Abstract
Elderly adults often exhibit performance deficits during goal-directed movements of the dominant arm compared with young adults. Recent studies involving hemispheric lateralization have provided evidence that the dominant and non-dominant hemisphere-arm systems are specialized for controlling different movement parameters and that hemispheric specialization may be reduced during normal aging. The purpose was to examine age-related differences in the movement structure for the dominant (right) and non-dominant (left) during goal-directed movements. Young and elderly adults performed 72 aiming movements as fast and as accurately as possible to visual targets with both arms. The findings suggest that previous research utilizing the dominant arm can be generalized to the non-dominant arm because performance was similar for the two arms. However, as expected, the elderly adults showed shorter relative primary submovement lengths and longer relative primary submovement durations, reaction times, movement durations, and normalized jerk scores compared to the young adults.
Keywords: Aging, Feedback, Hand, Laterality, Submovement
1. Introduction
Goal-directed aiming movements to visual targets consist of an initial impulse toward the target (primary submovement) and often a late corrective adjustment (secondary submovement) near the target (Milner, 1992; Woodworth, 1899). Traditionally, the presence and incidence of secondary submovements have been thought to be a reflection of the magnitude of neuromotor noise in the pre-programmed primary submovement (Meyer, Abrams, Kornblum, Wright, & Smith, 1988; Pratt, Chasteen, & Abrams, 1994; van Donkelaar & Franks, 1991; Walker, Philbin, & Fisk, 1997). Accordingly, secondary submovements serve to compensate for initial trajectory errors by correcting the position of the hand near the target, thereby allowing for accurate target acquisition. Parsing movements into submovements is important in motor control because submovements may elucidate the basic building blocks of voluntary movement (Rohrer et al., 2004; Thomas, Yan, & Stelmach, 2000; von Hofsten, 1991) and have been shown to be altered in the elderly (Ketcham, Seidler, Van Gemmert, & Stelmach, 2002; Pratt et al., 1994; Romero, Van Gemmert, Adler, Bekkering, & Stelmach, 2003a; Seidler, Alberts, & Stelmach, 2002) and various patient populations (Rohrer et al., 2004; Romero, Van Gemmert, Adler, Bekkering, & Stelmach, 2003b).
One limitation of the aforementioned studies on movement structure in young and elderly adults is that the vast majority only examined the dominant (often the right) arm. However, accumulating evidence suggests that there are differences in the control of various aspects of movement between arms, at least in right-handed young adults (Bagesteiro & Sainburg, 2002, 2003; Haaland, 2006; Haaland & Harrington, 1996; Haaland, Prestopnik, Knight, & Lee, 2004; Sainburg & Kalakanis, 2000; Sainburg & Schaefer, 2004). Specifically, it has been shown that the dominant arm-hemisphere system is specialized in the feedforward control of trajectory and intersegmental dynamics, whereas the non-dominant arm-hemisphere system is specialized in final positional and proprioceptive feedback control (Sainburg, 2005).
Although it seems reasonable that the differences observed in movement specialization for the two arms would also hold true for elderly adults, behavioral and physiological evidence suggests that various parameters of limb movement control may be influenced by aging-related adaptations associated with a lifetime of preferential use of dominant limb (Porac, Coren, & Duncan, 1980; Porac & Friesen, 2000; Sale & Semmler, 2005). More importantly, several brain aging models suggest that these hemispheric differences in movement control may be compromised or reduced as a result of the normal aging process (Dolcos, Rice, & Cabeza, 2002; Kalisch, Wilimzig, Kleibel, Tegenthoff, & Dinse, 2006). For example, the right-hemisphere aging model predicts that aging-related declines in motor performance may be greater for the non-dominant hemisphere and limb (Brown & Jaffe, 1975; Gerhardstein, Peterson, & Rapcsak, 1998; Goldstein & Shelly, 1981; McDowell, Harrison, & Demaree, 1994). Alternatively, the Hemispheric Asymmetry Reduction in Old Adults (HAROLD) model proposes task-related reductions in the lateralization of activity as a compensatory mechanism for reduced cognitive performance in aging (Cabeza, 2002; Cabeza, Anderson, Locantore, & McIntosh, 2002; Raz et al., 1997; Reuter-Lorenz et al., 2000). Although this model is based on neuroimaging data in cognitive domains and might only occur in certain brain regions such as the prefrontal cortex (Cabeza, 2002), a recent study has implied that this phenomenon may also be manifested in motor system output (Kalisch et al., 2006). However, few studies have addressed the possibility of reduced lateralization of motor function with aging (Dolcos et al., 2002; Kalisch et al., 2006), in spite of the possibility that reduced hemispheric specialization could impair movement control (Sainburg, 2005).
Despite the significance of submovements in the study of motor behavior and the differences in the control strategies employed by the dominant and non-dominant hemisphere-arm systems (Sainburg, 2005), no studies have directly addressed the influence of limb on movement structure characteristics. Based on the recently described dominant hemisphere-arm advantage for feedforward and trajectory control (Sainburg, 2005) and the traditional view that the length of the primary submovement is a reflection of the magnitude of neuromotor noise motor command, we hypothesized that the right arm would exhibit a longer primary submovement length, longer primary submovement duration, and greater trajectory smoothness (less normalized jerk) compared to the left arm. Furthermore, these differences between the arms are suggested to change during the aging process, because of possible right hemispheric declines and/or a reduced lateralization of hemispheric specialization (Dolcos et al., 2002; Kalisch et al., 2006). Therefore, the purpose was to examine age-related differences in the movement structure for the dominant (right) and non-dominant (left) arms during goal-directed movements. A secondary objective was to determine if the large body of previous work from our laboratory (Alberts, Saling, Adler, & Stelmach, 2000; Ketcham, Dounskaia, & Stelmach, 2004; Ketcham et al., 2002; Leis et al., 2005; Romero et al., 2003a, 2003b; Seidler et al., 2002; Van Gemmert, Teulings, & Stelmach, 1998) involving movement structure and trajectory smoothness during visually guided movements in the dominant arm of young and elderly adults would generalize to the non-dominant arm.
2. Methods
2.1. Subjects
Thirteen young (24 ± 4.4 yrs) and thirteen elderly adults (73 ± 5.9 yrs) volunteered to participate in the study. The subjects were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971) and reported no known neurological impairments. All subjects had normal or corrected-to-normal vision. The protocol was approved by the Institutional Review Board of Arizona State University, and informed consent was obtained from each subject before participation in the study.
2.2. Experimental arrangement
Each subject was seated comfortably in a chair; facing a 53.34 cm computer monitor located approximately 70 cm in front of the subject at eye level. The height of the chair was adjusted so that the subject’s forearm rested comfortably on a table. A Wacom Intuos 12 × 18 digitizer tablet (sampling frequency 200 Hz; spatial resolution 0.001 cm) was positioned directly in front of each subject on the table and between the subject and the computer monitor (Fig. 1). Subjects were required to make point-to-point drawing movements with a digitizer pen between the start position and various targets displayed on the monitor. Vision of the entire arm and digitizer surface was occluded by means of an opaque shield placed over the digitizer. Thus, vision of the start position, movement trajectory, and target position was provided entirely by the computer monitor and subjects were able to view the complete trajectory of the pen tip on-line.
Fig. 1.
