(ɛγδβ)°-thalassemia is a very rare form of thalassemia only recognized in heterozygotes and caused by unique and sporadic deletions.1–3 The α/non α–globin chain ratio is imbalanced like in β thalassemia patients but without elevation of HbF or HbA2.4 They are characterized by a moderately severe neonatal hemolytic anemia with hypochromia but they distinguish from β-thalassemic patients because they may need red blood cell (RBC) transfusions for the first six months of life.2,3 The phenotype usually improves spontaneously during the first year of life in correlation with the progressive increase of β-globin chain production. As in other β-thalassemic conditions, 5,6 the α-globin genotype may modify the phenotype of (ɛγδβ)°-thalassemia. We report, for the first time, a novel (ɛγδβ)°-thalassemia deletion, associated with an alpha globin gene triplication, leading to an undescribed fetal thalassemic syndrome responsible for hydrops foetalis syndrome requiring multiple intra uterine RBC transfusions.
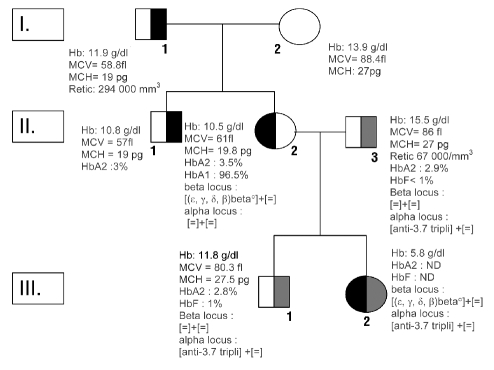
The probant’s mother (II. 2, Figure 1) was a 28-year-old Caucasian. She was transfused at birth because of a neonatal hemolysis with hypochromia. In adulthood her erythrocyte phenotype was: Hb: 10.7 g/dL; MCV: 61.0 fL; MCHC: 31 g/dL; MCH: 19.7 pg. During her first pregnancy, she presented with progressive worsening of her anemia (Hb: 5.8 g/dL) requiring RBC transfusions. The diagnosis of an (ɛ,γ,δ,β)°thal, removing 100 kb between the 3’ of HS2 5’LCR region and the 5’ of β-globin 3’, was made after delivery by DNA analysis (Figure 2). Before her second pregnancy, Hb value was 10.5 g/dL and the husband’s erythrocyte phenotype and electrophoresis of hemoglobin were normal (case II.3, Figure 1). During the first trimester of her second pregnancy, the mother was fine and the Hb value above 8.5/dL. The embryonic echography was normal. At week 20 of gestational age, the mother’s Hb decreased to 8.3 g/dL without aggravating factors for anemia besides the (ɛ,γ,δ,β)°thal. The fetal echography at week 20 of gestational age revealed a very severe life-threatening hydrops fetalis related to severe anemia the fetus’s Hb determined on umbilical cord blood sample was: 5.8g/dL. There were no other etiologies of hydrops fetalis (viral infection, immunization, organ dysfunction, autoimmunity). Genotyping of the globin loci revealed that the fetus had inherited the mother’s (ɛ,γ,δ,β)° thal and an anti -3.7 α-triplication from the father (Figure 1). Two intrauterine RBC transfusions were performed at weeks 22 and 29. Hydrops did not recur and growth was satisfactory. The baby was born by caesarean section at 35 weeks and was well without hepatosplenomegaly; his Hb was 9.3 g/dL, reticulocyte count 356 106/L, bilirubin 40 mg/L. RBC transfusions have been necessary every three weeks to maintain Hb value above 9 g/dL, the baby now being four months old.
Figure 1.
Family pedigree and hematologic data. Individuals indicated by half-black shaded symbol presented neonatal hemolytic anemia and microcytosis and those with a half grey shade symbol presented anti-3.7 triplication α gene. Erythrocyte parameters of patient III2 were determined during fetal life.
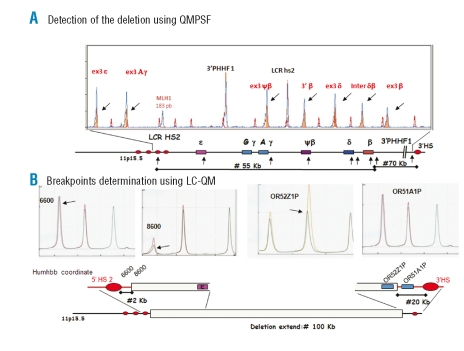
Figure 2.
(A) QMPSF. The screening of deletion is done by a multiplex semi-quantitative PCR (sQ-PCR) with a set of 9 primers located on various positions covering the extent of the β-globin gene cluster, from the 5’ locus control region (5’LCR) to a region approximately 90 kb 3’ of the β-gene. The MLh1 gene is used as internal controls. Fluorescent chromatograms from PCR product obtained with patient and normal control are compared after normalization using MLh1. Deleted markers have a pic value roughly half of that of pic in normal control.7 (B) LC-QM. In order to narrow the breakpoint location as much as possible, successive triplex sQ-PCR are tested using the same approach as QMPSF but with a DHPLC device.8 Displayed chromatograms show the closest markers in both sides of the breakpoint. HUMHBB co-ordinates refer to the position of the sense primer set used in LC-QM steps within the β-globin cluster sequence (genbank ac code: HUMHBB). “6600” is located 5’ to the 5’HS2 and 8600 is located 2kb downstream. ORxxxxx refers to olfactory receptor genes located down-stream from β-globin cluster. Settings are available upon request.
