JAK2 (V617F) and various exon 12 mutations are mplicated in the pathogenesis of myeloproliferative neoplasms. It is known that JAK2 (V617F) causes phosphorylation of SOCS3, and the authors demonstrate that this is also true for the JAK2 exon 12 mutants. Data are presented that propose SOCS3 tyrosine phosphorylation as a novel bio-marker of JAK2 mutation positive myeloproliferative neoplasms.
Keywords: myeloproliferative neoplasms, JAK2, V617F, exon 12, SOCS3
Abstract
JAK2 V617F, identified in the majority of patients with myeloproliferative neoplasms, tyrosine phosphorylates SOCS3 and escapes its inhibition. Here, we demonstrate that the JAK2 exon 12 mutants described in a subset of V617F-negative MPN cases, also stabilize tyrosine phosphorylated SOCS3. SOCS3 tyrosine phosphorylation was also observed in peripheral blood mononuclear cells and granulocytes isolated from patients with JAK2 H538QK539L or JAK2 F537-K539delinsL mutations. JAK kinase inhibitors, which effectively inhibited the proliferation of cells expressing V617F or K539L, also caused a dose-dependent reduction in both mutant JAK2 and SOCS3 tyrosine phosphorylation. We propose, therefore, that SOCS3 tyrosine phosphorylation may be a novel bio-marker of myeloproliferative neoplasms resulting from a JAK2 mutation and a potential reporter of effective JAK2 inhibitor therapy currently in clinical development.
Introduction
Myeloproliferative neoplasms (MPNs) like polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF), are clonal stem cell disorders attributed to a somatic mutation in Janus kinase (JAK) 2, resulting in a valine-to-phenylalanine substitution at position 617 (V617F).1–5 Recently, other mutations within JAK2 have been identified in patients with V617F-negative PV.6–8 Like JAK2 V617F, these exon 12 mutations render JAK2 constitutively active and confer cytokine-independent growth in vitro.6 Suppressor of cytokine signaling (SOCS) 3 is a negative regulator of the erythropoietin receptor (EPOR) and the receptor-associated JAK2 kinase. SOCS3 contains a SOCS-box region at the C-terminus allowing it to form a multi-subunit E3 ligase complex consisting of ElonginBC, Cullin5 and Rbx1.9,10 In doing so, SOCS3 can target associated proteins for ubiquitination and degradation.11–14 We have reported that, in contrast to wild-type JAK2, JAK2 V617F cannot be regulated by SOCS3. Furthermore, turnover of SOCS3 is inhibited by JAK2 V617F and this correlates with marked tyrosine phosphorylation of SOCS3 protein.15 The aim of this study was to establish if SOCS3 was stabilized and phosphorylated by the other MPN-associated mutant JAK2 kinases. Our observations suggest that SOCS3 tyrosine phosphorylation may be a novel MPN bio-marker and a potential mechanism enabling the mutant JAK2 kinases to overcome SOCS3 inhibition.
Design and Methods
Constructs, reagents and transfections
293T cells were transfected using calcium phosphate.16 FLAG-tagged SOCS3 and JAK2 were expressed in pME18S and pRK respectively. JAK2 mutations were created in pRK-JAK2 and pMXI-JAK2-IRES-GFP using the QuikChange® site-directed mutagenesis kit (Stratagene, CA, USA). BaF3 cells were retrovirally infected with supernatants containing EPOR and JAK2, V617F or K539L expressed in pMXI-IRES-CD2 and pMXI-IRES-GFP, respectively.17 Prof. Terry Lappin (CCRCB, QUB) provided erythropoietin (EPO). AG490 and JAK inhibitor 1 were obtained from Calbiochem.
Immunoprecipitations and Western Blotting
293T cells were maintained and lysed as described.16 Lysates were immunoprecipitated with anti-JAK2 (Upstate Biotechnology, NY, USA) or anti-SOCS3 (clone 008) (Fusion Antibodies, Belfast). Western blots were probed with anti-phosphotyrosine (clone 4G10) (Upstate Biotechnology), anti-Flag (M2) or anti-γ-tubulin (Sigma Aldrich, UK), anti-JAK2 or anti-SOCS3.