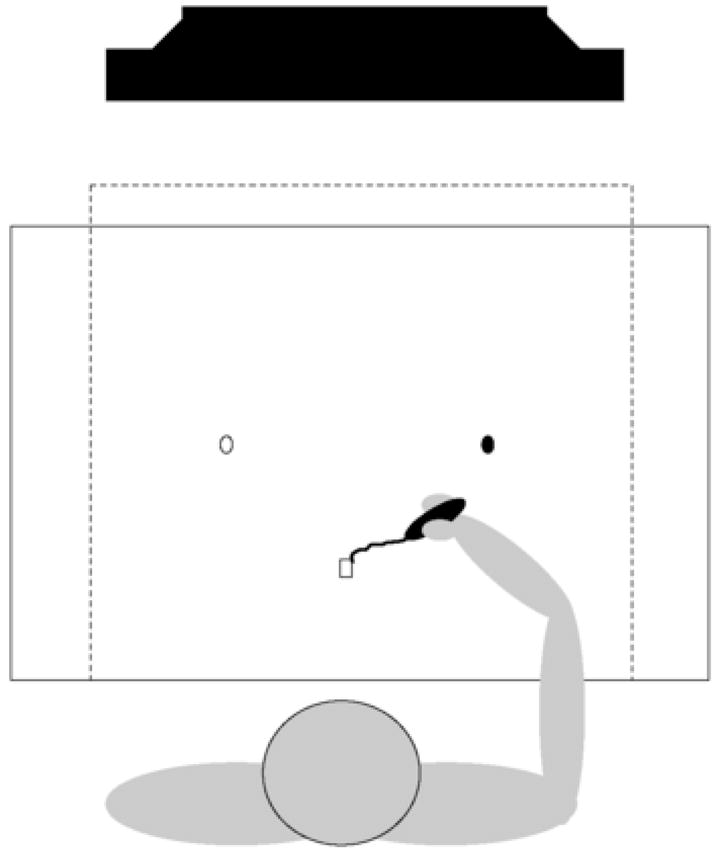
Experimental arrangement. Subjects were seated and facing a monitor with a digitizer tablet positioned on a table between the subject and the monitor. Vision of the entire arm was occluded by an opaque shield (dashed square) placed over the arm and digitizer. Subjects were required to make point-to-point drawing movements with an inkless pen between the start position and various targets displayed on the monitor. Visual feedback of the target, pen position, and movement trajectory were provided via the computer monitor during each trial.
2.3. Experimental procedures
Subjects reported to the laboratory for a single experimental session. Prior to beginning the experimental session, subjects received a written description of the project and signed informed consent forms. In addition, all subjects were given a visual demonstration of the experimental procedures by one of the investigators and were required to demonstrate that they understood how to interpret the visual feedback (described below) that was provided after each trial. All experimental sessions lasted approximately 1 hour.
Half of the subjects started with the left (non-dominant) hand, whereas the other half started with the right (dominant) hand. Participants held the digitizer pen in a normal pen grip (dynamic tripod grasp) and performed a linear drawing movement away from the body midline in the horizontal plane. At the start of each trial, subjects were required to position the digitizer pen in the start target (red square) for 0.5 s and to maintain the limb in a static position. Once this requirement was satisfied, the start position (red square) turned black or white after a random delay of 300 to 1000ms. At the same time that the start position changed color, two visual targets (one black, one white) appeared in a choice reaction time paradigm. Subjects were required to move to the target that corresponded to the same color as the start square had turned to (black or white). The subjects were instructed to perform the point-to-point drawing movement as fast and as accurately as possible and to terminate the movement within the end target. Online visual feedback of the movement trajectory was provided during each trial. The trial ended when the subject stopped within the target.
In order to make the position of the target unpredictable and to cover a large workspace, a total of six target positions were employed (2 locations × 3 directions). Three target positions were at angles of 5, 45, and 85 degrees to the left (left location) or to the right (right location) of the subject’s midline (Fig. 2). All targets were located 13.4 cm from the start position and a very small target size (0.6 cm diameter) was used to ensure that visual feedback-based corrections would be needed on each trial. The target size and distance between the home position and target location remained constant throughout all experimental conditions. Thus, the index of difficulty for the aiming task was constant (5.48) throughout the experiment. Data was collapsed over each of the three movement positions on each side of the subject’s midline because there was no age by target position interaction within each location. All possible targets were presented in randomized order determined by the computer program as one block of 72 trials per arm.
Fig. 2.
Experimental presentation. A. The start of the experiment. B. Execution of a trial. C. Possible left and right target locations were at angles of 5, 45, and 85 degrees of the subject’midline. Thus, a total of six target positions were employed (left 5, 45, 85 degrees, and right 5, 45, and 85 degrees). All targets were located 13.4 cm from the start position (same index of difficulty for all targets). The target had a diameter of 0.6 cm diameter and remained constant throughout all experimental conditions.
2.4. Data analysis
All data collected during the experiments were acquired using custom computer programs written in the OASIS (KIKO Software, Doetinchem, The Netherlands) and analyzed off-line using custom written programs in MatLab (Mathworks Inc., Natick, Massachusetts, USA). The position signals were sampled at 200 Hz and smoothed using a 4th order Butterworth dual pass digital filter with an optimal cut-off frequency of 7 Hz. The onsets and offsets of pen tip recording were estimated by a fixed criterion of 5% of the peak velocity for absolute velocity. After estimation, these segmentation points were modified by a search for the point in time corresponding to the nearest zero crossing or local minimum of the acceleration profile for movement onset and offset, before or after, respectively, the 5% criterion (Romero et al., 2003a, 2003b).
2.5. Dependent variables
The reaction time was calculated as the time elapsed between target presentation and the onset of the movement, whereas movement time was calculated as the time between the movement onset and offset times. In addition, the peak velocity and average velocity were quantified for the entire movement. Movement structure characteristics were quantified after parsing each movement into primary and secondary submovements (Meyer et al., 1988). The primary submovement duration was calculated as the time elapsed between movement onset and to the second zero crossing of the acceleration profile. Similarly, the primary submovement length was calculated as the magnitude of pen displacement between these two time points. Secondary, or corrective submovements, were considered as the time from the second zero crossing of the acceleration profile until movement offset. Relative length and duration of the primary submovement were calculated as a percentage of the total movement amplitude and duration, respectively (Romero et al., 2003b). These relative measures were used for analysis due to the fact that differences in movement times between elderly and young adults would render absolute measures less meaningful. The smoothness of the movement trajectory was quantified as the normalized jerk (Romero et al., 2003a; Van Gemmert et al., 1998). Normalized jerk is a unitless measure, because it is normalized for both the amplitude and duration of movement (Teulings et al., 1997).
2.6. Statistical analysis
The dependent variables were analyzed with a mixed three-factor ANOVAs (2 age × 2 arm × 2 location) with repeated measures on arm and location. When ANOVAs yielded significant interactions, post hoc comparisons using the Bonferroni adjustment for the multiple comparisons were performed to locate significant differences. Significance level for all statistical tests was set at p < 0.05, and if appropriate Bonferroni corrected. Data are indicated as means ± SE in the figures and means ± SD within the text and tables.
3. Results
3.1. Reaction time
There was a significant [F(1,24) = 46.39; p < 0.001] main effect for age, indicating that reaction time was greater for the elderly (648 ± 161 ms) compared with the young adults (411 ± 53 ms; Fig. 3). The reaction time was similar [F(1,24) = 0.15] for the left (527 ± 163 ms) and right arms (531 ± 175 ms). There was also a significant [F(1,24) = 5.86; p = 0.023] main effect for location, which indicated that the reaction time was greater for the left (543 ± 170 ms) compared with the right target location (515 ± 167 ms). The arm × age, location × age, arm × location, and arm × age × location interactions were all non-significant.
