The carcinogenic risk induced by low doses of ionizing radiation is controversial. It cannot be assessed with epidemiologic methods alone because at low doses the data are imprecise and often conflicting. Since the 1970s, the radiation protection community has estimated the risk of low doses by means of extrapolation from the risk assessed at high doses, generally by using the linear no-threshold (LNT) model.
The LNT relationship implies proportionality between dose and cancer risk. This approach is based on one set of data and two hypotheses: (a) The relationship between dose and DNA damage in vivo seems linear from 1 mGy to 100 Gy with use of H2AX foci as a marker for DNA double-strand breaks (DSBs)—however, this marker is not specific (1); (b) each DSB is hypothesized to have the same probability of inducing cell transformation, irrespective of the quantity of DSBs present simultaneously in the cell; and (c) each transformed cell is hypothesized to have the same probability of developing into an invasive cancer, irrespective of the dose delivered to the tissue. The advances during the past 2 decades in radiation biology, the understanding of carcinogenesis, and the discovery of defenses against carcinogenesis challenge the LNT model, which appears obsolete (2–6).
Life developed in a bath of ionizing radiation and solar ultraviolet radiation and created aerobic organisms requiring (a) defenses against the metabolically induced reactive oxygen species, (b) DNA repair, and (c) elimination of damaged cells. Several sets of data show the efficacy of these defenses to be much higher at low than at high doses and for fractionated or protracted irradiation than for acute irradiation.
The LNT model was introduced as a concept to facilitate radiation protection (7). But the use of this model led to the claim that even the smallest dose (one electron traversing a cell) may initiate carcinogenesis—for instance, from diagnostic x-ray sources (8,9). This claim is highly hypothetical and has resulted in medical, economic, and other societal harm.
The French Academies report (10) concluded that the LNT model and its use for assessing the risks associated with low doses are not based on scientific evidence. In contrast, the Biological Effects of Ionizing Radiation (BEIR) VII report (11) and that of the International Commission on Radiological Protection (ICRP) (12) recommended the use of the LNT model. We wish to update this debate by using recent radiation biologic and epidemiologic data.
THE RADIATION BIOLOGIC DATA
Ionizing radiation interacts randomly with molecules along charged particle tracks and may damage DNA either through direct events in the molecule (ionization or excitation) or, more frequently, through indirect mechanisms mediated by reactive oxygen species produced by radiogenic hydrolysis. Reactive oxygen species also derive abundantly from oxygen metabolism. The life of aerobic organisms would have been impossible without defenses against reactive oxygen species, as shown by the study of patients with congenital diseases.
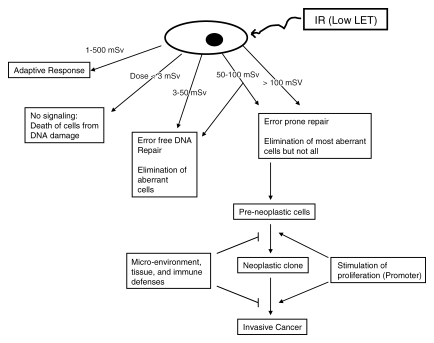
Irradiated cells protect themselves (a) by immediate defense, repair, and damage removal mechanisms and (b) by delayed and temporary protection also against renewed DNA damage, irrespective of its causes—that is, through adaptive responses (Figure).
Figure 1.
Diagram shows that effects of low linear energy transfer (LET) ionizing radiation (IR) on mammalian cells are dose dependent. With doses of less than 3 mSv at high or low dose rates, no signaling occurs, and mitotic cell death ensues. A dose of 3–50 mSv results in primarily error-free DNA repair, along with elimination of aberrant cells through apoptosis or other types of mitotic death. At doses between 50 and 100 mSv, dose rate and cell type play a role in whether a cell undergoes error-free repair or substantial levels of DNA misrepair. With doses greater than 100 mSv, error-prone DNA repair is substantial. Fortunately, the majority of aberrant cells are eliminated through cellular death pathways. Most of the preneoplastic cells are eliminated by immune defenses, inhibited by the microenvironment (and tissue), or become senescent. Cancer promoters may transform a preneoplastic clone to a neoplastic clone to eventual invasive cancer. Again, immune mechanisms and the microenvironment (and tissue) may eliminate and/or suppress the neoplastic cells. Furthermore, after irradiation (1–500 mSv), cells develop adaptive responses that counteract, in hit and non-hit cells, insults from endogenous toxins (eg, reactive oxygen species produced from aerobic metabolism) or exogenous oxidative stresses (eg, renewed irradiation, chemicals). The LNT hypothesis or slight modifications of it with the dose and dose rate effectiveness factor (DDREF) is not compatible with the scientific evidence. The concept of a practical threshold for carcinogenesis is plausible.
Immediate Protections at Cell Level
Three types of immediately operating defense mechanisms have been identified:
Defenses against reactive oxygen species.—Oxygen metabolism, ionizing radiation, ultraviolet radiation, and other insults, such as an infection or physical exercise, can cause reactive oxygen species, with oxidative stress vitally counteracted by scavengers and antioxidant molecules (13,14). These defenses are less effective when the dose is high. Endogenous reactive oxygen species may cause up to about eight DSBs per cell each day, similar to that induced by 200 mGy (or 0.14 mGy/min) (15,16), but these are less severe than radiation-induced DSBs.
DNA repair.—The repair mechanisms present in mammalian cells have existed in yeast for 800 million years. Deinococcus radiodurans bacteria have error-free repair mechanisms that can tolerate doses of 7 kGy (17); in multicellular organisms, the tolerance dose is much lower. Sensor molecules may detect DNA damage and activate signaling factors. In turn, these factors may induce cell cycle arrest and facilitate DNA repair and other defense mechanisms (18–24). The two main repair systems for DSBs are homologous recombination and nonhomologous end joining (NHEJ). The signaling network and the choice of the repair system are influenced by dose, dose rate, nature of radiation, and position of the cell in the cell cycle. At low doses of x-rays, homologous recombination is error free, while NHEJ is low error prone. The efficacy and fidelity of DNA repair diminishes with increasing amounts of DNA damage simultaneously present in a cell. Indeed, the carcinogenic risk is negligible (or nonexistent) at low doses and dose rates (6,21,25) and increases with higher doses and dose rates (21,24–26).
Checkpoints throughout the cell cycle allow for DNA repair or apoptosis and decrease the likelihood of aberrations and genomic instability in a dose-dependent manner (27,28). Also, and contrary to former beliefs, the magnitude of the mutagenic effect (per unit dose) varies with dose rate (29,30), reaching a minimum in the range of 1–10 mGy/min, which corresponds approximately to the rate of reactive oxygen species–inducing DNA damage during oxidative stress (16). The probability of error during the repair of DSBs is low when DSBs are widely separated in space or time but increases drastically when multiple breaks are present simultaneously (26). In humans, intrachromosomal inversions and deletions are not induced by doses less than 100 mSv or at low dose rates (31–33). Also, large studies performed in vivo or in vitro have failed to reveal an increased incidence of chromosomal aberration at doses less than 20 mSv (34).
DNA repair also differs with type of damage, dose, and dose rate through signals from adjacent cells. Human fibroblasts repair DSBs more efficiently after a dose of 20 mGy than after a dose of 5 mGy, with no repair after a dose of 1.2 mGy in confluent cell cultures (1). Low-dose cellular hyperradiosensitivity (HRS) (35,36) decreases with increasing dose and disappears at doses higher than 0.5 Gy because of full repair activation. HRS eliminates at low doses potentially mutant cells, thereby reducing the carcinogenic risk. Defects in DNA repair systems are associated with a higher cancer incidence in animals and in humans. An example is ataxia telangiectasia (37).