Patient samples
Peripheral blood mononuclear cells (PBMCs) and granulocytes were isolated1,15 and analyzed as described15 from healthy donors or MPN patients who gave informed consent in accordance with the Declaration of Helsinki. Approval was obtained from the Research Ethics Committees, Northern Ireland and Addenbrooke’s National Health Service Trust Research Ethics Committee.
Proliferation assays
BaF3-EPOR cell populations were seeded in triplicate at 3 × 104 per mL in the presence or absence of 5 u/mL EPO. Viable cells were counted in quadruplicate by try-pan exclusion at 24h, 48h and 72h. Statistical analysis was carried out using a Student’s t test.
Results and Discussion
JAK2 V617F is resistant to the inhibitory effects of SOCS3.15 In the presence of EPOR, wild-type JAK2 induced a loss of SOCS3 protein while JAK2 V617F tyrosine phosphorylated and stabilized both endogenous and exogenous SOCS3 (Figure 1A). Recently, a number of mutations within JAK2 (including JAK2 K539L, JAK2 N542-E543del, JAK2 H538QK539L and JAK2 F537-K539delinsL)6–8 have been identified in a subset of V617F-negative MPN patients. We wished to determine whether these mutations also induced SOCS3 tyrosine phosphorylation. Co-expression studies of SOCS3 and wild-type or mutant JAK2 were performed in 293T cells, in the presence of the EPOR.18 Compared to wild-type JAK2, expression of K539L or N542-E543del stabilized SOCS3 protein, as did JAK2 V617F (Figure 1B). Additionally, endogenous and exogenous SOCS3 was tyrosine phosphorylated in the presence of these MPN-associated mutant JAK2 kinases (Figure 1A and 1B). Similar results were obtained when JAK2 H538QK539L and JAK2 F537-K539delinsL were tested (Figure 1C). We next investigated if SOCS3 tyrosine phosphorylation was observed in JAK2 V617F-negative MPN patients. PBMCs and granulocytes were isolated from a healthy individual and 2 patients, with either H538QK539L or F537-K539delinsL mutations. Protein concentration was estimated and equalized for each sample. Compared with controls, both MPN patients showed greater levels of tyrosine phosphorylated SOCS3 in both PBMCs and granulocytes, while SOCS3 protein levels were similar (Figure 1D). We next investigated whether SOCS3 tyrosine phosphorylation is specific to the MPN-associated V617F or exon 12 mutations in JAK2. To investigate this we determined whether other activating mutations in JAK2 resulted in stabilization of tyrosine phosphorylated SOCS3. To do this we created the JAK2 T875N mutation identified in an acute megakaryoblastic leukemia cell line and expressed this mutation in the presence of SOCS3. As before, V617F caused stabilization of tyrosine phosphorylated SOCS3, while both wild-type JAK2 and JAK2 T875N failed to do so in the absence (Figure 1E, left hand panels) or presence (Figure 1E, right hand panels) of EPO stimulation. These data demonstrated that SOCS3 tyrosine phosphorylation may be a useful bio-marker specific to the MPN-associated V167F or exon 12 mutations.
Figure 1.
SOCS3 tyrosine phosphorylation by the MPN-associated mutant JAK2 kinases. (A) 293T cells were transfected with cDNA encoding EPOR with JAK2 or JAK2 V617F both in the presence and absence of FLAG-SOCS3. Whole cell lysates (WCLs) were immunoblotted with anti-JAK2 (top panel), anti-FLAG (panel 2) to detect SOCS3 or anti-γ-tubulin (panel 3). The remaining lysates were immunoprecipitated with anti-SOCS3 and immunoblotted with anti-phosphotyrosine (pY) (bottom panel). (B) 293T cells were transfected with EPOR and either JAK2, JAK2 V617F, JAK2 K539L or JAK2 N542-E543del in the presence and absence of FLAG-SOCS3. Lysates were analyzed as in (A). (C) 293T cells were transfected with cDNA encoding EPOR with JAK2 wild-type, H538QK539L or F537-K539delinsL in the absence and presence of SOCS3. Lysates were analyzed as in (A). (D) PBMCs and granulocytes were isolated from a control patient or MPN patients with a JAK2 H538QK539L mutation (patient 1) or a F537-K539delinsL mutation (patient 2). Lysates were immunoprecipitated with anti-SOCS3 and immunoblotted with anti-pY (top panel) and reprobed with anti-SOCS3 (middle panel). WCLs were also immunoblotted with anti-γ-tubulin (bottom panel). (E) 293T cells were transfected with cDNA encoding EPOR, SOCS3 and either JAK2 wild-type, JAK2 V617F or JAK2 T875N. Cells were left untreated (left hand panels) or treated with EPO (50 u/mL) for 15min prior to lysis (right hand panels). Lysates were analyzed as in (A).
