Abstract
Accumulating evidence has shown that marrow failure in some patients with myelodysplastic syndrome is associated with autoimmunity, T-cell mediated myelosuppression and cytokine-induced cytopenias. In this perspective article, Drs. Barrett and Sloand expound on auto-immunity in myelodysplastic syndromes, in particular focusing on what has been learned from study of patients with trisomy 8. See related article on page 496.
Myelodysplastic syndromes (MDS), first clearly defined as an entity by the pioneering studies of Bernard Dreyfus and others, have long been perceived as pre-leukemic hematopoietic stem cell abnormalities which cause bone marrow failure and ultimately transform to acute leukemia.
The bone marrow failure, resulting in the transfusion dependence and neutropenic infection that characterizes the disease, was assumed to be an intrinsic stem cell defect causing defective maturation. However, accumulating evidence has shown that marrow failure in some MDS is associated with autoimmunity, T-cell mediated myelosuppression and cytokine-induced cytopenias. The novel study by Chamuleau et al.1 in this issue suggests that the innate immune system may play a role in MDS pathophysiology. Study of the autoimmune component in MDS pathophysiology has led to better definition of patient subgroups where immune processes are the dominant cause of the cytopenia, and has led to the development of immunosuppressive treatments which have improved the quality of life and extended survival of selected patients with MDS.
Immune mechanisms in the pathophysiology of cytopenia in myelodysplastic syndromes and the response to immunosuppression
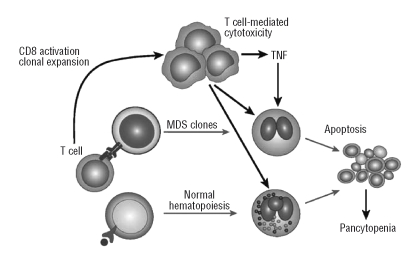
Evidence for autoimmune abnormalities in MDS comes from clinical and experimental sources.2–6 Hamblin et al.7 first drew attention to an association between MDS and the autoimmune diseases. MDS is sometimes seen in conjunction with Reynaud’s syndrome, rheumatoid arthritis and polymyalgia rheumatica. Furthermore, MDS is associated with aplastic anemia, a disease with an established autoimmune pedigree: patients with AA can develop MDS and overlap syndromes of severe aplastic anemia with features of MDS also exist and frequently give rise to diagnostic confusion. Tichelli et al. were the first to describe inhibition of erythroid colony growth by autologous T cells in some patients with MDS.8 Based on the success of anti-lymphocyte globulin to treat AA and anecdotal accounts of cytopenic patients with clear features of MDS responding to immunosuppressive treatment (IST), we treated a series of transfusion-dependent MDS patients with ATG and achieved sustained red cell transfusion independence in 20%.9 In several patients studied, we demonstrated that ATG abrogated the T-cell mediated suppression of granulocyte colony growth in responding patients. Subsequently, other investigators confirmed that ATG can lead to improved marrow function and loss of transfusion dependence, accompanied by a normalization of a skewed T-cell repertoire.6 These findings strongly supported T-cell mediated marrow suppression as the cause of the cytopenia in about 20–30% of MDS patients. Figure 1 illustrates the working hypothesis regarding the relationship between the immune system and cytopenias in MDS. The highest response to immunosuppression occurred in the less common group of younger MDS patients (under the age of 60) with refractory anemia (RA)10.
Figure 1.
Model of immune interactions with myelodysplastic syndromes and the response to treatment. Myelodysplastic syndromes clones express a neoantigen or overexpress an antigen. This triggers expansion of T cell clones cytotoxic for the myelodysplastic syndromes cell. Activated T cells secrete cytokines, TNFα and IFNγ, which promote apoptosis of normal progenitor cells suppressing hematopoiesis.
Although several factors such as the PNH abnormality, RA, and degree of cytopenia have been identified in various series as predictive factors for response, only age and the presence of HLA DR15 emerged as an independent prognostic factor in multivariate analysis in a large NIH study of 120 patients.10 Such young MDS responders to ATG included a preponderance of female patients with RA and an over-representation of trisomy 8.
