NO and
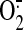 released during
reperfusion (Ferdinandy, 2006),
peroxynitrite easily permeates lipid bilayers (permeability coefficient of
∼8.0 × 10-4 cm/s, nearly 400-fold that of
released during
reperfusion (Ferdinandy, 2006),
peroxynitrite easily permeates lipid bilayers (permeability coefficient of
∼8.0 × 10-4 cm/s, nearly 400-fold that of
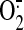 )
(Marla et al., 1997). Its
toxic effects include peroxidation of membrane lipids and nitrative
inactivation of enzymes, such as prostacyclin synthase and superoxide
dismutase. ONOO- inhibits tyrosine phosphorylation and,
consequently, affects signal transduction pathways. With the ability to induce
single- and/or double-strand DNA breaks, peroxynitrite plays a critical role
in reperfusion injury.
)
(Marla et al., 1997). Its
toxic effects include peroxidation of membrane lipids and nitrative
inactivation of enzymes, such as prostacyclin synthase and superoxide
dismutase. ONOO- inhibits tyrosine phosphorylation and,
consequently, affects signal transduction pathways. With the ability to induce
single- and/or double-strand DNA breaks, peroxynitrite plays a critical role
in reperfusion injury.
Efforts have been made in the laboratory setting to reduce reperfusion
injury, but no clinical cardioprotective strategy exists. Scavengers or
inhibitors of NO (Li et al.,
2006) or 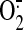 (Hoffman et al., 2003) can
reduce peroxynitrite production and subsequent cardiac injury, but both NO and
(Hoffman et al., 2003) can
reduce peroxynitrite production and subsequent cardiac injury, but both NO and
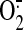 have important
physiological roles (Hampton et al.,
1998). Therefore, an agent directly inhibitive of ONOO-
may be more biologically relevant and advantageous.
have important
physiological roles (Hampton et al.,
1998). Therefore, an agent directly inhibitive of ONOO-
may be more biologically relevant and advantageous.
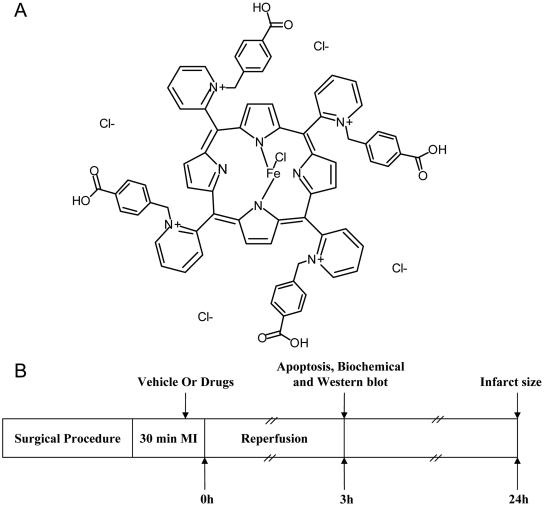
INO-4885 is one of the recently synthesized pyridyl-substituted porphyrin compounds (Fig. 1A). It is a potent peroxynitrite decomposition catalyst with a rate constant of ∼107 M/s in the degradation of peroxynitrite to benign species. Although its in vitro biochemical properties have been well characterized (Williams and Southan, 2008), whether this compound may attenuate in vivo tissue injury caused by peroxynitrite overproduction has not been investigated previously. In the current study, we determined the cardioprotective effects of INO-4885 and explored the mechanisms involved. In addition, we compared the cardioprotective efficacy of INO-4885 with other agents known to block ONOO- overproduction through different mechanisms.
Fig. 1.
A, chemical structure of INO-4885. B, experimental protocol. Male adult mice were anesthetized with 2% isoflurane. MI was produced by temporarily exteriorizing the heart via a left thoracic incision and placing a 6-0 silk suture slipknot around the left anterior descending coronary artery. After 30 min of MI, the slipknot was released, and the myocardium was reperfused for 3 or 24 h (for infarct size assessment only). Ten minutes before reperfusion, mice were randomized to receive vehicle, INO-4885, INO-C, Tiron, apocynin, and 1400W by intraperitoneal injection.
Materials and Methods
Experimental Protocols. Male adult mice were anesthetized with 2% isoflurane. Myocardial ischemia (MI) was produced by temporarily exteriorizing the heart via a left thoracic incision and placing a 6-0 silk suture slipknot around the left anterior descending coronary artery. After 30 min of MI, the slipknot was released, and the myocardium was reperfused for 3 (for apoptosis assay) or 24 (for infarct size assessment) h. Ten minutes before reperfusion, mice were randomized to receive vehicle (saline, n = 12), INO-4885 (3, 10, 30, 100, 300 μg/kg dose-response study, n = 6-8; 30 μg/kg for other assays, n = 12), INO-C (agent identical to INO-4885 sans active site, 300 μg/kg, n = 12), Tiron (superoxide scavenger, 500 mg/kg, n = 11), apocynin (NADPH oxidase inhibitor, 5 mg/kg, n = 11), and 1400W (iNOS inhibitor, 5 mg/kg, n = 11) by intraperitoneal injection. Sham operation control mice (Sham MI/R) underwent the same surgical procedures, except that the suture placed under the left coronary artery was not tied (n = 8). At the conclusion of reperfusion period, the left anterior ascending ligature was retied, and 2% Evans blue dye was injected into the left ventricular cavity. Circulated dye was uniformly distributed except in the heart region supplied by the occluded coronary artery [i.e., area at risk (AAR)]. The heart was quickly excised, and the ischemic/reperfused tissue (Evans blue negative area) was isolated and processed according to the procedures described below (Fig. 1). The experiments were performed with adherence to National Institutes of Health Guidelines on the Use of Laboratory Animals and were approved by the Thomas Jefferson University Committee on Animal Care.
