Abstract
Background and Purpose:
Magnesium sulfate is used extensively for prevention of eclamptic seizures. Empirical and clinical evidence supports the effectiveness of magnesium sulfate; however, questions remain as to its safety and mechanism. This review summarizes current evidence supporting the possible mechanisms of action and several controversies for magnesium sulfate treatment.
Summary of Review:
Several mechanisms are presented, including the effects of magnesium sulfate on peripheral and cerebral vasodilation, blood-brain barrier protection, and as an anticonvulsant.
Conclusions:
Though the specific mechanisms of action remain unclear, the effect of magnesium sulfate in the prevention of eclampsia is likely multi-factorial. Magnesium sulfate may act as a vasodilator, with actions in the peripheral vasculature or the cerebrovasculature, to decrease peripheral vascular resistance and/or relieve vasoconstriction. Additionally, magnesium sulfate may also protect the blood-brain barrier and limit cerebral edema formation, or it may act through a central anticonvulsant action.
Keywords: Eclampsia, Magnesium sulfate, Vasodilation, Blood-brain barrier, Anticonvulsant
Introduction
Magnesium sulfate (MgSO4) has been used throughout the 20th century for prevention of eclamptic seizures1, 2 and continues to be used extensively3-5. Empirical evidence supports the effectiveness of MgSO4 in preventing and treating eclamptic seizures1, 6-8, in addition to recent controlled clinical trials5, 9, 10. For eclamptic seizure prophylaxis in preeclamptic women, MgSO4 is superior to phenytoin11, 12, nimodipine13, diazepam14, and placebo9. In the multinational Collaborative Eclampsia Trial, MgSO4 reduced the risk of recurrent seizures in eclamptic women by 52% when compared to diazepam and by 67% when compared to phenytoin15. The publication of these clinical trials significantly increased the use of magnesium sulfate versus other anticonvulsants in the United Kingdom and Ireland where the reported use in preeclampsia increased from 2% to 40%16. In addition, 60% of providers surveyed indicated they would use magnesium as an anticonvulsant for eclampsia in 1998, up from only 2% of eclamptic women who received magnesium sulfate in 199216, 17.
Although the effectiveness of MgSO4 in treating and preventing eclampsia has been established, questions still exist as to its safety. There are concerns regarding the possibility of hypermagnesemia toxicity in eclampsia treatment. Normal serum concentrations of Mg+2 are 1.5-2.5 mEq/L (1.8-3.0 mg/dL), with one-third to one-half bound to plasma proteins18, 19. Total magnesium serum concentrations advocated for the treatment of eclamptic convulsions are 3.5-7 mEq/L (4.2-8.4 mg/dL)2, 20, 21, which can be obtained by administering it intramuscularly (6 g loading dose followed by 2 g/h), intravenously (2-4 g dose up to 1 g/min) or a combination of both6, 18, 22. Areflexia, particularly loss of the patellar deep tendon reflex, has been observed at 8-10 mEq/L, and respiratory paralysis seen at >13 mEq/L6, 18, 22. Progressively higher serum magnesium levels can ultimately lead to cardiac arrest18, 22, 23. Some suggest that using standard infusion protocols may not lead to therapeutic serum magnesium levels in all patients, with 36.2% of patients found to have total serum magnesium lower than 4 mEq/L at 30 minutes after treatment initiation in one study24, though no eclamptic seizures were reported during MgSO4 treatment. In addition, there are reports that in some patients eclamptic seizures do not cease even with elevated levels of MgSO46, 7, 25, suggesting that MgSO4 is not effective in treating all cases of eclampsia.
As technologic advances allow for ionized magnesium to be more readily measured, questions have arisen as to whether it is more appropriate to monitor total serum magnesium or the ionized, physiologically active, form. Studies have shown little correlation between total and ionized magnesium levels, either at baseline prior to treatment or during MgSO4 treatment for preeclampsia19, 24. In preeclamptic patients treated with a loading dose of 4 g intravenously followed by 2 g per hour infusion, it was found that both total and ionized Mg+2 concentrations increased quickly after infusion, but steady-state concentrations for total magnesium were 4.84 ± 0.24 mg/dL, whereas for ionized magnesium it was 2.04 ± 0.14 mg/dL19. Similar results have been found by other groups using the same infusion protocol24. Interestingly, as MgSO4 infusion caused significant increases in ionized Mg+2 levels, serum ionized calcium (Ca+2) concentrations were unchanged26, suggesting that the effect of MgSO4 is not exerted through modulations of ionized calcium levels.
Though the use of MgSO4 is wide-spread and effective, its mechanism of action remains unclear. Several possible mechanisms of action have been proposed, including acting as a vasodilator, with actions either peripherally or in the cerebral circulation to relieve vasoconstriction, protecting the blood-brain barrier (BBB) to decrease cerebral edema formation, and acting as a central anticonvulsant. Each of these possible mechanisms of action are discussed below.
Magnesium-induced Vasodilation
Magnesium is a unique calcium antagonist as it can act on most types of calcium channels in vascular smooth muscle27 and as such would be expected to decrease intracellular calcium. One major effect of decreased intracellular calcium would be inactivation of calmodulin-dependent myosin light chain kinase activity and decreased contraction27, causing arterial relaxation that may subsequently lower peripheral and cerebral vascular resistance, relieve vasospasm, and decrease arterial blood pressure. The vasodilatory effect of MgSO4 has been investigated in a wide variety of vessels. For example, both in vivo and in vitro animal studies have shown that it is a vasodilator of large conduit arteries such as the aorta28, 29, as well as smaller resistance vessels including mesenteric27, 30-32, skeletal muscle27, uterine33, and cerebral arteries27, 30, 34. However, the importance of magnesium-induced vasodilation in the treatment and prevention of eclampsia is not completely understood.
