Abstract
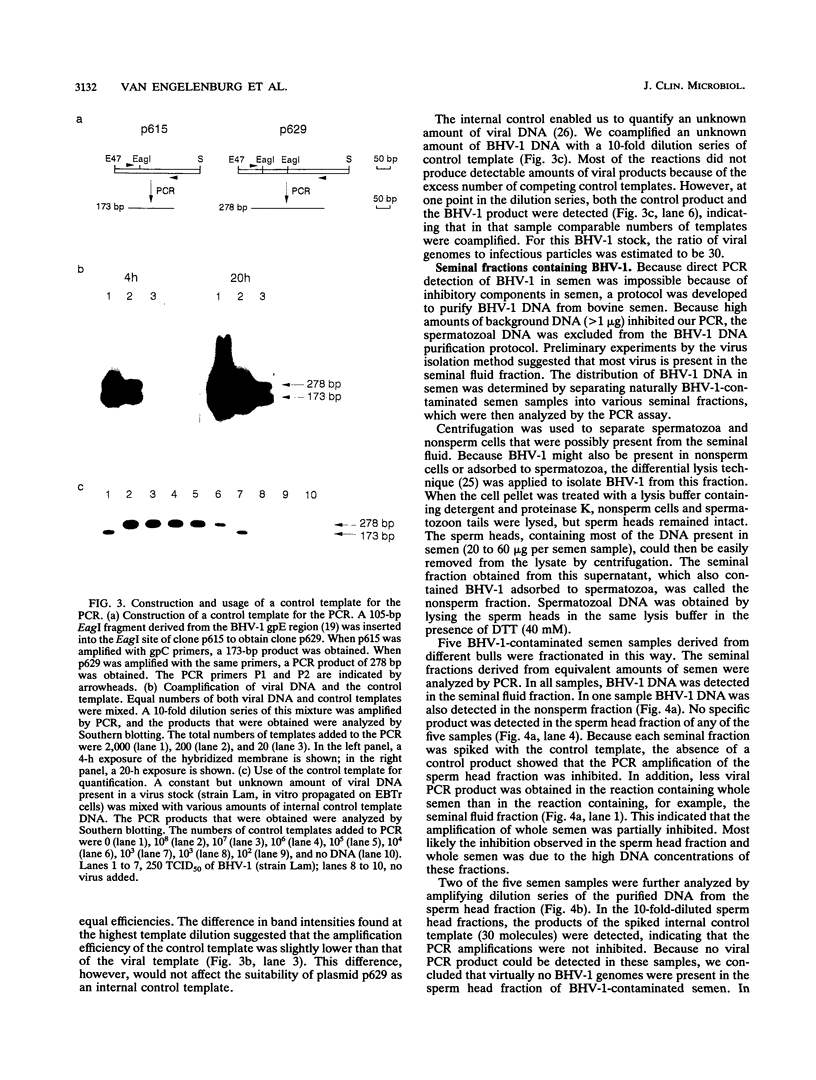
We developed a polymerase chain reaction (PCR) assay to detect bovine herpesvirus type 1 (BHV-1) in bovine semen. Since bovine semen contains components that inhibit PCR amplification, a protocol was developed to purify BHV-1 DNA from bovine semen. To identify failures of PCR amplification, we used an internal control template that was coamplified by the same PCR primers. When separated fractions of BHV-1-contaminated semen were analyzed by the PCR, we found that more than 90% of the BHV-1 DNA was present in a pooled fraction consisting of seminal fluid, nonsperm cells, and virus adsorbed to spermatozoa. By using this fraction, three to five molecules of BHV-1 DNA in 50 microliters of bovine semen could be detected. A pilot study to compare this PCR assay with the routinely used virus isolation method showed that this PCR assay is 2- to 100-fold more sensitive. In addition, the results of the PCR assay are available in 1 day, whereas the virus isolation method takes 7 days. Therefore, the PCR assay may be a good alternative to the virus isolation method.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Wyler R. The DNA of an IPV strain of bovid herpesvirus 1 in sacral ganglia during latency after intravaginal infection. Vet Microbiol. 1984 Feb;9(1):53–63. doi: 10.1016/0378-1135(84)90078-6. [DOI] [PubMed] [Google Scholar]
- Bielanski A., Loewen K. G., Hare W. C. Inactivation of bovine herpesvirus-1 (BHV-I) from in vitro infected bovine semen. Theriogenology. 1988 Oct;30(4):649–657. doi: 10.1016/0093-691x(88)90300-7. [DOI] [PubMed] [Google Scholar]
- Cone R. W., Hobson A. C., Huang M. L. Coamplified positive control detects inhibition of polymerase chain reactions. J Clin Microbiol. 1992 Dec;30(12):3185–3189. doi: 10.1128/jcm.30.12.3185-3189.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aquila R. T., Bechtel L. J., Videler J. A., Eron J. J., Gorczyca P., Kaplan J. C. Maximizing sensitivity and specificity of PCR by pre-amplification heating. Nucleic Acids Res. 1991 Jul 11;19(13):3749–3749. doi: 10.1093/nar/19.13.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennett D. P., Barasa J. O., Johnson R. H. Infectious bovine rhinotracheitis virus: studies on the venereal carrier status in range cattle. Res Vet Sci. 1976 Jan;20(1):77–83. [PubMed] [Google Scholar]