These observations raise many questions concerning the mechanism underlying the immune dysfunction of MDS. What are the antigens on MDS cells recognized by auto-reactive T cells? What is the role of cytokines such as TNF-α in the bystander effect on residual normal hematopoiesis? Does the immune-mediated myelosuppression serve as a form of immune surveillance to retard the evolution to leukemia? Much insight into these questions has been provided by studies of the cytopenia associated with trisomy 8 MDS. The use of fluorescent in situ hybridization (FISH) to identify the trisomy 8 marker allows the investigator to separately identify T-cell interactions with normal and MDS cells and define their specificity in vitro. Using FISH techniques we showed that cytotoxic T cells specifically targeted trisomy 8 bearing cells.11 Because the Wilm’s tumor antigen, WT1, is over-expressed on CD34 cells in many patients with MDS, it is an obvious candidate antigen for an MDS specific T cell response.12,13 We found that patients, particularly those with trisomy 8 (who have a high probability of response to IST), over-express WT1. Furthermore, T cells from these patients show repetitive skewing of restricted Vβ 3.2 CD8 T cell and several other Vβ subsets, which diminishes after successful IST.11 CD8 cells isolated from the Vβ 3.2 fraction contain the preponderance of the myelosuppressive activity of autologous T cells and specifically block proliferation of trisomy 8 hematopoietic progenitors.
Furthermore, the Vβ CD8+ lymphocytes, which are expanded in patients over-expressing WT1 (with or without trisomy 8), contain a high frequency of WT1126 peptide-HLA tetramer positive CD8+ T cells which over-express TNF-α (Sloand et al., unpublished data, 2008). These cells are capable of suppressing growth of trisomy 8 cells. These findings strongly indicate that WT1 is at least one of the antigens driving the myelosuppressive T-cell response in MDS.
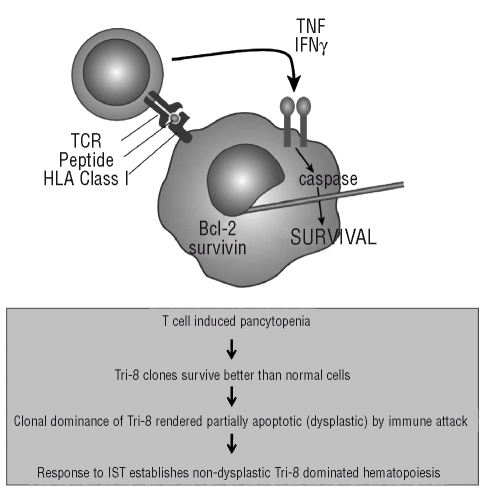
Thus far, the detailed picture of MDS pathophysiology emerging at the molecular level seems straightforward. However, clinical data is puzzling. Patients with trisomy 8 who recover their blood counts after immunosuppressive treatment revert to normal marrow morphology yet do not lose the trisomy 8 population in the marrow; indeed the percentage of trisomy 8 cells measured by karyotyping or by FISH can actually increase. Despite this increase in karyotypically abnormal cells these patients are stable for extensive periods of time11 Thus it appears that the removal of T-cell control permits hematologic recovery with a further expansion of the MDS clone. In untreated patients, the persistence of the trisomy 8 clone in the presence of the T-cell response suggests that the MDS cells are adapted to survive T-cell attack. Experimental data support this idea: trisomy 8 cells show signs of apoptotic induction with increased caspase and BCL-2 but appear to avoid destruction from T-cell mediated apoptosis by up-regulating survivin.14 This stalled apoptotic process results in “living dead” cells with dysplastic morphology. After IST, the loss of MDS specific cytotoxic T cells permits the return to normal morphology and expansion of the now unrestrained trisomy 8 clone. (Figure 2). This scenario would suggest that IST should be used with great caution for fear of removing immune surveillance, which although inconveniently causing cytopenia, might be the only barrier to leukemic progression. Fortunately, from a long-term follow-up of IST treated patients data already exist to refute this concern. We found no increase in progression to leukemia in either responders or non-responders after as much as ten years of follow-up.10 Responders treated with IST actually had a decreased rate of leukemic progression10 In addition, apart from patients with trisomy 8, serial FISH studies demonstrated a significant decrease in most karyotypic abnormalities following IST.11
Figure 2.