Fig. 3.
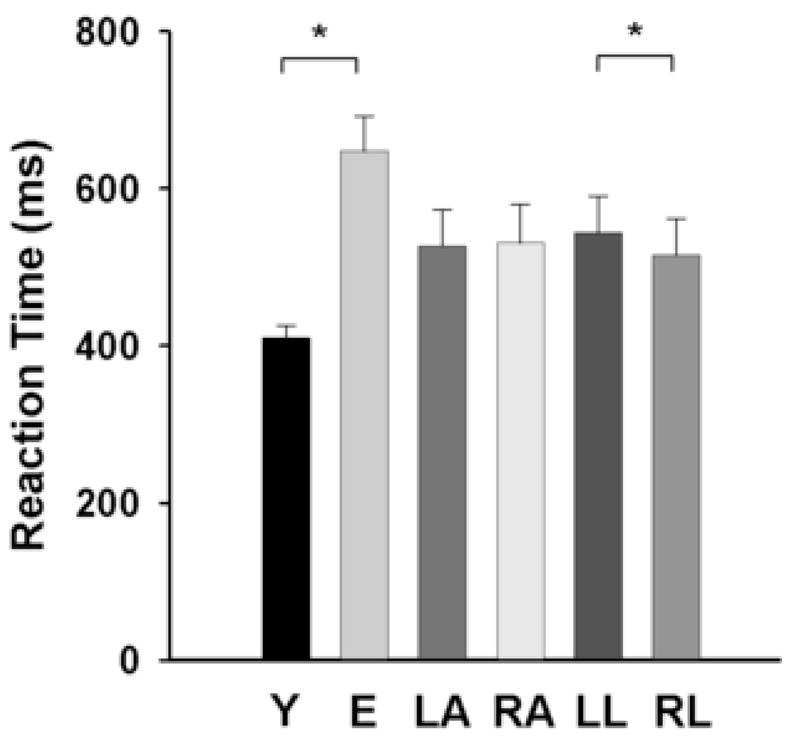
Reaction time for young and elderly adults, the left and right hands, and the left and right locations. Values are means ± SE. Reaction time was longer for the elderly compared with the young adults, similar for the left and right arms, and longer for the left compared with the right location. *indicates significant (p < 0.05) between groups or locations.
3.2. Movement time
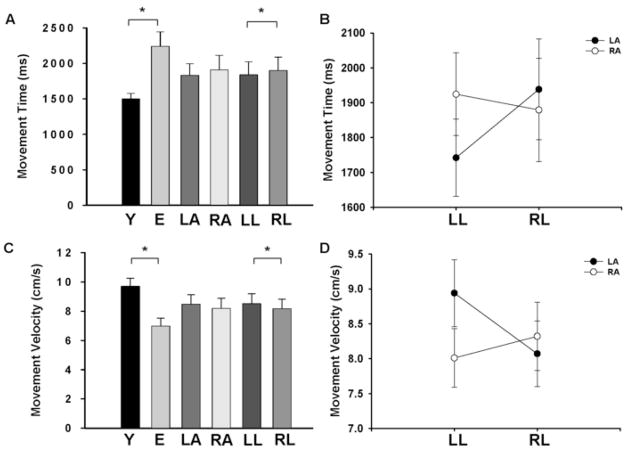
There was a significant [F(1,24) = 12.01; p = 0.002] main effect for age indicating that movement time was greater for elderly compared with young adults (2242 ± 731 ms vs. 1500 ± 282 ms; Fig. 4A). There was no main effect for arm [F(1,24) = 0.75], which indicated that movement time was similar for the left (1833 ± 588 ms) and right arms (1909 ± 739 ms). There was a significant [F(1,24) = 4.82; p < 0.038] main effect for location, indicating that movement time was greater for the right (1902 ± 678 ms) compared to the left target location (1840 ± 658 ms). There was a significant [F(1,24) = 24.83; p < 0.001; Fig. 4B] arm × location interaction, which indicated that the movement time was greater when each arm moved to the corresponding contralateral location (i.e. left location for the right arm and vice versa). The arm × age, location × age, and arm × age × location interactions were all non-significant.
Fig. 4.
Movement time and movement velocity for young and elderly adults, the left and right hands, and the left and right locations. A. Movement time was greater for the elderly compared with the young adults, similar for the left and right arms, and greater for the right compared with the left location. B. Movement velocity was greater for the elderly compared with the young adults, similar for the left and right arms, and greater for the left compared with the right location. C. There was a significant arm × location interaction, which indicated that the movement time was greater when each arm moved to the corresponding contralateral location. D. There was a significant arm × location interaction, which indicated that the movement velocity was lower when each arm moved to the corresponding contralateral location. *indicates significant (p < 0.05) between groups or locations
3.3. Movement velocity
The average and peak velocities were calculated for each trial. Since the results were qualitatively the same, only the results for average velocity are reported. There was a significant [F(1,24) = 12.29; p = 0.002] main effect for age indicating that movement velocity was greater for the young compared with the elderly adults (9.7 ± 2.0 cm/s vs 7.0 ± 2.0 cm/s; Fig. 4C). The movement velocity was similar [F(1,24) = 0.28] for the left (8.5 ± 2.3 cm/s) and right arms (8.2 ± 2.4 cm/s). There was also a significant [F(1,24) = 9.32; p = 0.005] main effect for location, indicating that movement velocity was greater for the left (8.5 ± 2.4 cm/s) compared with the right target location (8.2 ± 2.3 cm/s). There was a significant [F(1,24) = 33.84; p < 0.001; Fig. 4D] arm × location interaction, which indicated that the movement velocity was lower when each arm moved to the corresponding contralateral location (i.e. left location for the right arm and vice versa). The arm × age, location × age, and arm × age × location interactions were all non-significant.
3.4. Relative primary submovement length
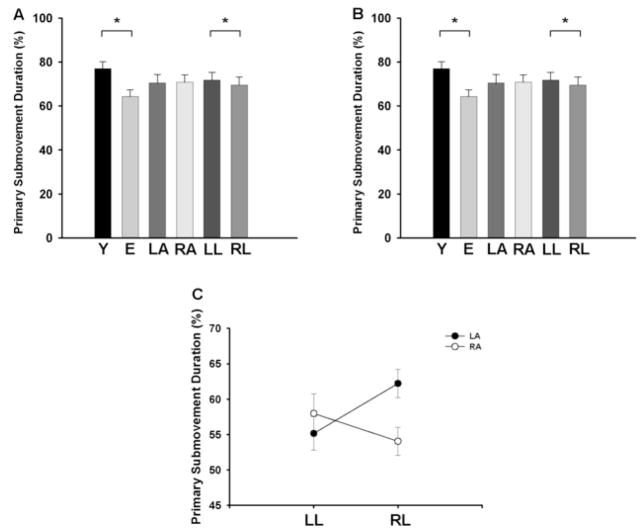
There was a significant main effect for age [F(1,24) = 8.96; p = 0.006], which indicated that the relative primary submovement length was greater for the young compared with the elderly adults (77.0 ± 11.3 % vs. 64.2 ± 11.3 %; Fig. 5A). The relative primary submovement length was similar [F(1,24) = 0.85] for the left (70.5 ± 14.0 %) and right arms (70.8 ± 12.0 %). There was a significant [F(1,24) = 6.82; p = 0.015] main effect for location, which indicated that the relative primary submovement length was greater for the left (71.7 ± 12.9 %) compared with the right target locations (69.5 ± 13.0 %). The arm × age, arm × location, location × age, arm × location, and arm × age × location interactions were all non-significant.