We next established if SOCS3 could regulate the phosphorylation of the exon 12 JAK2 mutants. Following EPO stimulation, SOCS3 potently inhibited wild-type JAK2 phosphorylation, with a concomitant reduction in SOCS3 protein levels, while SOCS3 was unable to significantly down-modulate mutant JAK2 phosphorylation and in all cases SOCS3 protein was stabilized (Figure 2). This may indicate that tyrosine phosphorylation of SOCS3 abrogated its ability to negatively regulate JAK2 K539L, H538QK539L, or F537-K539delinsL.
Figure 2.
SOCS3 cannot down modulate phosphorylation of V617F or the exon 12 mutants. 293T cells were transfected with EPOR and either wild-type JAK2, V617F, K539L, H538QK539L or F537-K539delinsL mutation alone or in the presence of SOCS3. Cells were treated with EPO (50 u/mL) for 15 min prior to lysis. Whole cell lysates (WCLs) were immunoprecipitated with anti-JAK2 and immunoblotted with anti-phosphotyrosine (pY) (top panel) and reprobed with anti-JAK2 (panel 2). WCLs were immunoblotted with anti-FLAG to detect SOCS3 (panel 3) or antiγ-tubulin (bottom panel).
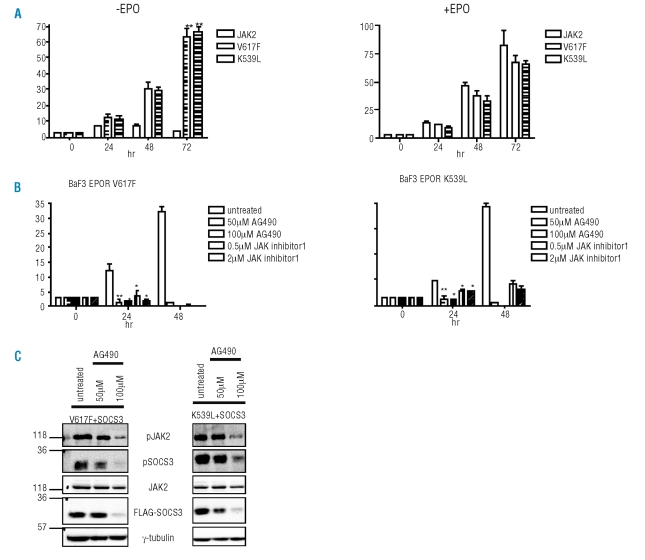
To investigate if SOCS3 could be a novel bio-marker of MPN, we analyzed the effect of JAK inhibitors on SOCS3 tyrosine phosphorylation. Firstly, we created stable BaF3 cells lines expressing EPOR along with wild-type JAK2, V617F or K539L. Trypan exclusion counts confirmed that although BaF3 cells expressing wild-type JAK2 were unable to proliferate in the absence of EPO, cells expressing V617F or K539L continued to proliferate (Figure 3A). Next we investigated the effects of AG490 or JAK inhibitor 1 on the proliferation of BaF3 cells expressing V617F and K539L in the absence of EPO stimulation. Both AG490 and JAK inhibitor 1 severely impaired the proliferation of BaF3 cells expressing V617F or K539L in a dose-dependent manner (Figure 3B). Since AG490 gave the most pronounced growth inhibitory effects, we investigated the impact of AG490 on SOCS3 tyrosine phosphorylation (Figure 3C). 293T cells expressing EPOR and SOCS3 along with V617F (Figure 3C, left panels) or K539L (Figure 3C, right panels) were treated with AG490 for 24h. AG490 caused a dose-dependent reduction in SOCS3 tyrosine phosphorylation correlating with a reduction in mutant JAK2 phosphorylation. These findings suggest that SOCS3 tyrosine phosphorylation mirrors JAK2 activity and may highlight SOCS3 tyrosine phosphorylation as a marker of MPN and a potential therapeutic read-out of JAK2 inhibitor therapy currently in clinical trials.
Figure 3.