Elimination of damaged cells by death or proliferation arrest.—Elimination of cells with altered DNA, a crucial defense mechanism (38–42), may occur by apoptosis shortly after irradiation with doses ranging from a few to about 200 mSv. However, this mechanism of death is less important in most cell types at high doses when the number of cell deaths may cause tissue dysfunction.
At less than a few millisieverts, DNA repair is not activated, and mitotic cell death occurs on resumption of proliferation (1,41). For doses higher than 5 mSv, DNA repair is observed—for example, after computed tomographic (CT) scanning (doses of 10–20 mGy) (42).
Senescence is an alternative pathway for eliminating genetically defective cells without sacrifice of functional advantages before cell death (43–45).
Adaptive Responses
Besides immediate defenses against detrimental effects in irradiated cells, a stimulation of defenses is observed in neighboring hit and non-hit cells, including reactive oxygen species scavenging, DNA repair, and damaged cell removal by apoptosis and cell senescence. These may occur in mammalian cells with a delay of up to hours after low doses and may operate against renewed exposure to radiogenic and nonradiogenic genotoxins for hours to months, depending on the type of upregulation (46–49).
Such adaptive responses are observed in cultured cells and in rodents after doses of 1–500 mGy and disappear with higher doses. Moreover, spontaneously transformed cells may die because of cytokines released by nontransformed cells (50,51). Low-dose irradiation hours or days after a high dose also reduces some of the high-dose genotoxic effects (52). Adaptive responses exist in humans (53–55).
Bystander Effects
Irradiated cells may damage neighboring cells because of signals or products (bystander effects) that have been claimed to cause supralinear dose-effect relationships. However, recent results (56–58) suggest that on the contrary, irradiated cells also protect neighbors, thus acting as an adaptive response. Irradiated fish release products into the water that enhance the defenses against carcinogenesis of other fish swimming in the same water (59).
Furthermore, individuals contaminated with radium (60) or thorium (61) did not show excess cancer when the cumulative dose was less than a few gray. No carcinogenic bystander effect has been observed in these individuals (60,61). The proliferation and promotion of potentially mutant cells seems to be inhibited by normal cells surrounding irradiated cells, and only doses greater than a few gray with resulting massive cell damage promote carcinogenesis.
Genomic Instability
Persistent DNA damage causing chromosomal aberrations or aneuploidy may lead to genomic instability in cell progeny (62–65). However, doses less than 250 mSv of x or γ radiation yield no genetic instability (65–67). This also appears to pertain to low tissue doses from α particles in humans (10,16). The argument that low-dose irradiation can induce a genomic instability, which can in turn induce a cancer, is not based on convincing data. Moreover, some experimental data, such as those of Boulton et al (68), show that in mice there is no significant correlation between sensitivity to radiation-induced genomic instability and cancer induction.
Defenses at Tissue Level
Neighboring cells control the proliferation of each other (69–72). Several factors, such as infection and inflammation (71,73), facilitate the emergence of fully transformed clonal cells or enhance malignant clonal proliferation. Precancerous cells can acquire the capacity to overcome and also to manipulate protective mechanisms, in order to be recognized as a “friend” instead of being fought as a “foe” (72). Such situations occur after impairment of the microenvironment (74–76) or massive cell death following exposure to large amounts of any genotoxic agent (physical or chemical), with subsequent compensatory cell proliferation by homeostatic mechanisms (10,16). Tissue disorganization through disease also facilitates the escape of preneoplastic subclones from the barriers of the microenvironment. For example, liver cirrhosis and lung fibrosis increase the likelihood of cancer in the liver and lung.
Immune mechanisms counteract disease-promoting agents and kill abnormal cells (77–79). Immunosuppression often precedes cancer (80). Immune competence may be upregulated by low-dose radiation and reduce cancer risk (78,81).
When few cells are damaged, cell death seems the simplest way to avoid the presence of transformed cells (10,78). However, when the dose becomes higher than about 200 mGy, cellular impairment and death increase tissue endangerment; thus, DNA repair becomes mandatory despite the risk of misrepair and mutation. This conclusion is consistent with microarray data: (a) The sets of genes that are activated or repressed are not the same after a low or a high dose (82–84) or dose rate (85) and (b) the temporal gene expressions are also not the same after high doses (3 hours after a 2-Gy dose) and low doses (only after 48 hours for a 10-mGy dose) (84). This is consistent with proteomics and transcriptional changes being different at high and low doses, not only quantitatively but also qualitatively (86).
Hormesis
Upregulation of protective mechanisms at the cell and tissue levels by low doses likely also operates against carcinogenic factors other than ionizing radiation and against spontaneous cancer, as demonstrated in various experiments in vitro and in vivo (46,87–93), Indeed, a dose of 10 mGy reduces the rate of spontaneous transformation in culture cells below the background level. Some epidemiologic data suggest that hormesis also exists in humans (94).
Summary
Biologic data demonstrate that the defense mechanisms against radiation-induced carcinogenesis are powerful and diverse (Figure) (10,16,78,95,96). This is not surprising, because organisms have been subjected to reactive oxygen species from physiologic processes and environmental insults during evolution. Life is characterized by the ability to build defenses against toxic agents, whether internal or environmental. The defenses are overwhelmed at high doses and are stimulated at low doses, which is incompatible with the LNT model.
EPIDEMIOLOGIC DATA
Analysis of the main studies reported on radiation-induced carcinogenesis leads us to conclude that there is no convincing evidence of a carcinogenic effect in humans or experimental animals for doses of less than 100 mGy of low linear energy transfer radiation (10,12,97). Little et al (98) have reached another conclusion, because their interpretations of some surveys differ.
Japanese Atomic Bomb Survivors
Leukemia.—Little and Muirhead (99,100) have shown that the dose response is not linear at doses less than 150 mSv. In fact, a quasi-threshold, or even a hormetic effect, may exist below this dose.
Solid tumors.—Preston et al (101,102) analyzed all solid tumors together. Because histologically distinct human tumors have widely different dose responses (103), mixing tumors leads to debatable results.
As epidemiologic data are inaccurate at low doses (104,105), one may fit them with a linear response relationship, a linear quadratic response relationship, a quadratic response relationship, a threshold somewhere between 40 and 60 mSv (101,102), or even a hormetic response relationship. Heidenreich et al (104) concluded that there was no significant increase in cancer incidence or mortality at doses less than 200 mSv, whereas other authors believe that this threshold is less than 100 mSv (9,11).
The concept that cancer induction proceeds similarly after low and high doses and dose rates is inconsistent with biologic evidence. Hence, assessments of low-dose risks in cohorts of atomic bomb survivors, radiation workers, or people exposed to radon at home included individuals who received more than 100 mSv and thus are subject to bias (105). When investigating risks at doses less than 100 mSv only, cancer excess is not seen. This does not exclude the possibility of an effect too small to be detectable, but suggests that the carcinogenic risk, if it exists, should be very low and of a debatable importance.
Atomic bomb survivors were also exposed to neutrons, of which small doses are potentially carcinogenic. A relative biological effectiveness (RBE) of 10 was used for calculating the dose equivalent (in sieverts). An RBE of 30 would have been more appropriate. Neutrons may explain the putative carcinogenic effect of low doses (104,106,107).