Fig. 5.
Relative primary submovement length and duration for young and elderly adults, the left and right hands, and the left and right locations. Values are means ± SE. A. Relative primary submovement length was greater for the young compared with the elderly adults, similar for the left and right arms, and greater for the left compared with the right location. B. Relative primary submovement duration was greater for the young compared with the elderly adults, similar for the left and right arms, and greater for the left compared with the right location. C. There was a significant arm × location interaction, which indicated that the relative primary submovement duration was greater when each arm moved to the corresponding contralateral location. *indicates significant (p < 0.05) between groups or locations.
3.5. Relative primary submovement duration
Since the total movement time was greater for the elderly compared with the young adults, the relative primary submovement duration was used for analysis because large differences in movement times between elderly and young adults render absolute measures less meaningful. There was a significant [F(1,24) = 10.95; p = 0.003] main effect for age indicating that the relative primary submovement duration was greater for the young compared with the elderly adults (63.9 ± 11.4 % vs. 50.8 ± 10.2 %; Fig. 5C). The relative primary submovement duration was similar [F(1,24) = 4.19] for the left (56.6 ± 13.2 %) and right arms (58.1 ± 12.0 %). There was a significant [F(1,24) = 11.84; p = 0.002] main effect for location, which indicated that the relative primary submovement duration was greater for the left (58.7 ± 12.7 %) compared with the right target locations (56.0 ± 12.5 %). There was a significant [F(1,24) = 7.20; p = 0.013; Fig. 5D] arm × location interaction which indicated that the relative primary submovement duration was greater when each arm moved to the corresponding contralateral location (i.e. left location for the right arm and vice versa). The arm × age, location × age and arm × age × location interactions were all non-significant.
3.6. Normalized Jerk
There was a significant [F(1,24) = 17.63; p < 0.001] main effect for age, indicating that normalized jerk was greater for elderly (290 ± 190) compared with young adults (90 ± 33; Fig. 6). The normalized jerk was similar [F(1,24) = 1.25] for the left (199 ± 177) and right arms (180 ± 161). In addition, the normalized jerk was similar [F(1,24) = 1.36] for the left (183 ± 166) and right locations (197 ± 173). There was a significant [F(1,24) = 6.01; p = 0.022] arm × location interaction. However, Bonferronni corrected post hoc comparisons within each arm did not show significant differences, indicating that arm-related difference was the primary effect. The arm × age, location × age, and arm × age × location interactions were all non-significant.
Fig. 6.
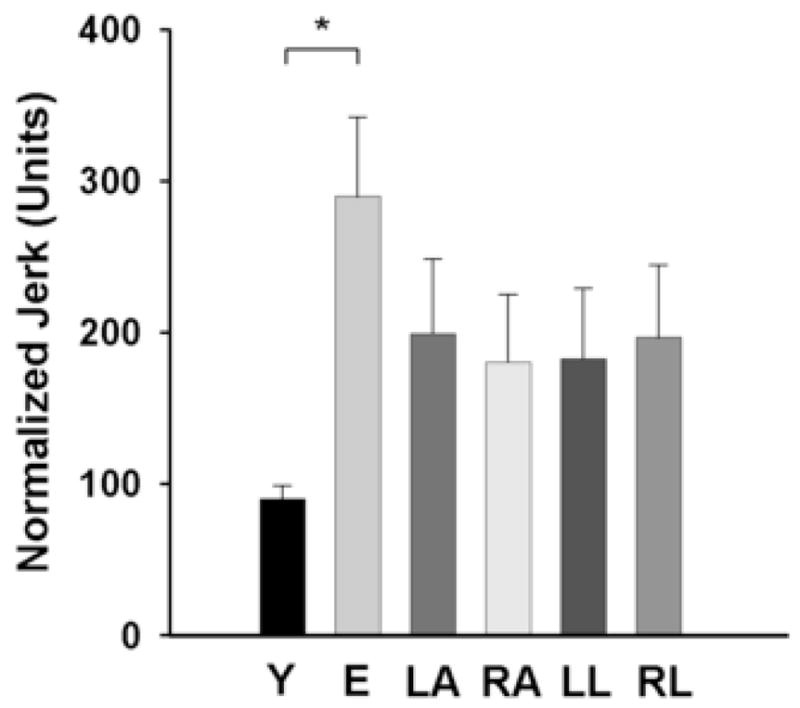
Normalized jerk for young and elderly adults, the left and right hands, and the left and right locations. Values are means ± SE. The normalized jerk was greater for the elderly compared with the young adults, similar for the left and right arms, and similar for the left and right locations. *indicates significant (p < 0.05) between groups.
4. Discussion
The purpose was to examine possible age-related differences in the movement structure for the right and left arm during goal-directed aiming movements to determine if previous work involving movement structure and trajectory smoothness in the dominant arm of young and elderly adults would generalize to the non-dominant arm. The study produced two main groups of findings. First, the reaction time, movement time, relative primary submovement length, relative primary submovement duration, and normalized jerk were all greater for the elderly adults. Second, there were no differences in any of these variables between the left and right arm for either of the two age groups. Thus, movement structure was different for the two age groups, but similar for the two arms. These findings suggest that previous work involving movement structure and trajectory smoothness in the dominant arm can be generalized to the non-dominant arm.
4.1. Reaction time
Although its use is currently controversial (Derakhshan, 2006; Goble, 2007; Gonzalez, Ganel, & Goodale, 2006), reaction time has classically been used in the study of motor control as a metric of hemisphere dominance for the preparation and initiation of movements (Bradshaw, Bradshaw, & Nettleton, 1990; Carson, Chua, Elliott, & Goodman, 1990; Carson, Goodman, Chua, & Elliott, 1993; Goodman & Kelso, 1980). The reaction time of the young adults was much shorter than the reaction time of the elderly adults. These are not novel findings as many studies have found large differences in reaction time between elderly and young adults (Amrhein, Stelmach, & Goggin, 1991; Amrhein & Theios, 1993; Larish & Stelmach, 1982; Salthouse, 1979; Salthouse & Somberg, 1982; Stelmach, Goggin, & Amrhein, 1988; Warabi, Noda, Yanagisawa, Tashiro, & Shindo, 1986). Interestingly, reaction time was greater for the left location compared with the right location, independent of limb or age group. This finding may be related to right visual field dominance, which has been shown for linguistic and pattern stimuli (Efron, Yund, & Nichols, 1990; Yund, Efron, & Nichols, 1990). Furthermore, the phenomenon of a right location dominance for reaction time has been observed before in both arm and finger movements (Boulinguez, Ferrois, & Graumer, 2003; Carson et al., 1990; Elliott et al., 1993), even though, the neural basis for this observation is not well-understood (Carson, 1996).
The reaction time for the left arm was shorter than the right arm; however, this difference did not reach statistical significance. This observation differs from most previous investigations, which showed significant longer reaction times for the dominant arm (Barthelemy & Boulinguez, 2001, 2002; Boulinguez & Nougier, 1999; Bradshaw et al., 1990; Carson et al., 1990; Carson et al., 1993; Goodman & Kelso, 1980; Velay & Benoit-Dubrocard, 1999; Velay, Daffaure, Raphael, & Benoit-Dubrocard, 2001). Nevertheless, Gonzalez and colleagues (2006b) found similar reaction times for the two arms in a reach-to-grasp task. They suggested that the lack of a difference for the two arms was due to the relatively long and variable reaction times in that study. Similarly, the accuracy demands and choice-reaction time paradigm of the current task was rather difficult and also lead to relatively long and variable reaction times.
