Bortezomib is a synthetic small molecule inhibitor of the chymotryptic activity of the 26S proteasome. Effects of bortezomib on normal immune cells have also been previously reported. This study adds to observations on the effects of bortezomib on natural killer (NK) cells. Effects include induction of apoptosis in resting NK cells, together with suppression of NK-mediated cytotoxicity via the NKp46 pathway. The concern is raised that treatment with bortezomib could interfere with NK cell-mediated attack on tumor cells, or on infected cells.
Keywords: natural killer cells, bortezomib, apoptosis, NK receptor
Abstract
Background
Bortezomib is a selective and potent inhibitor of the proteasome and has prominent effects in vitro and in vivo against tumors. Very recently, cytotoxic effects of bortezomib on immune-competent cells such as T cells and dendritic cells were also revealed. The aim of the study was to investigate the effects of this agent on natural killer cell survival and function.
Design and Methods
We investigated cytotoxic properties of bortezomib on natural killer cell apoptosis and function. Primary resting natural killer cells were purified from peripheral blood mononuclear cells of healthy donors by negative selection. The apoptotic cells were quantified by dual labeling of recombinant annexin V and propidium iodide. Mitochondrial membrane potential and expression of natural killer cell activating receptors were also quantified by flow cytometry. Natural killer cell cytotoxicity against murine and human tumor cells was tested by chromium 51 release assay.
Results
Our results demonstrate that bortezomib induces apoptosis in resting natural killer cells in a dose- and time-dependent manner. Glutathione, a reactive oxygen species scavenger, prevented the loss of mitochondrial membrane potential and conferred protection against bortezomib-induced apoptosis in resting natural killer cells, indicating a role for oxidative stress. Additionally, bortezomib significantly decreased expression of the natural killer activating receptor NKp46 in non-apoptotic resting natural killer cells in a dose-dependent manner, and as a result the redirected cytotoxicity mediated via NKp46 activation was diminished. Bay 11-7082, a pharmacological inhibitor of NF-κB activation, also reduced NKp46 expression and suppressed redirected cytotoxicity.
Conclusions
Bortezomib induces apoptosis in primary resting natural killer cells in a dose- and time-dependent manner, and reduces NKp46 receptor expression as well as natural killer cell cytotoxicity mediated by the NKp46 activation pathway, suggesting that bortezomib may disrupt natural killer cell-mediated immunity through at least two different mechanisms: induction of natural killer cell apoptosis, and suppression of NKp46 receptor-mediated cytotoxicity.
Introduction
Natural killer (NK) cells are large granular lymphocytes, principally found in peripheral blood and lymphoid tissues. Unlike T cells, NK cells are capable of rapid recognition and elimination of tumor cells and pathogen infected cells without pre-priming steps, providing early host response to infections and transformed or malignant cells.1 NK cells utilize perforin/granzyme exocytosis and Fas/Fas ligand pathways as well as secreted tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) to kill their targets, and the granule exocytosis pathway is the major mechanism of killing used by NK cells.1 Apart from their cytolytic activity, NK cells produce cytokines and interact directly with other immune components such as dendritic cells (DC), thereby regulating also the adaptive immune system.2
The proteasome is a multi-catalytic enzyme complex localized in the cytosol and nucleus of all eukaryotic cells and its principle function is to degrade ubiquitinated proteins for maintenance of normal cell function.3,4 Several intracellular proteins such as p53 and IκB, the inhibitor of nuclear transcription factor kappa B (NF-κB) that govern cell growth and survival are regulated by the ubiquitin-mediated proteasome pathway.4,5
Bortezomib selectively inhibits the proteolytic activity of 26S proteasome and thus blocks the degradation of these poly-ubiquitinated proteins destined for catalysis by the proteasome.4 Owing to its potent cytotoxic activity against tumor cells, bortezomib has recently received much attention from hematologists/oncologists and has been applied in treatment of various malignancies including solid tumors.5,6 In addition to its direct cytotoxic (pro-apoptotic) effects on tumor cells of various origin, proteasome inhibition can also sensitize tumor cells to NK cell mediated-lysis through TRAIL and/or Fas/Fas ligand pathways.7,8
Recent studies have demonstrated that proteasome inhibition by bortezomib induced apoptosis in activated human T cells and suppressed expression of activation-associated cell surface receptors and cytokines,9,10 highlighting that proteasome inhibition may suppress immune function of T cells. Moreover, bortezomib effectively inhibited cytotoxicity of interleukin (IL)-2 stimulated human NK cells without affecting the viability of NK cells.11 Here, we show that proteasome inhibition by bortezomib induces apoptosis in primary human resting NK (rNK) cells in a dose- and time-dependent manner. Moreover, bortezomib selectively decreases NKp46 expression on rNK cells, resulting in a suppression of NK cell cytotoxicity mediated through the NKp46 activation pathway, as evaluated in a redirected NK cytotoxicity assay. Thus, our findings highlight that bortezomib may impede NK cell-mediated immunity against transformed or infected cells.
Design and Methods
Cell culture medium
RPMI-1640 medium (Gibco, Paisley, Scotland, United Kingdom) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Paisley, United Kingdom), 2 mmol/L L-glutamine, and 50 units/mL penicillin-streptomycin (Gibco).
Inhibitors
Bortezomib (Velcade) (Millennium Pharmaceuticals Cambridge, MA) was prepared as a stock solution (1 mg/mL) in 0.9% saline. Pan-caspase inhibitor, Z-VAD-FMK (zVAD) and glutathione (GSH) were purchased from R&D Systems (Minneapolis, MN, USA) and Sigma-Aldrich Sweden AB (Stockholm, Sweden) respectively. Bay 11-7082, an NF-κB inhibitor, was purchased from Calbiochem (San Diego, CA, USA).
