After successful treatment of malignant diseases, therapy-related myelodysplastic syndrome and acute myeloid leukemia have emerged as significant problems. This study shows that allogeneic stem cell transplantation can cure a significant portion of these patients. See related perspective article on page 542.
Keywords: allogeneic stem cell transplantation, therapy-related, myelodysplastic syndrome, acute myelogenous leukemia, cytogenetic abnormalities
Abstract
Background
After successful treatment of malignant diseases, therapy-related myelodysplastic syndrome and acute myeloid leukemia have emerged as significant problems.
Design and Methods
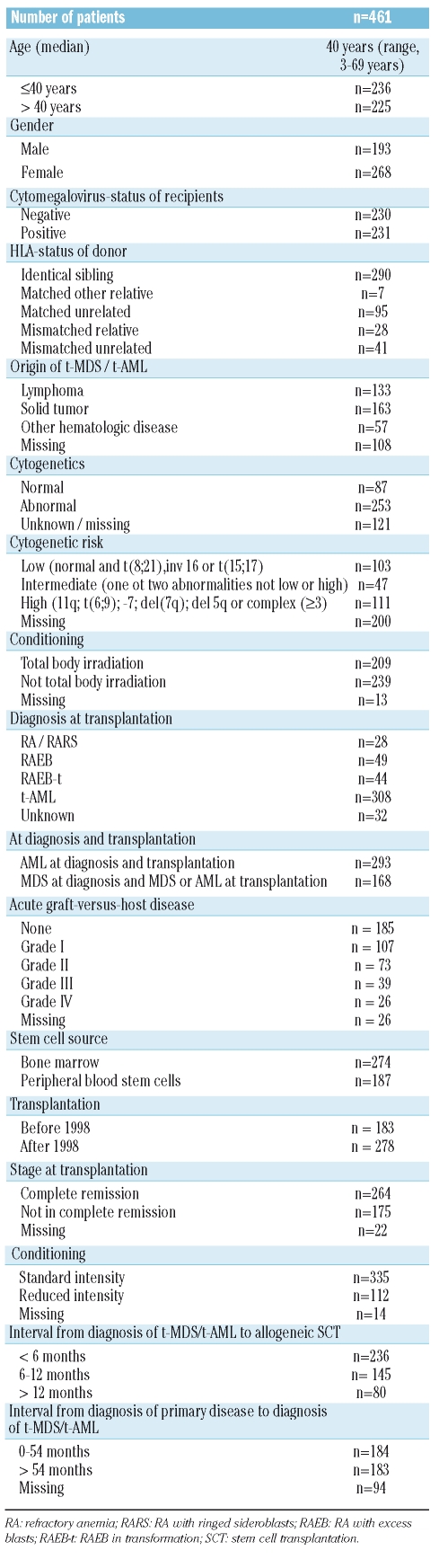
The aim of this study was to investigate outcome and risk factors in patients with therapy-related myelodysplastic syndrome or acute myeloid leukemia who underwent allogeneic stem cell transplantation. Between 1981 and 2006, 461 patients with therapy-related myelodysplastic syndrome or acute myeloid, a median age of 40 years and a history of solid tumor (n=163), malignant lymphoma (n=133), or other hematologic diseases (n=57) underwent stem cell transplantation and their data were reported to the European Group for Blood and Marrow Transplantation.
Results
The cumulative incidence of non-relapse mortality and relapse at 3 years was 37% and 31%, respectively. In a multivariate analysis significant factors for relapse were not being in complete remission at the time of transplantation (p=0.002), abnormal cytogenetics (p=0.005), higher patients’ age (p=0.03) and therapy-related myelodysplastic syndrome (p=0.04), while higher non-relapse mortality was influenced by higher patients’ age. Furthermore, there was a marked reduction in non-relapse mortality per calendar year during the study period (p<0.001). The 3-year relapse-free and overall survival rates were 33% and 35%, respectively. In a multivariate analysis significant higher overall survival rates were seen per calendar year (p<0.001), for younger age (<40 years) and normal cytogenetics (p=0.05). Using age (<40 years), abnormal cytogenetics and not being in complete remission at the time of transplantation as risk factors, three different risk groups with overall survival rates of 62%, 33% and 24% could be easily distinguished.
Conclusions
Allogeneic stem cell transplantation can cure patients with therapy-related myelodysplastic syndrome and acute myeloid leukemia and has markedly improved over time. Non-complete remission, abnormal cytogenetics and higher patients’ age are the most significant factors predicting survival.