Confounding factors.—The atomic bomb survivors were exposed to radiation and to other carcinogenic agents. For example, the September 11, 2001, World Trade Center attack in New York City released into the environment a variety of toxins, causing first responders to suffer from acute aerodigestive symptoms with persistent respiratory inflammation and possibly granulomatous disease (108). Long-term effects are being investigated (109). The atomic bombs released far greater levels of nonradioactive toxins and caused burns, with chronic inflammation being potentially carcinogenic. Firefighters have increased cancer risks, suggesting that inhalation of carcinogenic and toxic compounds brings an occupational cancer risk (110–115).
In summary, the analyses of the atomic bomb data do not provide solid arguments for the LNT model.
In Utero Irradiation
There is the claim that prenatal irradiation with x-rays delivering a dose of 10–20 mGy increases cancer risk during childhood by about 40% (116). Results of a large case-control study did not support this finding (117). Among the confounding factors are the more frequent antenatal examinations with x-rays in older mothers and in mothers of high socioeconomic status, which are both factors known to be associated with an increased risk of childhood cancer (118). Underlying diseases leading to increased examinations with x-rays cannot be excluded as risks of childhood cancer (16). A Swedish population-based study matched children with leukemia with database records of instances of antenatal irradiation with x-rays (without recourse to interviews) and found no association between in utero irradiation and leukemias (119). Twins have a higher probability of in utero irradiation than singletons, and there was no increased leukemia risk (120,121). No increased cancer risk has been observed after in utero irradiation in atomic bomb survivors (122) or in experimental animals. Hence, the International Agency for Research on Cancer expert committee did not accept antenatal irradiation with x-rays as an established cancer risk factor (118).
Chernobyl Accident
Contrary to previous claims, there was no increase in leukemia or other cancers (except thyroid cancer) in regions contaminated after the Chernobyl accident where thyroid doses ranged up to 1 Sv (123). The increase in thyroid cancer among young children is correlated with dose (124), and a threshold at 200 mSv is compatible with data (125).
Occupational Exposure
An excess of skin cancer and leukemia was detected prior to 1950 in radiologists and radiologic technicians. Following the implementation of the recommendations of the ICRP in 1930, this excess decreased and disappeared among those beginning their practice after 1950, despite many receiving annual doses greater than 50 mSv (126–129). Similar data exist for airplane crews (130).
Among workers at nuclear power plants, a first study in 85 000 workers (131) did not reveal any increase in solid tumors but found a small increase in leukemia for those exposed to doses greater than 400 mSv. In a larger cohort (407 391 workers) studied subsequently (132), the cancer incidence after a cumulative dose of less than about 150 mSv was indistinguishable from that in the control group. Questionably elevated cancer incidences were observed only in Canadian workers and in those at the Oak Ridge Laboratory (Oak Ridge, Tenn); these two cohorts included workers who were active during World War II, when dosimetry was not a main concern. Another study of Canadian workers who worked in later years (133) found a cancer mortality rate of 0.7 of that in the matched control population.
For large numbers of luminescent dial workers contaminated with radium (60) and patients contaminated with thorium (61), follow-up was as long as that for atomic bomb survivors. Cancers (osteosarcoma or liver cancers) were not observed for doses less than a few gray (60,61,134).
High Background Radiation
Most studies around nuclear plants (135) have not detected any excess cases of cancers and leukemia or other diseases. Around some plants, a small excess of leukemia cases among children has been observed (136). However, this excess does not appear to be related to irradiation of children or their parents but rather to have been caused by a virus and population mixing (137–141).
People living in Kerala, India, experience lifetime terrestrial irradiation of up to 70 mSv a year, much higher than other populations in India, without an increased risk of carcinogenesis. In China (Yangjiang and its surrounding area), people are exposed to two levels of radiation per year—6.4 and 2.4 mSv, respectively. There was no increase in cancer or mortality (142,143), although the higher level of background radiation was confirmed by a higher incidence of chromosomal aberration (144). The existence of these aberrations despite the absence of a cancer excess is an argument in favor of the absence of a causal relationship between a chromosomal aberration and cancers.
Irradiation with X-rays for Diagnostic Examinations
Several studies in patients after x-ray–based examinations (145–147) have not detected any increase in leukemia or solid tumors. The only positive studies were in girls or young women after repeated chest fluoroscopic procedures for chronic tuberculosis (148,149) or scoliosis (150). Among these patients, excess breast cancer was detected only for cumulative doses greater than about 0.5 Gy. No other excess cancer appeared after cumulative doses up to 1 Gy. There was also no increased cancer after cardiac catheterization (147).
Several studies stressed the risk of cancer after diagnostic irradiation with x-rays by using the LNT model (8,9). However, several investigators (151–153) have questioned these estimates because of their doubtful assumptions. An overestimate of the diagnostic radiology risk may deprive patients from adequate treatment (154,155).
Radiation Therapy
One million patients each year undergo radiation therapy. By using cancer registries, investigators have indicated that the incidence of second primary cancers is incompatible with the LNT model and with the risk coefficients derived from Hiroshima and Nagasaki (103,156,157). The cumulative doses outside the target volume (158) ranged from a few milligrays to 60 Gy. A low dose rate (<15 mGy/min) reduced carcinogenesis not only in experimental animals (97,103) but also in patients (103,159,160). A dose per fraction of less than 120–160 mGy cumulating to about 3 Gy caused less carcinogenesis than higher doses per fraction (103,154,157). A threshold has been reported at about 0.6 Gy (corresponding to doses per fraction of 20 mGy); above this threshold, the dose-effect relationship appears to be quadratic (154).
An excess of solid tumors and leukemia has been observed in patients after treatment with several hundred millicuries of iodine 131 (131I) for thyroid cancer (161). No excess cancer was detected among the large number of patients after they received 10–20 mCi (370–740 MBq) of 131I for the treatment of hyperthyroidism (162). Moreover, a preliminary meta-analysis in 415 000 individuals exposed to less than 100 mSv showed no cancer excess (163).
α Particles are at least as carcinogenic as electrons. Therefore, the absence of cancer for doses less than a few grays, such as in patients who received Thorotrast and who have large numbers of cells that have been traversed by an α particle, cannot be overlooked (61,134).
CONCLUSION
There are potent defenses against the carcinogenic effects of ionizing radiation. Their efficacy is much higher for low doses and dose rates; this is incompatible with the LNT model but is consistent with current models of carcinogenesis (16). The data suggest that a combination of error-free DNA repair and elimination of preneoplastic cells furnishes practical thresholds (Figure).
For low linear energy transfer radiation, experimental animal data show the absence of carcinogenic effects for acute irradiation at doses less than 100 mSv and for chronic irradiation at doses less than 500 Sv (97,103,164).
Among humans, there is no evidence of a carcinogenic effect for acute irradiation at doses less than 100 mSv and for protracted irradiation at doses less than 500 mSv (10,103,147,163). Surveys of second primary malignancies in patients who have undergone radiation therapy should provide more information (103,154,157).
The fears associated with the concept of LNT and the idea that any dose, even the smallest, is carcinogenic lack scientific justification (10,16,78,163).
The Chernobyl accident showed that overestimating radiation risks could be more detrimental than underestimating them. Misinformation partially led to traumatic evacuations of about 200 000 individuals, an estimated 1250 suicides, and between 100 000 and 200 000 elective abortions outside the Union of Soviet Socialist Republics (164,165).
