Abstract
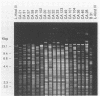
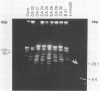
Twenty-nine Borrelia burgdorferi isolates, obtained from dusky-footed wood rats (Neotoma fuscipes) and California kangaroo rats (Dipodomys californicus) in California, were analyzed genetically. Chromosomal DNA was examined by restriction endonuclease analysis (REA) and gene probe restriction fragment length polymorphism. Pulsed-field gel electrophoresis was used to analyze the plasmid profiles of the isolates. REA, the method with the greatest discrimination, disclosed 24 distinct restriction patterns among the 29 isolates. These restriction patterns were sorted into four restriction fragment length polymorphism groups on the basis of their gene hybridization patterns. Results of the REA and plasmid profile analysis supported this grouping. The degree of genetic diversity among Californian isolates demonstrated by our findings is greater than that previously reported among other groups of North American isolates and is similar or greater than the diversity reported among European isolates.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam T., Gassmann G. S., Rasiah C., Göbel U. B. Phenotypic and genotypic analysis of Borrelia burgdorferi isolates from various sources. Infect Immun. 1991 Aug;59(8):2579–2585. doi: 10.1128/iai.59.8.2579-2585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. F., Magnarelli L. A., LeFebvre R. B., Andreadis T. G., McAninch J. B., Perng G. C., Johnson R. C. Antigenically variable Borrelia burgdorferi isolated from cottontail rabbits and Ixodes dentatus in rural and urban areas. J Clin Microbiol. 1989 Jan;27(1):13–20. doi: 10.1128/jcm.27.1.13-20.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranton G., Postic D., Saint Girons I., Boerlin P., Piffaretti J. C., Assous M., Grimont P. A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992 Jul;42(3):378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Garon C. F. The genes encoding major surface proteins of Borrelia burgdorferi are located on a plasmid. Ann N Y Acad Sci. 1988;539:144–153. doi: 10.1111/j.1749-6632.1988.tb31847.x. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Heiland R. A., Howe T. R. Heterogeneity of major proteins in Lyme disease borreliae: a molecular analysis of North American and European isolates. J Infect Dis. 1985 Sep;152(3):478–484. doi: 10.1093/infdis/152.3.478. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. J Clin Microbiol. 1988 Mar;26(3):475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril C., Richaud C., Baranton G., Saint Girons I. S. Linear chromosome of Borrelia burgdorferi. Res Microbiol. 1989 Oct;140(8):507–516. doi: 10.1016/0923-2508(89)90083-1. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Bosler E. M., Hanrahan J. P., Coleman J. L., Habicht G. S., Bast T. F., Cameron D. J., Ziegler J. L., Barbour A. G., Burgdorfer W. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983 Mar 31;308(13):740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Bissett M. L., Hill W. Characterization of Borrelia burgdorferi strains isolated from Ixodes pacificus ticks in California. J Clin Microbiol. 1987 Dec;25(12):2296–2301. doi: 10.1128/jcm.25.12.2296-2301.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerlin P., Peter O., Bretz A. G., Postic D., Baranton G., Piffaretti J. C. Population genetic analysis of Borrelia burgdorferi isolates by multilocus enzyme electrophoresis. Infect Immun. 1992 Apr;60(4):1677–1683. doi: 10.1128/iai.60.4.1677-1683.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce W. M., Brown R. N., Zingg B. C., Lefebvre R. B., Lane R. S. First isolation of Borrelia burgdorferi in southern California. J Med Entomol. 1992 May;29(3):496–500. doi: 10.1093/jmedent/29.3.496. [DOI] [PubMed] [Google Scholar]
- Brown R. N., Lane R. S. Lyme disease in California: a novel enzootic transmission cycle of Borrelia burgdorferi. Science. 1992 Jun 5;256(5062):1439–1442. doi: 10.1126/science.1604318. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Denny T. P., Gilmour M. N., Selander R. K. Genetic diversity and relationships of two pathovars of Pseudomonas syringae. J Gen Microbiol. 1988 Jul;134(7):1949–1960. doi: 10.1099/00221287-134-7-1949. [DOI] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsey M. S., Jr, Amundson T. E., Burgess E. C., Schell W., Davis J. P., Kaslow R., Edelman R. Lyme disease ecology in Wisconsin: distribution and host preferences of Ixodes dammini, and prevalence of antibody to Borrelia burgdorferi in small mammals. Am J Trop Med Hyg. 1987 Jul;37(1):180–187. doi: 10.4269/ajtmh.1987.37.180. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Hinnebusch J., Barbour A. G. Linear- and circular-plasmid copy numbers in Borrelia burgdorferi. J Bacteriol. 1992 Aug;174(16):5251–5257. doi: 10.1128/jb.174.16.5251-5257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. A., Kodner C. B., Johnson R. C. DNA analysis of Borrelia burgdorferi NCH-1, the first northcentral U.S. human Lyme disease isolate. J Clin Microbiol. 1992 Mar;30(3):698–703. doi: 10.1128/jcm.30.3.698-703.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R. S., Brown R. N. Wood rats and kangaroo rats: potential reservoirs of the Lyme disease spirochete in California. J Med Entomol. 1991 May;28(3):299–302. doi: 10.1093/jmedent/28.3.299. [DOI] [PubMed] [Google Scholar]