Introduction
Chemotherapy and/or radiotherapy as part of a multimodal treatment approach in cancer patients has led to an increase in the cure rate. After successful treatment of cancer, late effects such as therapy-related myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) have emerged as significant problems.1 With the increasing cure rate for some malignancies due to intensified chemotherapies including total body irradiation and autologous stem cell support, there seems to be an increasing incidence of therapy-related MDS (t-MDS) and therapy-related AML (t-AML).2 Primary MDS, AML after MDS and t-MDS/t-AML share biological and clinical features, but t-MDS/t-AML are more rapidly progressive diseases and respond less well chemotherapy.3–6
Two different types of treatment-related leukaemia can be distinguished. The first type results from prior therapy with alkylating agents or radiation therapy and occurs after a latency period of 5 to 7 years. This type of AML is often preceded by a preleukemic period of myelodysplasia. Up to 90% of the patients with alkylating-agent-related MDS or AML show clonal chromosomal aberrations including monosomy or deletions on chromosomes 5 and/or 7 or complex aberrations involving chromosomes 3, 12, 17, and 21.7 The second type of therapy-related leukemia is induced by topoisomerase II-targeted drugs, such as etoposide, anthracyclines, and anthracenedione.8–10 This type of AML usually occurs after a median of 2 years, and is not preceded by a MDS. According to the French-American-British (FAB) classification, M4 and M5 subtypes are observed more frequently, and cytogenetic analysis shows a high frequency of rearrangements of chromosome band 11q23, t(8;21), t(15;17), inv(16) or t(8;16) as in de novo-AML.10,11 Importantly the small number of patients with t-AML who have favorable cytogenetic abnormalities, such as t(8;21), t(15;17) or inv(16), have a considerably better outcome, not markedly different from that observed in patients with de novo AML with the same abnormalities.12
In a recent analysis of autologous stem cell transplantation in 65 patients with t-AML/t-MDS of the MDS-Subcommittee of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT), 3-year overall and event-free rates of 35% and 32%, respectively, were reported. Younger age and transplantation in first complete remission were significant factors for improved survival.13 In a French series of allogeneic stem cell transplantation in patients with secondary MDS/AML, 2-year event-free and overall survival rates of 28% and 30%, respectively, were reported.14 More recently, Chang reported on 257 patients with secondary MDS or t-AML who underwent allogeneic stem cell transplantation. They found that relapse-free survival correlated significantly with disease stage and karyotype.15 The authors compared the results in patients with secondary MDS/t-AML with those in patients with de novo-MDS/secondary AML and found no differences in outcome after adjustment for diseases status and cytogenetics, suggesting that outcome is determined mainly by disease characteristics rather than by etiology. Other studies indicated that cytogenetic abnormalities represent a major prognostic factor for outcome of patients with t-MDS;16 after adjustment for cytogenetic risk factors, no major difference between de novo- and t-MDS could be observed following allogeneic stem cell transplantation.17
In order to shed light on these issues, we conducted an analysis of the outcome and risk factors in patients with t-MDS/t-AML who underwent allogeneic stem cell transplatation.
Design and Methods
Between 1981 and 2006, 461 patients with t-MDS or t-AML underwent first allogeneic stem cell transplantation, and their data were reported to the EBMT registry. The median age of the patients was 40 years (range, 3–69), and their primary disease was a solid tumor (n=163), malignant lymphoma (n=133), or other hematologic disease (n=57). For those patients with information about treatment of primary disease (n=108) this treatment consisted of chemotherapy (50%), chemotherapy plus radiotherapy (32%), only radiotherapy (10%) or high-dose chemotherapy followed by autologous stem cell support (7%). The time from primary diagnosis to t-MDS/t-AML was 54 months (range, 1–417 months) and from t-MDS/t-AML to allogeneic stem cell transplantation 6 months (range, 1–278 months). The number of older patients increased over time: between 1981 and 1989 only 3% of the patients were older than 50 years of age, between 1990 and 1999 this population increased to 18% and between 2000 and 2006 39% of the patients were older than 50 years of age. t-AML without preceding MDS was noted in 293 patients, whereas 168 patients had MDS at diagnosis. Cytogenetic data were available for 340 patients, who could be divided into having normal cytogenetics (n=83) or abnormal cytogenetics (n=253).
The median follow-up of the surviving patients is 21 months (range, 1–177). The patients’ characteristics are shown in Table 1.
Table 1.
Patients’ characteristics.