Working hypothesis for induction of an immune response to MDS and the selection of trisomy 8 clones. Trisomy 8 cells over-express normal antigens such as WT1 which results in induction of an immune response. Cytotoxic T cells, which recognize the antigen, expand. However, these cells are ineffective in eliminating the trisomy 8 clone. Apoptosis is triggered in the trisomy 8 cell but is blocked upstream of caspase 8 activation because trisomy 8 cells overexpress survivin.
This suggests that although the immune response to MDS causes cytopenias, such immune surveillance may be unrelated to what appears to be an intrinsic tendency of MDS stems cell to progress to leukemia. Another possibility is that the immune system fosters development of genomic instability. Provocative evidence in other inflammatory states suggests that aneuploidy may result from oxidative stress and nitric oxide (NO)-induced cell cycle arrest. Conditions such as Barrett’s esophagitis, hepatitis, graft versus host disease of the skin, and ulcerative colitis result in aneuploid and tetraploid lesions which can precede malignant development.15–18 This mechanism could account for the high frequency of clonal progression in patients with aplastic anemia and for the decrease in leukemic progression among responders to IST.10
Given the existence of a natural T-cell response to WT1 in MDS, the use of WT1 vaccines to treat MDS appears difficult to justify. The induction of a T-cell response to WT1 by vaccination could provoke undesired marrow suppression. Currently there are not enough data on the use of WT1 vaccine in MDS to confirm or refute these concerns. We chose to select for WT1 vaccine treatment only those patients with progressing MDS with excess of blasts, because prevention or delay of overt leukemia could outweigh the disadvantage of inducing cytopenia.
Finally it remains a possibility that the entire T-cell response to MDS is a side-show, and we should direct our attention more to myelosuppression by NK cells shown by Chamuleau et al.1 to be strongly and specifically cytotoxic to MDS cells and perhaps in some patients (e.g. non-responders to ATG) responsible for myelosuppression and immune surveillance. In conclusion, the relationship between the immune system, marrow suppression and MDS remains confusing. Comprehensive studies in a large group of patients as performed by Chamuleau et al. are critical steps forward in trying to establish a global view of competing mechanisms contributing to the two major outcome determinants of MDS – marrow failure and leukemic progression.
References
- 1.Chamuleau MED, Westers TM, van Dreunen L, Groenland J, Zevenbergen A, Eeltink CM, et al. Immune mediated autologous cytotoxicity against hematopoietic precursor cells in patients with myelodysplastic syndrome. Haematologica. 2009;94:496–506. doi: 10.3324/haematol.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okada M, Okamoto T, Takemoto Y, Kanamaru A, Kakishita E. Function and X chromosome inactivation analysis of B lymphocytes in myelodysplastic syndromes with immunological abnormalities. Acta Haematol. 2000;102:124–30. doi: 10.1159/000040985. [DOI] [PubMed] [Google Scholar]
- 3.Mufti G, List AF, Gore SD, Ho AYL. Myelodysplastic Syndrome. Hematology. 2003;2003:176–99. doi: 10.1182/asheducation-2003.1.176. [DOI] [PubMed] [Google Scholar]
- 4.Stadler M, Germing U, Kliche KO, Josten KM, Kuse R, Hofmann WK, et al. A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia. 2004;18:460–5. doi: 10.1038/sj.leu.2403239. [DOI] [PubMed] [Google Scholar]
