Abstract
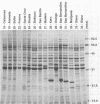
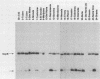
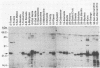
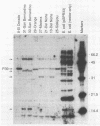
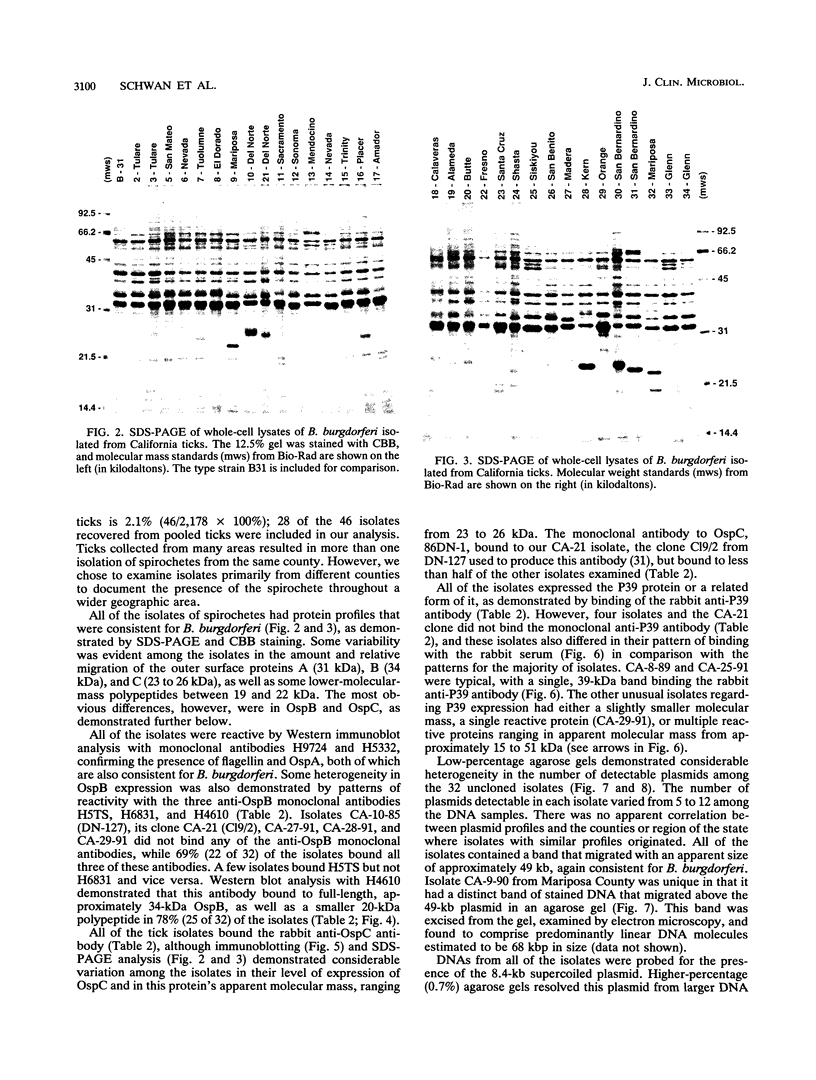
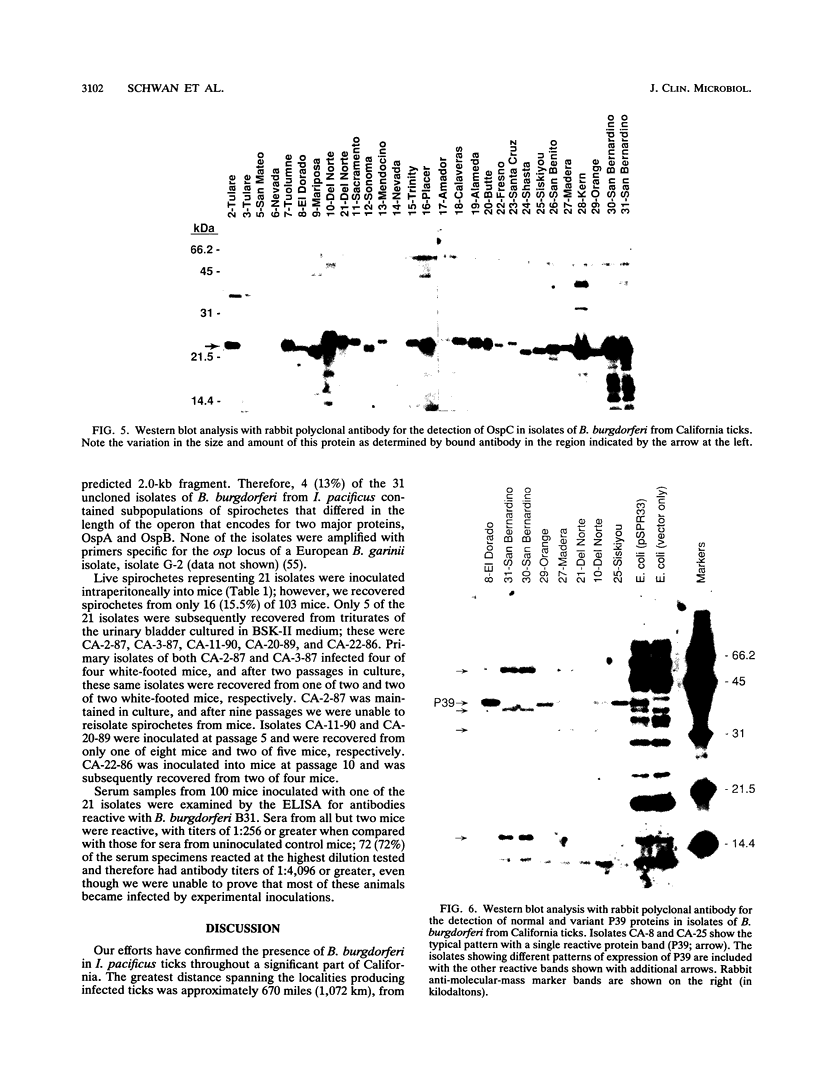
Previous studies describing the occurrence and molecular characteristics of Lyme disease spirochetes, Borrelia burgdorferi, from California have been restricted primarily to isolates obtained from the north coastal region of this large and ecologically diverse state. Our objective was to look for and examine B. burdorferi organisms isolated from Ixodes pacificus ticks collected from numerous regions spanning most parts of California where this tick is found. Thirty-one isolates of B. burgdorferi were examined from individual or pooled I. pacificus ticks collected from 25 counties throughout the state. One isolate was obtained from ticks collected at Wawona Campground in Yosemite National Park, documenting the occurrence of the Lyme disease spirochete in an area of intensive human recreational use. One isolate from an Ixodes neotomae tick from an additional county was also examined. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunoblot analysis, agarose gel electrophoresis, Southern blot analysis, and the polymerase chain reaction were used to examine the molecular and genetic determinants of these uncloned, low-passage-number isolates. All of the isolates were identified as B. burgdorferi by their protein profiles and reactivities with monoclonal and polyclonal antibodies, and all the isolates were typed by the polymerase chain reaction as North American-type spirochetes (B. burgdorferi sensu stricto). Although products of the ospAB locus were identified in protein analyses in all of the isolates, several isolates contained deleted forms of this locus that would result in the expression of chimeric OspA-OspB proteins. The analysis of OspC demonstrated that this protein was widely conserved among the isolates but was also quite variable in its molecular mass and the amount of it that was expressed.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam T., Gassmann G. S., Rasiah C., Göbel U. B. Phenotypic and genotypic analysis of Borrelia burgdorferi isolates from various sources. Infect Immun. 1991 Aug;59(8):2579–2585. doi: 10.1128/iai.59.8.2579-2585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranton G., Postic D., Saint Girons I., Boerlin P., Piffaretti J. C., Assous M., Grimont P. A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992 Jul;42(3):378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. Linear plasmids of the bacterium Borrelia burgdorferi have covalently closed ends. Science. 1987 Jul 24;237(4813):409–411. doi: 10.1126/science.3603026. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Immunobiology of relapsing fever. Contrib Microbiol Immunol. 1987;8:125–137. [PubMed] [Google Scholar]