The theory of cerebrovascular vasospasm as the etiology of eclampsia seemed to be reinforced by transcranial Doppler (TCD) studies which suggested that MgSO4 treatment caused dilation in the cerebral circulation35-37 as well as in animal studies that used large cerebral arteries34. However, a vasodilator such as MgSO4 would seem to be a paradoxical treatment choice for eclamptic encephalopathy. Eclampsia is thought to be a form of posterior reversible encephalopathy syndrome (PRES) and similar to hypertensive encephalopathy, in which acute elevations in blood pressure cause forced dilatation of the myogenic vasoconstriction of cerebral arteries and arterioles, increased BBB permeability and edema formation38-40. Studies from our lab have shown that MgSO4 causes concentration-dependent vasodilatation in both cerebral and mesenteric resistance arteries; however, mesenteric arteries were significantly more sensitive to MgSO4, particularly during pregnancy30. The finding of a modest vasodilatory effect in the cerebral circulation are consistent with other findings that MgSO4 treatment caused no significant change in cerebral blood flow (CBF), large cerebral artery diameter, or mean middle cerebral artery velocity as determined by magnetic resonance imaging (MRI)41 and TCD42, 43. Together, these results suggest that the effects of MgSO4 as an eclamptic seizure prophylaxis may be more closely related to an effect on peripheral vascular resistance and lowering of systemic blood pressure than to a direct effect on CBF (Table 1 and Figure 1).
Table 1.
Vascular Effects of Magnesium Sulfate.
| Cellular Target | Mode of Action | Possible Mechanism(s) |
|---|---|---|
|
Smooth Muscle Uterine +++ Mesenteric +++ Aorta +++ Cerebral + |
Relaxation ↓ Vasodilation ↓ Decreased Vascular Resistance |
Calcium Antagonism Decreased Voltage-operated Calcium Channel (VOCC) Activity Decreased [Ca+2]i Release From Sarcoplasmic Reticulum |
| Endothelium | Decreased Platelet Aggregation | Increased Prostaglandin I2 (PGI2) |
| Vasodilation | Increased Nitric Oxide (NO, Gestation Dependent) |
|
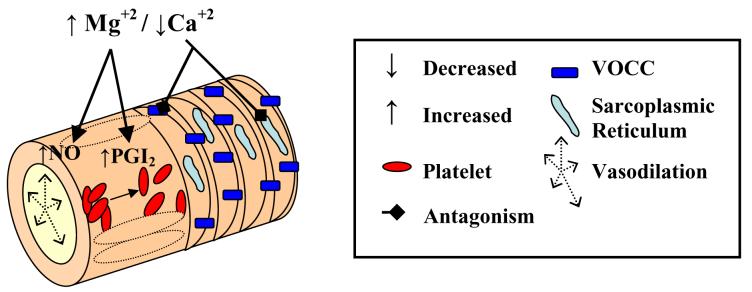
Figure 1. Vascular Effects of Magnesium Sulfate.
Magnesium is a potent vasodilator of uterine and mesenteric arteries, and aorta, but has minimal effect on cerebral arteries. In vascular smooth muscle, magnesium competes with calcium for binding sites, in this case for voltage-operated calcium channels (VOCC). Decreased calcium channel activity lowers intracellular calcium, causing relaxation and vasodilation. In endothelium, magnesium has been shown to increase production of prostaglandin I2 (through unknown mechanisms), which in turn decreases platelet aggregation. Magnesium also increases NO production causing vasodilation.
Reports of the effects of MgSO4 treatment on arterial blood pressure have been mixed. Hypotensive effects have been noted in various studies particularly with bolus injections2, 36, 44, though the duration of decreased blood pressure was varied. In pregnant rats treated with the nitric oxide synthase inhibitor L-NAME to induce hypertension, combination treatment with MgSO4 resulted in significantly lower blood pressures at term and better neonatal outcomes versus animals treated with L-NAME alone45. However, it has been cautioned that MgSO4 should not be considered primarily an anti-hypertensive agent, as there are other drugs better suited for that purpose in eclampsia, including hydralazine, labetalol, and nifedipine 20, 22.
Several reports have suggested that gestation may influence vascular reactivity to MgSO4 and that sensitivity varies with vascular bed28-30, 33. Human uterine arteries from pregnant patients are three-fold more reactive to MgSO4 than uterine arteries from non-pregnant patients33. In aorta from pregnant and non-pregnant rats, both increased and decreased sensitivity to MgSO4-induced vasodilation have been shown based on the preconstriction agent used for in vitro studies. These studies also suggest that pregnancy may differentially affect receptor versus voltage-operated calcium channels in aortic smooth muscle28. In another study of rat aortic rings, the effect of MgSO4 was dependent on gestation and nitric oxide production such that vasodilation was less at term than during late pregnancy29. Our studies found that while mesenteric resistance arteries showed no change in sensitivity with gestation, posterior cerebral resistance arteries from late-pregnant and postpartum animals were significantly less sensitive to MgSO4-induced vasodilation versus those from nonpregnant animals30. This may be due to gestation-induced changes in the cerebral endothelial vaodilatory mechanisms that have been demonstrated during pregnancy and the postpartum state46.
MgSO4 may have other effects within the vasculature that could also explain its effectiveness in eclampsia (included in Figure 1). Magnesium may act by stimulating production of prostacyclin by endothelial cells causing vasodilation47, or by inhibiting platelet aggregation47, 48. In patients with pregnancy-induced hypertension, MgSO4 treatment significantly decreased circulating levels of angiotensin-converting enzyme49. These actions may attenuate the endothelial dysfunction associated with (pre)eclampsia50-52.
Effects on the Blood-brain Barrier and Cerebral Edema Formation
The cerebral endothelium that forms the BBB has unique features compared to the peripheral endothelium including a lack of capillary fenestrations53, a low basal rate of pinocytosis54, 55, and the presence of high electrical resistance tight junctions between adjacent endothelial cells54, 56. Disruption of the BBB can result in vasogenic edema formation, an important component in the clinical picture of eclampsia57, 58. Decreased BBB permeability with MgSO4 treatment has been reported in a variety of animal models of BBB disruption including traumatic brain injury59, septic encephalopathy60, hypoglycemia61, and mannitol injection62. We recently reported MgSO4 treatment decreased BBB permeability in response to acute hypertension in late-pregnant rats63. In addition, several studies have shown that MgSO4 decreases cerebral edema formation after brain injury59, 62, 64-67. Together, these studies importantly suggest that one mechanism by which MgSO4 is effective in eclampsia treatment may be through protection of the BBB and decreased cerebral edema formation.