Antibodies
Monoclonal antibodies (mab) used for flow cytometric analysis were FITC-conjugated anti-human CD3, PE-conjugated anti-human CD3, PECy5-conjugated anti-human CD56, PE-conjugated anti-human NKG2D, FITC-conjugated anti-human DNAM-1, PE-conjugated anti-human NKp46, PE-conjugated anti-human NKp30 and relevant isotype control antibodies (BD Pharmingen, San Jose, CA, USA). FITC-conjugated anti-human perforin mab was purchased from Ancell Corporation (Bayport, MN, USA). Purified anti-human NKp46 mab for redirected NK cell cytotoxicity assay was purchased from R&D systems (Minneapolis, MN, USA).
Isolation of primary human natural killer cells
Human peripheral blood mononuclear cells (PBMCs) from adult blood donors (Blood Bank of Karolinska University Hospital, Stockholm, Sweden) were separated by Ficoll-Paque (Amersham Biosciences AB, Uppsala, Sweden) gradient centrifugation, and NK cells were isolated from PBMCs using the RosetteSep NK cell purification kit (Stemcell Technologies, Vancouver, Canada) and following the method described previously12 with minor modifications. Briefly, 20×106 PBMC were mixed with autologous red blood cells (RBC) with an RBC/PBMC ratio of 40:1, suspended in 1 ml complete RPMI-1640 medium and incubated with 50 μL RosetteSep cocktail for 20 min at RT. Then, the sample was diluted 2 times with complete medium before loading on Ficoll-Hypaque for separation. After centrifugation, NK cells were recovered from the interface. Purity of NK cells (CD3−CD56+) was approximately 90%, as determined by staining with CD3 and CD56 mabs.
Phosphatidylserine exposure
Phosphatidylserine (PS) exposure, a hallmark of apoptotic cells, can be detected by using recombinant annexin V (AV). In the present study, apoptotic cells were quantified by propidium iodide (PI) (Sigma) and AV (Annexin-V-Fluos staining kit, Roche Diagnostics GmbH, Mannheim, Germany) dual labeling according to the protocol provided by the manufacturer. Briefly, cells were cultured in 24-well plates in the presence or absence of bortezomib at the indicated concentrations for 12h, 24h, and 48h. After washing twice with cold PBS, the cells were resuspended in 100 μL of 1 × binding buffer containing AV and PI, and incubated for 15 min at RT. Then, 400 μL of incubation buffer was added, and cells were analyzed by flow cytometry within 30 min after staining on a FACS Calibur (Becton Dickinson, San Jose, CA, USA). AV+PI− and AV+PI+ cells were defined as early and late apoptotic cells respectively.
Caspase-3-like activity
Caspase-3-like activity was assessed using the method described previously.13 In brief, cell lysates of 1.0×106 treated or untreated rNK cells were combined with DEVD-AMC, a fluorogenic substrate, in 1 × HEPES buffer (20 mM HEPES, pH 7.5, 10% glycerol, and 2 mM dithiothreitol) and real-time release of AMC catalyzed by caspase-3-like enzymes was measured using a Fluoroscan II plate reader (Labsystems, Stockholm, Sweden). Fluorescence values for each sample were converted to picomoles of AMC release using a standard curve generated with free AMC and the maximum rate of AMC release (pmol/min) was calculated.
Mitochondrial membrane potential
Functional mitochondria were labeled by Mitotracker Red CMXRos (Mitotracker Red) (Molecular Probes, Eugene, OR) as described previously14 with minor modifications. In brief, a total of 1.0×106 rNK cells were pelleted and suspended in 1 mL of pre-warmed complete medium with a final concentration of 125 nM Mitotracker Red for 35 min at 37°C. Excess dye was removed with 2 washes in pre-warmed complete medium at 37°C, and finally the labeled cells were resuspended in PBS for mitochondrial membrane potential (MMP) measurement. Mitotracker Red fluorescence was detected using a FACS Calibur (Becton Dickinson).
Natural killer cell cytotoxicity
To compare differences in cytotoxicity of bortezomib-treated and untreated rNK cells, a standard 4h chromium 51 (51Cr)-release assay was performed using protocols described previously.15,16 Briefly, rNK cells were incubated with 51Cr (Amersham Biosciences, Uppsala, Sweden) labeled target cells in triplicates at various effector to target (E/T) ratios. K562, a human leukemia cell line, or FcγR+ P815, a murine mastocytoma cell line were employed as target cells, as indicated. For assessment of redirected cytotoxicity, 51Cr-labeled FcγR+ P815 cells were first incubated with anti-human NKp46 ab (2.5 μg/mL) for 30 min before adding rNK cells into wells at different E/T ratios. After 4h incubation at 37°C, 100 μL of cell-free supernatants were collected and the amount of 51Cr released into the supernatants was measured using an automatic gamma counter (Wallac, Upplands Väsby, Sweden). Specific lysis was calculated by the method described previously.16
Natural killer cell protein expression
For surface staining, cells were first incubated with 10% FCS-PBS buffer for 15 min at RT to block non-specific binding of antibodies to Fc receptors. The cells were then stained with fluorescence labeled antibodies in 10% FBS-PBS buffer for 30 min at 4°C, washed in PBS and finally resuspended in 1% PFA-PBS buffer prior to analysis on a FACS cytometer (FACSCalibur) (Becton Dickinson). Detection of intracellular perforin expression in purified NK cells was performed by using the Intrastain Kit (DAKO, Glostrup, Denmark) according to instructions provided by the manufacturer. Briefly, NK cell surface marker (CD56) was stained first as described above and cells were then fixed using reagent A for 15 min at RT. After washing, cells were resuspended in reagent B for cell membrane permeabilization, and the anti-human perforin (Ancell Corporation) or corresponding isotype control mab was added and cells were incubated for 15 min at RT. Finally, cells were washed with PBS and resuspended in 400 μl PBS prior to analysis by flow cytometry. The collected data were analyzed using CELLQUEST software (Becton Dickinson).