Effect of JAK inhibitors on mutant JAK2-induced proliferation and SOCS3 tyrosine phosphorylation. (A) BaF3 cells expressing EPOR along with wild-type JAK2, V617F or K539L were seeded at 3 × 104 cells/mL in triplicate. Proliferation was measured in the absence or presence of EPO (5 u/mL) by tyrpan blue exclusion over 72 hr. Samples were taken in quadruplicate and statistical analysis was carried out using a Student’s t test. **p<0.01. Error bars represent standard deviation. (B) BaF3-EPOR cells expressing V617F or K539L were seeded at 3 × 104 cells/mL in triplicate in the absence of EPO stimulation. Cells were untreated or treated with AG490 (50 μM or 100 μM) or JAK inhibitor 1 (0.5 μM or 2 μM). Proliferation was measured over 48hr by trypan exclusion. **p<0.01 *p<0.05. Error bars represent standard deviation. Statistical analysis was carried out using a Student’s t test. (C) 293T cells were transfected with EPOR, SOCS3 and either V617F (left panels) or K539L (right panels). Cells were untreated or treated with AG490 (50μM or 100 μM) for 24hr prior to lysis. Whole cell lysates (WCLs) were immunoprecipitated with anti-JAK2 (top panels) or anti-SOCS3 (panel 2) and immunoblotted with anti-phosphotyrosine (pY). JAK2, FLAG-0SOCS3 and γ-tubulin protein levels are shown in panels 3, 4 and 5 respectively.
SOCS3 tyrosine phosphorylation may also hint at a mechanism by which these mutant JAK2 kinases cause transformation. The inhibitory function of SOCS3 is attributed to the C-terminal SOCS-box which enables assembly of a multi-subunit E3 ligase complex.9,10 We have previously reported that SOCS3 is tyrosine phosphorylated in response to many cytokines and that tyrosine phosphorylation of SOCS3 in vitro prevents the SOCS3-ElonginC binding.19–21 Constitutive SOCS3 tyrosine phosphorylation may prevent the SOCS3-ElonginC interaction thereby inhibiting SOCS3 E3 ligase activity. In other words, the MPN-associated mutant JAK2 kinases may constitutively phosphorylate SOCS3 and could indicate a mechanism by which the MPN-associated JAK2 mutations may induce transformation. Regulation of SOCS proteins by phosphorylation is not restricted to SOCS3, since v-ABL-mediated phosphorylation of SOCS1 disrupts the interaction with the ElonginBC complex, thus stabilizing SOCS1 and blocking JAK degradation.22,23 If the MPN-associated mutants bypass the suppressive function of SOCS3 by inhibiting E3 ligase recruitment through tyrosine phosphorylation, SOCS3 may not only be a suitable bio-marker of MPN but may be a potential therapeutic target of MPN arising from these JAK2 mutations.
Footnotes
Authorship and Disclosures
JE designed, performed research and wrote the paper. YS, LMS and KN performed research, ARG and MFM provided patient samples and advised on research. SC provided vital reagents and contributed to the manuscript preparation. JAJ advised on research and contributed to manuscript preparation.
The authors reported no potential conflicts of interest.
Funding: JE, YS, KN and JAJ are supported by the Health Research Board Ireland, the Leukaemia Research Fund, Marie Curie and the Research and Development Office at HPSS.