- Elazhary M. A., Lamothe P., Silim A., Roy R. S. Bovine herpesvirus type 1 in the sperm of a bull from a herd with fertility problems. Can Vet J. 1980 Dec;21(12):336–339. [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D. R., Babiuk L. A., Zamb T. J. Nucleotide sequence of bovine herpesvirus type 1 glycoprotein gIII, a structural model for gIII as a new member of the immunoglobulin superfamily, and implications for the homologous glycoproteins of other herpesviruses. Virology. 1989 Nov;173(1):46–57. doi: 10.1016/0042-6822(89)90220-1. [DOI] [PubMed] [Google Scholar]
- Kasza L., Shadduck J. A., Christofinis G. J. Establishment, viral susceptibility and biological characteristics of a swine kidney cell line SK-6. Res Vet Sci. 1972 Jan;13(1):46–51. [PubMed] [Google Scholar]
- Kupferschmied H. U., Kihm U., Bachmann P., Müller K. H., Ackermann M. Transmission of IBR/IPV virus in bovine semen: A case report. Theriogenology. 1986 Mar;25(3):439–443. doi: 10.1016/0093-691x(86)90052-x. [DOI] [PubMed] [Google Scholar]
- Liang X. P., Babiuk L. A., van Drunen Littel-van den Hurk S., Fitzpatrick D. R., Zamb T. J. Bovine herpesvirus 1 attachment to permissive cells is mediated by its major glycoproteins gI, gIII, and gIV. J Virol. 1991 Mar;65(3):1124–1132. doi: 10.1128/jvi.65.3.1124-1132.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loparev V. N., Cartas M. A., Monken C. E., Velpandi A., Srinivasan A. An efficient and simple method of DNA extraction from whole blood and cell lines to identify infectious agents. J Virol Methods. 1991 Sep;34(1):105–112. doi: 10.1016/0166-0934(91)90126-k. [DOI] [PubMed] [Google Scholar]
- Lowe T., Sharefkin J., Yang S. Q., Dieffenbach C. W. A computer program for selection of oligonucleotide primers for polymerase chain reactions. Nucleic Acids Res. 1990 Apr 11;18(7):1757–1761. doi: 10.1093/nar/18.7.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Van Dyke M. W. A rapid method for the purification of deprotected oligodeoxynucleotides. Nucleic Acids Res. 1991 Feb 11;19(3):674–674. doi: 10.1093/nar/19.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oirschot J. T., Straver P. J., van Lieshout J. A., Quak J., Westenbrink F., van Exsel A. C. A subclinical infection of bulls with bovine herpesvirus type 1 at an artificial insemination centre. Vet Rec. 1993 Jan 9;132(2):32–35. doi: 10.1136/vr.132.2.32. [DOI] [PubMed] [Google Scholar]