- Barbour A. G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988 Mar;26(3):475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Schrumpf M. E. Polymorphisms of major surface proteins of Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):83–91. doi: 10.1016/s0176-6724(86)80107-9. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril C., Richaud C., Baranton G., Saint Girons I. S. Linear chromosome of Borrelia burgdorferi. Res Microbiol. 1989 Oct;140(8):507–516. doi: 10.1016/0923-2508(89)90083-1. [DOI] [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Bissett M. L., Hill W. Characterization of Borrelia burgdorferi strains isolated from Ixodes pacificus ticks in California. J Clin Microbiol. 1987 Dec;25(12):2296–2301. doi: 10.1128/jcm.25.12.2296-2301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce W. M., Brown R. N., Zingg B. C., Lefebvre R. B., Lane R. S. First isolation of Borrelia burgdorferi in southern California. J Med Entomol. 1992 May;29(3):496–500. doi: 10.1093/jmedent/29.3.496. [DOI] [PubMed] [Google Scholar]
- Brown R. N., Lane R. S. Lyme disease in California: a novel enzootic transmission cycle of Borrelia burgdorferi. Science. 1992 Jun 5;256(5062):1439–1442. doi: 10.1126/science.1604318. [DOI] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Lane R. S., Barbour A. G., Gresbrink R. A., Anderson J. R. The western black-legged tick, Ixodes pacificus: a vector of Borrelia burgdorferi. Am J Trop Med Hyg. 1985 Sep;34(5):925–930. doi: 10.4269/ajtmh.1985.34.925. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Schwan T. G. Lyme borreliosis: a relapsing fever-like disease? Scand J Infect Dis Suppl. 1991;77:17–22. [PubMed] [Google Scholar]
- Campagna J., Lavoie P. E., Birnbaum N. S., Furman D. P. Lyme disease in northern California. West J Med. 1983 Sep;139(3):319–323. [PMC free article] [PubMed] [Google Scholar]
- Czub S., Duray P. H., Thomas R. E., Schwan T. G. Cystitis induced by infection with the Lyme disease spirochete, Borrelia burgdorferi, in mice. Am J Pathol. 1992 Nov;141(5):1173–1179. [PMC free article] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R., Jauris S., Lottspeich F., Preac-Mursic V., Wilske B., Soutschek E. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22 kDa protein (pC) in Escherichia coli. Mol Microbiol. 1992 Feb;6(4):503–509. doi: 10.1111/j.1365-2958.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Marek N., Kodner C. Infection of Syrian hamsters with Lyme disease spirochetes. J Clin Microbiol. 1984 Dec;20(6):1099–1101. doi: 10.1128/jcm.20.6.1099-1101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriuchechnikov V. N., Korenberg E. I., Shcherbakov S. V., Kovalevskii Iu V., Levin M. L. Identifikatsiia borrelii, izolirovannykh v SSSR ot kleshchei Ixodes persulcatus Schulze. Zh Mikrobiol Epidemiol Immunobiol. 1988 Dec;(12):41–44. [PubMed] [Google Scholar]
