Abstract
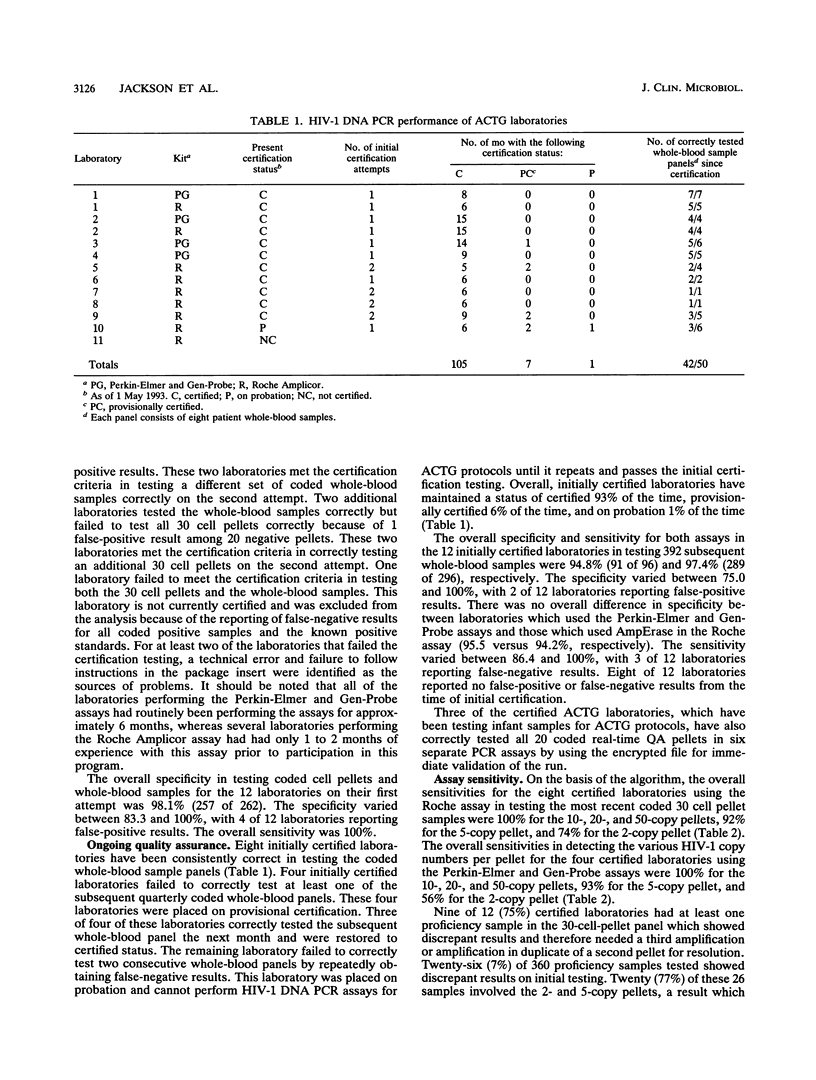
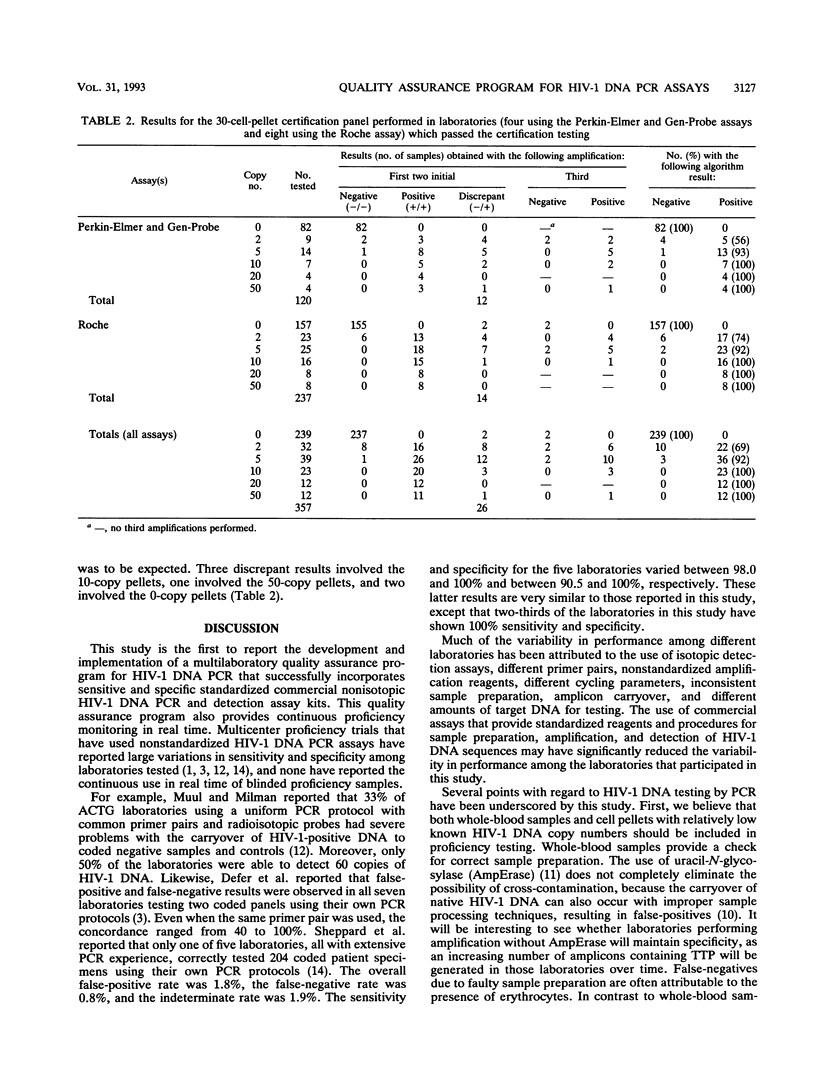
An independent quality assurance program has been established by the Virology Committee of the AIDS Clinical Trials Group in the Division of AIDS, National Institute of Allergy and Infectious Diseases, for monitoring polymerase chain reaction (PCR) assays for human immunodeficiency virus type 1 (HIV-1) DNA that are performed by 11 laboratories participating in multicenter clinical trials in the United States. To perform HIV-1 DNA PCR for patients in AIDS Clinical Trials Group protocols, each laboratory was initially certified by correctly testing a coded certification panel consisting of eight well-defined clinical whole-blood specimens and 30 cell pellets containing 0, 2, 5, 10, 20, or 50 8E5/LAV cells per 125,000 uninfected peripheral blood mononuclear cells. PCR was performed by one of two standardized commercial assays for amplification and nonisotopic detection of HIV-1 proviral DNA. For continuing certification, each laboratory must correctly test eight coded whole-blood samples per quarter and run three or four coded cell pellets and HIV-1 DNA copy standards with every PCR assay in real time. The PCR results for the coded pellets on each run are entered into an encrypted computer file, which immediately assesses the validity of the run. To date, 10 of 11 laboratories have correctly tested all HIV-1-positive and -negative samples in the initial certification panel on their first or second attempt. Subsequently, 9 of these 11 laboratories have continued to maintain their certified status. The use of commercial HIV-1 DNA PCR assays and an external quality assurance program have ensured that results from different laboratories are comparable and that problems with sensitivity and specificity are quickly identified.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busch M. P., Henrard D. R., Hewlett I. K., Mehaffey W. F., Epstein J. S., Allain J. P., Lee T. H., Mosley J. W. Poor sensitivity, specificity, and reproducibility of detection of HIV-1 DNA in serum by polymerase chain reaction. The Transfusion Safety Study Group. J Acquir Immune Defic Syndr. 1992;5(9):872–877. [PubMed] [Google Scholar]
- Comeau A. M., Hsu H. W., Schwerzler M., Mushinsky G., Grady G. F. Detection of HIV in specimens from newborn screening programs. N Engl J Med. 1992 Jun 18;326(25):1703–1703. doi: 10.1056/NEJM199206183262515. [DOI] [PubMed] [Google Scholar]
- De Rossi A., Ometto L., Mammano F., Zanotto C., Giaquinto C., Chieco-Bianchi L. Vertical transmission of HIV-1: lack of detectable virus in peripheral blood cells of infected children at birth. AIDS. 1992 Oct;6(10):1117–1120. [PubMed] [Google Scholar]