Quantitative Determination of Myocardial Apoptosis by Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling. Hearts were perfused with 0.9% NaCl for 5 min and 4% paraformaldehyde in PBS, pH 7.4, for 20 min. Four longitudinal sections from ischemic regions were cut and further fixed in 4% paraformaldehyde in PBS for 24 h at room temperature. Fixed tissues were embedded in a paraffin block, and two slides of 4- to 5-μm thickness were cut from each tissue block. Immunohistochemical procedures for apoptosis assessment were performed using a detection kit (Roche Diagnostics, Indianapolis, IN) as described previously (Tao et al., 2004). After rinsing with PBS, slides were coverslipped with mounting medium containing 4,6-diamidino-2-phenylindole (DAPI) to permit total nuclei counting. The tissue slides (eight slides per heart) were digitally photographed with a QICAM-Fast Digital Camera using a 20× objective lens mounted atop an Olympus BX51 Fluorescence Microscope (Olympus, Tokyo, Japan. Total nuclei (DAPI staining) and the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-positive nuclei were counted by IP Lab Imagine Analysis Software (version 3.5; BD Biosciences Bioimaging, Rockville, MD) utilizing custom script (BioVision, Mountain View, CA). The index of apoptosis (number of TUNEL positively stained myocytes/total number of myocytes × 100) was automatically calculated and exported to Microsoft Excel (Microsoft, Redmond, WA) for further analysis. Eight slides per heart were observed, and the results were averaged as n = 1. Assays were performed in a blinded manner.
Quantitative Determination of Myocardial Apoptosis by Caspase-3 Activation. Cardiac caspase-3 activity was performed by using caspase colorimetric assay kits (Millipore Bioscience Research Reagents, Temecula, CA) as described in our previous study (Gao et al., 2004). In brief, ischemic/reperfused myocardial tissue was homogenized in ice-cold lysis buffer for 30 s using a PRO 200 homogenizer. The homogenates were centrifuged for 5 min at 10,000g at 4°C. Supernatants were collected, and protein concentrations were measured by the bicinchoninic acid method (Pierce Chemical, Rockford, IL). Two hundred micrograms of supernatant protein was loaded to each well of a 96-well plate and incubated with 25 μg of N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide at 37°C for 1.5 h. pNA was cleaved from Asp-Glu-Val-Asp by active caspase 3, and free pNA was quantified using a SpectraMax-Plus microplate spectrophotometer (Molecular Devices, Sunnyvale, CA) at 405 nm. The results were expressed as nanomoles of pNA per hour per milligram of protein.
Quantitative Determination of Myocardial Infarct Size. At the conclusion of the 24-h reperfusion period, the LAD ligature was retied, and 2% Evans blue dye was injected into the left ventricular cavity. The heart was quickly excised, frozen at -20°C, and sectioned in 1-mm planes perpendicular to the long axis of the heart. Slices were incubated individually using a 24-well culture plate in 1% TTC in phosphate buffer at pH 7.4, 37°C for 10 min and photographed with a digital camera. The Evans blue-stained area (area not at risk), the TTC-stained area (red color, ischemic but viable tissue), and the TTC stain-negative area (white color, infarcted tissue) were digitally measured using an IP Lab Imagine Analysis Software (version 3.6; Scanalytics) utilizing custom-made script (BioVision). The myocardial infarct size was expressed as a percentage of infarct area over AAR.
Determination of Total NOx Content in Cardiac Tissue. Cardiac tissue samples from AAR were rinsed, homogenized in deionized water [1:10 (w/v)], and centrifuged at 14,000g for 10 min. The tissue NO and its in vivo metabolic products (NO2 and NO3), collectively known as NOx, were determined using a chemiluminescence NO detector (SIEVER 280i NO Analyzer), as described in our previous study (Gao et al., 2002).
Quantitation of Tissue Nitrotyrosine Content. Nitrotyrosine content in the I/R cardiac tissue, a footprint of in vivo ONOO- formation and a reliable index for nitrative stress (Aulak et al., 2004), was determined using an enzyme-linked immunosorbent assay method described in our previous publication (Ma et al., 2001). Results are presented as nitrotyrosine nanomoles per milligram of protein.
Quantification of Superoxide Production. Superoxide production in I/R heart tissue was measured by lucigenin-enhanced chemiluminescence as described previously (Lund et al., 2000). Superoxide production was expressed as relative light units per second per milligram of heart weight.
Immunoblotting. Tissue homogenate protein was separated on SDS-polyacrylamide electrophoresis gels, transferred to nitrocellulose membranes, and Western blotted with polyclonal antibody against iNOS (Millipore, Billerica, MA) and monoclonal antibody against gp91phox, a major component of NADPH oxidase (Transduction Laboratories, Lexington, KY). Nitrocellulose membranes were then incubated with horseradish peroxide-conjugated anti-rabbit or anti-mouse IgG antibody (1:2000; Cell Signaling Technology Inc., Danvers, MA) respectively for 1 h. Blots were developed with a Supersignal chemiluminescence detection kit (Pierce Chemical). The immunoblotting was visualized with a Kodak Image Station 400, and the blot densities were analyzed with Kodak 1D software (Eastman Kodak, Rochester, NY).
Immunohistochemistry. Formalin-fixed sections were routinely rehydrated and treated with unmasking solution (H3300; Vector Laboratories, Burlingame, CA). Sections were blocked by 5% normal horse serum for 30 min and then placed in levamisole solution for 30 min to neutralize endogenous alkaline phosphatase activity. Sections were incubated with primary antibody against nitrotyrosine (Cell Signaling Technology Inc.) at 4°C overnight in a humidified chamber followed by incubation with the secondary antibody (1% goat biotinylated anti-rabbit IgG-biotin). The sections were incubated with Vector red substrate solution (Vector Red Substrate Kit; Vector Laboratories) for 10 min at room temperature for red color development.