- Kurashige S., Bissett M., Oshiro L. Characterization of a tick isolate of Borrelia burgdorferi that possesses a major low-molecular-weight surface protein. J Clin Microbiol. 1990 Jun;28(6):1362–1366. doi: 10.1128/jcm.28.6.1362-1366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Brown R. N. Wood rats and kangaroo rats: potential reservoirs of the Lyme disease spirochete in California. J Med Entomol. 1991 May;28(3):299–302. doi: 10.1093/jmedent/28.3.299. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Burgdorfer W. Potential role of native and exotic deer and their associated ticks (Acari: Ixodidae) in the ecology of Lyme disease in California, USA. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):55–64. doi: 10.1016/s0176-6724(86)80103-1. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Burgdorfer W. Spirochetes in mammals and ticks (Acari: Ixodidae) from a focus of Lyme borreliosis in California. J Wildl Dis. 1988 Jan;24(1):1–9. doi: 10.7589/0090-3558-24.1.1. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Burgdorfer W. Transovarial and transstadial passage of Borrelia burgdorferi in the western black-legged tick, Ixodes pacificus (Acari: Ixodidae). Am J Trop Med Hyg. 1987 Jul;37(1):188–192. doi: 10.4269/ajtmh.1987.37.188. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Lavoie P. E. Lyme borreliosis in California. Acarological, clinical, and epidemiological studies. Ann N Y Acad Sci. 1988;539:192–203. doi: 10.1111/j.1749-6632.1988.tb31853.x. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Pascocello J. A. Antigenic characteristics of Borrelia burgdorferi isolates from ixodid ticks in California. J Clin Microbiol. 1989 Oct;27(10):2344–2349. doi: 10.1128/jcm.27.10.2344-2349.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R. S., Piesman J., Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- LeFebvre R. B., Lane R. S., Perng G. C., Brown J. A., Johnson R. C. DNA and protein analyses of tick-derived isolates of Borrelia burgdorferi from California. J Clin Microbiol. 1990 Apr;28(4):700–707. doi: 10.1128/jcm.28.4.700-707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi R. T., Garon C. F. Development of polymerase chain reaction primer sets for diagnosis of Lyme disease and for species-specific identification of Lyme disease isolates by 16S rRNA signature nucleotide analysis. J Clin Microbiol. 1992 Nov;30(11):2830–2834. doi: 10.1128/jcm.30.11.2830-2834.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather T. N., Telford S. R., 3rd, Adler G. H. Absence of transplacental transmission of Lyme disease spirochetes from reservoir mice (Peromyscus leucopus) to their offspring. J Infect Dis. 1991 Sep;164(3):564–567. doi: 10.1093/infdis/164.3.564. [DOI] [PubMed] [Google Scholar]