Statistical analysis
Non-parametric Mann-Whitney test or Student’s t test was used to compare differences between the groups studied. A difference was considered to be statistically significant when the two-sided p value was less than 0.05.
Results
Bortezomib induces apoptosis in primary resting natural killer cells
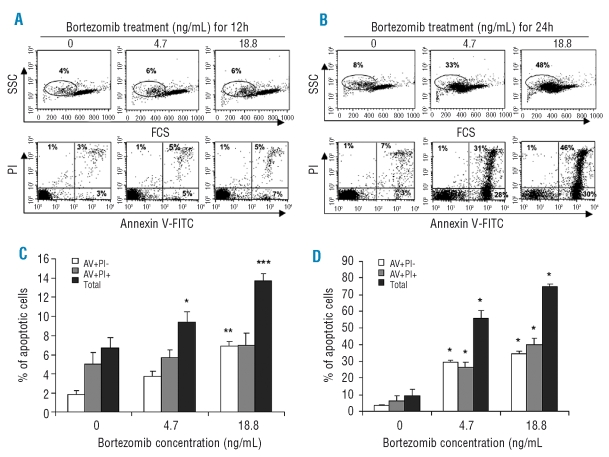
To evaluate cytotoxic effects of bortezomib on NK cells, highly purified resting NK cells (rNK) were cultured in the presence of bortezomib for 12 and 24h. We found that bortezomib markedly induced apoptosis in rNK cells in a dose- and time-dependent manner (Figure 1A and B). The percentages of total apoptotic cells (AV+PI− + AV+PI+) were significantly increased in the rNK cells treated by bortezomib with the doses of 4.7 ng/mL and 18.8 ng/mL for 12h (Figure 1C) and 24h (Figure 1D). Consistently, flow cytometric assessment of light scattering properties of rNK cells demonstrated a reduction in forward scatter (FSC), indicative of apoptosis, in response to bortezomib (Figure 1A, B).
Figure 1.
Resting human natural killer cells are sensitive to bortezomib-induced apoptosis. Highly purified resting NK cells obtained from PBMCs from healthy blood donors were harvested after 12 h and 24 h of culture in the presence or absence of bortezomib at the indicated doses, and apoptosis was quantified by flow cytometry with dual staining of annexin V, to detect phosphatidylserine (PS) exposure, and propidium iodide (PI), to detect cells with a loss of plasma membrane integrity. Percentages of early (AV+PI−), late (AV+PI+) and total (early + late) apoptotic cells were calculated. Dot plots of one representative experiment showing apoptotic cell detection after 12 h (A) and 24 h (B) of treatment with bortezomib. The percentages of the cells positive for AV/PI or of cells with decreased Forward Scatter (FSC) and increased Side Scatter (SSC) are indicated in the dot plots. (C) Comparisons of percentages of apoptotic cells among resting NK cells cultured with bortezomib at the indicated doses for 12 h. The values presented are the mean ± SEM (n=6–12). *p<0.05, **p<0.002 and ***p<0.001 versus untreated group, by the Mann-Whitney test. (D) Comparisons of apoptotic cell percentages among resting NK cells cultured with bortezomib at the indicated doses for 24 h. Data expressed as mean ± SEM (n=10–15). *p<0.0001 versus untreated group, by the Mann-Whitney test.
Bortezomib treatment for 12 and 24h at the dose of 18.8 ng/mL induced more pronounced apoptosis in rNK cells than 4.7 ng/mL, respectively (p<0.01 for 12h and p<0.001 for 24h, respectively, by Mann-Whitney test) (Figure 1C and D). When the same dose of bortezomib (4.7 ng/mL or 18.8 ng/mL) was applied, 24h treatment triggered more apoptosis in rNK cells than 12h treatment (p<0.0001 for both comparisons by Mann-Whitney test).
Reactive oxygen species-dependent bortezomib-induced apoptosis in resting natural killer cells
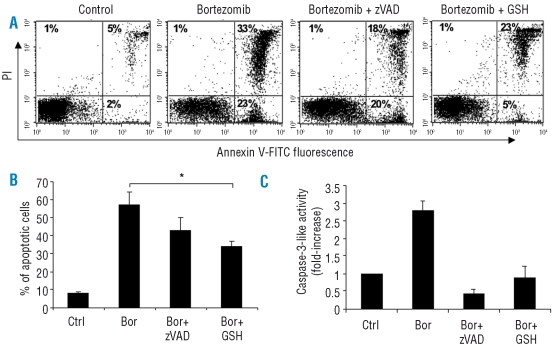
Next, we asked whether reactive oxygen species (ROS) and/or caspase activation contribute to bortezomib-induced NK cell apoptosis. Our results demonstrated that GSH, a potent ROS scavenger, markedly abolished total (AV+PI− + AV+PI+) apoptosis induced by bortezomib at 4.7 ng/mL for 24h (Figure 2A and B). Blocking caspase activation by zVAD, a pan-caspase inhibitor, did not effectively block total apoptosis in rNK cells induced by bortezomib (Figure 2B). For comparison, zVAD completely prevented caspase-3 activation of rNK cells induced by bortezomib at the same time-point, demonstrating that the inhibitor was functional under these conditions (Figure 2C). Moreover, GSH almost completely prevented the caspase-3 activation induced by bortezomib, indicating that caspase activation is ROS-dependent in this model (Figure 2C). Together, these data indicate that ROS generation contributes to apoptosis in rNK cells induced by bortezomib at the doses tested.