Statistical analyses
For the Kaplan-Meier curves (used in univariate descriptions) and Cox models (used to estimate hazard ratios, [HS]) the relapsed patients were censored for transplant-related mortality at the time of relapse and vice versa. Univariate comparison of Kaplan-Meier curves was performed using the two-tailed log-rank test. For ordered categorical variables, the trend version of the log-rank test was used. The association of various risk factors such as age, remission status at transplantation, stem cell source, time from primary diagnosis to diagnosis of t-MDS/t-AML, type of treatment-related disease, presence of cytogenetic abnormalities, and total body irradiation-containing conditioning with the outcomes (overall survival, relapse-free survival, relapse incidence and transplant-related mortality) was quantified using the hazard ratios estimated in the Cox models.
Relapse incidence and therapy-related mortality were calculated using cumulative incidence estimates. Calculations were performed with SPSS version 12.0. The cumulative incidences were calculated with SPSS 14.0 using a macro developed at the Department of Medical Statistics (LUMC).
Results
Non-relapse mortality
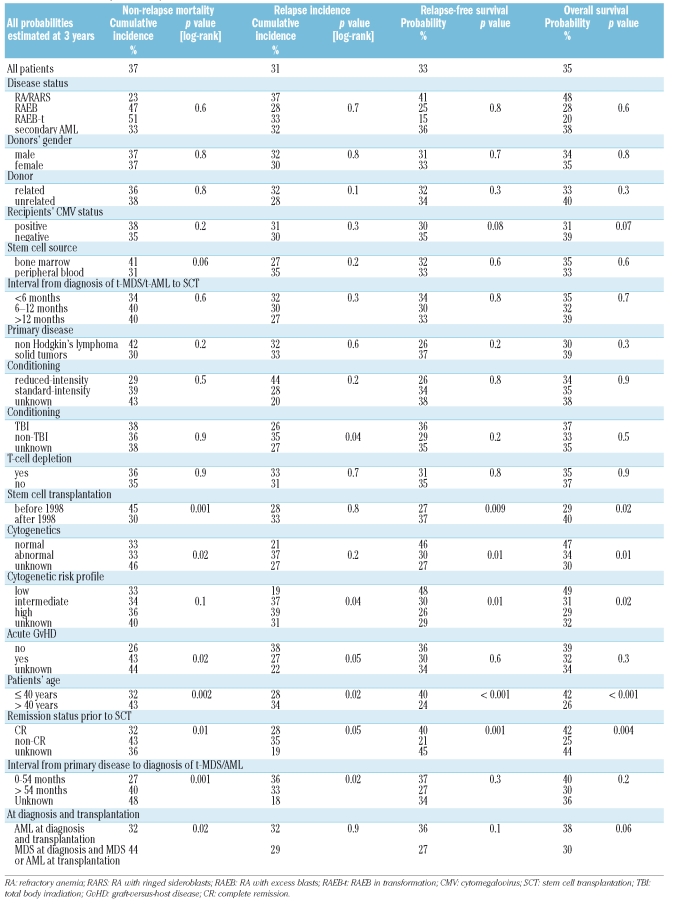
The non-relapse mortality rate at 1 year was 32%, and at 3 years 37%. Significant factors for higher non-relapse mortality were transplantation before 1998 (p=0.001), occurrence of acute graft-versus-host disease (p=0.02), patient’s age (>40 years; p=0.002), not being in complete remission at the time of transplantation (p=0.01), interval of more than 54 months from diagnosis of primary disease to diagnosis of t-MDS/t-AML (p=0.001) and t-MDS at diagnosis (in comparison to t-AML at diagnosis) (p=0.02) (Table 2).
Table 2.
Univariate analysis of relapse-free and overall survival.
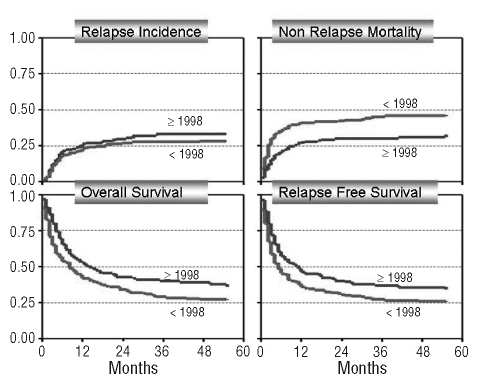
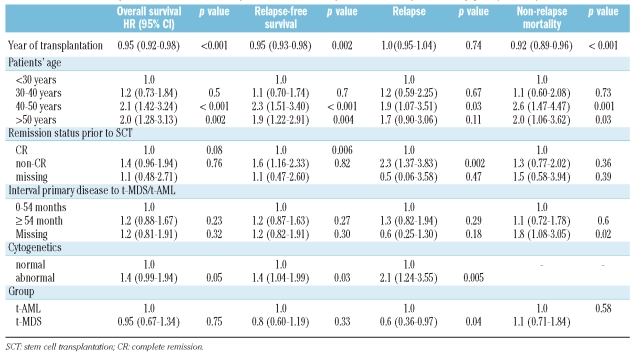
In a multivariate analysis, a continuous improvement per year in non-relapse mortality was noted [HR: 0.92; 95% confidence interval (CI): 0.89–0.96, p<0.001] (Figure 1). The age of the patient also significantly influenced non-relapse mortality: 40–50 years (HR: 2.6; 95% CI: 1.47–4.47, p=0.001) vs. over 50 years (HR: 2.0; 95% CI: 1.06–3.62, p=0.03). An unknown time-interval from diagnosis of primary disease to diagnosis of t-MDS/t-AML was significant in the multivariate analysis (HR: 1.8; 95% CI: 1.08–3.05, p=0.02) (Table 3).