- 5.Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, et al. CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS) Blood. 2007;110:847–50. doi: 10.1182/blood-2007-01-067546. [DOI] [PubMed] [Google Scholar]
- 6.Kochenderfer JN, Kobayashi S, Wieder ED, Su C, Molldrem JJ. Loss of T-lymphocyte clonal dominance in patients with myelodysplastic syndrome responsive to immunosuppression. Blood. 2002;100:3639–45. doi: 10.1182/blood-2002-01-0155. [DOI] [PubMed] [Google Scholar]
- 7.Hamblin TJ. Myelodysplasia. Br J Hosp Med. 1987;38:558–61. [PubMed] [Google Scholar]
- 8.Tichelli A, Gratwohl A, Wuersch A, Nissen C, Speck B. Antilymphocyte globulin for myelodysplastic syndrome. Br J Haematol. 1988;68:139–40. doi: 10.1111/j.1365-2141.1988.tb04194.x. [DOI] [PubMed] [Google Scholar]
- 9.Molldrem JJ, Jiang YZ, Stetler-Stevenson M, Mavroudis D, Hensel N, Barrett AJ. Haematological response of patients with myelodysplastic syndrome to antithymocyte globulin is associated with a loss of lymphocyte-mediated inhibition of CFU-GM and alterations in T-cell receptor Vbeta profiles. Br J Haematol. 1998;102:1314–22. doi: 10.1046/j.1365-2141.1998.00920.x. [DOI] [PubMed] [Google Scholar]
- 10.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–11. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloand EM, Mainwaring L, Fuhrer M, Ramkissoon S, Risitano AM, Keyvanafar K, et al. Preferential suppression of trisomy 8 compared with normal hematopoietic cell growth by autologous lymphocytes in patients with trisomy 8 myelodysplastic syndrome. Blood. 2005;106:841–51. doi: 10.1182/blood-2004-05-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Zeng W, Miyazato A, Billings E, Maciejewski JP, Kajigaya S, et al. Distinctive gene expression profiles of CD34 cells from patients with myelodysplastic syndrome characterized by specific chromosomal abnormalities. Blood. 2004;104:4210–8. doi: 10.1182/blood-2004-01-0103. [DOI] [PubMed] [Google Scholar]
- 13.Oka Y, Tsuboi A, Murakami M, Hirai M, Tominaga N, Nakajima H, et al. Wilms tumor gene peptide-based immunotherapy for patients with overt leukemia from myelodysplastic syndrome (MDS) or MDS with myelofibrosis. Int J Hematol. 2003;78:56–61. doi: 10.1007/BF02983241. [DOI] [PubMed] [Google Scholar]
- 14.Sloand EM, Pfannes L, Chen G, Shah S, Solomou EE, Barrett J, Young NS. CD34 cells from patients with trisomy 8 myelodysplastic syndrome (MDS) express early apoptotic markers but avoid programmed cell death by up-regulation of antiapoptotic proteins. Blood. 2007;109:2399–405. doi: 10.1182/blood-2006-01-030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ying L, Marino J, Hussain SP, Khan MA, You S, Hofseth AB, et al. Chronic inflammation promotes retinoblastoma protein hyperphosphorylation and E2F1 activation. Cancer Res. 2005;65:9132–6. doi: 10.1158/0008-5472.CAN-05-1358. [DOI] [PubMed] [Google Scholar]
- 16.Goodman JE, Hofseth LJ, Hussain SP, Harris CC. Nitric oxide and p53 in cancer-prone chronic inflammation and oxyradical overload disease. Environ Mol Mutagen. 2004;44:3–9. doi: 10.1002/em.20024. [DOI] [PubMed] [Google Scholar]
- 17.O’Sullivan JN, Bronner MP, Brentnall TA, Finley JC, Shen WT, Emerson S, et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat Genet. 2002;32:280–4. doi: 10.1038/ng989. [DOI] [PubMed] [Google Scholar]
- 18.Svendsen LB, Søndergaard JO, Hegnhøj J, Højgård L, Lauritsen KB, Bülow S, et al. In vitro tetraploidy in patients with ulcerative colitis. Scand J Gastroenterol. 1987;22:601–5. doi: 10.3109/00365528708991905. [DOI] [PubMed] [Google Scholar]