The DDREF attempts to overcome discrepancies between experimental or epidemiologic data and LNT predictions; it implies that for low doses and/or dose rates, the probability for DNA damage to be carcinogenic is reduced by half. However, the DDREF leaves unchanged the highly debatable concept that even the smallest dose can induce cancer.
Overestimating the risks from diagnostic irradiation with x-rays may harm patients when appropriate studies are withheld for fear of potential malpractice (155).
The LNT model (with or without the DDREF) is inconsistent with biologic and experimental data, which show the nature and the effectiveness of immediate and delayed defense systems to vary widely with dose and dose rate. No convincing epidemiologic data support the LNT relationship. It has been said that for low doses, epidemiology faces its limits (166).
Defenses against the oxidative radicals created by water radiolysis are very effective for doses that create a number of radicals similar to those observed during oxidative stress. These defenses are poorer against high doses or dose rates greater than 0.5 Gy/min. DNA repair systems are very effective at low doses or dose rates (about 5–10 mGy/min) and become more error prone with increasing dose and dose rate.
The elimination of mutant cells by death or proliferation arrest is a crucial defense. Most human cancers display defects in apoptosis or other means of eliminating mutant cells (167). Damaged cells can be eliminated after low doses by means of death, senescence, or immune response.
Low-dose-rate irradiation (approximately 10 mGy/min) is less carcinogenic (per unit dose) than high-dose-rate irradiation (1 Gy/min). Fractionated irradiation is much less carcinogenic than acute irradiation owing to DNA repair during the time interval between fractions. Doses to normal tissue less than 150 mSv per radiation therapy fraction appear to be much less carcinogenic than higher doses (154,157).
All data suggest the existence of practical thresholds for carcinogenesis. This concept means that below the dose threshold, the carcinogenic risk, if it exists, is so small that it is without clinical importance. The fear of carcinogenesis from diagnostic x-ray examinations (eg, CT) that has been propagated is unjust. It is unethical to fuel anxiety with debatable hypotheses. A balance should be made between the risk, if any, of an x-ray examination and the medical information it provides (155). More human data are urgently needed and could be assembled by studying patients undergoing radiation therapy.
LNT was a useful model half a century ago. But current radiation protection concepts should be based on facts and on concepts consistent with current scientific results and not on opinions. Preconceived concepts impede progress; in the case of the LNT model, they have resulted in substantial medical, economic, and other societal harm.
Discuss this article online at www.rsna.org/radiology/discuss
The views expressed in this article do not represent the views of or endorsement by the United States Goverment or the National Institutes of Health.
See also the article by Little et al in this issue.
Authors stated no financial relationship to disclose.
Funding: J.M.K. is an employee of the National Institutes of Health.
References
- 1.Rothkamm K, Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A 2003;100(9):5057–5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg RA. The biology of cancer. New York, NY: Taylor & Francis, 2006.
- 4.Park CC, Henshall-Powell RL, Erickson AC, et al. Ionizing radiation induces heritable disruption of epithelial cell interactions. Proc Natl Acad Sci U S A 2003;100(19):10728–10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med 2004;10(8):789–799. [DOI] [PubMed] [Google Scholar]
- 6.Trosko JE, Chang CC, Upham BL, Tai MH. Ignored hallmarks of carcinogenesis: stem cells and cell-cell communication. Ann N Y Acad Sci 2004;1028:192–201. [DOI] [PubMed] [Google Scholar]
- 7.Kathren RL. Pathway to a paradigm: the linear non-threshold dose-response model in historical context. The American Academy of Health Physics 1995;Radiology Centennial Hartman Oration. Health Phys 1996;70(5):621–635. [DOI] [PubMed]
- 8.Berrington de González A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet 2004;363(9406):345–351. [DOI] [PubMed] [Google Scholar]
- 9.Brenner DJ, Hall EJ. Computed tomography: an increasing source of radiation exposure. N Engl J Med 2007;357(22):2277–2284. [DOI] [PubMed] [Google Scholar]
- 10.Tubiana M, Aurengo A, Averbeck D, et al, eds. Dose-effect relationships and the estimation of the carcinogenic effects of low doses of ionizing radiation. Academy of Medicine (Paris) and Academy of Science (Paris) Joint Report No. 2, March 30, 2005.
- 11.National Research Council, Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Health risks from low levels of ionizing radiation: BEIR VII, Phase 2. Washington, DC: The National Academies Press, 2006. [PubMed]
- 12.International Commission on Radiological Protection. Low-dose extrapolation of radiation-related cancer risk. Publication 99. Amsterdam, the Netherlands: Elsevier, 2006.
- 13.De Bont R, Van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis 2004;19(3):169–185. [DOI] [PubMed] [Google Scholar]
- 14.Spitz DR, Azzam EI, Li JJ, Gius D. Cancer Metastasis Rev 2004;23(3–4):311–322. [DOI] [PubMed] [Google Scholar]
- 15.Burkart W, Jung T, Frasch G. Damage pattern as a function of radiation quality and other factors. C R Acad Sci III 1999;322(2–3):89–101. [DOI] [PubMed]
- 16.Tubiana M. The linear no-threshold relationship and advances in our understanding of carcinogenesis. Int J Low Radiat 2008;5(3):173–204. [Google Scholar]
- 17.Zahradka K, Slade D, Bailone A, et al. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature 2006;443(7111):569–573. [DOI] [PubMed] [Google Scholar]
- 18.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 2004;73:39–85. [DOI] [PubMed] [Google Scholar]
- 19.Shiloh Y, Lehmann AR. Maintaining integrity. Nat Cell Biol 2004;6(10):923–928. [DOI] [PubMed] [Google Scholar]
- 20.Bakkenist CJ, Kastan MB. Initiating cellular stress responses. Cell 2004;118(1):9–17. [DOI] [PubMed] [Google Scholar]
- 21.Dikomey E, Brammer I. Relationship between cellular radiosensitivity and non-repaired double-strand breaks studied for different growth states, dose rates and plating conditions in a normal fibroblast line. Int J Radiat Biol 2000;76(6):773–781. [DOI] [PubMed] [Google Scholar]
- 22.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double strand break repair pathway choice. Cell Res 2008;18(1):134–147. [DOI] [PubMed] [Google Scholar]
- 23.Zha S, Alt FW, Cheng HL, Brush JW, Li G. Defective DNA repair and increased genomic instability in Cernunnos-XLF-deficient murine ES cells. Proc Natl Acad Sci U S A 2007;104(11):4518–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong WM, Cortes U, Hande MP, et al. Synergistic role of Ku80 and poly(ADP-ribose) polymerase in suppressing chromosomal aberrations and liver cancer formation. Cancer Res 2002;62(23):6990–6996. [PubMed] [Google Scholar]
- 25.Boucher D, Hindo J, Averbeck D. Increased repair of gamma-induced DNA double-strand breaks at lower dose-rate in CHO cells. Can J Physiol Pharmacol 2004;82(2):125–132. [DOI] [PubMed] [Google Scholar]
- 26.Rothkamm K, Löbrich M. Misrepair of radiation-induced DNA double-strand breaks and its relevance for tumorigenesis and cancer treatment (review). Int J Oncol 2002;21(2):433–440. [PubMed] [Google Scholar]
- 27.Löbrich M, Jeggo PA. The impact of a negligent G2/M checkpoint on genomic instability and cancer induction. Nat Rev Cancer 2007;7(11):861–869. [DOI] [PubMed] [Google Scholar]
- 28.Krueger SA, Joiner MC, Weinfeld M, Piasentin E, Marples B. Role of apoptosis in low-dose hyper radiosensitivity. Radiat Res 2007;167(3):260–267. [DOI] [PubMed] [Google Scholar]
- 29.Vilenchik MM, Knudson AG. Inverse radiation dose-rate effects on somatic and germ-line mutations and DNA damage rates. Proc Natl Acad Sci U S A 2000;97(10):5381–5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vilenchik MM, Knudson AG. Endogenous DNA double-strand break production, fidelity of repair and induction of cancer. Proc Natl Acad Sci U S A 2003;100(22):12871–12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooker AM, Bhat M, Day TK, et al. The linear no-threshold model does not hold for low-dose ionizing radiation. Radiat Res 2004;162(4):447–452. [DOI] [PubMed] [Google Scholar]
- 32.Zeng G, Day TK, Hooker AM, et al. Non-linear chromosomal inversion response in prostate after low dose X-radiation exposure. Mutat Res 2006;602(1–2):65–73. [DOI] [PubMed]
- 33.Loucas BD, Eberle R, Bailey SM, Cornforth MN. Influence of dose rate on the induction of simple and complex chromosome exchanges by gamma rays. Radiat Res 2004;162(4):339–349. [DOI] [PubMed] [Google Scholar]
- 34.UNSCEAR: United Nations Scientific Committee on the effects of atomic radiation. Sources, effects and risks of ionising radiation. New York, NY: United Nations, 2000.