Figure 2.
Bortezomib induces apoptosis in resting NK cells in a ROS-dependent manner. Resting NK cells were treated with zVAD (20 μM) or GSH (2 mM) for 30 min prior to adding bortezomib (4.7 ng/mL) for 24 h. Apoptosis was monitored by flow cytometric detection of annexin V (AV)/ propidium iodide (PI) staining. (A) Apoptosis of resting NK cells induced by bortezomib in the presence or absence of zVAD or GSH. Dot plots of one representative experiment are shown. Percentages of positive cells for AV and/or PI are indicated in the plots. (B) Apoptosis of NK cells treated with bortezomib in the presence or absence of zVAD or GSH. The results are expressed as mean percentage ± SEM of total (AV+PI− + AV+PI+) apoptotic cells from five independent experiments. *p<0.05 by Student’s t test. (C) Caspase-3-like enzyme activity of the untreated and treated rNK cells was determined as described in the Design and Methods section, and fold increase of caspase-3-like activity is shown. The values are presented as mean ± SEM (n=4).
Bortezomib decreases mitochondrial membrane potential in primary resting natural killer cells
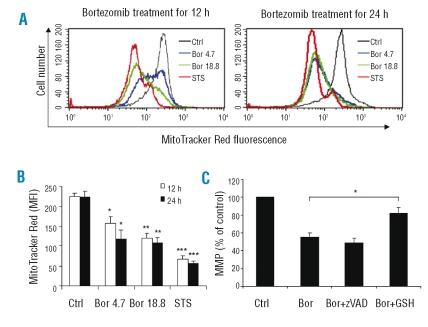
Mitochondria play pivotal roles in the process of apoptosis. To determine the role of mitochondria in the bortezomib-induced apoptosis in resting NK cells, mitochondrial membrane potential (MMP) of rNK cells cultured with bortezomib for 12 or 24 h, respectively, was measured. As shown in Figure 3A, bortezomib markedly induced a loss of Mitotracker Red fluorescence intensity in rNK cells in a dose-dependent manner. Statistical analysis showed that the differences in mean fluorescence intensity of Mitotracker Red between bortezomib (4.7 ng/mL or 18.8 ng/mL)-treated and untreated rNK cells were significant (Figure 3B). Furthermore, in line with our finding showing that GSH suppressed apoptosis in rNK cells (Figure 2), our studies showed that GSH partially and significantly prevented the loss of MMP induced by bortezomib (4.7 ng/mL) (Figure 3C). In contrast, zVAD did not show any protective effects on MMP, suggesting that the dissipation of MMP is upstream of caspase activation in bortezomib-treated rNK cells (Figure 3C).
Figure 3.
Bortezomib triggers a ROS-dependent drop in mitochondrial membrane potential of resting NK cells. The changes in fluorescence intensity of Mitotracker Red, reflecting MMP, were compared between untreated (Ctrl) and bortezomib (Bor) (4.7 ng/mL and 18.8 ng/mL) treated resting NK cells at 12h and 24h, by flow cytometry. (A) Histograms of one representative experiment depicting Mitotracker Red fluorescence intensity in untreated and bortezomib-treated resting NK cells. Staurosporine (STS) (2 μM) was applied as a positive control in the assay. ( B ) Mean fluorescence intensity (MFI) of Mitotracker Red staining results are presented as mean ± SEM (n=3–4). *p<0.02, **p<0.005 and ***p<0.0001 versus untreated group, by Student’s t test. (C) Resting NK cells were pre-incubated with GSH (2 mM) or zVAD (20 μM) for 30 min prior to adding bortezomib (4.7 ng/mL) for 24 h. MMP was quantified as mentioned above. Data are shown as mean ± SEM (n=3–8). The differences in MFI between resting NK cells treated by bortezomib alone and bortezomib plus GSH or zVAD was compared (*p<0.001, by Student’s t test).