References
- 1.Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 2.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 3.Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 4.Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7:387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280:22788–92. doi: 10.1074/jbc.C500138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–68. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pietra D, Li S, Brisci A, Passamonti F, Rumi E, Theocharides A, et al. Somatic mutations of JAK2 exon 12 in patients with JAK2 (V617F)-negative myeloproliferative disorders. Blood. 2007;111:1686–9. doi: 10.1182/blood-2007-07-101576. [DOI] [PubMed] [Google Scholar]
- 8.Percy MJ, Scott LM, Erber WN, Harrison CN, Reilly JT, Jones FG, et al. The frequency of JAK2 exon 12 mutations in idiopathic erythrocytosis patients with low serum erythropoietin levels. Haematologica. 2007;92:1607–14. doi: 10.3324/haematol.11643. [DOI] [PubMed] [Google Scholar]
- 9.Kamura T, Sato S, Haque D, Liu L, Kaelin WG, Jr, Conaway RC, et al. The Elongin BC complex interacts with the conserved SOCS-box motif present in members of the SOCS, ras, WD-40 repeat, and ankyrin repeat families. Genes Dev. 1998;12:3872–81. doi: 10.1101/gad.12.24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamura T, Maenaka K, Kotoshiba S, Matsumoto M, Kohda D, Conaway RC, et al. VHL-box and SOCS-box domains determine binding specificity for Cul2-Rbx1 and Cul5-Rbx2 modules of ubiquitin ligases. Genes Dev. 2004;18:3055–65. doi: 10.1101/gad.1252404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Sepulveda P, Ilangumaran S, Rottapel R. Suppressor of cytokine signaling-1 inhibits VAV function through protein degradation. J Biol Chem. 2000;275:14005–8. doi: 10.1074/jbc.c000106200. [DOI] [PubMed] [Google Scholar]
- 12.Kawazoe Y, Naka T, Fujimoto M, Kohzaki H, Morita Y, Narazaki M, et al. Signal transducer and activator of transcription (STAT)-induced STAT inhibitor 1 (SSI-1)/suppressor of cytokine signaling 1 (SOCS1) inhibits insulin signal transduction pathway through modulating insulin receptor substrate 1 (IRS-1) phosphorylation. J Exp Med. 2001;193:263–9. doi: 10.1084/jem.193.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–8. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 14.Ungureanu D, Saharinen P, Junttila I, Hilton DJ, Silvennoinen O. Regulation of Jak2 through the ubiquitin-proteasome pathway involves phosphorylation of Jak2 on Y1007 and interaction with SOCS-1. Mol Cell Biol. 2002;22:3316–26. doi: 10.1128/MCB.22.10.3316-3326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hookham MB, Elliott J, Suessmuth Y, Staerk J, Ward AC, Vainchenker W, et al. The myeloproliferative disorder-associated JAK2 V617F mutant escapes negative regulation by suppressor of cytokine signaling 3. Blood. 2007;109:4924–9. doi: 10.1182/blood-2006-08-039735. [DOI] [PubMed] [Google Scholar]
- 16.Elliott J, Lynch OT, Suessmuth Y, Qian P, Boyd CR, Burrows JF, et al. Respiratory syncytial virus NS1 protein degrades STAT2 by using the Elongin-Cullin E3 ligase. J Virol. 2007;81:3428–36. doi: 10.1128/JVI.02303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tannahill GM, Elliott J, Barry AC, Hibbert L, Cacalano NA, Johnston JA. SOCS2 can enhance interleukin-2 (IL-2) and IL-3 signaling by accelerating SOCS3 degradation. Mol Cell Biol. 2005;25:9115–26. doi: 10.1128/MCB.25.20.9115-9126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, et al. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F-mediated transformation. Proc Natl Acad Sci USA. 2005;102:18962–7. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cacalano NA, Sanden D, Johnston JA. Tyrosine-phosphorylated SOCS-3 inhibits STAT activation but binds to p120 RasGAP and activates Ras. Nat Cell Biol. 2001;3:460–5. doi: 10.1038/35074525. [DOI] [PubMed] [Google Scholar]
- 20.Sommer U, Schmid C, Sobota RM, Lehmann U, Stevenson NJ, Johnston JA, et al. Mechanisms of SOCS3 phosphorylation upon interleukin-6 stimulation. Contributions of Src-and receptor-tyrosine kinases. J Biol Chem. 2005;280:31478–88. doi: 10.1074/jbc.M506008200. [DOI] [PubMed] [Google Scholar]
- 21.Haan S, Ferguson P, Sommer U, Hiremath M, McVicar DW, Heinrich PC, et al. Tyrosine phosphorylation disrupts elongin interaction and accelerates SOCS3 degradation. J Biol Chem. 2003;278:31972–9. doi: 10.1074/jbc.M303170200. [DOI] [PubMed] [Google Scholar]
- 22.Limnander A, Danial NN, Rothman PB. v-Abl signaling disrupts SOCS-1 function in transformed pre-B cells. Mol Cell. 2004;15:329–41. doi: 10.1016/j.molcel.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 23.Chen JL, Limnander A, Rothman PB. Pim-1 and Pim-2 kinases are required for efficient pre-B-cell transformation by v-Abl oncogene. Blood. 2008;111:1677–85. doi: 10.1182/blood-2007-04-083808. [DOI] [PubMed] [Google Scholar]