The severe hydrops foetalis was due in this case to inheritance of an (ɛγδβ)°-thalassemia and an anti -3.7 α-globin gene triplication. Typically, patients heterozygous for an (ɛγδβ)°-thalassemia deletion alone exhibit at birth a hypochromic anemia with various degrees of hemolysis. Blood transfusion in the neonatal period is sometimes necessary in (ɛγδβ)°-thalassemia like in the probant’s mother’s case II.2.2,3 However contrasting phenotypes have been reported in one family.1,9 The occurrence of early manifestations in this case is explained by the increasing imbalance between the αand non α-globin chains ratio during fetal life. In our case III.2, the association with the triplicated α-genes increased the imbalanced α/non α-globin ratio which explains the “fetal thalassemia intermedia” requiring blood transfusions during the intra-uterine period. Thalassemia intermedia is characterized by an unstable thalassemic erythropoiesis needing transfusion when an erytroid stress occurs. In our case, it is very likely that the transition from embryonic to fetal erythropoiesis was the “erythroid stress” causing a greater sensitivity to the effect of globin imbalance and the hydrops foetalis. Intra uterine transfusion in (ɛγδβ)°-thalassemia related to the presence of such a deletion has been previously cited twice; it could be hypothesized that triplication alpha might also be involved in these cases (α-triplication not evaluated).2,3 The frequency of α-triplication varies according to population origin10 and cannot be identified with routine parameters, as attested by the father’s normal phenotype (II.3).4 However the impact of such an association is extremely important and could threaten the fetus’s life.
Our observation emphasizes the absolute necessity of systematically looking at α-gene status in partners of (ɛγδβ)°-thalassemia carriers before conception considering the severity of such an association. Careful follow-up of both the fetus and the mother, if carrier of the (ɛγδβ)°-thalassemia deletion, is mandatory.
References
- 1.Furuya C, Yamashiro Y, Hattori Y, Hino M, Nishioka H, Shimizu Y, et al. A novel epsilon gamma delta beta thalassemia of 1.4 Mb deletion found in a Japanese patient. Am J Hematol. 2008;83:84–6. doi: 10.1002/ajh.21040. [DOI] [PubMed] [Google Scholar]
- 2.Game L, Bergounioux J, Close JP, Marzouka BE, Thein SL. A novel deletion causing (epsilon gamma delta beta) degrees thalassaemia in a Chilean family. Br J Haematol. 2003;123:154–9. doi: 10.1046/j.1365-2141.2003.04564.x. [DOI] [PubMed] [Google Scholar]
- 3.Harteveld CL, Osborne CS, Peters M, van der Werf S, Plug R, Fraser P, et al. Novel 112 kb (ɛγδ) δβ-thalassaemia deletion in a Dutch family. Br J Haematol. 2003;122:855–8. doi: 10.1046/j.1365-2141.2003.04505.x. [DOI] [PubMed] [Google Scholar]
- 4.Giambona A, Passarello C, Vinciguerra M, Li Muli R, Teresi P, Anza M, et al. Significance of borderline hemoglobin A2 values in an Italian population with a high prevalence of β-thalassemia. Haematologica. 2008;93:1380–4. doi: 10.3324/haematol.12840. [DOI] [PubMed] [Google Scholar]
- 5.Rund D, Rachmilewitz E. β-thalassemia. N Engl J Med. 2005;353:1135–46. doi: 10.1056/NEJMra050436. [DOI] [PubMed] [Google Scholar]
- 6.Thein SL. Genetic modifiers of the β-haemoglobinopathies. Br J Haematol. 2008;141:357–66. doi: 10.1111/j.1365-2141.2008.07084.x. [DOI] [PubMed] [Google Scholar]
- 7.Casilli F, Di Rocco ZC, Gad S, Tournier I, Stoppa-Lyonnet D, Frebourg T, et al. Rapid detection of novel BRCA1 rearrangements in high-risk breast-ovarian cancer families using multiplex PCR of short fluorescent fragments. Hum Mutat. 2002;20:218–26. doi: 10.1002/humu.10108. [DOI] [PubMed] [Google Scholar]
- 8.Houdayer C, Gauthier-Villars M, Lauge A, Pages-Berhouet S, Dehainault C, Caux-Moncoutier V, et al. Comprehensive screening for constitutional RB1 mutations by DHPLC and QMPSF. Hum Mutat. 2004;23:193– 202. doi: 10.1002/humu.10303. [DOI] [PubMed] [Google Scholar]
- 9.Trent RJ, Williams BG, Kearney A, Wilkinson T, Harris PC. Molecular and hematologic characterization of Scottish- Irish type (ɛγδβ)0 thalassemia. Blood. 1990;76:2132–8. [PubMed] [Google Scholar]
- 10.Goossens M, Dozy AM, Embury SH, Zachariades Z, Hadjiminas MG, Stamatoyannopoulos G, et al. Triplicated α-globin loci in humans. Proc Natl Acad Sci USA. 1980;77:518–21. doi: 10.1073/pnas.77.1.518. [DOI] [PMC free article] [PubMed] [Google Scholar]