- 35.Chalmers A, Johnston P, Woodcock M, Joiner M, Marples B. PARP-1, PARP-2, and the cellular response to low doses of ionizing radiation. Int J Radiat Oncol Biol Phys 2004;58(2):410–419. [DOI] [PubMed] [Google Scholar]
- 36.Krueger SA, Collis SJ, Joiner MC, Wilson GD, Marples B. Transition in survival from low dose hyper-radiosensitivity to increased radioresistance is independent of activation of ATM Ser 1981 activity. Int J Radiat Oncol Biol Phys 2007;69(4):1262–1271. [DOI] [PubMed] [Google Scholar]
- 37.Löbrich M, Kühne M, Wetzel J, Rothkamm K. Joining of correct and incorrect double-strand break ends in normal human and ataxia telangiectasia fibroblasts. Genes Chromosomes Cancer 2000;27(1):59–68. [PubMed] [Google Scholar]
- 38.Bursch W, Lauer B, Timmermann-Trosiener I, Barthel G, Schuppler J, Schulte-Hermann R. Controlled death (apoptosis) of normal and putative preneoplastic cells in rat liver following withdrawal of tumor promoters. Carcinogenesis 1984;5(4):453–458. [DOI] [PubMed] [Google Scholar]
- 39.Columbano A, Endoh T, Denda A, et al. Effects of cell proliferation and cell death (apoptosis and necrosis) on the early stages of rat hepatocarcinogenesis. Carcinogenesis 1996;17(3):395–400. [DOI] [PubMed] [Google Scholar]
- 40.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med 2000;29(3–4):323–333. [DOI] [PubMed]
- 41.Collis SJ, Schwaninger JM, Ntambi AJ, et al. Evasion of early cellular response mechanisms following low level radiation induced DNA damage. J Biol Chem 2004;279(48):49624–49632. [DOI] [PubMed] [Google Scholar]
- 42.Löbrich M, Rief N, Kühne M, et al. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci U S A 2005;102(25):8984–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartkova J, Rezaei N, Liontos M, et al. Oncogene induced senescence is part of the tumorigenesis barrier imposed by the DNA damage checkpoints. Nature 2006;444(7119):633–637. [DOI] [PubMed] [Google Scholar]
- 44.Campisi J. Senescent cells, tumor suppression and organism aging. Cell 2005;120(4):513–522. [DOI] [PubMed] [Google Scholar]
- 45.Schmitt CA. Cellular senescence and cancer treatment. Biochim Biophys Acta 2007;1775(1):5–20. [DOI] [PubMed] [Google Scholar]
- 46.Feinendegen LE, Pollycove M. Biologic responses to low doses of ionizing radiation: detriment versus hormesis. I. Dose responses of cells and tissues. J Nucl Med 2001;42(7):17N–27N. [PubMed] [Google Scholar]
- 47.Rigaud O, Moustacchi E. Radioadaptation for gene mutation and the possible molecular mechanisms of the adaptive response. Mutat Res 1996;358(2):127–134. [DOI] [PubMed] [Google Scholar]
- 48.Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect 1998;106(suppl 1):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Broome EJ, Brown DL, Mitchel RE. Dose responses for adaption to low dose of (60)Co gamma rays and (3)H beta particles in normal human fibroblasts. Radiat Res 2002;158(2):181–186. [DOI] [PubMed] [Google Scholar]
- 50.Portess DI, Bauer G, Hill MA, O'Neill P. Low-dose irradiation on nontransformed cells stimulates the selective removal of precancerous cells via intercellular induction of apoptosis. Cancer Res 2007;67(3):1246–1253. [DOI] [PubMed] [Google Scholar]
- 51.Bauer G. Low dose radiation and intercellular induction of apoptosis: potential implications for the control of oncogenesis. Int J Radiat Biol 2007;83(11–12):873–888. [DOI] [PubMed]
- 52.Day TK, Zeng G, Hooker AM, et al. Adaptive response for chromosomal inversions on pKZ1 mouse prostate induced by low doses of X radiation delivered after a high dose. Radiat Res 2007;167(6):682–692. [DOI] [PubMed] [Google Scholar]
- 53.Barquinero JF, Barrios L, Caballin MR, et al. Occupational exposure to radiation induces an adaptive response in human lymphocytes. Int J Radiat Biol 1995;67(2):187–191. [DOI] [PubMed] [Google Scholar]
- 54.Stoilov LM, Mullenders LH, Darroudi F, Natarajan AT. Adaptive response to DNA and chromosomal damage induced by X-rays in human blood lymphocytes. Mutagenesis 2007;22(2):117–122. [DOI] [PubMed] [Google Scholar]
- 55.Ghiassi-nejad M, Mortazavi SM, Cameron JR, Niroomand-rad A, Karam PA. Very high background radiation areas of Ramsar, Iran: preliminary biological studies. Health Phys 2002;82(1):87–93. [DOI] [PubMed] [Google Scholar]
- 56.Mothersill C, Seymour CB. Radiation-induced bystander effects and the DNA paradigm: an “out of field” perspective. Mutat Res 2006;597(1–2):5–10. [DOI] [PubMed]
- 57.Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. Bystander induced differentiation: a major response to targeted irradiation of a urothelial explant model. Mutat Res 2006;597(1–2):43–49. [DOI] [PubMed]
- 58.Schettino G, Folkard M, Michael BD, Prise KM. Low-dose binary behavior of bystander cell killing after microbeam irradiation of a single cell with focused c(k) x rays. Radiat Res 2005;163(3):332–336. [DOI] [PubMed] [Google Scholar]
- 59.Smith RW, Wang J, Bucking CP, Mothersill CE, Seymour CB. Evidence for a protective response by the gill proteome of rainbow trout exposed to X-ray induced bystander signals. Proteomics 2007;7(22):4171–4180. [DOI] [PubMed] [Google Scholar]
- 60.Carnes BA, Groer PG, Kotek TJ. Radium dial workers: issues concerning dose response and modeling. Radiat Res 1997;147(6):707–714. [PubMed] [Google Scholar]
- 61.van Kaick G, Dalheimer A, Hornik S, et al. The German Thorotrast Study: recent results and assessment of risks. Radiat Res 1999;152(6 suppl):S64–S72. [PubMed] [Google Scholar]
- 62.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation. I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res 2003;159(5):567–580. [DOI] [PubMed] [Google Scholar]
- 63.Cahill DP, Kinzler KW, Vogelstein B, Lengauer C. Genetic instability and Darwinian selection in tumors. Trends Cell Biol 1999;9(12):M57–M61. [PubMed] [Google Scholar]
- 64.Stoler DL, Chen N, Basik M, et al. The onset and extent of genomic instability in sporadic colorectal tumor progression. Proc Natl Acad Sci U S A 1999;96(26):15121–15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Streffer C. Bystander effects, adaptive response and genomic instability induced by prenatal irradiation. Mutat Res 2004;568(1):79–87. [DOI] [PubMed] [Google Scholar]
- 66.Kadhim MA, Hill MA, Moore SR. Genomic instability and the role of radiation quality. Radiat Prot Dosimetry 2006;122(1–4):221–227. [DOI] [PubMed]