Bortezomib treatment down-regulates NKp46 expression on resting natural killer cells
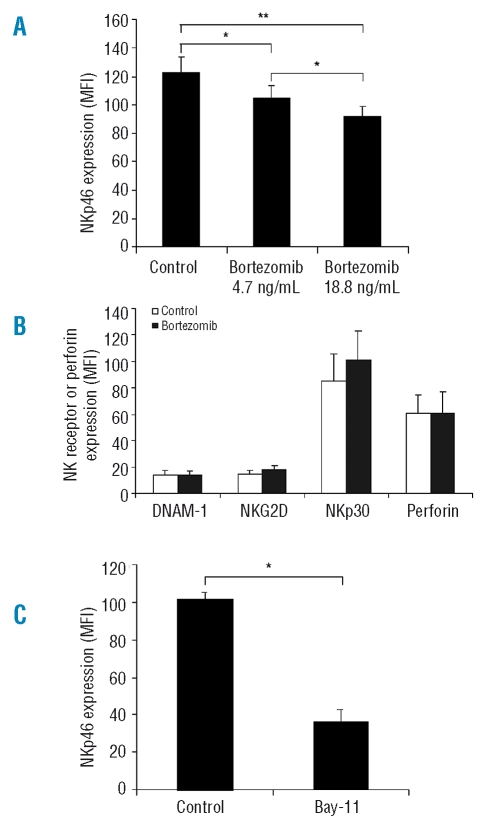
To examine whether proteasome inhibition affects expression of NK cell activation receptors (NKp30, NKp46, NKG2D and DNAM-1), rNK cell were exposed to bortezomib (4.7 ng/mL or 18.8 ng/mL) for 12h and the expression of these receptors was then quantified by flow cytometry. Additionally, expression of these receptors was analyzed only in non-apoptotic NK cells, as determined by forward and side scatter with more than 95% AV-cells (data not shown). As shown in Figure 4A, NKp46 expression in rNK cells was significantly reduced upon treatment with bortezomib in a dose-dependent manner. The same trend was seen in experiments performed in each of the 3 separate donors, i.e. NKp46 expression (MFI) on rNK cells treated with bortezomib was reduced to 88.7% (MFI: 110 vs. 124), 83.8% (MFI: 88 vs. 105), and 82.3% (MFI: 116 vs. 141), respectively. By contrast, expression of DNAM-1, NKG2D and NKp30 on bortezomib (4.7 ng/mL)-treated rNK cells was not significantly different from untreated rNK cells (Figure 4B). Moreover, expression of intracellular perforin was compared between bortezomib (4.7 ng/mL)-treated and untreated rNK cells, and no difference was found between these two groups (Figure 4B).
Figure 4.
Bortezomib and Bay 11-7082 downregulate NK cell activating receptor NKp46 expression in resting NK cells. Primary rNK cells treated by bortezomib (4.7 ng/mL or 18.8 ng/mL) or the NF-κB inhibitor, Bay 11-7082 (1.25 μM) for 12h and untreated rNK cells (control) were stained with fluorescence conjugated antibody specific for NKp46 and the expression of NKp46 (mean fluorescence intensity, MFI) was quantified by flow cytometry. (A) Comparison of NKp46 expression (MFI) between bortezomib-treated and untreated rNK cells. The results presented are mean ± SEM (n=3). *p<0.05 and **p<0.02 by Student’s t test. (B) Comparison of cell surface expression of DNAM-1, NKG2D and NKp30, as well as intracellular perforin expression (MFI) between bortezomib (4.7 ng/mL)-treated and untreated rNK cells. Expression of the NK cell activating receptors (DNAM-1, NKG2D and NKp30) and perforin was quantified by flow cytometry as described in the Design and Methods section. Data are expressed as mean ± SEM (n=3). *p<0.01 by Student’s t test. (C) Comparison of NKp46 expression (MFI) between Bay 11-7082-treated and untreated rNK cells.
To uncover a role of NF-κB in regulation of NKp46 expression, we further tested NKp46 expression on rNK cells after blocking NF-κB activity by a NF-κB blocker Bay 11-7082 and found that blocking NF-κB activity induced significant loss of NKp46 expression on rNK cells (Figure 4B), indicating that the bortezomib-induced decrease in NKp46 expression may be, at least in part, attributable to the known inhibitory effects of this agent on NF-κB function.
Bortezomib treatment impairs NKp46-dependent cytotoxicity
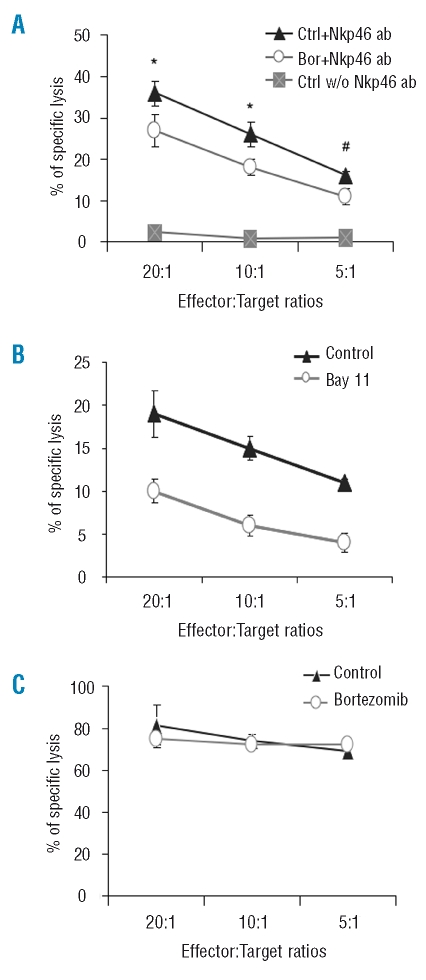
NKp46 is one of the important activating receptors that are required for NK cell function. To investigate the functional consequence of decreased NKp46 expression on rNK cells following bortezomib treatment, we employed a redirected NK cell cytotoxicity assay using FcγR+ P815 as target cells in the presence of anti-NKp46 antibody, and found that the lysis of FcγR+ P815 cells by the rNK cells exposed to a low dose of bortezomib (4.7 ng/mL) for 12h was significantly decreased when compared with controls at E:T ratios of 20:1 and 10:1 (Figure 5A). Moreover, blocking NF-κB activation by Bay 11-7082 for 12h substantially inhibited rNK cell-mediated lysis of FcγR+ P815 cells in the presence of anti-NKp46 antibody (Figure 5B). In the absence of this antibody, the rNK cells showed no or limited lysis of FcγR+ P815 cells (Figure 5A), demonstrating that the NK cell-mediated killing of FcγR+ P815 cells was dependent upon the anti-NKp46 antibody. Importantly, very limited percentages of apoptosis (4–9% vs. 3–6% apoptotic cells in bortezomib-treated and untreated rNK cells, respectively) were observed under these conditions, implying that the bortezomib-induced impairment of NK cell killing mediated through the NKp46 activation pathway is not related to the apoptosis inducing-properties of bortezomib. Consistently, rNK cells treated by bortezomib (4.7 ng/mL) for 12h were able to lyse K562 target cells as efficiently as the untreated rNK cells (Figure 5C).