Figure 1.
Overall survival, relapse-free survival, non-relapse mortality and relapse incidence for patients with t-MDS/t-AML by category of transplant year: before 1998 (<1998; n=183), 1998 onwards (≥1998; n=278).
Table 3.
Multivariate analysis of overall survival, relapse-free survival, relapse and non-relapse mortality [HR: (95% CI:).
Relapse
The cumulative incidence of relapse at 3 years was 31%. Significant factors for a higher relapse rate at 3 years were intermediate and high-risk cytogenetics (p=0.04), non-occurrence of acute graft-versus-host disease (p=0.05), not being in complete remission at the time of transplantation (p=0.05), a conditioning regimen not cintaining total body irradiation (p=0.04), an interval of less than 54 months between primary diagnosis and diagnosis of t-MDS/t-AML (p=0.02), and patient’s age greater than 40 years (p=0.02) (Table 3).
In a multivariate analysis, abnormal cytogenetics (HR: 2.1; 95% CI: 1.24–3.55, p=0.005), not being in complete remission at the time of transplantation (HR: 2.3; 95% CI: 1.37–3.83, p=0.002), age 40–50 years (HR: 1.9; 95% CI: 1.07–3.51, p=0.03) and t-MDS (HR: 0.6; 95% CI: 0.36–0.99, p=0.04) were the independent significant factors for predicting the incidence of relapse (Table 3).
Relapse-free survival
The estimated 3-year relapse-free survival was 33%. In the univariate analysis, significant factors for an improved 3-year relapse-free survival rate were stem cell transplantation after 1998 (p=0.009), normal or low-risk cytogenetics (p=0.01), patient’s age under 40 years (p<0.001), and stem cell transplantation in complete remission (p=0.001). (Table 3). In a multivariate analysis, year of transplantation, as a continuous variable, was highly significant for improved relapse-free survival (HR: 0.95; 95% CI: 0.93–0.98, p=0.002). Further independent factors for an improved relapse-free survival in multivariate analysis were abnormal cytogenetics (HR: 1.4; 95% CI: 1.04–1.99, p=0.03), not being in complete remission at the time of transplantation (HR: 1.6; 95% CI: 1.16–2.33, p=0.006), patients’ age of 40–50 years (HR: 2.3; 95% CI: 1.51–3.40, p<0.001) and over 50 years old (HR: 1.9; 95% CI: 1.22–2.91, p=0.004) (Table 3).
Figure 2.
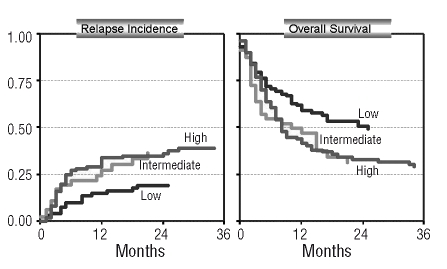
Relapse incidence (A) and overall survival (B) in 261 patients with known cytogenetics classified as low (n=103), intermediate (n=47), and high risk (n=111), (for risk categories see text) (competing risk model).
Overall survival
The estimated overall survival at 3 years was 35%. Significant factors for improved overall survival at 3 years were stem cell transplantation after 1998 (p=0.02), patient’s age less than 40 years (p<0.001), being in complete remission at the time of transplantation (p=0.004) and low-risk cytogenetics (p=0.02). A trend for improved survival at 3 years was seen in patients with t-AML without preceding MDS (p=0.06), and in patients who were cytomegalovirus-negative (p=0.07) (Table 3). In multivariate analysis, a marked improvement in survival was observed over time (per calendar year) (HR: 0.95; 95% CI: 0.92–0.98, p<0.001). Independent factors for impaired survival in the multivariate analysis were age 40–50 years (HR: 2.1; 95% CI: 1.42–3.24, p<0.001), and greater than 50 years (HR: 2.0; 95% CI: 1.28–3.13, p=0.002) and abnormal cytogenetics (HR: 1.4; 95% CI: 0.99–1.94, p=0.05), (Table 3).