- Defer C., Agut H., Garbarg-Chenon A., Moncany M., Morinet F., Vignon D., Mariotti M., Lefrère J. J. Multicentre quality control of polymerase chain reaction for detection of HIV DNA. AIDS. 1992 Jul;6(7):659–663. doi: 10.1097/00002030-199207000-00007. [DOI] [PubMed] [Google Scholar]
- Folks T. M., Powell D., Lightfoote M., Koenig S., Fauci A. S., Benn S., Rabson A., Daugherty D., Gendelman H. E., Hoggan M. D. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med. 1986 Jul 1;164(1):280–290. doi: 10.1084/jem.164.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger F. B., Bremer J. W., Myers L. E., Gold J. W., McQuay L. Standardization of sensitive human immunodeficiency virus coculture procedures and establishment of a multicenter quality assurance program for the AIDS Clinical Trials Group. The NIH/NIAID/DAIDS/ACTG Virology Laboratories. J Clin Microbiol. 1992 Jul;30(7):1787–1794. doi: 10.1128/jcm.30.7.1787-1794.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. B., Ndugwa C., Mmiro F., Kataaha P., Guay L., Dragon E. A., Goldfarb J., Olness K. Non-isotopic polymerase chain reaction methods for the detection of HIV-1 in Ugandan mothers and infants. AIDS. 1991 Dec;5(12):1463–1467. doi: 10.1097/00002030-199112000-00008. [DOI] [PubMed] [Google Scholar]
- Krivine A., Firtion G., Cao L., Francoual C., Henrion R., Lebon P. HIV replication during the first weeks of life. Lancet. 1992 May 16;339(8803):1187–1189. doi: 10.1016/0140-6736(92)91131-q. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Longo M. C., Berninger M. S., Hartley J. L. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene. 1990 Sep 1;93(1):125–128. doi: 10.1016/0378-1119(90)90145-h. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., McDonough S. H., Cabanas D., Ryder T. B., Harper M., Moore J., Schochetman G. Rapid and quantitative detection of enzymatically amplified HIV-1 DNA using chemiluminescent oligonucleotide probes. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1323–1329. doi: 10.1089/aid.1990.6.1323. [DOI] [PubMed] [Google Scholar]
- Sheppard H. W., Ascher M. S., Busch M. P., Sohmer P. R., Stanley M., Luce M. C., Chimera J. A., Madej R., Rodgers G. C., Lynch C. A multicenter proficiency trial of gene amplification (PCR) for the detection of HIV-1. J Acquir Immune Defic Syndr. 1991;4(3):277–283. [PubMed] [Google Scholar]
- Whetsell A. J., Drew J. B., Milman G., Hoff R., Dragon E. A., Adler K., Hui J., Otto P., Gupta P., Farzadegan H. Comparison of three nonradioisotopic polymerase chain reaction-based methods for detection of human immunodeficiency virus type 1. J Clin Microbiol. 1992 Apr;30(4):845–853. doi: 10.1128/jcm.30.4.845-853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