Statistical Analysis. All text and figure values are presented as means ± SE. All data were subjected to analysis of variance, followed by Bonferroni correction for post hoc Student's t test. Probabilities of 0.05 or less were considered to be statistically significant.
Results
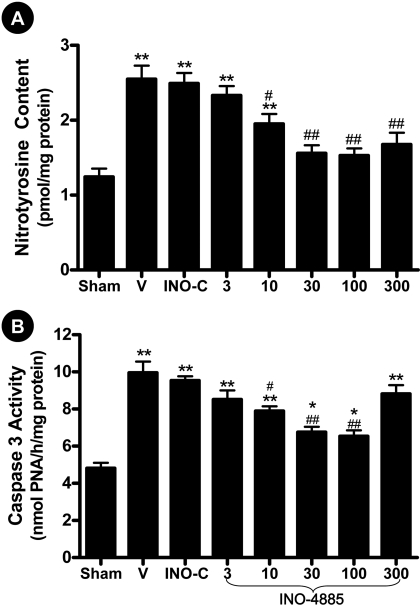
Dose-Dependent Effect of INO-4885 on Myocardial Nitrotyrosine Content and Caspase 3 Activity. To identify the optimal dose regimen of INO-4885 treatment, mice were subjected to 30 min of ischemia, followed by 3 h of reperfusion and treated with 3, 10, 30, 100, or 300 μg/kg INO-4885 10 min before reperfusion. The cardiac nitrotyrosine content and caspase 3 activity observed in INO-4885-treated mice (of varying doses) were compared with those animals treated with vehicle (0.9% NaCl) or INO-C, an agent containing all the structural components of INO-4885 sans active metal center. As illustrated in Fig. 2A, treatment with INO-4885 reduced reperfusion-induced nitrotyrosine production in a dose-dependent fashion; the minimal effective dose was 10 μg/kg, and the maximal protective dose was 100 μg/kg. Likewise, treatment with INO-4885 reduced caspase-3 activity in a dose-dependent manner in the range of 3 to 100 μg/kg. However, at the INO-4885 dose of 300 μg/kg, caspase 3 activity increased and no longer conformed to a dose-dependent trend (Fig. 2B). This result suggests that a nonspecific effect occurs at a high INO-4885 dose. Because there was no significant difference in nitrotyrosine content and caspase 3 activity between the animals treated with 30 and 100 μg/kg INO-4885, the 30 μg/kg INO-4885 dosing regimen was considered the optimal dose and used for the remainder of experiments.
Fig. 2.
Dose-dependent effect of INO-4885 on cardiac nitrotyrosine content (A) and caspase-3 activity (B). Mice were subjected to sham MI/R (Sham) or MI/R treated with vehicle (V), inactive control compound (INO-C), or INO-4885 at the dosage indicated. *, p < 0.05; **, p < 0.01 versus Sham group; #, p < 0.05; ##, p < 0.01 versus vehicle group.
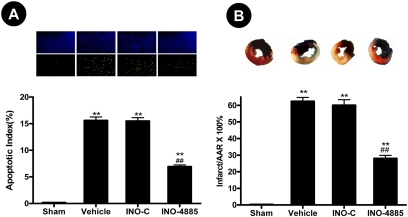
INO-4885 Treatment Reduced Cardiomyocyte Apoptosis and Decreased Infarct Size. Thirty minutes of left anterior descending coronary artery occlusion followed by 3 h of reperfusion resulted in significant myocardial apoptotic death manifested by a 2.1-fold increase in caspase 3 activity (Fig. 2B) and a 15-fold increase in TUNEL-positive cell labeling (Fig. 3A). Administration of INO-4885 (dose, 30 μg/kg) significantly decreased myocardial apoptosis, as evidenced by decreased caspase 3 activity (6.75 ± 0.29 versus 9.96 ± 0.6 nmol pNA/h/mg protein, p < 0.01) and reduction in TUNEL-positive cells (6.95 ± 0.31% versus 15.6 ± 0.63%, p < 0.01, Fig. 3A). In contrast, administration of an inactive compound with identical chemical structure lacking catalytic metal center neither reduced caspase 3 activity (9.54 ± 0.22 nmol pNA/h/mg protein) nor decreased TUNEL-positive labeling (15.5 ± 0.7%) (Figs. 2 and 3).
Fig. 3.
A, treatment with INO-4885 (30 μg/kg) decreased myocardial apoptosis determined at 3 h after reperfusion. Inserts, representative photomicrographs of TUNEL labeling. Blue (DAPI) dots, total nuclei; green dots, TUNEL-positive nuclei. The index of apoptosis (number of positively stained myocytes/total number of myocytes × 100) was calculated and presented as bar graph. B, treatment with INO-4885 decreased myocardial infarct size determined at 24 h after reperfusion. Inserts, representative pictures for infarct size determination. Blue area, area not subjected to MI/R; red area, ischemia/reperfused but viable; blue/red negative area, infarct area. **, p < 0.01 versus Sham group; ##, p < 0.01 versus vehicle group.