Cytogenetic subanalysis
In 261 out of 340 patients with available cytogenetic data a detailed cytogenetic sub-analysis could be performed. This subgroup of patients was divided into a low-risk group (n= 103) who had either (t(8;21), inv t(16), t(15;17), (n=16) or a normal karyotype (n=87), an intermediate risk group (n=47) (who had one or two abnormalities), and a high-risk group (n=111) who had 11q23, t(6;9), -7, del (7q), del (5q), or complex (≥3) abnormalities.
In this analysis, the cumulative incidence of relapse at 3 years was 19% for low-risk patients, 37% for intermediate risk patients, and 39% for high-risk patients (p=0.04), while the non-relapse mortality did not differ at 3 years (33%, 34%, 36% respectively; p=0.1) resulting in an improved relapse-free and overall survival at 3 years for the low-risk group in comparison to the intermediate and high-risk patients [48% vs. 30% vs. 26% (p=0.01) and 49% vs. 31% vs. 29% (p=0.02)]. (Figure 3).
Figure 3.
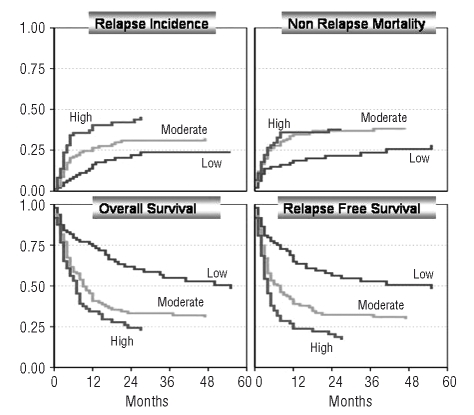
Outcome (overall survival, relapse-free survival, non-relapse mortality and relapse incidence) according to the simplified risk score model including age, complete remission and abnormal cytogenetics (for details see text)(competing risk model).
Risk score
To develop a simple risk score for patients with t-MDS/t-AML, we used the significant factors age, stage, and cytogenetics. This risk score was based on a series of Cox proportional hazards models. First, for each of four possible outcomes (overall survival, relapse-free survival, non-relapse mortality and relapse), using a stepwise backwards approach, a model was fitted eliminating non-significant factors among age, stage, cytogenetics, interval treatment-diagnosis, total body irradiation and disease. After comparing the four resulting models, four new models were fitted each containing those factors that were significant in at least one of the stepwise-models. This ensures that the same factors are used for all outcomes. For each outcome the coefficients were compared among the predictors in one model. The goal was to obtain a simple-to-use system of penalty-points by rounding the coefficients to integers without a specific statistical underlying criterion for optimality. The three factors age, stage and cytogenetics were found to have the highest weights and were taken into account in the four reduced models, using integer coefficients only. The relative weights (effects) of age and stage were different depending on the outcome modeled, as described below; hence a compromise is needed if one wishes to use just one set of penalty points for all four outcomes. For overall survival and non-relapse mortality, points were given as follows: age over 40 years: +2; not in complete remission: +1; and abnormal cytogenetics: +1. For relapse were given: age over 40 years: +2; not in complete remission: +2; and abnormal cytogenetics: +2. For relapse-free survival the points asigned were: age over 40 years: +2; not in complete remission: +2; and abnormal cytogenetics: +1. Patients were classified into groups at low-risk, moderate-risk and high-risk as follows: overall survival and non-relapse-mortality (low: 0, 1; moderate: 2; high: 3, 4); relapse (low: 0, 2; moderate: 4; high: 6); relapse-free survival (low: 0, 1; moderate: 2, 3, high: 4, 5). This risk model enables a clear classification into the following risk groups: overall survival at 2 years: low: n=109 (60%); moderate: n=78 (39%) and high: n=150 (25%)(p<0.001); relapse-free survival at 2 years: low: n=146 (58%); moderate: n=220 (32%) and high: n=95 (20%) (p<0.001); non-relapse mortality at 2 years: low: n=109 (20%); moderate: n=78 (39%) and high: n=150 (39%) (p=0.001); relapse at two years: low: n=143 (23%); moderate: n=137 (28%) and high: n=57 (52%) (p<0.001). To simplify this model, we used identical risk scores for all outcome variables (overall survival, relapse-free survival, non-relapse mortality, and relapse). For this optimized score, we used the relapse-free survival score because it affects both non-relapse mortality as well as relapse. This simplified score also allows a clear separation of risk categories regarding overall survival at 2 years: low: n=96 (62%); moderate: n=168 (33%) and high: n=73 (24%) (p<0.001); relapse-free survival at 2 years: low: n=96 (58%); moderate: n=168 (32%) and high: n=73 (20%) (p<0.001); non-relapse mortality at 2 years: low: n=96 (22%); moderate: n= 168 (37%) and high: n=73 (38%) (p=0.001); relapse at 2 years: low: n=96 (20%); moderate: n=168 (31%) and high: n=73 (42%) (p<0.001) (Figure 3).