Several mechanisms of action have been proposed to explain the neuroprotective effects of MgSO4 (Table 2 and Figure 2). Magnesium is a calcium antagonist that acts both intracellularly and extracellularly68, and may act directly on cerebral endothelial cells. It is possible that by acting as a calcium antagonist at the level of the endothelial cell actin cytoskeleton, MgSO4 opposes paracellular movement of solutes through the tight junctions (Figure 2). This hypothesis is supported by several studies which demonstrated that inhibition of myosin light chain (MLC) phosphorylation decreases agonist-induced permeability by inhibiting actin stress fiber contraction69-71. Alternatively, pinocytosis is induced by acute hypertension and may contribute to increased BBB permeability during elevated intravascular pressure.72 MgSO4 treatment may therefore decrease pinocytosis caused by acute hypertension and restrict the movement of water and solutes into the brain by transcellular transport, thereby limiting edema formation and improving clinical outcomes in eclampsia.
Table 2.
Effect of Magnesium Sulfate on Cerebral Edema and the Blood-brain Barrier.
| Cellular Target | Mode of Action | Possible Mechanism |
|---|---|---|
|
Cerebral Endothelium |
Decreased Blood-brain Barrier (BBB) disruption ↓ Limited Cerebral Edema Formation Via Paracellular Transport |
Calcium Antagonism ↓ Decreased Cell Contraction ↓ Decreased Tight Junction Permeability |
| Limited Transcellular Transport | Decreased Pinocytosis | |
| Astrocyte | Limited Cerebral Edema | Decreased Aquaporin 4 (AQP4) Expression |
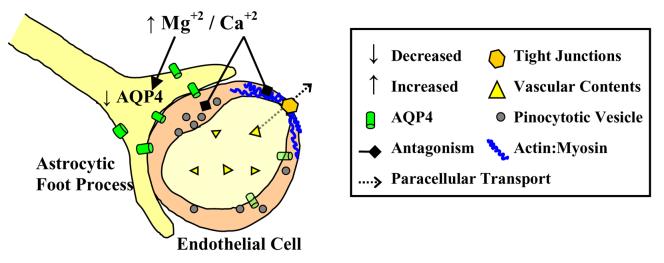
Figure 2. Effect of Magnesium Sulfate on Cerebral Edema and the Blood-brain Barrier.
The calcium antagonistic effects of magnesium can also affect the cerebral endothelium that forms the blood-brain barrier. Decreased cell calcium inhibits endothelial contraction and opening of tight junctions that are linked to the actin cytoskeleton. Decreased tight junction permeability limits paracellular transport of vascular contents, ions and proteins, which can promote vasogenic edema and seizures. It is also possible that magnesium sulfate diminishes transcellular transport by limiting pinocytosis that is known to occur rapidly during acute hypertension. Magnesium may also downregulate aquaporin 4 (AQP4), a water channel protein localized to astrocytic endfeet, and possibly cerebral endothelium, that is associated with cerebral edema formation (through unknown mechanisms).
Anticonvulsant Activity
Although widely used, there is controversy regarding the use of MgSO4 treatment for neurological conditions, such as eclamptic seizures. Concerns have been raised that MgSO4 treatment may mask the outward signs of convulsions through its action at the neuromuscular junction without treating the cause of the seizure in the central nervous system18, 73. Dose-related depression of neuromuscular transmission has been shown in preeclamptic women receiving traditional MgSO4 therapy74. Studies have also shown that there is little to no change in electroencephalograms obtained during MgSO4 treatment, and minimal signs of central nervous system depression in both normal75 and eclamptic patients25, and in animals76. However, clinical trials have demonstrated the efficacy of MgSO4 in the treatment and prevention of eclamptic seizures versus more traditional anticonvulsant drugs, including phenytoin and diazepam 12, 14, 15.
The possible anticonvulsant activity of magnesium may be related to its role as an N-methyl-d-aspartate (NMDA) receptor antagonist77-79, shown in Table 3 and Figure 3. Seizures are thought to be mediated at least in part by stimulation of glutamate receptors, such as the NMDA receptor79, 80. In rats, systemic magnesium treatment results in a resistance to both electrically stimulated81 and NMDA-induced hippocampal seizures82. In addition, systemic treatment with MgSO4 causes a significant reduction in the NMDA receptor binding capacity in the brain78. Animal studies have also shown that MgSO4 reduces epileptic seizure activity83, though these findings have been challenged due to inadequate controls76.
Table 3.
Anticonvulsant Activity of Magnesium Sulfate.
| Cellular Target | Mode of Action | Possible Mechanism |
|---|---|---|
| Neurons | Increased Seizure Threshold | N-Methyl-d-Aspartate (NMDA) Receptor Antagonism ↓ Decreased Effect of Glutamate, Limiting Massive Neuronal Depolarization |
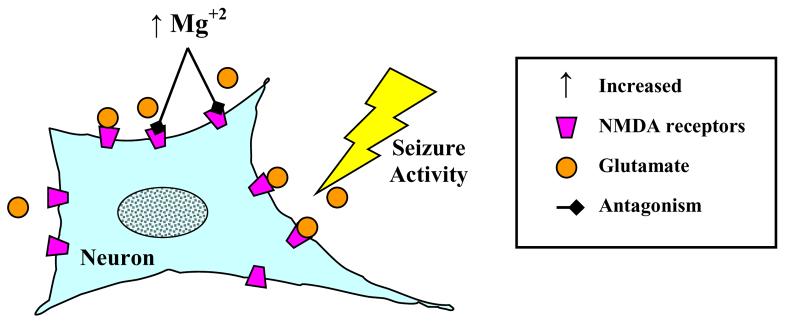
Figure 3. Anticonvulsant Activity of Magnesium Sulfate.