Figure 5.
Bortezomib inhibits cytotoxicity of resting NK cells via the NKp46-mediated pathway. Resting NK cells selected from healthy blood donors were cultured with bortezomib (4.7 ng/mL) or NF-κB inhibitor Bay 11-7082 for 12 h. Standard 4h-51Cr release assay was applied using FcR+ P815 (A and B) or K562 (C) as target cells, respectively, to determine suppressive properties of bortezomib on resting NK cell cytotoxicity. For the redirected cytotoxicity assay (A and B), FcR+ P815 cells were pre-incubated with a purified anti-human NKp46 monoclonal antibody (ab) (2.5 μg/mL) for 30 min prior to incubation with bortezomib-treated (Bor) and untreated resting NK cells (Ctrl) (A), or Bay 11-7082 (1.25 μM) and solvent (DMSO)-treated resting NK cells (B) at different effector:target (E:T) ratios. Ctrl NK cells incubated with FcR+ P815 without (w/o) NKp46 ab were used as an internal control ( A ). *p<0.05, #p=0.05 by Student’s t test. Data shown in A and C are expressed as percentages (mean ± SEM) of specific lysis of target cells at different E/T ratios indicated from three independent experiments. Results presented in B are mean ± SEM of specific lysis (%) at different E/T ratios, generated from triplicate samples from two independent donors.
Discussion
Our studies reported herein show for the first time that primary human resting NK cells (rNK) undergo apoptosis in response to proteasome inhibition by bortezomib, a novel anti-cancer agent. Moreover, bortezomib treatment selectively decreased NKp46 expression on rNK cells resulting in the inhibition of NK cell killing of target cells mediated through the NKp46 activating pathway. Importantly, we selected bortezomib doses of 4.7 ng/mL (12.2 nM) and 18.8 ng/mL (48.8 nM), which were in the dose range mostly applied in previous studies on tumor cells and cell lines.8,17,18
Generation of ROS triggered by proteasome inhibition appears to play a critical role in bortezomib-induced tumor cell apoptosis.17,19,20 Interestingly, our results demonstrated that GSH, a ROS scavenger, significantly abolished apoptosis and prevented the dissipation of MMP in rNK cells induced by bortezomib, indicating that increased ROS generation is associated with bortezomib-mediated apoptosis. However, our findings do not exclude a role for additional, ROS-independent pathways in proteasome inhibition-mediated apoptosis of rNK cells. Bortezomib-induced caspase activation has been observed in tumor cells.17,18 Our results obtained in primary human NK cells also showed that bortezomib triggers caspase-3 activation in a dose-dependent manner. However, the pan-caspase inhibitor, zVAD did not significantly reduce apoptosis in rNK cells incubated with bortezomib. Additionally, such caspase inhibition did not protect rNK cells from bortezomib-mediated loss of mitochondrial membrane integrity. Taken together, the current findings suggest that ROS generation rather than caspase activation is a key triggering factor for bortezomib-induced apoptosis in rNK cells. Consistently, our results showed that GSH almost completely prevented caspase activation induced by bortezomib. Previous studies on the effects of bortezomib in cancer cells, including one recent report on malignant NK cell lines21 have also implicated mitochondria in bortezomib-triggered apoptosis. Thus, bortezomib may act via similar mechanism(s), including a disruption of mitochondrial membrane integrity, to trigger apoptosis in both malignant NK cells and primary resting NK cells.
NK cell functions are finely regulated by a balance between activating and inhibiting signaling originated from engagements of activation and inhibition receptors expressed on NK cells with corresponding ligands expressed on the target cells.22,23 We found that bortezomib selectively down-regulated the expression of NKp46 but not other NK receptors tested (NKp30, NKG2D and DNAM-1) and this effect was not attributable to the apoptosis inducing-property of bortezomib. We cannot exclude the possibility that bortezomib treatment could affect other downstream signaling activities in resting NK cells. Nevertheless, NK cell cytotoxicity mediated by the NKp46 activation pathway was impaired, and these results were in accordance with the decreased NKp46 expression observed after administration of bortezomib. By contrast, NK cells treated with bortezomib killed the NK cell sensitive target cells, K562 cells, as efficiently as untreated NK cells. NK cell activity is controlled by the expression of activating and inhibiting receptors on NK cells and the presence or absence of major histocompatibility complex (MHC) class I molecules on target cells.24,25 Human K562 leukemic cells are commonly used as targets in NK killing assays and are known to lack expression of MHC class I molecules26 thereby rendering these cells especially sensitive to lysis by NK cells. NKp46 signaling is only one of the known pathways triggering NK cell activation,22 and selective reduction of NKp46 expression on NK cells induced by bortezomib may thus not affect NK cell cytotoxicity against all target cells. Nevertheless, previous studies27 have shown that the destruction of certain virus-infected and tumor cells is mediated, in part, via NKp46, suggesting that the bortezomib-induced suppression of NKp46-mediated killing may impact on these processes. Additionally, we found that there was no defect in NK cell expression of perforin or cytolytic granule degranulation28 in bortezomib-treated NK cells (C. Zheng et al., unpublished data, 2008). Bortezomib is thus likely to inhibit NK cell cytotoxicity by suppressing NKp46 activation pathway-associated NK cell cytolytic activity rather than interfering with the cytolytic granule exocytosis machinery. One recent study claimed that clinically relevant concentrations of bortezomib (10 nM) did not affect NK cell viability or function whereas MHC class I expression was down-regulated in multiple myeloma cell lines.29 However, the authors did not present such data in the manuscript and no information was provided on the specific assays used to monitor NK cell function upon treatment with bortezomib. In contrast, the present study provides conclusive evidence that primary human rNK cells are susceptible to bortezomib-induced apoptosis at doses ranging from 4.7 ng/mL (corresponding to 12.2 nM).