- 67.Okada M, Okabe A, Uchihori Y, et al. Single extreme low dose/low dose rate irradiation causes alteration in lifespan and genome instability in primary human cells. Br J Cancer 2007;96(11):1707–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boulton E, Cleary H, Papworth D, Plumb M. Susceptibility to radiation-induced leukaemia/lymphoma is genetically separable from sensitivity to radiation-induced genomic instability. Int J Radiat Biol 2001;77(1):21–29. [DOI] [PubMed] [Google Scholar]
- 69.Bhowmick NA, Chytil A, Plieth D, et al. TGF-beta signalling in fibroblasts modulates the oncogenic potential of adjacent epithelium. Science 2004;303(5659):848–851. [DOI] [PubMed] [Google Scholar]
- 70.Radisky DC, Bissell MJ. Cancer: respect thy neighbor! Science 2004;303(5659):775–777. [DOI] [PubMed] [Google Scholar]
- 71.Radisky ES, Radisky DC. Stromal induction of breast cancer: inflammation and invasion. Rev Endocr Metab Disord 2007;8(3):279–287. [DOI] [PubMed] [Google Scholar]
- 72.Mueller MM, Fusenig NE. Friends or foes: bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2004;4(11):839–849. [DOI] [PubMed] [Google Scholar]
- 73.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 2007;117(5):1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res 2000;60(5):1254–1260. [PubMed] [Google Scholar]
- 75.Barcellos-Hoff MH. Integrative radiation carcinogenesis: interactions between cell and tissue responses to DNA damage. Semin Cancer Biol 2005;15(2):138–148. [DOI] [PubMed] [Google Scholar]
- 76.Jaffe LF. Epigenetic theories of cancer initiation. Adv Cancer Res 2003;90:209–230. [DOI] [PubMed] [Google Scholar]
- 77.Pardoll DM. Immunology: stress, NK receptors, and immune surveillance. Science 2001;294(5542):534–536. [DOI] [PubMed] [Google Scholar]
- 78.Feinendegen LE, Pollycove M, Neumann RD. Whole body responses to low-level radiation exposure: new concepts in mammalian radiobiology. Exp Hematol 2007;35(4 suppl 1):37–46. [DOI] [PubMed] [Google Scholar]
- 79.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 2008;371(9614):771–783. [DOI] [PubMed] [Google Scholar]
- 80.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med 2003;348(17):1681–1691. [DOI] [PubMed] [Google Scholar]
- 81.Liu SZ. Cancer control related to stimulation of immunity by low dose irradiation. Dose Response 2006;5(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amundson SA, Lee RA, Koch-Paiz CA, et al. Differential responses of stress genes to low dose-rate gamma irradiation. Mol Cancer Res 2003;1(6):445–452. [PubMed] [Google Scholar]
- 83.Mercier G, Berthault N, Mary J, Peyre J, et al. Biological detection of low radiation doses by combining results of two microarray analysis methods. Nucleic Acids Res 2004;32(1):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Franco N, Lamartine J, Frouin V, et al. Low-dose exposure to gamma rays induces specific gene regulations in normal human keratinocytes. Radiat Res 2005;163(6):623–635. [DOI] [PubMed] [Google Scholar]
- 85.Sokolov MV, Smirnova NA, Camerini-Otero RD, Neumann RD, Panyutin IG. Microarray analysis of differentially expressed genes after exposure of normal human fibroblasts to ionizing radiation from an external source and from DNA-incorporated iodine-125 radionuclide. Gene 2006;382:47–56. [DOI] [PubMed] [Google Scholar]
- 86.Yang F, Stenoien DL, Strittmatter EF, et al. Phosphoproteome profiling of human skin fibroblast cells in response to low- and high-dose irradiation. J Proteome Res 2006;5(5):1252–1260. [DOI] [PubMed] [Google Scholar]
- 87.Duport P. A database of cancer induction by low dose radiation in mammals: overview and initial observations. Int J Low Radiat 2003;1(1):120–131. [Google Scholar]
- 88.Calabrese EJ. Hormesis: from marginalization to mainstream—a case for hormesis as the default dose-response model in risk assessment. Toxicol Appl Pharmacol 2004;197(2):125–136. [DOI] [PubMed] [Google Scholar]
- 89.Feinendegen LE. Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol 2005;78(925):3–7. [DOI] [PubMed] [Google Scholar]
- 90.Azzam EI, de Toledo SM, Raaphorst GP, Mitchell RE. Low-dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T1/2. Radiat Res 1996;146(4):369–373. [PubMed] [Google Scholar]
- 91.Redpath JL. Radiation induced neoplastic transformation in vitro: evidence for a protective effect at low doses of low LET. Cancer Metastasis Rev 2004;23(3–4):333–339. [DOI] [PubMed]
- 92.Ko M, Lao XY, Kapadia R, Elmore E, Redpath JL. Neoplastic transformation in vitro by low doses of ionizing radiation: role of adaptive response and bystander effects. Mutat Res 2006;597(1–2):11–17. [DOI] [PubMed]
- 93.Elmore E, Lao XY, Kapadia R, Redpath JL. The effect of dose rate on radiation-induced neoplastic transformation in vitro by low doses of low-LET radiation. Radiat Res 2006;166(6):832–838. [DOI] [PubMed] [Google Scholar]
- 94.Kaiser J. Hormesis: a healthful dab of radiation? Science 2003;302(5644):378. [DOI] [PubMed] [Google Scholar]
- 95.Feinendegen LE, Neumann RD. Physics must join with biology in better assessing risk from low dose irradiation. Radiat Prot Dosimetry 2005;117(4):346–356. [DOI] [PubMed] [Google Scholar]
- 96.Jeggo P, Löbrich M. Radiation-induced DNA damage responses. Radiat Prot Dosimetry 2006;122(1–4):124–127. [DOI] [PubMed]
- 97.Tanooka H. Threshold dose-response in radiation carcinogenesis: an approach from chronic beta-irradiation experiments and a review of non tumour doses. Int J Radiat Biol 2001;77(5):541–551. [DOI] [PubMed] [Google Scholar]
- 98.Little MP, Wakeford R, Tawn EJ, Bouffler SD, Berrington de Gonzalez A. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology 2009;251(1):6–12. [DOI] [PMC free article] [PubMed]
- 99.Little MP, Muirhead CR. Evidence for curvilinearity in the cancer incidence dose-response in the Japanese atomic bomb survivors. Int J Radiat Biol 1996;70(1):83–94. [DOI] [PubMed] [Google Scholar]
- 100.Little MP, Muirhead CR. Derivation of low dose extrapolation factors from analysis of the curvature in the cancer incidence dose response in Japanese atomic bomb survivors. Int J Radiat Biol 2000;76(7):939–953. [DOI] [PubMed] [Google Scholar]