Seizures consist of an excessive release of excitotoxic neurotransmitters including glutamate. Excessive glutamate can activate the N-methyl-d-aspartate (NMDA) receptor, leading to massive depolarization of neuronal networks and bursts of action potentials. Magnesium may act to increase the seizure threshold by inhibiting NMDA receptors, thereby limiting the effect of glutamate.
Magnesium ions must cross the BBB in order to elicit a central anticonvulsant effect. It has been demonstrated in animals that MgSO4 can cross the intact BBB and enter the central nervous system in correlation with the level of serum hypermagnesemia81. Interestingly, seizure activity increases the movement of magnesium into the brain81. Human studies have also shown small but significant increases in cerebrospinal fluid concentrations of MgSO4 after systemic administration2, 84. Conversely, other work has suggested that the BBB prevents changes in brain and cerebrospinal fluid magnesium concentrations85. However, this same group later suggested that even a small amount of magnesium in the central nervous system may suppress cortical neuronal activity86. The possibility remains that acute hypertension that leads to convulsions and BBB disruption may permit MgSO4 to enter the brain parenchyma and act as an anticonvulsant during eclampsia.
Future Directions
A better understanding of the mechanisms of action of MgSO4 could allow for more directed use in the treatment of eclampsia and other brain injury disorders. An interesting area for future studies is the relationship between MgSO4 and cerebral edema formation, as it has been proposed that MgSO4 may limit cerebral edema formation through an effect on aquaporin (AQP) expression. Aquaporin-4 (AQP4) is a water channel protein that has been localized to astrocytic endfeet87, 88 and has also been reported to have a perivascular domain89. Cerebral edema in response to brain injury is associated with an upregulation of AQP4 in the brain90, 91, and it has been suggested that MgSO4 treatment attenuates cerebral edema formation via downregulation of AQP4 expression in astrocytes65, though the mechanism of action has not been delineated. This idea is particularly interesting with respect to eclampsia as cerebral AQP4 expression is significantly increased during pregnancy92.
One of the difficulties in studying preeclampsia and eclampsia is the lack of appropriate animal models, particularly as (pre)eclampsia is a disease specific to bipedal species93. In our lab, we have used a rat model of hypertensive encephalopathy during pregnancy to study the neurologic outcomes of eclampsia, specifically how acute elevations in blood pressure lead to forced dilatation of myogenic vasoconstriction, causing increased blood-brain barrier permeability and subsequent edema formation63, 94. Other animal models of preeclampsia and eclampsia exist, including reduced uterine placental perfusion (RUPP), Dahl Salt-Sensitive rats, nitric oxide synthase inhibition, and exogenous soluble fms-like tyrosine receptor kinase-1 (sFlt-1). These models focus on different aspects of the disease including the impact of placental perfusion, preexisting hypertension, and the significance of endothelial dysfunction, oxidative stress and circulating anti-angiogenic factors. The pros and cons of the different models have been reviewed elsewhere93, 95, all of which provide opportunities to further study the specific actions of MgSO4 for seizure prophylaxis.
Conclusion
MgSO4 has been shown to be an effective treatment option for the prevention of eclampsia. Its mechanism of action is likely multi-factorial, encompassing both vascular and neurological mechanisms. Being a calcium antagonist, its effect on vascular smooth muscle to promote relaxation and vasodilation may have a role in lowering total peripheral vascular resistance. In addition, MgSO4 may have an effect on the cerebral endothelium to limit vasogenic edema by decreasing stress fiber contraction and paracellular permeability via calcium-dependent second messenger systems such as MLC kinase. Lastly, MgSO4 may also act centrally to inhibit NMDA receptors, providing anticonvulsant activity by increasing the seizure threshold. A more complete understanding of the effects of MgSO4 will likely promote safer and more effective treatments of eclampsia.
Acknowledgements and Funding
We gratefully acknowledge the support of the American Heart Association Established Investigator Award (0540081N to M.J.C.), the American Heart Association Northeast Affiliate Research Committee Predoctoral Fellowship (000019871 to A.G.E.), the National Institute of Neurological Disorders and Stroke (R01 NS045940 to M.J.C.), the Totman Medical Research Trust, and the University of Vermont College of Medicine MD/PhD Program.
Footnotes
Author Disclosures
Anna G. Euser: Research Grant: AHA Northeast Affiliate Predoctoral Fellowship, Amount: >= $10,000
Marilyn J. Cipolla: Research Grant: NIH NS045849, Amount: >= $10,000
AHA EI 0540083, Amount: >= $10,000
Conflicts of Interest and Disclosures: None.