- Lane R. S., Pascocello J. A. Antigenic characteristics of Borrelia burgdorferi isolates from ixodid ticks in California. J Clin Microbiol. 1989 Oct;27(10):2344–2349. doi: 10.1128/jcm.27.10.2344-2349.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFebvre R. B., Lane R. S., Perng G. C., Brown J. A., Johnson R. C. DNA and protein analyses of tick-derived isolates of Borrelia burgdorferi from California. J Clin Microbiol. 1990 Apr;28(4):700–707. doi: 10.1128/jcm.28.4.700-707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFebvre R. B., Perng G. C., Johnson R. C. Characterization of Borrelia burgdorferi isolates by restriction endonuclease analysis and DNA hybridization. J Clin Microbiol. 1989 Apr;27(4):636–639. doi: 10.1128/jcm.27.4.636-639.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre R. B., Perng G. C., Johnson R. C. The 83-kilodalton antigen of Borrelia burgdorferi which stimulates immunoglobulin M (IgM) and IgG responses in infected hosts is expressed by a chromosomal gene. J Clin Microbiol. 1990 Jul;28(7):1673–1675. doi: 10.1128/jcm.28.7.1673-1675.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. F., Wilson M. L., Spielman A. Mice as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg. 1985 Mar;34(2):355–360. doi: 10.4269/ajtmh.1985.34.355. [DOI] [PubMed] [Google Scholar]
- Mather T. N., Wilson M. L., Moore S. I., Ribeiro J. M., Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). Am J Epidemiol. 1989 Jul;130(1):143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- Owen R. J. Chromosomal DNA fingerprinting--a new method of species and strain identification applicable to microbial pathogens. J Med Microbiol. 1989 Oct;30(2):89–99. doi: 10.1099/00222615-30-2-89. [DOI] [PubMed] [Google Scholar]
- Perng G. C., LeFebvre R. B., Johnson R. C. Further characterization of a potent immunogen and the chromosomal gene encoding it in the Lyme disease agent, Borrelia burgdorferi. Infect Immun. 1991 Jun;59(6):2070–2074. doi: 10.1128/iai.59.6.2070-2074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic D., Edlinger C., Richaud C., Grimont F., Dufresne Y., Perolat P., Baranton G., Grimont P. A. Two genomic species in Borrelia burgdorferi. Res Microbiol. 1990 May;141(4):465–475. doi: 10.1016/0923-2508(90)90072-x. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W. Antigenic changes of Borrelia burgdorferi as a result of in vitro cultivation. J Infect Dis. 1987 Nov;156(5):852–853. doi: 10.1093/infdis/156.5.852-a. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Burgdorfer W., Garon C. F. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect Immun. 1988 Aug;56(8):1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal L. H., Curran A. S. Lyme disease: a multifocal worldwide epidemic. Annu Rev Public Health. 1991;12:85–109. doi: 10.1146/annurev.pu.12.050191.000505. [DOI] [PubMed] [Google Scholar]
- Simor A. E., Shames B., Drumm B., Sherman P., Low D. E., Penner J. L. Typing of Campylobacter pylori by bacterial DNA restriction endonuclease analysis and determination of plasmid profile. J Clin Microbiol. 1990 Jan;28(1):83–86. doi: 10.1128/jcm.28.1.83-86.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsky R. J., Piesman J. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J Clin Microbiol. 1989 Aug;27(8):1723–1727. doi: 10.1128/jcm.27.8.1723-1727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Stålhammar-Carlemalm M., Jenny E., Gern L., Aeschlimann A., Meyer J. Plasmid analysis and restriction fragment length polymorphisms of chromosomal DNA allow a distinction between Borrelia burgdorferi strains. Zentralbl Bakteriol. 1990 Oct;274(1):28–39. doi: 10.1016/s0934-8840(11)80972-2. [DOI] [PubMed] [Google Scholar]
- Timms P., Eaves F. W., Girjes A. A., Lavin M. F. Comparison of Chlamydia psittaci isolates by restriction endonuclease and DNA probe analyses. Infect Immun. 1988 Jan;56(1):287–290. doi: 10.1128/iai.56.1.287-290.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R., Helmes C., Schaible U. E., Lobet Y., Moter S. E., Kramer M. D., Simon M. M. Evaluation of genetic divergence among Borrelia burgdorferi isolates by use of OspA, fla, HSP60, and HSP70 gene probes. Infect Immun. 1992 Nov;60(11):4856–4866. doi: 10.1128/iai.60.11.4856-4866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Schierz G., Kühbeck R., Barbour A. G., Kramer M. Antigenic variability of Borrelia burgdorferi. Ann N Y Acad Sci. 1988;539:126–143. doi: 10.1111/j.1749-6632.1988.tb31846.x. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]