Myocardial infarction represents the total myocardial injury caused by necrosis and apoptosis. Although data presented in Figs. 2 and 3 clearly demonstrate INO-4885 treatment reduced cardiomyocyte apoptosis 3 h after reperfusion, it remains to be determined whether this treatment merely delayed the cell death process or possibly converted cellular apoptosis to necrosis. To directly address this issue, an additional group of animals was subjected to 30 min of myocardial ischemia followed by 24-h reperfusion. The effect of INO-4885 on myocardial infarct size was determined. As summarized in Fig. 3B, treatment with INO-4885 (dose, 30 μg/kg) significantly reduced myocardial infarct size (28 ± 1.8 versus 62 ± 2.3%, p < 0.01). In contrast, administration of INO-C had no significant effect on myocardial infarct size (60 ± 3.3%). Taken together, these results provided firm evidence that INO-4885 is a cardioprotective molecule that reduces myocardial reperfusion injury when administered shortly before reperfusion.
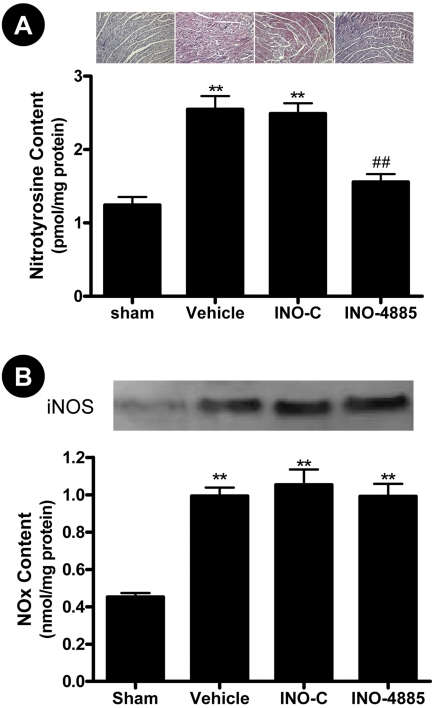
INO-4885 Treatment Markedly Reduced Nitrotyrosine Content in I/R Hearts. Peroxynitrite generation has been implicated in a variety of pathology such as reperfusion injury, heart failure, and diabetes. Because of its short half-life, the direct measurement of peroxynitrite is extremely difficult; its nitrative product nitrotyrosine is now widely accepted as a biomarker for peroxynitrite production. We employed two methods in this study to detect nitrotyrosine production (enzyme-linked immunosorbent assay and immunohistochemistry). I/R significantly increased nitrotyrosine content compared with sham-operated animals (2.55 ± 0.18 versus 1.24 ± 0.11 pmol/mg protein, p < 0.01). Treatment with INO-4885 resulted in a significant reduction of nitrotyrosine compared with vehicle (1.56 ± 0.11, p < 0.01 versus vehicle), whereas treatment with control compound (INO-C) had no effect (2.49 ± 0.14) (Fig. 4A). Cardiomyocytes treated with vehicle or INO-C displayed strong positive staining in the immunohistochemical assay, indicating most peroxynitrite-induced protein nitration occurs within cardiomyocytes. INO-4885 treatment attenuates the protein nitration, evidenced by much weaker nitrotyrosine staining (Fig. 4A).
Fig. 4.
INO-4885 treatment reduced nitrotyrosine production (A) determined by immunohistochemistry and enzyme-linked immunosorbent assay but had no effect on iNOS expression and NO production (B). **, p < 0.01 versus Sham group; ##, p < 0.01 versus vehicle group.
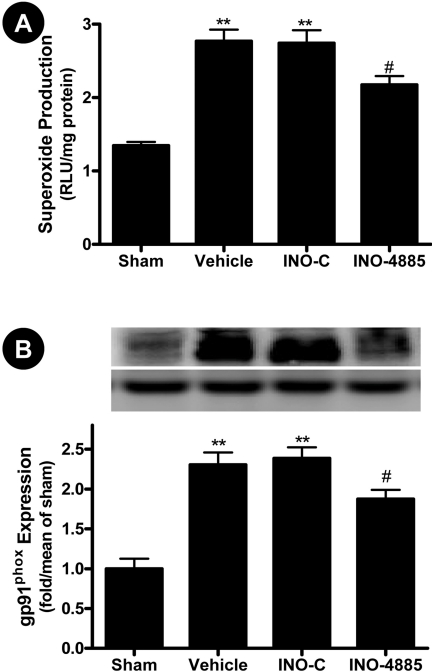
INO-4885 Had No Effect on NO Overproduction But Reduced Superoxide
Production and Inhibited gp91phox Expression. Peroxynitrite is
formed by combination of NO and
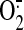 at a 1:1 ratio. To
ascertain whether reduced protein nitration observed in INO-treated animals
was because of the catalytic removal of ONOO-, total cardiac NO and
superoxide productions were determined. Consistent with previously published
results, MI/R caused iNOS expression and increased NO production
(Fig. 4B). As expected,
treatment with INO-4885 or its inactive control failed to inhibit MI/R-induced
iNOS expression and NO overproduction (Fig.
4B), indicating that the protective effects of INO-4885 are not
via regulation of the NO production pathway. However, to our surprise,
treatment with INO-4885, but not its inactive control, modestly reduced
superoxide overproduction in the ischemic/reperfused cardiac tissue
(Fig. 5A). Although INO-4885
has weak superoxide-scavenging ability, the reaction of superoxide with
INO-4885 is much slower than its reaction with NO (>100-fold). Therefore,
reduced superoxide content in the INO-4885-treated animals (30 μg/kg)
cannot be attributed to the superoxide-scavenging ability of INO-4885.
Considerable evidence exists that NADPH oxidase is the primary source for
superoxide production in the ischemic/reperfused heart. To further determine
the potential mechanism that might be responsible for reduced superoxide
content in the INO-4885-treated hearts, cardiac expression of
gp91phox (the essential component of NADPH oxidase) was determined.