Discussion
This large study of EBMT registry data shows that allogeneic stem cell transplantation is a curative therapy option for patients with t-MDS or t-AML resulting in 3-year event-free and overall survival rates of 33% and 35%, respectively. In an earlier study of the EBMT a 3-year-probability of disease-free survival of 35% was reported for patients transplanted for t-MDS/t-AML (n=67) from HLA-identical siblings, which did not differ significantly from the patients transplanted for primary MDS (37%, n=712) or secondary AML (32%, n=106).18 For 70 patients with t-MDS/t-AML the French Society of Bone Marrow Transplantation reported a 28% event-free survival at 2 years after allogeneic stem cell transplantation from related and unrelated donors.14 In a small single-center study including 18 patients with MDS, the disease-free survival after allogeneic stem cell transplantation was only 24% for patients with t-MDS in comparison to 43% for those with primary MDS.19 Several co-operative AML study groups reported a worse overall survival for patients with t-AML in comparison to those with de novo AML. An analysis of the Medical Research Council (MRC)20 found a significantly shorter overall survival for patients with t-AML (30% vs. 44%), which was in line with the results of the German AML Cooperative group, which reported a median survival of 10 months for patients with t-AML in comparison to 15 months for those with de novo AML (p=0.001).21
A recent update highlighted the impact of karyotype aberrations in patients with t-AML, since t-AML patients with favorable karyotype had a significantly better median survival than patients with an unfavorable karyotype (26.7 vs. 5.6 months; p=0.02).22 This is in line with two studies comparing t-MDS and de novo MDS. Neither study found a significant difference in outcome after allogeneic stem cell transplantation, when both groups were adjusted for disease status and cytogenetic abnormalities.15,17 The importance of cytogenetic abnormalities in t-MDS/t-AML is also highlighted in our analysis, and patients with t-MDS/t-AML and abnormal intermediate or high risk cytogenetics had a significantly higher risk of relapse (HR: 2.1) and reduced event-free survival (HR: 1.4) in multivariate analysis. In a subanalysis of 261 patients for whom complete cytogenetic data were available and who could be classified as being at low, intermediate, or high risk, those patients with low risk cytogenetics had a lower incidence of relapse, resulting in improved event-free and overall survival.
A major finding of the study was the importance of calendar year of transplantation on survival, which was due to a marked reduction in non-relapse mortality over time. This might be due in large part to improvements in unrelated transplants which exceeded 50% in older studies;23,24 however, in the present study the non-relapse mortality of patients transplanted from unrelated donors did not differ from that of patients undergoing related transplantation. Besides the marked improvement over time, age, disease group, and remission status were independent risk factors for overall and event-free survival, which were also found for de novo MDS and secondary AML.18 The median age of the patients was 40 years and age was an independent risk factor for survival. However, the number of older patients increased over time: between 1981 and 1989 only 3% of the patients were older than 50 years of age, between 1990 and 1999 this population increased to 18% and between 2000 and 2006 39% of the patients were older than 50 years of age. Despite this increase of older patients there was still a significant improvement in survival in recent years.
In contrast to the findings of an EBMT study, dose-reduced conditioning was not associated with significant differences compared to standard conditioning.25 Since cytogenetic data were not complete, we were not able to distinguish between alkylating- and topoisomerase II-inhibitor-induced t-MDS or t-AML, which might have an impact on outcome. We, therefore, tried to separate these forms of t-MDS/t-AML by grouping patients with AML at diagnosis and MDS at diagnosis and by using a cut-off of 54 months between diagnosis of the primary disease and diagnosis of t-MDS/t-AML. For patients with AML at diagnosis, which is suggestive of topoisomerase II-inhibitor-induced t-AML, a significantly higher incidence of relapse was found in the multivariate analysis, although this could be due to patients with AML not in complete remission at tha time of transplantation.