Interestingly, we also observed that blocking of NF-κB with a selective pharmacological inhibitor (Bay 11-7082) substantially decreased NKp46 expression on rNK cells and also suppressed rNK cell cytotoxicity mediated by the NKp46 activation pathway, suggesting a role of NF-κB in the regulation of NK cell activating receptor expression and function. In line with this hypothesis, NF-κB was shown to play an important role in regulating the expression of the NK surface molecule, Ly49 in mice.30 NF-κB is one of the main targets of proteasome inhibition3,4 and the blocking effects on NF-κB function mediated by bortezomib may thus be involved in the selective down-regulation of NKp46 observed in the present study.
Cytotoxic effects of proteasome inhibition on other components of the immune system have been reported in recent years. Bortezomib treatment thus induced apoptosis and diminished expression of several activation related-cell surface molecules on activated T cells,9,10 and resulted in a remarkable loss of T and B cell precursors in mice.31 Furthermore, proteasome inhibition induced apoptosis in DC,32 resulted in decreased expression of maturation and co-stimulatory molecules on DCs and consequently inhibited DC-induced CD4+ T cell proliferation and NK cell activation.33 These findings, in conjunction with the present data, highlight that proteasome inhibition may result in multiple suppressive effects on the immune system not only through direct induction of apoptosis but also by disrupting expression of specific cell surface molecules that are important for the functions of immune-competent cells, and also suggest a clinical potential of bortezomib in prevention of graft loss in patients who have undergone organ transplantation.
In conclusion, our results demonstrate that proteasome inhibition by bortezomib induces apoptosis of human primary resting NK cells through a ROS-dependent pathway. Moreover, bortezomib treatment decreases NKp46 expression on resting human NK cells and suppresses NKp46-mediated NK cell killing of target cells, suggesting a role of bortezomib in modulation of NK cell-mediated immunity through apoptosis induction and suppression of NKp46 expression.
Acknowledgments
XF wishes to thank Prof. Yibiao Wang, the Second Hospital of Shandong University, China, for continuous encouragement and support.
Footnotes
Authorship and Disclosures
XW, AO, and XF conducted experiments and analyzed data under the supervision of CZ and BF; CJ, MN, and J-IH were involved in conceptualizing the project and contributed resources; BF was involved in the design of the project and edited the final version of the manuscript; CZ designed the project, conducted parts of the research, and wrote the manuscript.
The authors reported no potential conflicts of interest.
Funding: the study was supported by grants from the Swedish Cancer Foundation, Chinese Nature Science Foundation (Grant No. 30670903), Swedish Research Council, and the Swedish Children’s Cancer Foundation.
References
- 1.Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, et al. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42:501–10. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 2.Moretta L, Ferlazzo G, Bottino C, Vitale M, Pende D, Mingari MC, Moretta A. Effector and regulatory events during natural killer-dendritic cell interactions. Immunol Rev. 2006;214:219–28. doi: 10.1111/j.1600-065X.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- 3.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 4.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–60. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 5.Barr P, Fisher R, Friedberg J. The role of bortezomib in the treatment of lymphoma. Cancer Invest. 2007;25:766–75. doi: 10.1080/07357900701579570. [DOI] [PubMed] [Google Scholar]
- 6.Milano A, Iaffaioli RV, Caponigro F. The proteasome: a worthwhile target for the treatment of solid tumours? Eur J Cancer. 2007;43:1125–33. doi: 10.1016/j.ejca.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Lundqvist A, Abrams SI, Schrump DS, Alvarez G, Suffredini D, Berg M, et al. Bortezomib and depsipeptide sensitize tumors to tumor necrosis factor-related apoptosis-inducing ligand: a novel method to potentiate natural killer cell tumor cytotoxicity. Cancer Res. 2006;66:7317–25. doi: 10.1158/0008-5472.CAN-06-0680. [DOI] [PubMed] [Google Scholar]
- 8.Hallett WH, Ames E, Motarjemi M, Barao I, Shanker A, Tamang DL, et al. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J Immunol. 2008;180:163–70. doi: 10.4049/jimmunol.180.1.163. [DOI] [PubMed] [Google Scholar]
- 9.Berges C, Haberstock H, Fuchs D, Miltz M, Sadeghi M, Opelz G, et al. Proteasome inhibition suppresses essential immune functions of human CD4(+) T cells. Immunology. 2008;124:234–46. doi: 10.1111/j.1365-2567.2007.02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco B, Perez-Simon JA, Sanchez-Abarca LI, Carvajal-Vergara X, Mateos J, Vidriales B, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood. 2006;107:3575–83. doi: 10.1182/blood-2005-05-2118. [DOI] [PubMed] [Google Scholar]