As illustrated in Fig. 5B, MI/R
caused greater than 2-fold increase in gp91phox expression, which
was modestly, but significantly, inhibited by treatment with INO-4885
(p < 0.05). In contrast, treatment with the inactive control had
no effect on gp91phox expression.
at a 1:1 ratio. To
ascertain whether reduced protein nitration observed in INO-treated animals
was because of the catalytic removal of ONOO-, total cardiac NO and
superoxide productions were determined. Consistent with previously published
results, MI/R caused iNOS expression and increased NO production
(Fig. 4B). As expected,
treatment with INO-4885 or its inactive control failed to inhibit MI/R-induced
iNOS expression and NO overproduction (Fig.
4B), indicating that the protective effects of INO-4885 are not
via regulation of the NO production pathway. However, to our surprise,
treatment with INO-4885, but not its inactive control, modestly reduced
superoxide overproduction in the ischemic/reperfused cardiac tissue
(Fig. 5A). Although INO-4885
has weak superoxide-scavenging ability, the reaction of superoxide with
INO-4885 is much slower than its reaction with NO (>100-fold). Therefore,
reduced superoxide content in the INO-4885-treated animals (30 μg/kg)
cannot be attributed to the superoxide-scavenging ability of INO-4885.
Considerable evidence exists that NADPH oxidase is the primary source for
superoxide production in the ischemic/reperfused heart. To further determine
the potential mechanism that might be responsible for reduced superoxide
content in the INO-4885-treated hearts, cardiac expression of
gp91phox (the essential component of NADPH oxidase) was determined.
As illustrated in Fig. 5B, MI/R
caused greater than 2-fold increase in gp91phox expression, which
was modestly, but significantly, inhibited by treatment with INO-4885
(p < 0.05). In contrast, treatment with the inactive control had
no effect on gp91phox expression.
Fig. 5.
INO-4885 treatment decreased superoxide production (A) and inhibited NADPH oxidase expression (B). **, p < 0.01 versus Sham group; #, p < 0.05 versus vehicle group.
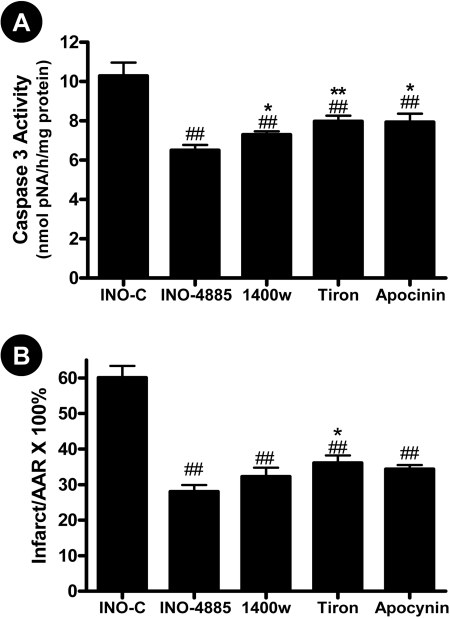
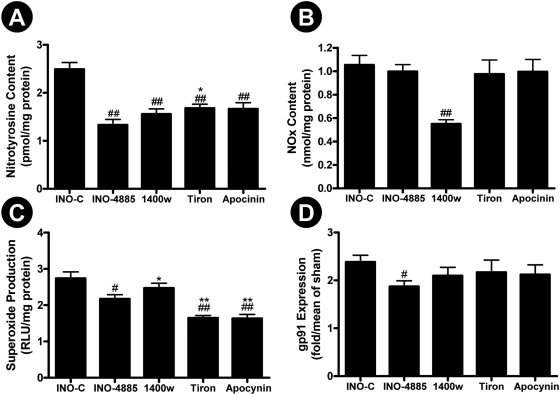
Comparison of the Cardioprotective Effect of INO-4885 with iNOS Inhibitor, NADPH Oxidase Inhibitor, and Superoxide Scavenger. The data presented above indicate that INO-4885 is a cardioprotective molecule that may attenuate peroxynitrite-induced tissue injury via multiple mechanisms, suggesting that INO-4885 may offer more cardioprotection than single-action compounds. To directly explore this possibility, an additional study was performed in which the cardioprotective effect of INO-4885 was compared with an iNOS inhibitor, 1400W, an NADPH oxidase inhibitor, apocynin, and a superoxide scavenger, Tiron. Consistent with previously published results by other investigators and our own laboratory, we demonstrate these agents are capable of providing protection against reperfusion injury, evidenced by decreased caspase-3 activity and reduced infarct size (Fig. 6). However, INO-4885 provided the best protection among all four compounds studied. Moreover, although blocking NO overproduction by 1400W (Fig. 7B) or reducing superoxide overproduction by Tiron or apocynin (Fig. 7C) were effective in reducing nitrotyrosine formation (Fig. 7A), INO-4885 was the only agent that reduced gp91phox expression (Fig. 7D) and reduced nitrotyrosine to a level comparable with sham-operated animals (no statistical difference, Fig. 7A).
Fig. 6.
Comparison of the effect of INO-4885, 1400W, apocynin and Tiron on caspase 3 activation (A) and infarct size (B). ##, p < 0.01 versus INO-C group; *, p < 0.05; **, p < 0.01 versus INO-4885 group.
Fig. 7.
Comparison of the effect of INO-4885, 1400W, apocynin and Tiron on nitrotyrosine formation (A), NOx content (B), superoxide production (C), and gp91phox expression (D). #, p < 0.05; ##, p < 0.01 versus INO-C group; *, p < 0.05 versus INO-4885 group.