References
- 1.Lazard EM. A preliminary report on the intravenous use of magnesium sulfate in puerperal eclampsia. Am J Obstet Gynecol. 1925;9:178–188. doi: 10.1016/s0002-9378(96)70690-7. [DOI] [PubMed] [Google Scholar]
- 2.Pritchard JA. The use of the magnesium ion in the management of eclamptogenic toxemias. Surg Gynecol Obstet. 1955;100:131–140. [PubMed] [Google Scholar]
- 3.Working Group on High Blood Pressure in Pregnancy National high blood pressure education program working group report on high blood pressure in pregnancy. Am J Obstet Gynecol. 1990;163:1689–1712. doi: 10.1016/0002-9378(90)90653-o. [DOI] [PubMed] [Google Scholar]
- 4.Sibai BM. Magnesium sulfate is the ideal anticonvulsant in preeclampsia-eclampsia. Am J Obstet Gynecol. 1990;162:1141–1145. doi: 10.1016/0002-9378(90)90002-o. [DOI] [PubMed] [Google Scholar]
- 5.Witlin AG, Sibai BM. Magnesium sulfate therapy in preeclampsia and eclampsia. Obstet Gynecol. 1998;92:883–889. doi: 10.1016/s0029-7844(98)00277-4. [DOI] [PubMed] [Google Scholar]
- 6.Pritchard JA, Cunningham FG, Pritchard SA. The Parkland Memorial Hospital protocol for treatment of eclampsia: Evalauation of 235 cases. Am J Obstet Gynecol. 1984;148:951–960. doi: 10.1016/0002-9378(84)90538-6. [DOI] [PubMed] [Google Scholar]
- 7.Sibai BM, McCubbin JH, Anderson GD, Lipshitz J, Dilts PV., Jr Eclampsia. I. Observations from 67 recent cases. Obstet Gynecol. 1981;58:609–613. [PubMed] [Google Scholar]
- 8.Sibai BM. Eclampsia VI. Maternal-perinatal outcome in 254 cases. Am J Obstet Gynecol. 1990;163:1049–1055. doi: 10.1016/0002-9378(90)91123-t. [DOI] [PubMed] [Google Scholar]
- 9.Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, Smith D, The Magpie Trial Collaboration Group Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: A randomised placebo-controlled trial. Lancet. 2002;359:1877–1890. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 10.Chien PFW, Khan KS, Arnott N. Magnesium sulphate in the treatment of eclampsia and pre-eclampsia: An overview of the evidence from randomised trials. Br J Obstet Gynaecol. 1996;103:1085–1091. doi: 10.1111/j.1471-0528.1996.tb09587.x. [DOI] [PubMed] [Google Scholar]
- 11.Duley L, Henderson-Smart D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane Database Syst Rev. 2003:4. doi: 10.1002/14651858.CD000128. [DOI] [PubMed] [Google Scholar]
- 12.Lucas MJ, Leveno KJ, Cunningham FG. A comparison of magnesium sulfate with phenytoin for the prevention of eclampsia. N Engl J Med. 1995;333:201–205. doi: 10.1056/NEJM199507273330401. [DOI] [PubMed] [Google Scholar]
- 13.Belfort MA, Anthony J, Saade GR, Allen JC, Jr., the Nimodipine Study Group A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. N Engl J Med. 2003;348:304–311. doi: 10.1056/NEJMoa021180. [DOI] [PubMed] [Google Scholar]
- 14.Duley L, Henderson-Smart D. Magnesium sulphate versus diazepam for eclampsia. Cochrane Database Syst Rev. 2003:4. doi: 10.1002/14651858.CD000127. [DOI] [PubMed] [Google Scholar]
- 15.The Eclampsia Trial Collaborative Group Which anticonvulsant for women with eclampsia? Evidence from the collaborative eclampsia trial. Lancet. 1995;345:1455–1463. [PubMed] [Google Scholar]
- 16.Gülmezoglu AM, Duley L. Use of anticonvulsants in eclampsia and pre-eclampsia: Survey of obstetricians in the United Kingdom and Republic of Ireland. BMJ. 1998;316:975–976. doi: 10.1136/bmj.316.7136.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douglas KA, Redman CWG. Eclampsia in the United Kingdom. BMJ. 1994;309:1395–1400. doi: 10.1136/bmj.309.6966.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donaldson JO. Does magnesium sulfate treat eclamptic convulsions? Clinical Neuropharmacology. 1986;9:37–45. doi: 10.1097/00002826-198602000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Taber EB, Tan L, Chao CR, Beall MH, Ross MG. Pharmacokinetics of ionized versus total magnesium in subjects with preterm labor and preeclampsia. Am J Obstet Gynecol. 2002;186:1017–1021. doi: 10.1067/mob.2002.122421. [DOI] [PubMed] [Google Scholar]
- 20.Leveno KJ, Cunningham FG. Management of preeclampsia. In: Lindheimer MD, Roberts JM, Cunningham FG, editors. Chesley's Hypertensive Disorders in Pregnancy. Appleton & Lange; Stamford, CT: 1999. pp. 543–580. [Google Scholar]
- 21.Sibai BM, Graham JM, McCubbin JH. A comparison of intravenous and intramuscular magnesium sulfate regimens in preeclampsia. Am J Obstet Gynecol. 1984;150:728–733. doi: 10.1016/0002-9378(84)90676-8. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JM. Pregnancy-related hypertension. In: Creasy RK, Resnik R, Iams JD, editors. Maternal-Fetal Medicine: Principles and Practice. Saunders; Philadelphia, PA: 2004. pp. 884–887. [Google Scholar]
- 23.McCubbin JH, Sibai BM, Abdella TN, Anderson GD. Cardiopulmonary arrest due to acute maternal hypermagnesaemia. Lancet. 1981;1:1058. doi: 10.1016/s0140-6736(81)92225-x. [DOI] [PubMed] [Google Scholar]
- 24.Aali BS, Khazaeli P, Ghasemi F. Ionized and total magnesium concentration in patients with severe preeclampsia-eclampsia undergoing magnesium sulfate therapy. J Obstet Gynaecol Res. 2007;33:138–143. doi: 10.1111/j.1447-0756.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 25.Sibai BM, Spinnato JA, Watson DL, Lewis JA, Anderson GD. Effect of magnesium sulfate on electroencephalographic finding in preeclampsia-eclampsia. Obstet Gynecol. 1984;64:261–266. [PubMed] [Google Scholar]