- 11.Markasz L, Stuber G, Vanherberghen B, Flaberg E, Olah E, Carbone E, et al. Effect of frequently used chemotherapeutic drugs on the cytotoxic activity of human natural killer cells. Mol Cancer Ther. 2007;6:644–54. doi: 10.1158/1535-7163.MCT-06-0358. [DOI] [PubMed] [Google Scholar]
- 12.Marcenaro S, Gallo F, Martini S, Santoro A, Griffiths GM, Arico M, et al. Analysis of natural killer-cell function in familial hemophagocytic lymphohistiocytosis (FHL): defective CD107a surface expression heralds Munc13–4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108:2316–23. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Ottosson A, Pervaiz S, Fadeel B. Smac-mediated sensitization of human B-lymphoma cells to staurosporine- and lactacystin-triggered apoptosis is apoptosome-dependent. Leukemia. 2007;21:1035–43. doi: 10.1038/sj.leu.2404660. [DOI] [PubMed] [Google Scholar]
- 14.Jayaraman S. Flow cytometric determination of mitochondrial membrane potential changes during apoptosis of T lymphocytic and pancreatic beta cell lines: comparison of tetramethylrhodamineethylester (TMRE), chloromethyl-X-rosamine (H2-CMX-Ros) and MitoTracker Red 580 (MTR580) J Immunol Methods. 2005;306:68–79. doi: 10.1016/j.jim.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Campbell KS, Yusa S, Kikuchi-Maki A, Catina TL. NKp44 triggers NK cell activation through DAP12 association that is not influenced by a putative cytoplasmic inhibitory sequence. J Immunol. 2004;172:899–906. doi: 10.4049/jimmunol.172.2.899. [DOI] [PubMed] [Google Scholar]
- 16.Zheng C, Ostad M, Andersson M, Celsing F, Holm G, Sundblad A. Natural cytotoxicity to autologous antigen-pulsed dendritic cells in multiple myeloma. Br J Haematol. 2002;118:778–85. doi: 10.1046/j.1365-2141.2002.03712.x. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107:257–64. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 18.Pham LV, Tamayo AT, Yoshimura LC, Lo P, Ford RJ. Inhibition of constitutive NF-κ B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol. 2003;171:88–95. doi: 10.4049/jimmunol.171.1.88. [DOI] [PubMed] [Google Scholar]
- 19.Feng R, Oton A, Mapara MY, Anderson G, Belani C, Lentzsch S. The histone deacetylase inhibitor, PXD101, potentiates bortezomib-induced anti-multiple myeloma effect by induction of oxidative stress and DNA damage. Br J Haematol. 2007;139:385–97. doi: 10.1111/j.1365-2141.2007.06772.x. [DOI] [PubMed] [Google Scholar]
- 20.Ling YH, Liebes L, Zou Y, Perez-Soler R. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic response to Bortezomib, a novel proteasome inhibitor, in human H460 non-small cell lung cancer cells. J Biol Chem. 2003;278:33714–23. doi: 10.1074/jbc.M302559200. [DOI] [PubMed] [Google Scholar]
- 21.Shen L, Au WY, Guo T, Wong KY, Wong ML, Tsuchiyama J, et al. Proteasome inhibitor bortezomib-induced apoptosis in natural killer (NK)-cell leukemia and lymphoma: an in vitro and in vivo preclinical evaluation. Blood. 2007;110:469–70. doi: 10.1182/blood-2007-02-072900. [DOI] [PubMed] [Google Scholar]
- 22.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. Embo J. 2004;23:255–9. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verheyden S, Demanet C. NK cell receptors and their ligands in leukemia. Leukemia. 2008;22:249–57. doi: 10.1038/sj.leu.2405040. [DOI] [PubMed] [Google Scholar]
- 25.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura M, Mitsunaga S, Akaza T, Mitomi Y, Tadokoro K, Juji T. Protection against natural killer cells by interferon-gamma treatment of K562 cells cannot be explained by augmented major histocompatibility complex class I expression. mmunology. 1994;83:75–80. [PMC free article] [PubMed] [Google Scholar]
- 27.Arnon TI, Achdout H, Lieberman N, Gazit R, Gonen-Gross T, Katz G, et al. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004;103:664–72. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]
- 28.Bryceson YT, Rudd E, Zheng C, Edner J, Ma D, Wood SM, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–15. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi J, Tricot GJ, Garg TK, Malaviarachchi PA, Szmania SM, Kellum RE, et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood. 2008;111:1309–17. doi: 10.1182/blood-2007-03-078535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascal V, Nathan NR, Claudio E, Siebenlist U, Anderson SK. NF-κ B p50/p65 affects the frequency of Ly49 gene expression by NK cells. J Immunol. 2007;179:1751–9. doi: 10.4049/jimmunol.179.3.1751. [DOI] [PubMed] [Google Scholar]
- 31.Maseda D, Meister S, Neubert K, Herrmann M, Voll RE. Proteasome inhibition drastically but reversibly impairs murine lymphocyte development. Cell Death Differ. 2008;15:600–12. doi: 10.1038/sj.cdd.4402297. [DOI] [PubMed] [Google Scholar]
- 32.Subklewe M, Sebelin-Wulf K, Beier C, Lietz A, Mathas S, Dorken B, et al. Dendritic cell maturation stage determines susceptibility to the proteasome inhibitor bortezomib. Hum Immunol. 2007;68:147–55. doi: 10.1016/j.humimm.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Straube C, Wehner R, Wendisch M, Bornhauser M, Bachmann M, Rieber EP, et al. Bortezomib significantly impairs the immunostimulatory capacity of human myeloid blood dendritic cells. Leukemia. 2007;21:1464–71. doi: 10.1038/sj.leu.2404734. [DOI] [PubMed] [Google Scholar]