4.2. Movement time and velocity
The movement times were longer and movement velocities were lower in the elderly adults, as has been repeatedly shown in the past (Amrhein et al., 1991; Bellgrove, Phillips, Bradshaw, & Gallucci, 1998; Cooke, Brown, & Cunningham, 1989; Pohl, Winstein, & Fisher, 1996; Stelmach et al., 1988; Strayer, Wickens, & Braune, 1987; Walker et al., 1997; Welford, 1984). More importantly, the movement times and movement velocities were similar for the left and right arms. These findings seem to be counterintuitive as it is generally believed that a right arm advantage exists for movement time and movement velocity (Annett, Annett, Hudson, & Turner, 1979; Carson et al., 1993; Todor & Cisneros, 1985). Nevertheless, several recent studies have found that movement velocity can be the same for the two arms despite between limb differences in other aspects of motor performance (Bagesteiro & Sainburg, 2002, 2003; Sainburg, 2002; Sainburg & Kalakanis, 2000; Wang & Sainburg, 2007).
Interestingly, movement time was longer and movement velocity was lower when each arm moved to the corresponding contralateral location, despite the same index of difficulty for targets at the two locations (Figs. 4B, 4D, and 5C). In the past, these contralateral performance differences have been attributed to superior performance of either arm when it moves in their own hemispace (Elliott & Chua, 1996). However, physiological evidence strongly suggests that these differences in movement speed are due to the fact that the inertial resistance is greatest when the arm moves diagonally across the body (Gordon, Ghilardi, Cooper, & Ghez, 1994).
4.3. Relative primary submovement length and duration
It has been generally accepted that the primary submovement length and duration reflect the amount of neuromotor noise in the descending command (Meyer et al., 1988; Pratt et al., 1994; van Donkelaar & Franks, 1991; Walker et al., 1997). Based on this rationale and the consistency of previous findings (Ketcham et al., 2002; Pratt et al., 1994; Romero et al., 2003b; Seidler-Dobrin & Stelmach, 1998; Seidler et al., 2002), it was expected that the primary submovement lengths and durations would be greater in young compared with elderly adults. Accordingly, the elderly adults had shorter relative primary submovement lengths and spent less relative time in the primary submovement compared with the young adults. Overall, these results provide consistent evidence that elderly adults exhibit alterations in component submovements compared with young adults. Although the exact origin of these differences remains to be elucidated, these aging-related deficits are usually attributed to impairments in motor planning (Ketcham et al., 2002; Seidler et al., 2002; Walker et al., 1997) or the processing of feedback information (Pratt et al., 1994; Romero et al., 2003a, 2003b). Since the timing of overlapping submovements and corrections to movement trajectories has been shown to depend on the prediction of the magnitude and direction of the movement error (Fishbach, Roy, Bastianen, Miller, & Houk, 2007), it is also possible that elderly adults utilize a more conservative cost function to initiate a corrective submovement to minimize movement error (Pratt et al., 1994; Walker et al., 1997).
The primary aim of the study was to compare the submovement structure of the left and right arms in young and elderly adults. Based on recent evidence that the dominant hemisphere-arm system shows an advantage for feedforward control (Sainburg, 2005), one possibility was that the relative length and duration of the primary submovement would be greater for the right arm. Presumably, this would be a result of a lower magnitude of noise in the descending command from the left hemisphere, which is thought to contribute to altered movement structure. Alternatively, differences in dominant and non-dominant limb performance may only be present when the regulation of joint interaction torque or the use of proprioceptive feedback control are crucial to task performance (Sainburg 2005). This possibility would suggest that interlimb differences in motor performance should not be present during tasks involving significant visual contributions (Grosskopf & Kuhtz-Buschbeck, 2006; Sainburg, 2002). The current results support the second possibility as there were no differences in the relative length or duration of the primary submovement between the two arms for both the young and elderly adults. Accordingly, although all aspects of motor performance exhibited a general age-related decline, the same pattern of lateralization was maintained with advancing age.
4.4. Movement Trajectory Smoothness
Movement trajectories during goal-directed reaching have been mathematically shown to be best described by a cost function that minimizes jerk (Hogan, 1984). Accordingly, normalized jerk has commonly been utilized as a measure of movement trajectory smoothness and has been shown to be greater in elderly adults (Contreras-Vidal et al., 1998; Yan, 2000) and in patient populations (Alberts et al., 2000; Leis et al., 2005; Romero et al., 2003a; Teulings et al., 1997; Van Gemmert et al., 1998). Consistent with these findings, normalized jerk was approximately four times greater for old adults in the current study. The vast majority of the studies that have modeled arm movement trajectories or quantified normalized jerk during arm movements in young and elderly adults have only studied the right arm in right-handed subjects (Alberts et al., 2000; Contreras-Vidal et al., 1998; Leis et al., 2005; Romero et al., 2003a; Teulings et al., 1997; Van Gemmert, Teulings, Contreras-Vidal, & Stelmach, 1999; Yan, 2000). Given the purported superiority of the right arm in trajectory control (Sainburg, 2005), it was expected that normalized jerk would be greater for the left arm. Contrary to these expectations, the normalized jerk was similar for the two arms for both age groups, which appear to be novel findings. However, the direct functional consequences of the greater normalized jerk in elderly adults, patient populations, or between young adults remains unknown. The available evidence suggests that increased trajectory variability, and presumably greater normalized jerk, may lead to impaired endpoint accuracy and greater endpoint variance (Christou, Shinohara, & Enoka, 2003; Enoka et al., 2003; Harris & Wolpert, 1998).
4.5. Lateralization and aging
The fact that young and elderly adults exhibited similar performance for all the movement parameters studied does not support the concept of a preferential decline in the non-dominant hemisphere-arm system in right-handed individuals (Dolcos et al., 2002; Mitrushina, Fogel, D’Elia, Uchiyama, & Satz, 1995). However, the findings do not completely rule out reductions in the lateralization of motor function as predicted by the HAROLD model (Cabeza, 2002) and its possible application to the motor system (Kalisch et al., 2006). This is due to the fact that none of the dependent variables measured in the current study were lateralized for the young adults. However, prior to this experiment it was uncertain if submovement regulation would be lateralized in young adults. Furthermore, the majority of literature on arm aiming movements suggested that this difference would be present. Specifically, recent evidence has strongly supported the idea that the left hemisphere-right arm system (dominant) is specialized for feedforward and trajectory control, whereas the right hemisphere-left arm system is specialized for feedback control and final positional accuracy (for reviews see Sainburg, 2005; Haaland et al. 2004). Based on the traditional viewpoints (Meyer et al. 1988) and numerous studies from our laboratory, we hypothesized that the length and duration of the primary submovement should be associated with the level of noise in the preprogrammed motor command. Taken together, these two theoretical frameworks led to our hypothesis that the submovement length and duration would be greater for the left hemisphere-right arm system in young adults. Although the results of the current study did not show this to be the case, the findings are novel in that they show that numerous previous studies on submovement characteristics for the left hemisphere-right arm system likely apply to the right hemisphere-left arm system. In addition, recent studies have found that only certain aspects of movement such as the regulation of intersegmental dynamics and proprioceptive feedback control demonstrate a lateralization of function, at least in young adults. Therefore, the possibility that increased bilateral activity in sensorimotor cortex (Riecker et al., 2006) may result in reduced lateralization of motor output cannot be completely discounted (Kalisch et al., 2006) and requires further investigation.