Discussion
Reperfusion or restoration of blood supply is the sole modality to save ischemic tissue from more permanent damage. However, growing evidence from clinical observation and animal studies indicates reperfusion itself causes injury to tissue, via distribution of toxic cytokines and free radicals. Though the specific mechanisms of reperfusion injury remain unclarified, oxidant stress, polymorphonuclear neutrophil infiltration, and calcium overload have been implicated in the process. Among these, oxidant stress is critically important and can directly induce membrane lipid, protein, and DNA oxidation. Furthermore, oxidant stress can trigger polymorphonuclear neutrophil activation and aggravate calcium overload by increasing cellular membrane permeability.
Under physiological conditions, peroxynitrite production is very low, and
its oxidative damage is minimized by endogenous antioxidant defenses. However,
even modest increases of the NO and
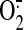 will greatly stimulate
peroxynitrite genesis; a 10-fold increase in NO and
will greatly stimulate
peroxynitrite genesis; a 10-fold increase in NO and
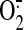 will increase
peroxynitrite formation 100-fold (Radi et
al., 2002). Excessive peroxynitrite production can induce lipid
peroxidation, protein nitration, and DNA oxidation and breakage
(Szabó and Bahrle,
2005; Pacher et al.,
2007), ultimately leading to dysfunction of critical cellular
processes, disruption of cell signaling pathways, and induction of cell death
through both necrosis and apoptosis. As such, peroxynitrite overproduction has
been implicated in the pathogenesis of many diseases including myocardial
infarction, diabetes, organ rejection, inflammatory disorders, and Parkinson's
disease. Thus, considerable effort has been given in the search for the
optimal therapeutic intervention capable of reduction of peroxynitrite
production or acceleration of its decomposition.
will increase
peroxynitrite formation 100-fold (Radi et
al., 2002). Excessive peroxynitrite production can induce lipid
peroxidation, protein nitration, and DNA oxidation and breakage
(Szabó and Bahrle,
2005; Pacher et al.,
2007), ultimately leading to dysfunction of critical cellular
processes, disruption of cell signaling pathways, and induction of cell death
through both necrosis and apoptosis. As such, peroxynitrite overproduction has
been implicated in the pathogenesis of many diseases including myocardial
infarction, diabetes, organ rejection, inflammatory disorders, and Parkinson's
disease. Thus, considerable effort has been given in the search for the
optimal therapeutic intervention capable of reduction of peroxynitrite
production or acceleration of its decomposition.
Peroxynitrite is formed by NO and superoxide reaction at 1:1 ratio. Therefore, interventions blocking either NO or superoxide overproduction may reduce peroxynitrite formation and attenuate tissue injury. Our current study demonstrated that administration of 1400W had no effect on superoxide production but significantly reduced NO production, reduced nitrotyrosine formation, and reduced myocardial ischemia/reperfusion injury. In contrast, administration of apocynin or Tiron had no effect on NO production but significantly reduced superoxide production, decreased nitrotyrosine formation, and reduced myocardial ischemia/reperfusion injury. These results underline the efficacy of blocking one of the arms of peroxynitrite formation for reducing peroxynitrite formation and protecting against ischemic/reperfusion injury. These results are consistent with previously published data by other investigators.
NO reacts with superoxide at a near diffusion-limiting rate. A superoxide scavenger that can override this reaction and completely block peroxynitrite formation is currently unavailable. On the other hand, NO plays multiple physiologic regulatory roles. Complete blockade of NO production for the purpose of peroxynitrite formation prevention may potentially cause significant adverse effects outweighing the advantages of inhibited peroxynitrite genesis. Although intracellular molecules, such as ascorbate and glutathione, can react with and detoxify peroxynitrite, their reaction rates are too slow to be effective in blocking peroxynitrite-induced tissue injury (Groves, 1995; Lee, 1997). The impetus has been great in recent years to identify compounds capable of reacting with peroxynitrite with the speed necessary to remove it from the environment before its reaction with cellular components. Pharmachemical research demonstrated that synthetic metalloporphyrins react very efficiently with peroxynitrite (Shimanovich and Groves, 2001), and compounds in this class have been investigated as peroxynitrite decomposition catalysis (Groves, 1999). Several agents of this class, such as FeTMPS (5,10,15,20-tetrakis(2,4,6-trimethyl-disulfonatophenyl)porphyrinato iron III) and FP-15 (Mabley et al., 2002), have been reported to remove peroxynitrite and reduce its injurious effects (Cuzzocrea et al., 2000; Mabley et al., 2002; Chirino et al., 2004; Lancel et al., 2004; Thiyagarajan et al., 2004). However, the reaction rate between these molecules and peroxynitrite is approximately 105 M/s, and relatively high dosages are required to achieve significant cellular protection.
INO-4885 is a second generation peroxynitrite decomposition catalyst, with a peroxynitrite degradation rate 5 × 107 M/s. This is at least 10 times faster than FP-15, the most extensively investigated first generation peroxynitrite decomposition catalyst (Lymar and Hurst, 1996). Its catalytic nature allows INO-4885 to be regenerated and to be potently active at very low therapeutic doses. Our current study provided the first direct evidence that INO-4885 can significantly reduce cardiomyocyte apoptosis and myocardial infarct size in an acute mouse ischemia/reperfusion model. We observed a dose-dependent relationship of the effects of INO-4885, with incremental increase of doses 3 to 100 μg/kg, yielding increasingly reduced cellular apoptosis and decreased caspase-3 activity. The optimal cardioprotective dosage of INO-4885 identified from the current study is 30 μg/kg, a dose 10 to 50× lower than the protective dose reported for first generation peroxynitrite decomposition catalysts (3-10 mg/kg) (Cuzzocrea et al., 2000; Mabley et al., 2002; Chirino et al., 2004; Lancel et al., 2004; Thiyagarajan et al., 2004).