- Mickel S., Arena V., Jr, Bauer W. Physical properties and gel electrophoresis behavior of R12-derived plasmid DNAs. Nucleic Acids Res. 1977;4(5):1465–1482. doi: 10.1093/nar/4.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Naversen D. N., Gardner L. W. Erythema chronicum migrans in America. Arch Dermatol. 1978 Feb;114(2):253–254. [PubMed] [Google Scholar]
- Norris S. J., Carter C. J., Howell J. K., Barbour A. G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992 Nov;60(11):4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. H., Jr, Owsley M. R., Hutcheson H. J., James A. M., Chen C., Irby W. S., Dotson E. M., McLain D. K. Conspecificity of the ticks Ixodes scapularis and I. dammini (Acari: Ixodidae). J Med Entomol. 1993 Jan;30(1):54–63. doi: 10.1093/jmedent/30.1.54. [DOI] [PubMed] [Google Scholar]
- Piesman J., Donahue J. G., Mather T. N., Spielman A. Transovarially acquired Lyme disease spirochetes (Borrelia burgdorferi) in field-collected larval Ixodes dammini (Acari: Ixodidae). J Med Entomol. 1986 Mar 31;23(2):219–219. doi: 10.1093/jmedent/23.2.219. [DOI] [PubMed] [Google Scholar]
- Péter O., Bretz A. G. Polymorphism of outer surface proteins of Borrelia burgdorferi as a tool for classification. Zentralbl Bakteriol. 1992 Jun;277(1):28–33. doi: 10.1016/s0934-8840(11)80867-4. [DOI] [PubMed] [Google Scholar]
- Rosa P. A., Hogan D., Schwan T. G. Polymerase chain reaction analyses identify two distinct classes of Borrelia burgdorferi. J Clin Microbiol. 1991 Mar;29(3):524–532. doi: 10.1128/jcm.29.3.524-532.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T. G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989 Dec;160(6):1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T., Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992 Oct;6(20):3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Sadziene A., Wilske B., Ferdows M. S., Barbour A. G. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993 May;61(5):2192–2195. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987 Nov;156(5):852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Schrumpf M. E., Karstens R. H. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J Clin Microbiol. 1988 May;26(5):893–895. doi: 10.1128/jcm.26.5.893-895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Karstens R. H., Schrumpf M. E., Simpson W. J. Changes in antigenic reactivity of Borrelia burgdorferi, the Lyme disease spirochete, during persistent infection in mice. Can J Microbiol. 