4.6. Factors responsible for lack of interlimb differences in motor performance
The absence of interlimb differences in motor performance seems to be in conflict with a series of recent studies involving investigation of manual asymmetries within the framework of the dynamic-dominance hypothesis (Sainburg, 2005). However, these conflicting findings are very likely due to differences in the task and dependent variables studied. The focus of the current study was on movement structure and trajectory smoothness during visually guided aiming. However, a key prediction of the dynamic-dominance hypothesis is that interlimb differences are only manifested under certain task conditions. Specifically, dominant hemisphere-arm advantages are mainly present when the predictive regulation of joint interaction torque is emphasized, whereas non-dominant hemisphere-arm advantages are usually realized when performance depends on the use of proprioceptive feedback control (Sainburg 2005). These findings would suggest that interlimb differences in motor performance should not be present during tasks involving visually guided aiming as in the present study. Several recent observations and lines of reasoning are consistent with this viewpoint. First, interlimb differences in motor performance were not present in response to a visuomotor rotation (Sainburg, 2002). Second, the kinematics of visually guided reach-to-grasp movements were similar between the two arms (Grosskopf & Kuhtz-Buschbeck, 2006). Third, hemispheric specialization for visual control does not depend on handedness (Gonzalez et al., 2006; Gonzalez, Whitwell, Morrissey, Ganel, & Goodale, 2007). Fourth, there are great differences in the neural mechanisms underlying the control of force in visual and non-visual conditions (Vaillancourt, Thulborn, & Corcos, 2003). Therefore, the similar movement structure and trajectory smoothness between the two arms in the present study were probably a consequence of the presence of visual feedback and the movement parameters studied.
4.7. Summary
In summary, the results demonstrated that the movement structure was different for the two age groups, but similar for the two arms. Therefore, two conclusions can be drawn: (1) previous work (Alberts et al., 2000; Contreras-Vidal et al., 1998; Ketcham et al., 2004; Ketcham et al., 2002; Leis et al., 2005; Pratt et al., 1994; Romero et al., 2003a, 2003b; Seidler et al., 2002; Teulings et al., 1997; Van Gemmert et al., 1998; Walker et al., 1997) involving the quantification of movement structure and trajectory smoothness during visually guided aiming in the dominant arm can likely be generalized to the non-dominant arm in both young and elderly adults; (2) the aging process is characterized by an altered movement substructure in elderly adults, suggesting aging-related increases in the magnitude of neuromotor noise leading to a reduction of the pre-programmed part of the voluntary movement.
Acknowledgments
Portions of the results of this study were presented at Progress in Motor Control VI in Santos, Brazil. Supported by NINDS 40266 and NIA 14676 awarded to G.E. Stelmach.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts JL, Saling M, Adler CH, Stelmach GE. Disruptions in the reach-to-grasp actions of Parkinson’s patients. Experimental Brain Research. 2000;134(3):353–362. doi: 10.1007/s002210000468. [DOI] [PubMed] [Google Scholar]
- Amrhein PC, Stelmach GE, Goggin NL. Age differences in the maintenance and restructuring of movement preparation. Psychology and Aging. 1991;6(3):451–466. doi: 10.1037//0882-7974.6.3.451. [DOI] [PubMed] [Google Scholar]
- Amrhein PC, Theios J. The time it takes elderly and young individuals to draw pictures and write words. Psychology and Aging. 1993;8(2):197–206. doi: 10.1037//0882-7974.8.2.197. [DOI] [PubMed] [Google Scholar]
- Annett J, Annett M, Hudson PT, Turner A. The control of movement in the preferred and non-preferred hands. Quarterly Journal of Experimental Psychology. 1979;31(Pt 4):641–652. doi: 10.1080/14640747908400755. [DOI] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. Journal of Neurophysiology. 2002;88(5):2408–2421. doi: 10.1152/jn.00901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro LB, Sainburg RL. Nondominant arm advantages in load compensation during rapid elbow joint movements. Journal of Neurophysiology. 2003;90(3):1503–1513. doi: 10.1152/jn.00189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy S, Boulinguez P. Manual reaction time asymmetries in human subjects: the role of movement planning and attention. Neuroscience Letters. 2001;315(1–2):41–44. doi: 10.1016/s0304-3940(01)02313-8. [DOI] [PubMed] [Google Scholar]
- Barthelemy S, Boulinguez P. Manual asymmetries in the directional coding of reaching: further evidence for hemispatial effects and right hemisphere dominance for movement planning. Experimental Brain Research. 2002;147(3):305–312. doi: 10.1007/s00221-002-1247-x. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Phillips JG, Bradshaw JL, Gallucci RM. Response (re-)programming in aging: a kinematic analysis. Journals Gerontology Series A Biological Sciences and Medical Sciences. 1998;53(3):222–227. doi: 10.1093/gerona/53a.3.m222. [DOI] [PubMed] [Google Scholar]
- Boulinguez P, Ferrois M, Graumer G. Hemispheric asymmetry for trajectory perception. Brain Research. Cognitive Brain Research. 2003;16(2):219–225. doi: 10.1016/s0926-6410(02)00276-8. [DOI] [PubMed] [Google Scholar]
- Boulinguez P, Nougier V. Control of goal-directed movements: the contribution of orienting of visual attention and motor preparation. Acta Psychologica (Amst) 1999;103(1–2):21–45. doi: 10.1016/s0001-6918(99)00022-0. [DOI] [PubMed] [Google Scholar]
- Bradshaw JL, Bradshaw JA, Nettleton NC. Abduction, adduction and hand differences in simple and serial movements. Neuropsychologia. 1990;28(9):917–931. doi: 10.1016/0028-3932(90)90108-z. [DOI] [PubMed] [Google Scholar]
- Brown JW, Jaffe J. Hypothesis on cerebral dominance. Neuropsychologia. 1975;13(1):107–110. doi: 10.1016/0028-3932(75)90054-8. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Carson RG. Putative right hemisphere contributions to the preparation of reaching and aiming movements. In: Elliott D, Roy EA, editors. Manual asymmetries in motor performance. Boca Raton: CRC Press; 1996. pp. 159–172. [Google Scholar]
- Carson RG, Chua R, Elliott D, Goodman D. The contribution of vision to asymmetries in manual aiming. Neuropsychologia. 1990;28(11):1215–1220. doi: 10.1016/0028-3932(90)90056-t. [DOI] [PubMed] [Google Scholar]
- Carson RG, Goodman D, Chua R, Elliott D. Asymmetries in the regulation of visually guided aiming. Journal of Motor Behavior. 1993;25(1):21–32. doi: 10.1080/00222895.1993.9941636. [DOI] [PubMed] [Google Scholar]
- Christou EA, Shinohara M, Enoka RM. Fluctuations in acceleration during voluntary contractions lead to greater impairment of movement accuracy in old adults. Journal of Applied Physiology. 2003;95(1):373–384. doi: 10.1152/japplphysiol.00060.2003. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal JL, Teulings HL, Stelmach GE. Elderly subjects are impaired in spatial coordination in fine motor control. Acta Psychologica (Amst) 1998;100(1–2):25–35. doi: 10.1016/s0001-6918(98)00023-7. [DOI] [PubMed] [Google Scholar]