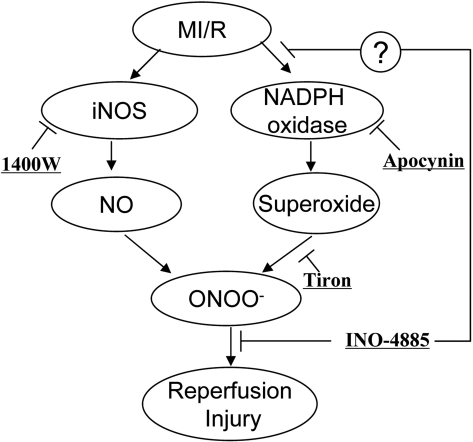
It must be indicated that although peroxynitrite formation and its resultant tissue injury can be blocked at multiple target sites (Fig. 8), our current study provided clear evidence that INO-4885 provided the best protection at dosage 10 to 1000 times lower than other drugs administered. Compounds 1400W, Tiron, and apocynin were used in this study at dosages previously reported as optimal by others and our laboratory. Therefore, a likely explanation for its superior cardioprotective action is that this compound not only functions as a peroxynitrite decomposition catalysts (thus facilitating peroxynitrite detoxification) but also has the ability to inhibit NADPH oxidase expression and superoxide production (thus blocking peroxynitrite formation). However, molecular mechanisms responsible for the inhibitory effect of INO-4885 on ischemia/reperfusion-induced NADPH oxidase overexpression remain unclear and will be investigated in our future studies. It should also be indicated that because of its multiple actions, this compound may not be the best choice if the purpose of a research is to determine peroxynitrite decomposition and tissue protection. In addition, our results demonstrated that although Tiron and apocynin are both more effective than INO-4885 in inhibiting superoxide production (Fig. 7C), INO-4885 is more effective in reducing protein nitration (Fig. 7A) and provided the greatest cardioprotection (Fig. 6). This additional evidence buttresses the notion that peroxynitrite, not superoxide or nitric oxide, is responsible for myocardial ischemia/reperfusion injury.
Fig. 8.
Schematic illustration of peroxynitrite-induced tissue injury in ischemic/reperfused heart and therapeutic interventions investigated in the current study.
In summary, we have demonstrated that INO-4885, a new peroxynitrite decomposition catalyst, provides significant cardioprotection against myocardial ischemia/reperfusion injury. Because peroxynitrite is now implicated in an ever-increasing list of diseases such as diabetes, hyperlipidemia, organ ischemia, Alzheimer's disease (Castegna et al., 2003), and methamphetamine-induced neurotoxicity (Imam et al., 2000), we have only begun to realize the exciting potential utility of peroxynitrite decomposition catalyst in a broad array of clinically relevant oxidant-induced pathology.
Acknowledgments
We thank Drs. William K. McVicar, Kanneganti Murthy, and Lewis Neville from Inotek for providing INO-4885 and INO-C for the study.
This work was supported by the American Diabetes Association [Grants 7-06-JF59 and 7-08-RA-98]; the National Institutes of Health [Grant 2R01HL-63828]; and the American Heart Association [Grant GIA0855554D].
doi:10.1124/jpet.108.144352.
ABBREVIATIONS: 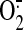 ,
superoxide; TTC, triphenyltetrazolium chloride; ONOO-,
peroxynitrite; NO, nitric oxide; I/R, ischemia/reperfusion; ONOO-,
peroxynitrite; INO-4885,
5,10,15,20-tetra[N-(benzyl-4′-carboxylate)-2-pyridinium]-21H,23H-porphine
iron(III) chloride; MI, myocardial ischemia; INO-C, INO-4885 without catalytic
moiety; iNOS, inducible nitric-oxide synthase; MI/R, myocardial
ischemia/reperfusion; AAR, area at risk; PBS, phosphate-buffered saline; DAPI,
4,6-diamidino-2-phenylindole; TUNEL, terminal deoxynucleotidyl transferase
dUTP nick-end labeling; pNA, p-nitroanilide; 1400W,
N-(3-(aminomethyl)benzyl)acetamidine.
,
superoxide; TTC, triphenyltetrazolium chloride; ONOO-,
peroxynitrite; NO, nitric oxide; I/R, ischemia/reperfusion; ONOO-,
peroxynitrite; INO-4885,
5,10,15,20-tetra[N-(benzyl-4′-carboxylate)-2-pyridinium]-21H,23H-porphine
iron(III) chloride; MI, myocardial ischemia; INO-C, INO-4885 without catalytic
moiety; iNOS, inducible nitric-oxide synthase; MI/R, myocardial
ischemia/reperfusion; AAR, area at risk; PBS, phosphate-buffered saline; DAPI,
4,6-diamidino-2-phenylindole; TUNEL, terminal deoxynucleotidyl transferase
dUTP nick-end labeling; pNA, p-nitroanilide; 1400W,
N-(3-(aminomethyl)benzyl)acetamidine.