To develop a simple risk score for patients with t-MDS/t-AML who underwent allogeneic stem cell transplantation we included the main risk factors of the multivariate analysis: age (>40 years), remission status (not complete), and abnormal cytogenetic features [excluding: inv(16), t(8;21), and t(15;17)]. This model enabled a clear separation of the patients into three risk groups (low, moderate and high), predicting non-relapse mortality, incidence of relapse as well as relapse-free and overall survival. This model highlighted that t-MDS/t-AML per se is not associated with a bad prognosis, but that the outcome depends heavily on age, remission status prior to stem cell transplantation, and cytogenetic abnormalities. For example, a patient aged less than 40 years in complete remission and without cytogenetic abnormalities who undergoes allogeneic stem cell transplantation from a related or unrelated donor has an estimated 2-year overall survival of 62%, and even if this patient has a cytogenetic abnormality or is not in complete remission at the time of transplantation, the estimated 2-year overall survival is still 62%. This low-risk category constituted 28% of the study population. In contrast, a patient aged more than 40 years with abnormal cytogenetics [excluding: inv(16), t(8;21) and t(15;17)] who is not in complete remission at the time of transplantation has a estimated 2-year overall survival of only 24%. This high-risk group constituted 22% of the study population. If the patient is aged more than 40 years, has normal cytogenetics and is in complete remission at the time of transplantation (moderate risk), the 3-year probability of overall survival increases to 33%.
We conclude that allogeneic stem cell transplantation for patients with t-MDS/t-AML improved per calendar year due to a marked reduction in non-relapse mortality. Not being in complete remission at the time of transplantation, abnormal intermediate or high risk cytogenetics and higher age of the patients are the most significant factors for survival, and are data that can be easily used in a risk model that predicts outcome.
Supplementary Material
Footnotes
We thank the physicians mentioned in the appendix for referring their patients' data to the EBMT Chronic Leukaemia Working Party registry
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
NK designed the study, analyzed and interpreted data and wrote the manuscript. AvB and RB analyzed and interpreted data; AZ, DN, AD, TR, JC, PL, AG, CC, DB, EC, AS contributed patients’ data and discussed data; JD gave cytogenetic advice; TdW analyzed and interpreted data.
The authors reported no potential conflicts of interest.
References
- 1.Levine EG, Bloomfield CD. Leukemias and myelodysplastic syndromes secondary to drug, radiation, and environmental exposure. Semin Oncol. 1992;19:47–84. [PubMed] [Google Scholar]
- 2.Milligan DW, Ruiz De Elvira MC, Kolb HJ, Goldstone AH, Meloni G, Rohatiner AZ, et al. Secondary leukaemia and myelodysplasia after autografting for lymphoma: results from the EBMT. EBMT Lymphoma and Late Effects Working Parties. European Group for Blood and Marrow Transplantation. Br J Haematol. 1999;106:1020–6. doi: 10.1046/j.1365-2141.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen-Bjergaard J, Philip P, Larsen SO, Jensen G, Byrsting K. Chromosome aberrations and prognostic factors in therapy-related myelodysplasia and acute nonlymphocytic leukemia. Blood. 1990;76:1083–91. [PubMed] [Google Scholar]
- 4.Picozzi VJ, Jr, Swanson GF, Morgan R, Hecht F, Greenberg PL. 13-cis retinoic acid treatment for myelodysplastic syndromes. J Clin Oncol. 1986;4:589–95. doi: 10.1200/JCO.1986.4.4.589. [DOI] [PubMed] [Google Scholar]
- 5.Ballen KK, Antin JH. Treatment of therapy-related acute myelogenous leukemia and myelodysplastic syndromes. Hematol Oncol Clin North Am. 1993;7:477–93. [PubMed] [Google Scholar]
- 6.Kantarjian HM, Estey EH, Keating MJ. Treatment of therapy-related leukemia and myelodysplastic syndrome. Hematol Oncol Clin North Am. 1993;7:81–107. [PubMed] [Google Scholar]
- 7.Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, et al. Clinical cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102:43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 8.Linassier C, Barin C, Calais G, Letortorec S, Bremond JL, Delain M, et al. Early secondary acute myelogenous leukemia in breast cancer patients after treatment with mitoxantrone, cyclophosphamide, fluorouracil and radiation therapy. Ann Oncol. 2000;11:1289–94. doi: 10.1023/a:1008375016038. [DOI] [PubMed] [Google Scholar]