1991 Jun;37(6):450–454. doi: 10.1139/m91-074. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Kime K. K., Schrumpf M. E., Coe J. E., Simpson W. J. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi). Infect Immun. 1989 Nov;57(11):3445–3451. doi: 10.1128/iai.57.11.3445-3451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Schrumpf M. E., Gage K. L., Gilmore R. D., Jr Analysis of Leptospira spp., Leptonema illini, and Rickettsia rickettsii for the 39-kilodalton antigen (P39) of Borrelia burgdorferi. J Clin Microbiol. 1992 Mar;30(3):735–738. doi: 10.1128/jcm.30.3.735-738.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan T. G., Simpson W. J. Factors influencing the antigenic reactivity of Borrelia burgdorferi, the Lyme disease spirochete. Scand J Infect Dis Suppl. 1991;77:94–101. [PubMed] [Google Scholar]
- Schwan T. G., Simpson W. J., Schrumpf M. E., Karstens R. H. Identification of Borrelia burgdorferi and B. hermsii using DNA hybridization probes. J Clin Microbiol. 1989 Aug;27(8):1734–1738. doi: 10.1128/jcm.27.8.1734-1738.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. J., Burgdorfer W., Schrumpf M. E., Karstens R. H., Schwan T. G. Antibody to a 39-kilodalton Borrelia burgdorferi antigen (P39) as a marker for infection in experimentally and naturally inoculated animals. J Clin Microbiol. 1991 Feb;29(2):236–243. doi: 10.1128/jcm.29.2.236-243.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. J., Garon C. F., Schwan T. G. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb Pathog. 1990 Feb;8(2):109–118. doi: 10.1016/0882-4010(90)90075-2. [DOI] [PubMed] [Google Scholar]
- Simpson W. J., Schrumpf M. E., Hayes S. F., Schwan T. G. Molecular and immunological analysis of a polymorphic periplasmic protein of Borrelia burgdorferi. J Clin Microbiol. 1991 Sep;29(9):1940–1948. doi: 10.1128/jcm.29.9.1940-1948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. J., Schrumpf M. E., Schwan T. G. Reactivity of human Lyme borreliosis sera with a 39-kilodalton antigen specific to Borrelia burgdorferi. J Clin Microbiol. 1990 Jun;28(6):1329–1337. doi: 10.1128/jcm.28.6.1329-1337.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsky R. J., Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J Clin Microbiol. 1989 Aug;27(8):1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steere A. C. Lyme disease. N Engl J Med. 1989 Aug 31;321(9):586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- Zingg B. C., Brown R. N., Lane R. S., LeFebvre R. B. Genetic diversity among Borrelia burgdorferi isolates from wood rats and kangaroo rats in California. J Clin Microbiol. 1993 Dec;31(12):3109–3114. doi: 10.1128/jcm.31.12.3109-3114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]