References
- Aulak KS, Koeck T, Crabb JW, and Stuehr DJ (2004) Dynamics of protein nitration in cells and mitochondria. Am J Physiol Heart Circ Physiol 286 H30-H38. [DOI] [PubMed] [Google Scholar]
- Beckman JS and Koppenol WH (1996) Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271 C1424-C1437. [DOI] [PubMed] [Google Scholar]
- Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, and Butterfield DA (2003) Proteomic identification of nitrated proteins in Alzheimer's disease brain. J Neurochem 85 1394-1401. [DOI] [PubMed] [Google Scholar]
- Chirino YI, Hernández-Pando R, and Pedraza-Chaverrí J (2004) Peroxynitrite decomposition catalyst ameliorates renal damage and protein nitration in cisplatin-induced nephrotoxicity in rats. BMC Pharmacol 4 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Misko TP, Costantino G, Mazzon E, Micali A, Caputi AP, Macarthur H, and Salvemini D (2000) Beneficial effects of peroxynitrite decomposition catalyst in a rat model of splanchnic artery occlusion and reperfusion. FASEB J 14 1061-1072. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P (2006) Peroxynitrite: just an oxidative/nitrosative stressor or a physiological regulator as well? Br J Pharmacol 148 1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Gao E, Yue TL, Ohlstein EH, Lopez BL, Christopher TA, and Ma XL (2002) Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation 105 1497-1502. [DOI] [PubMed] [Google Scholar]
- Gao F, Tao L, Yan W, Gao E, Liu HR, Lopez BL, Christopher TA, and Ma XL (2004) Early anti-apoptosis treatment reduces myocardial infarct size after a prolonged reperfusion. Apoptosis 9 553-559. [DOI] [PubMed] [Google Scholar]
- Groves JT (1999) Peroxynitrite: reactive, invasive and enigmatic. Curr Opin Chem Biol 3 226-235. [DOI] [PubMed] [Google Scholar]
- Groves JT and Marla SS (1995) Peroxynitrite-induced DNA strand scission mediated by a manganese porphyrin. J Am Chem Soc 117 9578. [Google Scholar]
- Hampton MB, Kettle AJ, and Winterbourn CC (1998) Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92 3007-3017. [PubMed] [Google Scholar]
- Hoffman A, Goldstein S, Samuni A, Borman JB, and Schwalb H (2003) Effect of nitric oxide and nitroxide SOD-mimic on the recovery of isolated rat heart following ischemia and reperfusion. Biochem Pharmacol 66 1279-1286. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Islam F, Itzhak Y, Slikker W Jr, and Ali SF (2000) Prevention of dopaminergic neurotoxicity by targeting nitric oxide and peroxynitrite: implications for the prevention of methamphetamine-induced neurotoxic damage. Ann N Y Acad Sci 914 157-171. [DOI] [PubMed] [Google Scholar]
- Lancel S, Tissier S, Mordon S, Marechal X, Depontieu F, Scherpereel A, Chopin C, and Neviere R (2004) Peroxynitrite decomposition catalysts prevent myocardial dysfunction and inflammation in endotoxemic rats. J Am Coll Cardiol 43 2348-2358. [DOI] [PubMed] [Google Scholar]
- Lee J, Hunt JA, and Groves JT (1997) Rapid decomposition of peroxynitrite by manganese porphyrin-antioxidant redox-couples. Bioorg Med Chem Lett 7 2913-2918. [Google Scholar]
- Li D, Qu Y, Tao L, Liu H, Hu A, Gao F, Sharifi-Azad S, Grunwald Z, Ma XL, and Sun JZ (2006) Inhibition of iNOS protects the aging heart against beta-adrenergic receptor stimulation-induced cardiac dysfunction and myocardial ischemic injury. J Surg Res 131 64-72. [DOI] [PubMed] [Google Scholar]
- Lund DD, Faraci FM, Miller FJ Jr, and Heistad DD (2000) Gene transfer of endothelial nitric oxide synthase improves relaxation of carotid arteries from diabetic rabbits. Circulation 101 1027-1033. [DOI] [PubMed] [Google Scholar]
- Lymar SV and Hurst JK (1996) Carbon dioxide: physiological catalyst for peroxynitrite-mediated cellular damage or cellular protectant? Chem Res Toxicol 9 845-850. [DOI] [PubMed] [Google Scholar]
- Ma XL, Gao F, Nelson AH, Lopez BL, Christopher TA, Yue TL, and Barone FC (2001) Oxidative inactivation of nitric oxide and endothelial dysfunction in stroke-prone spontaneous hypertensive rats. J Pharmacol Exp Ther 298 879-885. [PubMed] [Google Scholar]
- Mabley JG, Liaudet L, Pacher P, Southan GJ, Groves JT, Salzman AL, and SzabóC (2002) Part II: beneficial effects of the peroxynitrite decomposition catalyst FP15 in murine models of arthritis and colitis. Mol Med 8 581-590. [PMC free article] [PubMed] [Google Scholar]
- Marla SS, Lee J, and Groves JT (1997) Peroxynitrite rapidly permeates phospholipid membranes. Proc Natl Acad Sci U S A 94 14243-14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, and Liaudet L (2007) Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87 315-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R, Cassina A, Hodara R, Quijano C, and Castro L (2002) Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med 33 1451-1464. [DOI] [PubMed] [Google Scholar]
- Shimanovich R and Groves JT (2001) Mechanisms of peroxynitrite decomposition catalyzed by FeTMPS, a bioactive sulfonated iron porphyrin. Arch Biochem Biophys 387 307-317. [DOI] [PubMed] [Google Scholar]
- Szabó G and Bährle S (2005) Role of nitrosative stress and poly(ADP-ribose) polymerase activation in myocardial reperfusion injury. Curr Vasc Pharmacol 3 215-220. [DOI] [PubMed] [Google Scholar]
- Tao L, Gao E, Bryan NS, Qu Y, Liu HR, Hu A, Christopher TA, Lopez BL, Yodoi J, Koch WJ, et al. (2004) Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation. Proc Natl Acad Sci U S A 101 11471-11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiyagarajan M, Kaul CL, and Sharma SS (2004) Neuroprotective efficacy and therapeutic time window of peroxynitrite decomposition catalysts in focal cerebral ischemia in rats. Br J Pharmacol 142 899-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W and Southan G (2008) inventor; Inotek Pharmaceutical Corporation, assignee. Pyridyl-substituted porphyrin compounds and method of use. U.S. patent US7,432,369B2. 2008 October 7.