- 26.Aali S, Khazaeli P, Ghasemi F, Mehdizadeh A. Serum magnesium and calcium ions in patients with severe pre-eclampsia/eclampsia undergoing magnesium sulfate therapy. Med Sci Monit. 2007;13:CR191–CR194. [PubMed] [Google Scholar]
- 27.Altura BM, Altura BT, Carella A, Gebrewold A, Murakawa T, Nishio A. Mg2+ - Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can J Physiol Pharmacol. 1987;65:729–745. doi: 10.1139/y87-120. [DOI] [PubMed] [Google Scholar]
- 28.Aloamaka CP, Ezimokhai M, Morrison J, Cherian T. Effect of pregnancy on relaxation of rat aorta to magnesium. Cardiovascular Research. 1993;27:1629–1633. doi: 10.1093/cvr/27.9.1629. [DOI] [PubMed] [Google Scholar]
- 29.Longo M, Jain V, Vedernikov YP, Facchinetti F, Saade GR, Garfield RE. Endothelium dependence and gestational regulation of inhibition of vascular tone by magnesium sulfate in rat aorta. Am J Obstet Gynecol. 2001;184:971–978. doi: 10.1067/mob.2001.112587. [DOI] [PubMed] [Google Scholar]
- 30.Euser AG, Cipolla MJ. Resistance artery vasodilation to magnesium sulfate during pregnancy and the postpartum state. Am J Physiol Heart Circ Physiol. 2005;288:H1521–H1525. doi: 10.1152/ajpheart.00994.2004. [DOI] [PubMed] [Google Scholar]
- 31.Nishio A, Gebrewold A, Altura BT, Altura BM. Comparative vasodilator effects of magnesium salts on rat mesenteric arterioles and venules. Arch Int Pharmacodyn. 1989;298:139–163. [PubMed] [Google Scholar]
- 32.Villamor E, Perez-Vizcaino F, Ruiz T, Tamargo J, Moro M. In vitro effects of magnesium sulfate in isolated intrapulmonary and mesenteric arteries of piglets. Pediatr Res. 1996;39:1107–1112. doi: 10.1203/00006450-199606000-00029. [DOI] [PubMed] [Google Scholar]
- 33.Nelson SH, Suresh MS. Magnesium sulfate-induced relaxation of uterine arteries from pregnant and non-pregnant patients. Am J Obstet Gynecol. 1991;164:1344–1350. doi: 10.1016/0002-9378(91)90711-y. [DOI] [PubMed] [Google Scholar]
- 34.Perales AJ, Torregrosa G, Salom JB, Miranda FJ, Alabadi JA, Monleon J, Alborch E. In vivo and in vitro effects of magnesium sulfate in the cerebrovascular bed of the goat. Am J Obstet Gynecol. 1991;165:1534–1538. doi: 10.1016/0002-9378(91)90401-c. [DOI] [PubMed] [Google Scholar]
- 35.Belfort MA, Moise KJ., Jr. Effect of magnesium sulfate on maternal brain blood flow in preeclampsia: A randomized, placebo-controlled study. Am J Obstet Gynecol. 1992;167:661–666. doi: 10.1016/s0002-9378(11)91567-1. [DOI] [PubMed] [Google Scholar]
- 36.Belfort MA, Saade GR, Moise KJ., Jr. The effect of magnesium sulfate on maternal and fetal blood flow in pregnancy-induced hypertension. Acta Obstet Gynecol Scand. 1993;72:526–530. doi: 10.3109/00016349309058156. [DOI] [PubMed] [Google Scholar]
- 37.Naidu S, Payne AJ, Moodley J, Hoffmann M, Gouws E. Randomised study assessing the effect of phenytoin and magnesium sulphate on maternal cerebral circulation in eclampsia using transcranial doppler ultrasound. Br J Obstet Gynaecol. 1996;103:111–116. doi: 10.1111/j.1471-0528.1996.tb09660.x. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, Bravo SM, Klufas RA, Chai RY, Repke JT. Preeclampsia-eclampsia: Clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217:371–376. doi: 10.1148/radiology.217.2.r00nv44371. [DOI] [PubMed] [Google Scholar]
- 39.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas JL, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 40.Donaldson JO. Eclamptic hypertensive encephalopathy. Seminars in Neurology. 1988;8:230–233. doi: 10.1055/s-2008-1041383. [DOI] [PubMed] [Google Scholar]
- 41.Hatab MR, Zeeman GG, Twickler DM. The effect of magnesium sulfate on large cerebral artery blood flow in severe preeclampsia. J Maternal-Fetal Neonat Med. 2005;17:187–192. doi: 10.1080/14767050500073050. [DOI] [PubMed] [Google Scholar]
- 42.Belfort MA, Saade GR, Yared M, Grunewald C, Herd JA, Varner MA, Nisell H. Change in estimated cerebral perfusion pressure after treatment with nimodipine or magnesium sulfate in patients with preeclampsia. Am J Obstet Gynecol. 1999;181:402–407. doi: 10.1016/s0002-9378(99)70569-7. [DOI] [PubMed] [Google Scholar]
- 43.Sherman R, Armory P, Moody P, Hope T, Mahajan RP. Effects of magnesium sulphate on cerebral haemodynamics in healthy volunteers: A transcranial doppler study. British Journal of Anaesthesia. 2003;91:273–275. doi: 10.1093/bja/aeg170. [DOI] [PubMed] [Google Scholar]
- 44.Scardo JA, Hogg BB, Newman RB. Favorable hemodynamic effects of manesium sulfate in preeclampsia. Am J Obstet Gynecol. 1995;173:1249–1253. doi: 10.1016/0002-9378(95)91364-5. [DOI] [PubMed] [Google Scholar]
- 45.Standley CA, Batia L, Yueh G. Magnesium sulfate effectively reduces blood pressure in an animal model of preeclampsia. J Matern Fetal Neonatal Med. 2006;19:171–176. doi: 10.1080/14767050500448005. [DOI] [PubMed] [Google Scholar]
- 46.Cipolla MJ, Vitullo L, McKinnon J. Cerebral artery reactivity changes during pregnancy and the postpartum period: A role in eclampsia? Am J Physiol Heart Circ Physiol. 2004;286:H2127–H2132. doi: 10.1152/ajpheart.01154.2003. [DOI] [PubMed] [Google Scholar]