- 9.Kollmannsberger C, Hartmann JT, Kanz L, Bokemeyer C. Risk of secondary myeloid leukemia and myelodysplastic syndrome following standard-dose chemotherapy or high-dose chemotherapy with stem cell support in patients with potentially curable malignancies. J Cancer Res Clin Oncol. 1998;124:207–14. doi: 10.1007/s004320050156. [DOI] [PubMed] [Google Scholar]
- 10.Felix CA. Secondary leukemias induced by topoisomerase-targeted drugs. Biochim Biophys Acta. 1998;1400:233–55. doi: 10.1016/s0167-4781(98)00139-0. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen-Bjergaard J, Andersen MK, Johansson B. Balanced chromosome aberrations in leukemias following chemotherapy with DNA-topoisomerase II inhibitors. J Clin Oncol. 1998;16:1897–8. doi: 10.1200/JCO.1998.16.5.1897. [DOI] [PubMed] [Google Scholar]
- 12.Quesnel B, Kantarjian H, Bjergaard JP, Brault P, Estey E, Lai JL, et al. Therapy-related acute myeloid leukemia with t(8;21), inv(16), and t(8;16): a report on 25 cases and review of the literature. J Clin Oncol. 1993;11:2370–9. doi: 10.1200/JCO.1993.11.12.2370. [DOI] [PubMed] [Google Scholar]
- 13.Kröger N, Brand R, van Biezen A, Cahn JY, Slavin S, Blaise D, et al. Myelodysplastic Syndromes Subcommittee of The Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Autologous stem cell transplantation for therapy-related acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2006;37:183–9. doi: 10.1038/sj.bmt.1705226. [DOI] [PubMed] [Google Scholar]
- 14.Yakoub-Agha I, de La SP, Ribaud P, Sutton L, Wattel E, Kuentz M, et al. Allogeneic bone marrow transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia: a long-term study of 70 patients - report of the French society of bone marrow transplantation. J Clin Oncol. 2000;18:963–71. doi: 10.1200/JCO.2000.18.5.963. [DOI] [PubMed] [Google Scholar]
- 15.Chang C, Storer BE, Scott BL, Bryant EM, Shulman HM, Flowers ME, et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood. 2007;110:1379–87. doi: 10.1182/blood-2007-02-076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh ZN, Huo D, Anastasi J, Smith SM, Karrison T, Le Beau MM, et al. Therapy-related myelodysplastic syndrome: morphologic subclassification may not be clinically relevant. Am J Clin Pathol. 2007;127:197–205. doi: 10.1309/NQ3PMV4U8YV39JWJ. [DOI] [PubMed] [Google Scholar]
- 17.Armand P, Kim HT, DeAngelo DJ, Ho VT, Cutler CS, Stone RM, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13:655–64. doi: 10.1016/j.bbmt.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Witte T, Hermans J, Vossen J, Bacigalupo A, Meloni G, Jacobsen N, et al. Haematopoietic stem cell transplantation for patients with myelodysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2000;110:620–30. doi: 10.1046/j.1365-2141.2000.02200.x. [DOI] [PubMed] [Google Scholar]
- 19.Ballen KK, Gilliland DG, Guinan EC, Hsieh CC, Parsons SK, Rimm IJ, et al. Bone marrow transplantation for therapy-related myelodysplasia: comparison with primary myelodysplasia. Bone Marrow Transplant. 1997;20:737–43. doi: 10.1038/sj.bmt.1700971. [DOI] [PubMed] [Google Scholar]
- 20.Goldstone AH, Burnett AK, Avivi I, et al. Secondary acute myeloid leukemia has a worse outcome in MRC trials. Blood. 2002;100:88a . [Abstract 322] [Google Scholar]
- 21.Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18:120–5. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- 22.Kern W, Haferlach T, Schnittger S, Hiddemann W, Schoch C. Prognosis in therapy-related acute myeloid leukemia and impact of karyotype. J Clin Oncol. 2004;22:2510–1. doi: 10.1200/JCO.2004.99.301. [DOI] [PubMed] [Google Scholar]
- 23.Arnold R, de Witte T, van Biezen A, Hermans J, Jacobsen N, Runde V, et al. Unrelated bone marrow transplantation in patients with myelodysplastic syndromes and secondary acute myeloid leukemia: an EBMT survey. European Blood and Marrow Transplantation Group. Bone Marrow Transplant. 1998;21:1213–6. doi: 10.1038/sj.bmt.1701269. [DOI] [PubMed] [Google Scholar]
- 24.Castro-Malaspina H, Harris RE, Gajewski J, Ramsay N, Collins R, Dharan B, et al. Unrelated donor marrow transplantation for myelodysplastic syndromes: outcome analysis in 510 transplants facilitated by the National Marrow Donor Program. Blood. 2002;99:1943–51. doi: 10.1182/blood.v99.6.1943. [DOI] [PubMed] [Google Scholar]
- 25.Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J, et al. Myelodysplastic Syndrome Subcommittee of the Chronic Leukemia Working Party of the European Blood and Marrow Transplantation Group. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–46. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.