- 101.Preston DL, Pierce DA, Shimizu Y, et al. Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates. Radiat Res 2004;162(4):377–389. [DOI] [PubMed] [Google Scholar]
- 102.Preston DL, Ron E, Tokuoka S, et al. Solid tumors incidence in atomic bomb survivors 1958–1998. Radiat Res 2007;168(1):1–64. [DOI] [PubMed] [Google Scholar]
- 103.Suit H, Goldberg S, Niemierko A, et al. Secondary carcinogenesis in patients treated with radiation: a review of data on radiation-induced cancers in human, non-human primate, canine and rodent subjects. Radiat Res 2007;167(1):12–42. [DOI] [PubMed] [Google Scholar]
- 104.Heidenreich WF, Paretzke HG, Jacob P. No evidence for increased tumour rates below 200 mSv in atomic bomb survivors. Radiat Environ Biophys 1997;36(3):205–207. [DOI] [PubMed] [Google Scholar]
- 105.Breckow J. Linear-no-threshold is a radiation-protection standard rather than a mechanistic effect model. Radiat Environ Biophys 2006;44(4):257–260. [DOI] [PubMed] [Google Scholar]
- 106.Kellerer AM, Nekolla E. Neutrons versus gamma ray risk estimates: inferences from the cancer incidence and mortality data in Hiroshima. Radiat Environ Biophys 1997;36(2):73–83. [DOI] [PubMed] [Google Scholar]
- 107.Walsh L, Rhum W, Kellerer AM. Cancer risk estimates for gamma rays with regard to organ specific dose: all solid cancer combined. Radiat Environ Biophys 2004;43(3):145–151. [DOI] [PubMed] [Google Scholar]
- 108.Samet JM, Geyh AS, Utell MJ. The legacy of World Trade Center dust. N Engl J Med 2007;356(22):2233–2236. [DOI] [PubMed] [Google Scholar]
- 109.Moline J, Herbert R, Nguyen N. Health consequences of the September 11 World Trade Center attacks: a review. Cancer Invest 2006;24(3):294–301. [DOI] [PubMed] [Google Scholar]
- 110.Ma F, Fleming LE, Lee DJ, Trapido E, Gerace TA. Cancer incidence in Florida professional firefighters, 1981 to 1999. J Occup Environ Med 2006;48(9):883–888. [DOI] [PubMed] [Google Scholar]
- 111.LeMasters GK, Genaidy AM, Succop P, et al. Cancer risk among firefighters: a review and meta-analysis of 32 studies. J Occup Environ Med 2006;48(11):1189–1202. [DOI] [PubMed] [Google Scholar]
- 112.Kang D, Davis LK, Hunt P, Kriebel D. Cancer incidence among male Massachusetts firefighters 1987–2003. Am J Ind Med 2008;51(5):329–335. [DOI] [PubMed] [Google Scholar]
- 113.Bates MN. Registry-based case-control study of cancer in California firefighters. Am J Ind Med 2007;50(5):339–344. [DOI] [PubMed] [Google Scholar]
- 114.Guidotti TL. Evaluating causality for occupational cancers: the example of firefighters. Occup Med (Lond) 2007;57(7):466–471. [DOI] [PubMed] [Google Scholar]
- 115.Youakim S. Risk of cancer among firefighters: a quantitative review of selected malignancies. Arch Environ Occup Health 2006;61(5):223–231. [DOI] [PubMed] [Google Scholar]
- 116.Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol 1997;70:130–139. [DOI] [PubMed] [Google Scholar]
- 117.Shu XO, Potter JD, Linet MS, et al. Diagnostic X rays and ultrasound exposure and risk of childhood acute lymphoblastic leukemia by immunophenotype. Cancer Epidemiol Biomarkers Prev 2002;11(2):177–185. [PubMed] [Google Scholar]
- 118.International Agency for Research on Cancer. Monograph on the evaluation of carcinogenic risks to humans. Vol. 75, Ionizing radiation, part I: X and gamma radiation and neutrons. Lyon, France: International Agency for Research on Cancer, 2000.
- 119.Naumburg E, Bellocco R, Cnattingius S, Hall P, Boice JD Jr, Ekbom A. Intrauterine exposure to diagnostic X rays and risk of childhood leukemia subtypes. Radiat Res 2001;156(6):718–723. [DOI] [PubMed] [Google Scholar]
- 120.Inskip PD, Harvey EB, Boice JD, et al. Incidence of cancer in twins. Cancer Causes Control 1991;2(5):315–324. [DOI] [PubMed] [Google Scholar]
- 121.Rodvall Y, Hrubec Z, Pershagen G, Ahlbom A, Bjurman A, Boice JD Jr. Childhood cancer among Swedish twins. Cancer Causes Control 1992;3(6):527–532. [DOI] [PubMed] [Google Scholar]
- 122.Delongchamp RR, Mabuchi K, Yoshimoto Y, Preston DL. Cancer mortality among atomic bomb survivors exposed in utero or as young children. Radiat Res 1997;147(3):385–395. [PubMed] [Google Scholar]
- 123.Cardis E, Howe G, Ron E, et al. Cancer consequences of the Chernobyl accident: 20 years on. J Radiol Prot 2006;26(2):127–140. [DOI] [PubMed] [Google Scholar]
- 124.Cardis E, Kesminienne A, Ivanov V, et al. Risk of thyroid cancer after exposure to I-131 in childhood. J Natl Cancer Inst 2005;97(10):724–732. [DOI] [PubMed] [Google Scholar]
- 125.Scott BR. Re: Risk of thyroid cancer after exposure to (131)I in childhood. J Natl Cancer Inst 2006;98(8):561; author reply 561. [DOI] [PubMed] [Google Scholar]
- 126.Doll R, Berrington A, Darby SC. Low mortality of British radiologists. Br J Radiol 2005;78(935):1057–1058. [DOI] [PubMed] [Google Scholar]
- 127.Cameron JR. Radiation increased the longevity of British radiologists. Br J Radiol 2002;75(895):637–639. [DOI] [PubMed] [Google Scholar]
- 128.Mohan AK, Hauptmann M, Freedman DM, et al. Cancer and other causes of mortality among radiologic technologists in the United States. Int J Cancer 2003;103(2):259–267. [DOI] [PubMed] [Google Scholar]
- 129.Sigurdson AJ, Doody MM, Rao RS, et al. Cancer incidence in the US radiologic technologists health study, 1983–1998. Cancer 2003;97(12):3080–3089. [DOI] [PubMed] [Google Scholar]
- 130.Blettner M, Zeeb H, Auvinen A, et al. Mortality from cancer and other causes among male airline cockpit crew in Europe. Int J Cancer 2003;106(6):946–952. [DOI] [PubMed] [Google Scholar]
- 131.Cardis E, Gilbert ES, Carpenter L, et al. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiat Res 1995;142(2):117–132. [PubMed] [Google Scholar]
- 132.Cardis E, Vrijheid M, Blettner M, et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat Res 2007;167(4):396–416. [DOI] [PubMed] [Google Scholar]
- 133.Zablotska LB, Ashmore JP, Jowe GR. Analysis of mortality among Canadian nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat Res 2004;161(6):633–641. [DOI] [PubMed] [Google Scholar]
- 134.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation. II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res 2003;159(5):581–596. [DOI] [PubMed] [Google Scholar]
- 135.Hill C, Laplanche A. Overall mortality and cancer mortality around French nuclear sites. Nature 1990;347(6295):755–757. [DOI] [PubMed] [Google Scholar]