- 47.Watson KV, Moldow CF, Ogburn PL, Jacob HS. Magnesium sulfate: Rationale for its use in preeclampsia. PNAS. 1986;83:1075–1078. doi: 10.1073/pnas.83.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravn HB, Vissinger H, Kristensen SD, Wennmalm A, Thygesen K, Husted SE. Magnesium inhibits platelet activity - an infusion study in healthy volunteers. Thromb Haemostas. 1996;75:939–944. [PubMed] [Google Scholar]
- 49.Goldkrand JW, Fuentes AM. The relation of angiotensin-converting enzyme to the pregnancy-induced hypertension-preeclampsia syndrome. Am J Obstet Gynecol. 1986;154:792–800. doi: 10.1016/0002-9378(86)90460-6. [DOI] [PubMed] [Google Scholar]
- 50.Easton JD. Severe preeclampsia/eclampsia: Hypertensive encephalopathy of pregnancy? Cerebrovasc Dis. 1998;8:53–58. doi: 10.1159/000015818. [DOI] [PubMed] [Google Scholar]
- 51.Khan F, Belch JJF, MacLeod M, Mires G. Changes in endothelial function precede the clinical disease in women in whom preeclampsia develops. Hypertension. 2005;46:1123–1128. doi: 10.1161/01.HYP.0000186328.90667.95. [DOI] [PubMed] [Google Scholar]
- 52.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: An endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 53.Fenstermacher J, Gross P, Sposito N, Acuff V, Pettersen S, Gruber K. Structural and functional variations in capillary systems within the brain. Ann N Y Acad Sci. 1988;529:21–30. doi: 10.1111/j.1749-6632.1988.tb51416.x. [DOI] [PubMed] [Google Scholar]
- 54.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–217. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sedlakova R, Shivers RR, Del Maestro RF. Ultrastructure of the blood-brain barrier in the rabbit. J Submicrosc Cytol Pathol. 1999;31:149–161. [PubMed] [Google Scholar]
- 56.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaplan PW. Eclampsia. In: Kaplan PW, editor. Neurologic Disease in Women. Demos Medical Publishing, Inc; New York, NY: 2006. pp. 235–245. [Google Scholar]
- 58.Zunker P, Ley-Pozo J, Louwen F, Schuierer G, Holzgreve W, Ringelstein EB. Cerebral hemodynamics in pre-eclampsia/eclampsia syndrome. Ultrasound Obstet Gynecol. 1995;6:411–415. doi: 10.1046/j.1469-0705.1995.06060411.x. [DOI] [PubMed] [Google Scholar]
- 59.Esen F, Erdem T, Aktan D, Kalayci R, Cakar N, Kaya M, Telci L. Effects of magnesium administration on brain edema and blood-brain barrier breakdown after experimental traumatic brain injury in rats. Journal of Neurosurgical Anesthesiology. 2003;15:119–125. doi: 10.1097/00008506-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 60.Esen F, Erdem T, Aktan D, Orhan M, Kaya M, Eraksoy H, Cakar N, Telci L. Effect of magnesium sulfate administration on blood-brain barrier in a rat model of intraperitoneal sepsis: A randomized controlled experimental study. Critical Care. 2005;9:R18–R23. doi: 10.1186/cc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaya M, Kucuk M, Kalayci RB, Cimen V, Gurses C, Elmas I, Arican N. Magnesium sulfate attenuates increased blood-brain barrier permeability during insulin-induced hypoglycemia in rats. Can J Physiol Pharmacol. 2001;79:793–798. [PubMed] [Google Scholar]
- 62.Kaya M, Gulturk S, Elmas I, Arican N, Kocyildiz ZC, Kucuk M, Yorulmaz H, Sivas A. The effects of magnesium sulfate on blood-brain barrier disruption caused by intracarotid injection of hyperosmolar mannitol in rats. Life Sci. 2004;76:201–212. doi: 10.1016/j.lfs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Euser AG, Bullinger L, Cipolla MJ. Magnesium sulphate treatment decreases blood brain barrier permeability during acute hypertension in pregnant rats. Exp Physiol. 2008;93:254–261. doi: 10.1113/expphysiol.2007.039966. [DOI] [PubMed] [Google Scholar]
- 64.Feldman Z, Gurevitch B, Artru AA, Oppenheim A, Shohami E, Reichenthal E, Shapira Y. Effect of magnesium given 1 hour after head trauma on brain edema and neurological outcome. J Neurosurg. 1996;85:131–137. doi: 10.3171/jns.1996.85.1.0131. [DOI] [PubMed] [Google Scholar]
- 65.Ghabriel MN, Thomas A, Vink R. Magnesium restores altered aquaporin-4 immunoreactivity following traumatic brain injury to a pre-injury state. Acta Neurochir Suppl. 2006;96:402–406. doi: 10.1007/3-211-30714-1_83. [DOI] [PubMed] [Google Scholar]
- 66.Okiyama K, Smith DH, Gennarelli TA, Simon RP, Leach M, McIntosh TK. The sodium channel blocker and glutamate release inhibitor BW1003C87 and magnesium attenuate regional cerebral edema following experimental brain injury in the rat. J Neurochem. 1995;64:802–809. doi: 10.1046/j.1471-4159.1995.64020802.x. [DOI] [PubMed] [Google Scholar]
- 67.Turkoglu OF, Eroglu H, Okutan O, Tun MK, Bodur E, Sargon MF, Öner L, Beskonakli E. A comparative study of treatment for brain edema: Magnesium sulphate versus dexamethasone sodium phosphate. J Clin Neurosci. 2008;15:60–65. doi: 10.1016/j.jocn.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Fawcett WJ, Haxby EJ, Male DA. Magnesium: Physiology and pharmacology. British Journal of Anaesthesia. 1999;83:302–320. doi: 10.1093/bja/83.2.302. [DOI] [PubMed] [Google Scholar]
- 69.Yuan SY. Signal transduction pathways in enhanced microvascular permeability. Microcirculation. 2000;7:395–403. [PubMed] [Google Scholar]