- Cooke JD, Brown SH, Cunningham DA. Kinematics of arm movements in elderly humans. Neurobiology of Aging. 1989;10(2):159–165. doi: 10.1016/0197-4580(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Derakhshan I. Laterality of the command center in relation to handedness and simple reaction time: a clinical perspective. Journal of Neurophysiology. 2006;96(6):3556. doi: 10.1152/jn.00852.2006. author reply 3557. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Rice HJ, Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neuroscience and Biobehavial Reviews. 2002;26(7):819–825. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Efron R, Yund EW, Nichols DR. Visual detectability gradients: the effect of distractors in contralateral field. Brain and Cognition. 1990;12(1):128–143. doi: 10.1016/0278-2626(90)90009-d. [DOI] [PubMed] [Google Scholar]
- Elliott D, Chua R. Manual asymmetries in goal-directed movement. In: Elliott D, Roy EA, editors. Manual asymmetries in motor performance. New York: CRC Press; 1996. pp. 143–172. [Google Scholar]
- Elliott D, Roy EA, Goodman D, Carson RG, Chua R, Maraj BKV. Asymmetries in the preparation and control of manual aiming movements. Canadian Journal of Experimental Psychology. 1993;47:570–589. [Google Scholar]
- Enoka RM, Christou EA, Hunter SK, Kornatz KW, Semmler JG, Taylor AM, et al. Mechanisms that contribute to differences in motor performance between young and old adults. Journal of Electromyography and Kinesiology. 2003;13(1):1–12. doi: 10.1016/s1050-6411(02)00084-6. [DOI] [PubMed] [Google Scholar]
- Fishbach A, Roy SA, Bastianen C, Miller LE, Houk JC. Deciding when and how to correct a movement: discrete submovements as a decision making process. Experimental Brain Research. 2007;177(1):45–63. doi: 10.1007/s00221-006-0652-y. [DOI] [PubMed] [Google Scholar]
- Gerhardstein P, Peterson MA, Rapcsak SZ. Age-related hemispheric asymmetry in object discrimination. Journal of Clinical and Experimental Neuropsychology. 1998;20(2):174–185. doi: 10.1076/jcen.20.2.174.1162. [DOI] [PubMed] [Google Scholar]
- Goble D. Validity of using reaction time as a basis for determining motor laterality. Journal of Neurophysiology. 2007;97:1868. doi: 10.1152/jn.01221.2006. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Shelly C. Does the right hemisphere age more rapidly than the left? Journal of Clinical Neuropsychology. 1981;3(1):65–78. doi: 10.1080/01688638108403114. [DOI] [PubMed] [Google Scholar]
- Gonzalez CL, Ganel T, Goodale MA. Hemispheric specialization for the visual control of action is independent of handedness. Journal of Neurophysiology. 2006;95(6):3496–3501. doi: 10.1152/jn.01187.2005. [DOI] [PubMed] [Google Scholar]
- Gonzalez CL, Whitwell RL, Morrissey B, Ganel T, Goodale MA. Left handedness does not extend to visually guided precision grasping. Experimental Brain Research. 2007;182(2):275–279. doi: 10.1007/s00221-007-1090-1. [DOI] [PubMed] [Google Scholar]
- Goodman D, Kelso JA. Are movements prepared in parts? Not under compatible (naturalized) conditions. Journal of Experimental Psychology General. 1980;109(4):475–495. doi: 10.1037//0096-3445.109.4.475. [DOI] [PubMed] [Google Scholar]
- Gordon J, Ghilardi MF, Cooper SE, Ghez C. Accuracy of planar reaching movements. II. Systematic extent errors resulting from inertial anisotropy. Experimental Brain Research. 1994;99(1):112–130. doi: 10.1007/BF00241416. [DOI] [PubMed] [Google Scholar]
- Grosskopf A, Kuhtz-Buschbeck JP. Grasping with the left and right hand: a kinematic study. Experimental Brain Research. 2006;168(1–2):230–240. doi: 10.1007/s00221-005-0083-1. [DOI] [PubMed] [Google Scholar]
- Haaland KY. Left hemisphere dominance for movement. The Clinical Neuropsychologist. 2006;20(4):609–622. doi: 10.1080/13854040590967577. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Hemispheric asymmetry of movement. Current Opinion in Neurobiology. 1996;6(6):796–800. doi: 10.1016/s0959-4388(96)80030-4. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Prestopnik JL, Knight RT, Lee RR. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004;127(Pt 5):1145–1158. doi: 10.1093/brain/awh133. [DOI] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394(6695):780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- Hogan N. An organizing principle for a class of voluntary movements. Journal of Neuroscience. 1984;4(11):2745–2754. doi: 10.1523/JNEUROSCI.04-11-02745.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch T, Wilimzig C, Kleibel N, Tegenthoff M, Dinse HR. Age-related attenuation of dominant hand superiority. PLoS ONE. 2006;1:e90. doi: 10.1371/journal.pone.0000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketcham CJ, Dounskaia NV, Stelmach GE. Age-related differences in the control of multijoint movements. Motor Control. 2004;8(4):422–436. doi: 10.1123/mcj.8.4.422. [DOI] [PubMed] [Google Scholar]
- Ketcham CJ, Seidler RD, Van Gemmert AWA, Stelmach GE. Age-related kinematic differences as influenced by task difficulty, target size, and movement amplitude. Journals of Gerontology Series B Psychological Sciences and Social Sciences. 2002;57(1):P54–64. doi: 10.1093/geronb/57.1.p54. [DOI] [PubMed] [Google Scholar]
- Larish DD, Stelmach GE. Preprogramming, programming and reprogramming of aimed hand movements as a function of age. Journal of Motor Behavior. 1982;14(4):322–340. doi: 10.1080/00222895.1982.10735283. [DOI] [PubMed] [Google Scholar]
- Leis BC, Rand MK, Van Gemmert AWA, Longstaff MG, Lou JS, Stelmach GE. Movement precues in planning and execution of aiming movements in Parkinson’s disease. Experimental Neurology. 2005;194(2):393–409. doi: 10.1016/j.expneurol.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McDowell CL, Harrison DW, Demaree HA. Is right hemisphere decline in the perception of emotion a function of aging? International Journal of Neuroscience. 1994;79(1–2):1–11. doi: 10.3109/00207459408986063. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Abrams RA, Kornblum S, Wright CE, Smith JE. Optimality in human motor performance: ideal control of rapid aimed movements. Psychological Review. 1988;95(3):340–370. doi: 10.1037/0033-295x.95.3.340. [DOI] [PubMed] [Google Scholar]
- Milner TE. A model for the generation of movements requiring endpoint precision. Neuroscience. 1992;49(2):487–496. doi: 10.1016/0306-4522(92)90113-g. [DOI] [PubMed] [Google Scholar]
- Mitrushina M, Fogel T, D’Elia L, Uchiyama C, Satz P. Performance on motor tasks as an indication of increased behavioral asymmetry with advancing age. Neuropsychologia. 1995;33(3):359–364. doi: 10.1016/0028-3932(94)00113-4. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pohl PS, Winstein CJ, Fisher BE. The locus of age-related movement slowing: sensory processing in continuous goal-directed aiming. Journals of Gerontology Series B Psychological Sciences and Social Sciences. 1996;51(2):94–102. doi: 10.1093/geronb/51b.2.p94. [DOI] [PubMed] [Google Scholar]
- Porac C, Coren S, Duncan P. Life-span age trends in laterality. Journal of Gerontology. 1980;35(5):715–721. doi: 10.1093/geronj/35.5.715. [DOI] [PubMed] [Google Scholar]
- Porac C, Friesen IC. Hand preference side and its relation to hand preference switch history among old and oldest-old adults. Developmental Neuropsychology. 2000;17(2):225–239. doi: 10.1207/S15326942DN1702_05. [DOI] [PubMed] [Google Scholar]