- 136.Wheldon TE, Mairs RJ, Barret A. Causality of relationship between paternal radiation exposure and leukaemia incidence in the children of Sellafield workers. Int J Radiat Biol 1992;61(5):565–566. [DOI] [PubMed] [Google Scholar]
- 137.Darby SC, Doll R. Fallout, radiation doses near Dounreay, and childhood leukaemia. Br Med J (Clin Res Ed) 1987;294(6572):603–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kinlen L. Evidence for an infective cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain. Lancet 1988;2(8624):1323–1327. [DOI] [PubMed] [Google Scholar]
- 139.Boutou O, Guisard AV, Slama R, et al. Population mixing and leukaemia in young people around the La Hague nuclear waste processing plant. Br J Cancer 2002;87(7):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Evrard AS, Hemon D, Morin A, et al. Childhood leukaemia incidence around French nuclear installations using geographic zoning based on gaseous discharge dose estimates. Br J Cancer 2006;94(9):1342–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rudant J, Baccaini B, Ripert M, et al. Population-mixing at the place of residence at the time of birth and incidence of childhood leukaemia in France. Eur J Cancer 2006;42(7):927–933. [DOI] [PubMed] [Google Scholar]
- 142.Nair MK, Nambi KS, Amma NS, et al. Population study in the high natural background radiation area in Kerala, India. Radiat Res 1999;152(6 suppl):S145–S148. [PubMed] [Google Scholar]
- 143.Tao Z, Zha Y, Akiba S, et al. Cancer mortality in the high background radiation areas of Yangjiang, China during the period between 1979 and 1995. J Radiat Res (Tokyo) 2000;41(suppl):31–41. [DOI] [PubMed] [Google Scholar]
- 144.Hayata I, Wang C, Zhang W, et al. Effect of high level natural radiation on chromosomes of residents in southern China. Cytogenet Genome Res 2004;104(1–4):237–239. [DOI] [PubMed]
- 145.Boice JD Jr, Morin MM, Glass AG, et al. Diagnostic X-ray procedures and risk of leukemia, lymphoma and multiple myeloma. JAMA 1991;265(10):1290–1294. [PubMed] [Google Scholar]
- 146.Linos A, Gray JE, Orvis AL, et al. Low dose radiation and leukemia. N Engl J Med 1980;302(20):1101–1115. [DOI] [PubMed] [Google Scholar]
- 147.Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol 2006;36(suppl 14):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boice JD, Preston D, Davis FG, Monson RR. Frequent chest X-ray fluoroscopy and breast cancer incidence among tuberculosis patients in Massachusetts. Radiat Res 1991;125(2):214–222. [PubMed] [Google Scholar]
- 149.Miller AB, Howe GR, Sherman GJ, et al. Mortality from breast cancer after irradiation during fluoroscopic examinations in patients being treated for tuberculosis. N Engl J Med 1989;321(19):1285–1289. [DOI] [PubMed] [Google Scholar]
- 150.Morin Doody M, Lonstein JE, Stovall M, Hacker DG, Luckyanov N, Land CE. Breast cancer mortality after diagnostic radiography: findings from the U.S. Scoliosis Cohort Study. Spine 2000;25(16):2052–2063. [DOI] [PubMed] [Google Scholar]
- 151.Tubiana M. Computed tomography and radiation exposure [letter]. N Engl J Med 2008;358(8):850; author reply 852–853. [DOI] [PubMed] [Google Scholar]
- 152.Nagataki S. Computed tomography and radiation exposure [letter]. N Engl J Med 2008;358(8):850–851; author reply 852–853. [PubMed] [Google Scholar]
- 153.Feinendegen LE. Computed tomography and radiation exposure [letter]. N Engl J Med 2008;358(8):851; author reply 852–853. [PubMed] [Google Scholar]
- 154.Rubino C, de Vathaire F, Shamsaldin A, Lê MG. Radiation dose, chemotherapy, hormonal treatment and risk of second cancer after breast cancer treatment. Br J Cancer 2003;89(5):840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tubiana M, Aurengo A, Masse R, Valleron AJ. Risk of cancer from diagnostic X-rays. Lancet 2004;363(9424):1908. [DOI] [PubMed] [Google Scholar]
- 156.Little MP. Comparison of the risks of cancer incidence and mortality following radiation therapy for benign and malignant disease with cancer risks observed in the Japanese A-bomb survivors. Int J Radiat Biol 2001;77(4):431–464. [DOI] [PubMed] [Google Scholar]
- 157.Le Pogam MA, Rubino C, Diallo I, et al. Radiation dose fractionation and second cancer risks after breast cancer treatment. Radiat Prot Dosimetry [in press].
- 158.Diallo I, Lamon A, Shamsaldin A, Grimaud E, de Vathaire F, Chavaudra J. Estimation of the radiation dose delivered to any point outside the target volume per patient treated with external beam radiotherapy. Radiother Oncol 1996;38(3):269–271. [DOI] [PubMed] [Google Scholar]
- 159.Lundell M, Mattsson A, Karlsson P, Holmberg E, Gustafsson A, Holm LE. Breast cancer risk after radiotherapy in infancy. Radiat Res 1999;151(5):626–632. [PubMed] [Google Scholar]
- 160.Moon K, Stukenborg GJ, Keim J, Theodorescu D. Cancer incidence after localized therapy for prostate. Cancer 2006;107(5):991–998. [DOI] [PubMed] [Google Scholar]
- 161.Rubino C, Adjadj E, Doyon F, et al. Radiation exposure and familial aggregation of cancers as risk factors for colorectal cancer after radioiodine treatment for thyroid carcinoma. Int J Radiat Oncol Biol Phys 2005;62(4):1084–1089. [DOI] [PubMed] [Google Scholar]
- 162.Franklyn JA, Maisonneuve P, Sheppard M, Betteridge J, Boyle P. Cancer incidence and mortality after radioiodine treatment for hyperthyroidism: a population based study. Lancet 1999;353(9170):2111–2115. [DOI] [PubMed] [Google Scholar]
- 163.de Vathaire F. Données épidémiologiques sur les effets cancérigènes des faibles doses de rayonnements ionisants. Annexe 4. Relation dose-effet et l'estimation des effets cancérogènes des faibles doses de rayonnements ionisants. Rapport Conjoint n°2, Acad Sc et Acad Med (version Française). Nucleon édition–Paris 2005; 147–168.
- 164.Walinder G. Has radiation protection become a health hazard? The Swedish Nuclear Training and Safety Centre, Nykoping, Sweden. Madison, Wis: Med Phys Publishing, 1995;16–63, 95–117, 128–137.
- 165.Wigg DR. Radiation: facts, fallacies, and phobias. Australas Radiol 2007;51(1):21–25. [DOI] [PubMed] [Google Scholar]
- 166.Taubes G. Epidemiology faces its limits. Science 1995;269(5221):164–169. [DOI] [PubMed] [Google Scholar]
- 167.Brash DE. Sunlight and the onset of skin cancer. Trends Genet 1997;13(10):410–414. [DOI] [PubMed] [Google Scholar]