- 70.Yuan Y, Huang Q, Wu HM. Myosin light chain phosphorylation: Modulation of basal and agonist-stimulated venular permeability. Am J Physiol. 1997:272. doi: 10.1152/ajpheart.1997.272.3.H1437. [DOI] [PubMed] [Google Scholar]
- 71.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: Role of myosin light chain phosphorylation. J Cell Physiol. 1995;163:510–522. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- 72.Mayhan WG, Heistad DD. Permeability of blood-brain barrier to various sized molecules. Am J Physiol Heart Circ Physiol. 1985;248:H712–H718. doi: 10.1152/ajpheart.1985.248.5.H712. [DOI] [PubMed] [Google Scholar]
- 73.Kaplan PW, Lesser RP, Fisher RS, Repke JT, Hanley DF. No, magnesium sulfate should not be used in treating eclamptic seizures. Arch Neurol. 1988;45:1361–1364. doi: 10.1001/archneur.1988.00520360079017. [DOI] [PubMed] [Google Scholar]
- 74.Ramanathan J, Sibai BM, Pillai R, Angel JJ. Neuromuscular transmission studies in preeclamptic women receiving magnesium sulfate. Am J Obstet Gynecol. 1988;158:40–46. doi: 10.1016/0002-9378(88)90772-7. [DOI] [PubMed] [Google Scholar]
- 75.Somjen G, Hilmy M, Stephen CR. Failure to anesthetize human subjects by intravenous administration of magnesium sulfate. J Pharmac Exp Ther. 1966;154:652–659. [PubMed] [Google Scholar]
- 76.Koontz WL, Reid KH. Effect of parenteral magnesium sulfate on penicillin-induced seizure foci in anesthetized cats. Am J Obstet Gynecol. 1985;153:96–99. doi: 10.1016/0002-9378(85)90603-9. [DOI] [PubMed] [Google Scholar]
- 77.Goldman RS, Finkbeiner SM. Therapeutic use of magnesium sulfate in selected cases of cerebral ischemia and seizure. N Engl J Med. 1988;319:1224–1225. doi: 10.1056/NEJM198811033191813. [DOI] [PubMed] [Google Scholar]
- 78.Hallak M, Berman RF, Irtenkauf SM, Janusz C, Cotton DB. Magnesium sulfate treatment decreases N-methyl-d-aspartate receptor binding in the rat brain: An autoradiographic study. J Soc Gynecol Invest. 1994;1:25–30. doi: 10.1177/107155769400100106. [DOI] [PubMed] [Google Scholar]
- 79.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–620. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 80.Dingledine R, Hynes MA, King GL. Involvment of N-methyl-d-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol. 1986;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hallak M, Berman RF, Irtenkauf SM, Evans MI, Cotton DB. Peripheral magnesium sulfate enters the brain and increases the threshold for hippocampal seizures in rats. Am J Obstet Gynecol. 1992;167:1605–1610. doi: 10.1016/0002-9378(92)91749-z. [DOI] [PubMed] [Google Scholar]
- 82.Cotton DB, Hallak M, Janusz C, Irtenkauf SM, Berman RF. Central anticonvulsant effects of magnesium sulfate on N-methyl-d-aspartate-induced seizures. Am J Obstet Gynecol. 1993;198:974–978. doi: 10.1016/s0002-9378(12)90855-8. [DOI] [PubMed] [Google Scholar]
- 83.Borges LF, Gucer G. Effect of magnesium on epileptic foci. Epilepsia. 1978;19:81–91. doi: 10.1111/j.1528-1157.1978.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 84.Thurnau GR, Kemp DB, Jarvis A. Cerebrospinal fluid levels of magnesium in patients with preeclampsia after treatment with intravenous magnesium sulfate: A preliminary report. Am J Obstet Gynecol. 1987;157:1435–1438. doi: 10.1016/s0002-9378(87)80239-9. [DOI] [PubMed] [Google Scholar]
- 85.Hilmy MI, Somjen GG. Distribution and tissue uptake of magnesium related to its pharmacological effects. Am J Physiol. 1968;214:406–413. doi: 10.1152/ajplegacy.1968.214.2.406. [DOI] [PubMed] [Google Scholar]
- 86.Kato G, Somjen GG. Effects of micro-iontophoretic administration of magnesium and calcium on neurones in the central nervous system of cats. J Neurobiol. 1969;1:181–195. doi: 10.1002/neu.480010206. [DOI] [PubMed] [Google Scholar]
- 87.Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. PNAS. 2003;100:2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amiry-Moghaddam M, Xue R, Haug FM, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. FASEB J. 2004;18:542–544. doi: 10.1096/fj.03-0869fje. [DOI] [PubMed] [Google Scholar]
- 90.Papadopoulos MC, Verkman AS. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J Biol Chem. 2005;280:13906–13912. doi: 10.1074/jbc.M413627200. [DOI] [PubMed] [Google Scholar]
- 91.Taniguchi M, Yamashita T, Kumura E, Tamatani M, Kobayashi A, Yokawa T, Maruno M, Kato A, Ohnishi T, Kohmura E, Tohyama M, Yoshimine T. Induction of aquaporin-4 water channel mRNA after focal cerebral ischemia in rat. Molecular Brain Research. 2000;78:131–137. doi: 10.1016/s0169-328x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 92.Quick AM, Cipolla MJ. Pregnancy-induced up-regulation of aquaporin-4 protein in brain and its role in eclampsia. FASEB J. 2005;19:170–175. doi: 10.1096/fj.04-1901hyp. [DOI] [PubMed] [Google Scholar]
- 93.Podjarny E, Losonczy G, Baylis C. Animal models of preeclampsia. Semin Perinat. 2004;24:596–606. doi: 10.1016/s0270-9295(04)00131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Euser AG, Cipolla MJ. Cerebral blood flow autoregulation and edema formation during pregnancy in anesthetized rats. Hypertension. 2007;49:334–340. doi: 10.1161/01.HYP.0000255791.54655.29. [DOI] [PubMed] [Google Scholar]
- 95.Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension. 2007;50:14–24. doi: 10.1161/HYPERTENSIONAHA.106.079442. [DOI] [PubMed] [Google Scholar]