- Pratt J, Chasteen AL, Abrams RA. Rapid aimed limb movements: age differences and practice effects in component submovements. Psychology and Aging. 1994;9(2):325–334. doi: 10.1037//0882-7974.9.2.325. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7(3):268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12(1):174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Riecker A, Groschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A. Functional significance of age-related differences in motor activation patterns. Neuroimage. 2006;32(3):1345–1354. doi: 10.1016/j.neuroimage.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Fasoli S, Krebs HI, Volpe B, Frontera WR, Stein J, et al. Submovements grow larger, fewer, and more blended during stroke recovery. Motor Control. 2004;8(4):472–483. doi: 10.1123/mcj.8.4.472. [DOI] [PubMed] [Google Scholar]
- Romero DH, Van Gemmert AWA, Adler CH, Bekkering H, Stelmach GE. Altered aiming movements in Parkinson’s disease patients and elderly adults as a function of delays in movement onset. Experimental Brain Research. 2003a;151(2):249–261. doi: 10.1007/s00221-003-1452-2. [DOI] [PubMed] [Google Scholar]
- Romero DH, Van Gemmert AWA, Adler CH, Bekkering H, Stelmach GE. Time delays prior to movement alter the drawing kinematics of elderly adults. Human Movement Science. 2003b;22(2):207–220. doi: 10.1016/s0167-9457(02)00160-4. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Experimental Brain Research. 2002;142(2):241–258. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL. Handedness: differential specializations for control of trajectory and position. Exercise and Sport Sciences Reviews. 2005;33(4):206–213. doi: 10.1097/00003677-200510000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. Journal of Neurophysiology. 2000;83(5):2661–2675. doi: 10.1152/jn.2000.83.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg RL, Schaefer SY. Interlimb differences in control of movement extent. Journal of Neurophysiology. 2004;92(3):1374–1383. doi: 10.1152/jn.00181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. Journal of Applied Physiology. 2005;99(4):1483–1493. doi: 10.1152/japplphysiol.00371.2005. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Adult age and the speed-accuracy trade-off. Ergonomics. 1979;22(7):811–821. doi: 10.1080/00140137908924659. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Somberg BL. Isolating the age deficit in speeded performance. Journal of Gerontology. 1982;37(1):59–63. doi: 10.1093/geronj/37.1.59. [DOI] [PubMed] [Google Scholar]
- Seidler-Dobrin RD, Stelmach GE. Persistence in visual feedback control by the elderly. Experimental Brain Research. 1998;119(4):467–474. doi: 10.1007/s002210050362. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Alberts JL, Stelmach GE. Changes in multi-joint performance with age. Motor Control. 2002;6(1):19–31. doi: 10.1123/mcj.6.1.19. [DOI] [PubMed] [Google Scholar]
- Stelmach GE, Goggin NL, Amrhein PC. Aging and the restructuring of precued movements. Psychology and Aging. 1988;3(2):151–157. doi: 10.1037//0882-7974.3.2.151. [DOI] [PubMed] [Google Scholar]
- Strayer DL, Wickens CD, Braune R. Adult age differences in the speed and capacity of information processing: 2. An electrophysiological approach. Psychology and Aging. 1987;2(2):99–110. doi: 10.1037//0882-7974.2.2.99. [DOI] [PubMed] [Google Scholar]
- Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Experimental Neurology. 1997;146(1):159–170. doi: 10.1006/exnr.1997.6507. [DOI] [PubMed] [Google Scholar]
- Thomas JR, Yan JH, Stelmach GE. Movement substructures change as a function of practice in children and adults. Journal of Experimental Child Psychology. 2000;75(3):228–244. doi: 10.1006/jecp.1999.2535. [DOI] [PubMed] [Google Scholar]
- Todor JI, Cisneros J. Accommodation to increased accuracy demands by the right and left hands. Journal of Motor Behavior. 1985;17(3):355–372. doi: 10.1080/00222895.1985.10735354. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Thulborn KR, Corcos DM. Neural basis for the processes that underlie visually guided and internally guided force control in humans. Journal of Neurophysiology. 2003;90(5):3330–3340. doi: 10.1152/jn.00394.2003. [DOI] [PubMed] [Google Scholar]
- van Donkelaar P, Franks IM. Preprogramming vs. on-line control in simple movement sequences. Acta Psychologica (Amst) 1991;77(1):1–19. doi: 10.1016/0001-6918(91)90061-4. [DOI] [PubMed] [Google Scholar]
- Van Gemmert AWA, Teulings HL, Contreras-Vidal JL, Stelmach GE. Parkinson’s disease and the control of size and speed in handwriting. Neuropsychologia. 1999;37(6):685–694. doi: 10.1016/s0028-3932(98)00122-5. [DOI] [PubMed] [Google Scholar]
- Van Gemmert AWA, Teulings HL, Stelmach GE. The influence of mental and motor load on handwriting movements in parkinsonian patients. Acta Psychologica (Amst) 1998;100(1–2):161–175. doi: 10.1016/s0001-6918(98)00032-8. [DOI] [PubMed] [Google Scholar]
- Velay JL, Benoit-Dubrocard S. Hemispheric asymmetry and interhemispheric transfer in reaching programming. Neuropsychologia. 1999;37(8):895–903. doi: 10.1016/s0028-3932(98)00149-3. [DOI] [PubMed] [Google Scholar]
- Velay JL, Daffaure V, Raphael N, Benoit-Dubrocard S. Hemispheric asymmetry and interhemispheric transfer in pointing depend on the spatial components of the movement. Cortex. 2001;37(1):75–90. doi: 10.1016/s0010-9452(08)70559-8. [DOI] [PubMed] [Google Scholar]
- von Hofsten C. Structuring of early reaching movements: a longitudinal study. Journal of Motor Behavior. 1991;23(4):280–292. doi: 10.1080/00222895.1991.9942039. [DOI] [PubMed] [Google Scholar]
- Walker N, Philbin DA, Fisk AD. Age-related differences in movement control: adjusting submovement structure to optimize performance. Journals of Gerontology Series B Psychological Sciences and Social Sciences. 1997;52(1):40–52. doi: 10.1093/geronb/52b.1.p40. [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. The dominant and nondominant arms are specialized for stabilizing different features of task performance. Experimental Brain Research. 2007;178(4):565–570. doi: 10.1007/s00221-007-0936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warabi T, Noda H, Yanagisawa N, Tashiro K, Shindo R. Changes in sensorimotor function associated with the degree of bradykinesia of Parkinson’s disease. Brain. 1986;109(Pt 6):1209–1224. doi: 10.1093/brain/109.6.1209. [DOI] [PubMed] [Google Scholar]
- Welford AT. Between bodily changes and performance: some possible reasons for slowing with age. Experimental Aging Research. 1984;10(2):73–88. doi: 10.1080/03610738408258548. [DOI] [PubMed] [Google Scholar]
- Woodworth R. The accuracy of voluntary movement. Physiological Review Monographs. 1899;3(13):1–114. [Google Scholar]
- Yan JH. Effects of aging on linear and curvilinear aiming arm movements. Experimental Aging Research. 2000;26(4):393–407. doi: 10.1080/036107300750015778. [DOI] [PubMed] [Google Scholar]
- Yund EW, Efron R, Nichols DR. Target detection in one visual field in the presence or absence of stimuli in the contralateral field by right- and left-handed subjects. Brain and Cognition. 1990;12(1):117–127. doi: 10.1016/0278-2626(90)90008-c. [DOI] [PubMed] [Google Scholar]